Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.107639
Revised: May 26, 2025
Accepted: July 14, 2025
Published online: August 26, 2025
Processing time: 147 Days and 11.8 Hours
Inflammatory bowel disease (IBD), consisting primarily of ulcerative colitis and Crohn’s disease, is a chronic, relapsing inflammatory disorder of the gastro
Core Tip: Inflammatory bowel disease (IBD) is a chronic and relapsing inflammatory disorder of the gastrointestinal tract that severely compromises the quality of life of patients. Intestinal stem cell proliferation and differentiation underlie damaged mucosa repair. However, mucosal healing - a key IBD therapeutic target - remains elusive with current treatments. Emerging stem cell therapy promotes mucosal barrier restoration, offering IBD intervention. This review examines intestinal stem cell roles in IBD pathogenesis and discusses specifically engineered stem cell-based therapies for management.
- Citation: Zhang MJ, Chan SX, Jia ZG, Lv C, Chen JJ, Hong SC. Roles of intestinal stem cells in inflammatory bowel disease pathogenesis. World J Stem Cells 2025; 17(8): 107639
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/107639.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.107639
Inflammatory bowel disease (IBD) is a group of recurrent, chronic, non-specific inflammatory diseases of the bowel, including Crohn’s disease (CD) and ulcerative colitis (UC)[1,2]. An increasing number of patients are found not only in Western countries but also in newly industrialized regions such as Asia[3]. IBD is clinically characterized by diarrhea, mucous and bloody stools, abdominal pain. Many extraintestinal manifestations occur in patients with IBD commonly involve the musculoskeletal system, skin, hepatobiliary tract and eyes[4]. The pathogenesis of IBD is not fully understood and involves factors such as complex genetic, environmental, epithelial, microbial, and mucosal immunity[5,6]. The breakdown of the intestinal barrier, allowing microorganisms and other antigens to penetrate the bowel wall and trigger uncontrolled immune activation, is a hallmark of IBD[7-9].
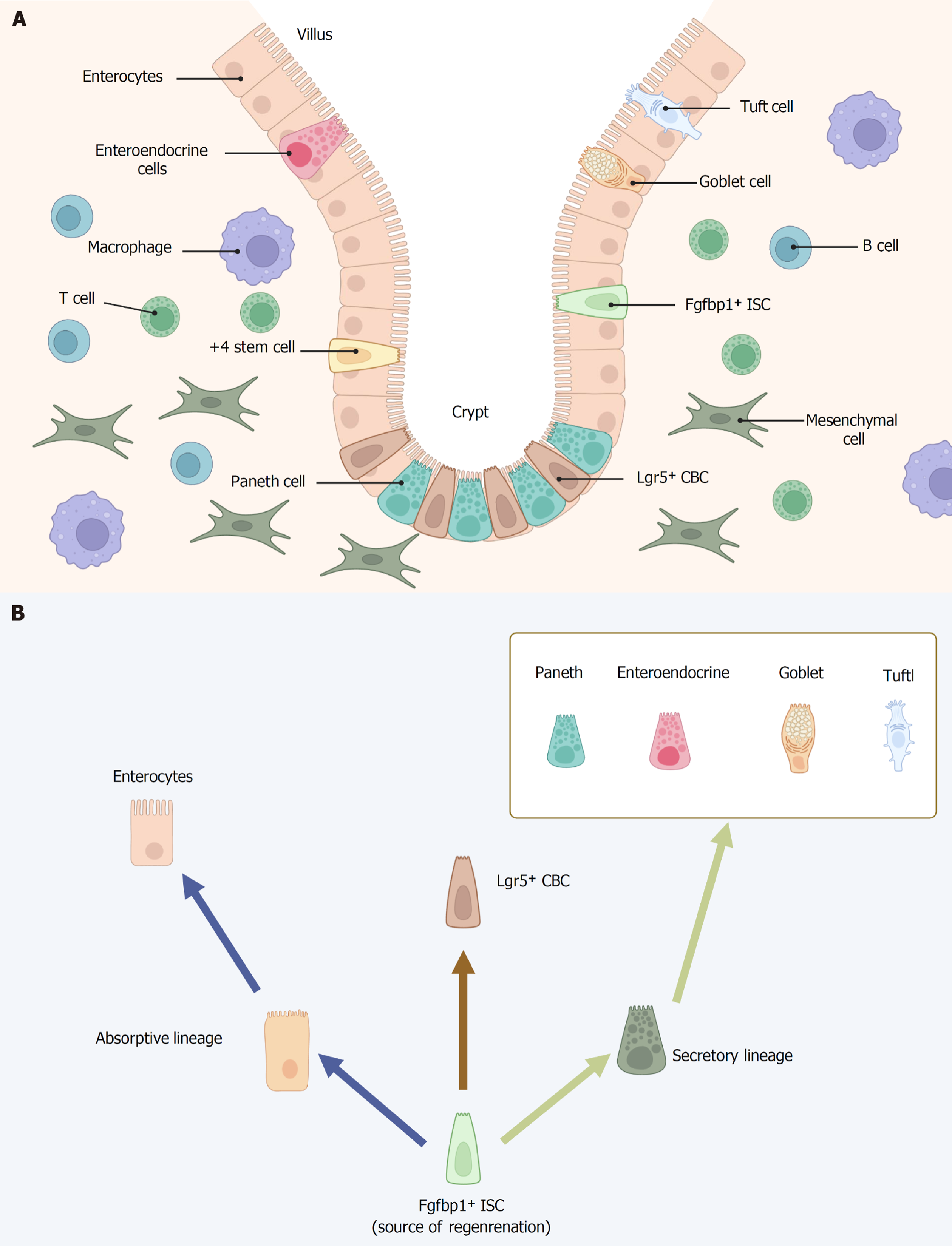
In mammals, the intestine is covered by a single layer of epithelial cells that is renewed every 96-120 hours[10]. Intestinal stem cells (ISCs) are important adult stem cells mainly located in intestinal crypts, which continuously differentiate and regenerate themselves to achieve intestinal epithelial cell renewal[11,12]. Within the crypt, constantly dividing stem cells give rise to progenitor cells (or transit-amplifying cells), which rapidly proliferate before differentiating into mature intestinal epithelial cells. These cells then continue to divide, giving rise to the two differentiated lineages present in the villus: Secretory cells and absorptive cells[13,14]. The two intestinal epithelial lineages underpin the primary physiological functions of the gastrointestinal tract. The secretory lineage includes four distinct cell types - mucus-secreting goblet cells, hormone-producing enteroendocrine cells (EECs), antimicrobial peptide-releasing Paneth cells (PCs) and immune regulating-tuft cells - that primarily function in maintaining the integrity of the epithelium[12]. In contrast, the absorptive lineage consists of enterocytes, which are responsible for nutrient absorption and represent the most prevalent cell type in the intestine[15]. Tissue homeostasis is dependent on the ISC niche[12].
Approaches to managing IBD are shifting from simply controlling symptoms to aiming for mucosal healing, with the goal of preventing disease progression and avoiding bowel damage[16,17]. Currently approved drugs for IBD include corticosteroids, thiopurine, methotrexate biologics and tiny chemical compounds[18-20]. Although new biologics and small molecule drugs have demonstrated promising efficacy, they are not without potential side effects, such as immu
Earlier discussions on ISC identity primarily centered around their physical location within the intestinal crypt. As early as 1974, it was suggested that the crypt base columnar cells (CBCs) might serve as the progenitor cells of the intestinal epithelium by Cheng and Leblond[24]. In a series of electron microscopy studies on small intestinal crypts, Cheng and Leblond[24] observed slender, immature, cycling cells situated between PCs at positions 1-4 of the crypt base with proliferation occurring roughly once every day. The absence of specific molecular markers to identify these cells has prevented enough definitive evidence to confirm that CBC cells are stem cells. In 2007, lineage-tracing experiments in adult mice were carried out using an inducible Cre knock-in allele and the Rosa26-lacZ reporter strain. Over a 60-day period, leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5)-positive CBCs generated all epithelial lineages, providing evidence that they function as the stem cells of the small intestine and colon[25]. In 2009, through a lineage tracing approach, Sato et al[26] demonstrated that ex vivo cultured Lgr5+ CBCs are capable of generating all intestinal epithelial lineages. Therefore, LGR5+ CBC cells satisfy both key criteria of stemness: The capacity to generate multiple lineages and maintain long-term self-renewal. Additionally, lineage tracing studies revealed that certain Lgr5+ cells also express prominin-1 (CD133), and these CD133+ cells have the capacity to give rise to the entire intestinal epithelium[27]. In recent years, additional potential markers of CBCs have been identified including olfactomedin-4 (Olfm4)[28], achaete-scute homolog 2[29] and SRY-related high-mobility group box 9[30], in addition to Lgr5 and CD133.
In addition to CBCs, a unique population of quiescent “reserve” ISCs is found at the fourth position from the bottom of the crypt, just above the differentiated PCs. These cells, termed +4 cells, were first identified by Potten et al[31,32]. Due to the limitations of contemporary technology, the stemness of this population could not be easily established, prompting several research teams to focus on identifying specific genetic markers. Several studies have since proposed alternative ISC markers, which are primarily enriched in the +4 cell population. Sangiorgi and Capecchi[33] identified that B cell-specific Moloney murine leukemia virus insertion site 1 (Bmi1) is expressed in a specific population of cells located near the crypt base in the small intestine, primarily at the +4 position, four cells above the base. These cells proliferate, expand, renew, and ultimately give rise to all the differentiated cell lineages of the small intestinal epithelium. In 2011, Takeda et al[34] showed that homeodomain only protein (Hopx), an atypical homeobox protein, serves as a specific marker for +4 cells. Hopx-expressing cells give rise to CBCs and all mature intestinal epithelial lineages, while CBCs can also differentiate into +4 Hopx-positive cells. These findings establish a bidirectional lineage relationship between active and quiescent stem cells within their niches. In addition to Bmi1 and Hopx, other +4 cells markers have been identified in the last years, including telomerase reverse transcriptase[35] and leucine-rich repeats and immunoglobulin-like domains containing protein 1[36]. Subsequent comprehensive expression analyses, employing single-molecule fluorescent in situ hybridization, transcriptomics, and proteomics, revealed that although these markers are predominantly enriched in +4 cells, they are also likely expressed in other crypt cell populations, including CBCs[37-39]. The +4 stem cell is now regarded as a reserve stem cell with high resistance to radiation, capable of replenishing the pool of continuously cycling CBC cells when necessary[40]. The question of whether +4 cells and CBCs represent two distinct ISC populations, and whether this distinction is an intrinsic property or a consequence of their different locations within the ISC niche, remains a topic of ongoing debate[41].
Lgr5+ cells have been widely accepted as the model for the only homeostatic ISCs supporting intestinal epithelial regeneration. But recently, Malagola et al[42] found that stemness potential extends beyond the crypt base, residing in the isthmus region, where undifferentiated cells contribute to intestinal homeostasis (Figure 1). By using a kinetic reporter for time-resolved fate mapping and fibroblast growth factor binding protein 1 (Fgfbp1)-CreERT2 lineage tracing, Capdevila et al[43] demonstrate that Fgfbp1+ cells in upper crypt are multipotent and can give rise to Lgr5+ cells, supporting their role as ISCs. Moreover, Fgfbp1+ cells are capable of sustaining epithelial regeneration following the depletion of Lgr5+ cells. Intestinal epithelial regeneration originates from the upper crypt rather than the crypt base, challenging previous understanding.
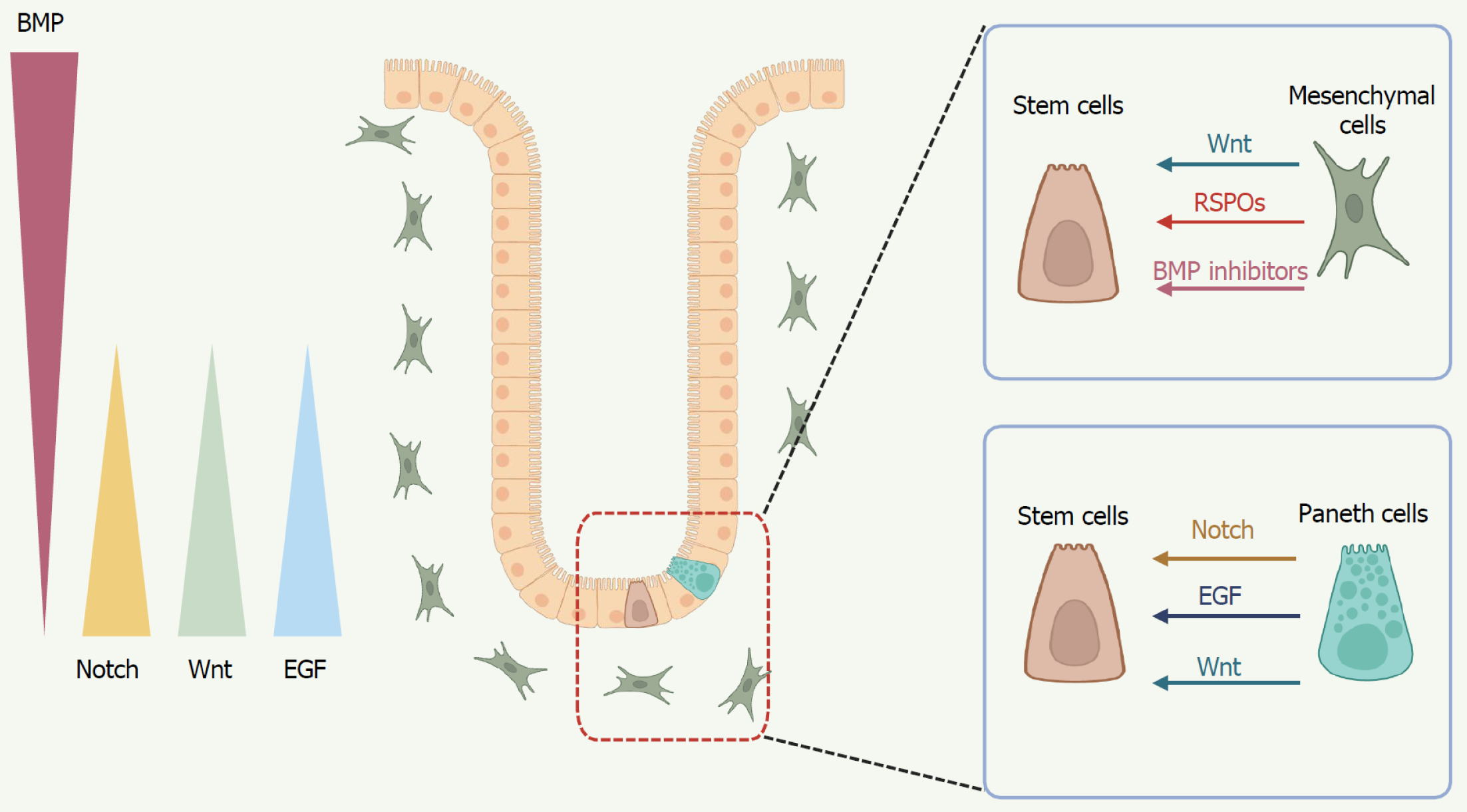
ISCs are sustained by the surrounding niche cells, which play a crucial role in maintaining their proliferative potential and self-renewal capacity (Figure 1). At the base of the crypt, ISCs are encased by a heterogeneous population of stromal cells, including pericryptal myofibroblasts, fibroblasts, endothelial cells, neural cells, immune cells, pericytes, and smooth muscle cells. In concert with other niche components such as PCs and EECs, these stromal cells modulate the proliferative activity of ISCs, the differentiation of mature intestinal epithelial cells, and their overall survival[44]. And the colon possesses a crypt-based ISC niche similar to that of the small intestine but lacks PCs. PCs produce antimicrobial substances, such as α-defensins, lysozyme, and phospholipase A2, along with the Wnt activator protein Wnt3 and transforming growth factor-alpha (TGF-α), which are essential for safeguarding ISC proliferation[45,46]. EECs, primarily enterochromaffin cells, have the ability to dedifferentiate into fully functional ISCs through asymmetric cell division and may also serve as a reserve pool of ISCs[47]. Stromal cells also constitute an integral component of the niche. In situ hybridization studies have demonstrated that several Wnt ligands, including Wnt-2b, Wnt-4, Wnt-5a, and Wnt-5b, as well as R-spondins (RSPOs), are expressed within the stromal compartment to support the function of ISCs[48]. Twist2+ stromal cells have recently been identified as a niche subpopulation that supports canonical ISCs by secreting Wnt ligands[49]. Immune cells are mainly located in the lamina propria, directly beneath the crypts[50]. Recent advances in organoid culture and single-cell sequencing have shown that intestinal immune cells not only mediate immune responses through cytokine release, but also function as key components of the ISC niche, regulating stem cell fate to maintain epithelial homeostasis and drive regeneration during tissue repair[51].
ISCs are regulated by multiple developmental signaling pathways, including Wnt, epidermal growth factor (EGF), Notch and bone morphogenetic protein (BMP), which are crucial for maintaining the balance between enterocyte proliferation, maturation, and migration, and play pivotal roles in governing the fate of ISCs (Figure 2). Below, we underscore the pivotal role of several key signaling pathways, coordinated between the epithelium and mesenchyme, in determining the identity and function of ISCs.
Wnt: The canonical Wnt/β-catenin signaling pathway serves as a crucial regulator of ISC proliferation. Wnt ligands comprise a family of 19 secreted glycoproteins that exert their signaling effects by binding to Frizzled receptors and lipoprotein receptor-related protein 5/6 coreceptors. They transduce the signal either via β-catenin in the canonical pathway or through a series of other proteins in the non-canonical pathway. Extracellular Wnt signaling triggers multiple intracellular signaling cascades, including the Wnt/β-catenin-dependent (canonical) pathway and the β-catenin-independent (non-canonical) pathway, the latter of which can be further subdivided into the planar cell polarity pathway and the Wnt/Ca2+ signaling pathway[52].
A hallmark of the Wnt/β-catenin canonical pathway is the accumulation and translocation of the adhesion-related protein β-catenin to the cell nucleus, where it binds to T-cell factor (TCF) family of transcription factors and directly regulates gene expression[53,54]. Systemic knockout of Tcf4 leads to the complete depletion of ISCs, followed by the disruption of the epithelial structure in neonatal mice[55]. Moreover, the deletion of Tcf7 L2 results in the loss of Lgr5+ ISCs, underscoring the essential role of this pathway in adult intestinal regeneration[56]. On the other hand, a finely tuned balance between Wnt agonists and antagonists within the ISC niche is crucial for preserving the integrity of the ISC compartment. Disruption of this balance, often caused by mutations in the tumor suppressor gene adenomatous poly
EGF: In organoid culture models, EGF, a key component secreted by PCs and essential for establishing ex vivo cultures, regulates organoid growth and differentiation toward the EEC lineage, and is a crucial part of the intestinal organoid culture medium[45,65]. Inhibiting EGF signaling in intestinal organoids causes proliferative LGR5+ ISCs to enter quie
Notch: The Notch signaling pathway is one of the most important pathways in determining cell fate. The canonical Notch signaling is initiated through the interaction between Notch ligands on neighboring cells and the Notch receptor, resulting in a series of proteolytic cleavages. This releases the Notch intracellular domain into the cytoplasm, from where it translocates to the nucleus. There, Notch intracellular domain binds to the DNA-binding transcription factor cellulose synthase-like, forming a complex that activates the transcription of downstream genes[69]. Notch receptor and ligand mRNAs have been identified in both epithelial and mesenchymal cells[70]. Notch signaling regulates the differentiation of CBCs into either absorptive or secretory cells. Activation of Notch promotes differentiation towards absorptive cells, whereas inhibition of Notch favors differentiation into secretory cells. Notch signaling is essential for maintaining the ISC pool, and its inhibition results in a reduction of Lgr5+ cell numbers and impaired proliferation[70]. Expression of Notch target genes in intestinal crypts, with hairy and enhancer of split 1 (Hes1) and Olfm4 Localized to CBC cells[71,72]. PCs, located adjacent to CBC stem cells at the crypt base, express the Notch ligand delta-like ligand 4 (DLL4). The expression of genes known to support ISC function (Dll4, Wnt3, EGF) in PCs suggests that these cells may act as niche cells for ISCs[73]. Deletion of either the Notch ligands (Dll1 and Dll4) or the Notch effectors (Hes1, Hes3, and Hes5) leads to the loss of crypt proliferation[72,74]. Atonal homolog 1 (Atoh1) is a key transcriptional activator that fully drives the differentiation of secretory cells. Inactivation of Notch in progenitor cells leads to the upregulation of Atoh1 expression, driving differentiation towards the secretory lineage[75]. And intestinal epithelial Atoh1 knockout results in the loss of the entire secretory lineage without affecting absorptive cell differentiation[76]. Furthermore, a recent study demonstrates that mitochondrial dynamics, governed by FOXO and Notch signaling, are essential in driving stem cell differentiation into secretory cell types, including goblet cells and PCs[77].
BMP: BMPs are members of the TGF-β superfamily. In mammals, over 12 BMP-related proteins have been identified, including BMP2, BMPs4-10, osteogenin-1, and growth differentiation factors 5-7, among others[78]. The BMP signaling pathway serves to antagonize the proliferative signals within the ISC niche, thereby inhibiting stem cell self-renewal and driving cellular differentiation. Inhibition of BMP signaling impairs the terminal differentiation of the secretory lineage, resulting in dysregulated goblet cell maturation within the intestinal epithelium[79]. In addition, different mesenchymal cell populations generate a crucial intestinal BMP signaling gradient. Platelet-derived growth factor alpha (high) telocytes, abundant at the villus base, serve as a BMP reservoir, while a CD81+ platelet-derived growth factor alpha (low) population located just beneath the crypts secretes the BMP antagonist Gremlin1[80]. These cells can expand ISCs in vitro without additional nutritional support and contribute to ISC maintenance in vivo[80]. Dysfunction of BMP signaling in stromal cells leads to excessive proliferation of mesenchymal cells and the excessive secretion of interleukins, which further promote abnormal goblet cell differentiation and excessive synthesis and accumulation of mucus, resulting in structural and functional abnormalities in the intestinal epithelial cells[81]. Kraiczy et al[82] conducted a classification analysis of subcryptal mesenchymal cells in the small intestine and were the first to demonstrate that the BMP signaling gradient within the intestinal crypt architecture governs the self-organization of the Wnt-secreting stem cell niche.
Signaling-mediated transcription regulation is key to ISC identity and fate, but growing evidence shows it works in tandem with immune cells, nutrition, and microbiome. ISC fate is regulated by cytokines, dietary factors, and microbial signals, which converge on core pathways that integrate niche signals.
Immune cells: IBD primarily results from dysregulation of the intestinal immune system. The intestinal epithelial barrier hosts a range of innate and adaptive immune cells, forming a key component of the gut mucosal immune system. Immune cells interact with ISCs both directly and through paracrine signaling. Pro-inflammatory cytokines such as interferon (IFN)-γ and IL-17 trigger Janus kinase/signal transducer and activator of the transcription 1 (STAT1) activation, leading to ISC apoptosis and impaired renewal. In contrast, regulatory T cells and IL-10 maintain ISCs viability via anti-apoptotic signaling[83]. Biton et al[51] used single-cell RNA sequencing to identify ISC subsets with high major histocompatibility complex II expression capable of interacting with CD4+ helper T cells and presenting antigens. Organoid studies showed that pro-inflammatory T helper type 1 (Th1), Th2, and Th17 cytokines (IFN-γ, IL-13, IL-17) drive Lgr5+ ISC differentiation, while regulatory T cells and IL-10 promote their self-renewal[51]. In vitro co-culture of intestinal organoids with activated T cells results in ISC depletion and compromised organoid viability. Furthermore, in the absence of PCs, IFN-γ derived from CD4+ T cells directly trigger ISC apoptosis via the Janus kinase/STAT signaling pathway[84]. Recent studies have revealed a novel mechanism whereby integrin αEβ7 on intestinal T cells engages E-cadherin on ISCs, modulating downstream adhesion signaling to control epithelial proliferation and differentiation, thereby sustaining intestinal homeostasis[85]. Innate lymphoid cells (ILCs) produce various cytokines even under steady-state conditions, many of which directly influence epithelial cell function - most notably IL-22. ILC3s, as a key source of IL-22, support ISC maintenance and differentiation while providing protection against DNA damage[86]. Meanwhile, under IL-22-independent injury conditions, ILC3s drive intestinal repair by activating the transcriptional regulator YAP in transit-amplifying cells, leading to intestinal mucosal repair[87]. ILC2s and ILC1s also exert significant regulatory effects on ISCs. For example, ILC2-derived IL-13 activates IL-13Rα1 signaling in crypt ISCs, inducing Foxp1 expression to facilitate β-catenin nuclear translocation and sustain Lgr5+ ISC maintenance[88]. Using gut organoid-ILC1 cocultures, Jowett et al[89] showed that murine and human ILC1 secrete TGF-β1 to promote CD44v6+ crypt expansion and express matrix metalloprotease 9 to drive extracellular matrix (ECM) remodeling. In addition to T cells and ILCs, coculture of gut organoids with macrophages and dendritic cells highlights their critical roles in maintaining ISCs homeostasis. In summary, the intricate regulatory interactions between immune cells and ISCs play a pivotal role in maintaining the intestinal barrier and overall gut homeostasis.
Nutrition: ISCs play an underappreciated but emerging role as sensors of dietary nutrients, adjusting their fate decisions in response to nutritional status to help maintain gut homeostasis[90]. Distinct nutritional intervention - such as caloric restriction, fat, and glucose - modulate ISC function and collectively regulate intestinal homeostasis. Caloric restriction amplifies the numbers and proliferation rates of Lgr5+ ISCs by suppressing mammalian target of rapamycin (mTORC) signaling in PCs, key components of the ISC niche, an effect that can be recapitulated by rapamycin[91,92]. Interesting, refeeding after fasting enhances ISC-associated tumor formation through the mTORC1-polyamine-protein synthesis axis[93]. Mattila et al[94] found that mTORC1 activation increases ISCs size in a region-specific manner, favoring the absorptive enteroblast lineage and inhibiting secretory EEC differentiation. Conversely, high-fat diet (60% fat) has been shown to enhance the proliferation and function of Lgr5+ ISCs and progenitor cells in the mammalian intestine[95]. Mechanistically, high-fat diet activates peroxisome proliferator-activated receptor delta, a ligand-dependent transcription factor that regulates metabolism and promotes self-renewal in ISCs and niche-associated progenitors. Short-term excess sucrose intake directly alters crypt cell metabolism and suppresses regenerative proliferation of ISCs and transit-amp
Microbiome: The maintenance of intestinal homeostasis depends on the coordinated interaction between ISCs and the gut microbiota. Recent studies have revealed a complex interplay between ISCs and the gut microbiota, with microbial communities and their metabolites regulating ISCs function through multiple mechanisms. Gut microbiota species, such as Lactobacillus and Bifidobacterium, modulate ISC self-renewal and differentiation to support intestinal homeostasis and host defense against bacterial infection[97]. Salmonella infection in intestinal organoids leads to a marked reduction in Lgr5 and Bmi1 expression, key markers of ISCs. In contrast, Limosilactobacillus reuteri preserves Lgr5+ cell populations and promotes epithelial proliferation through RSPO activation[98]. Meanwhile, recent studies further emphasize that gut microbial metabolites - including short-chain fatty acids, lactate, succinate, indoles and their derivatives, and bile acids - can directly or indirectly influence ISC fate[99]. Furthermore, ISC self-renewal is regulated by gut microbiota and enteric serotonergic neurons. Microbiota-derived valeric acid enhances tryptophan hydroxylase 2 expression by inhibiting nucleosome remodeling and deacetylase complex recruitment, leading to 5-hydroxytryptamine-mediated activation of prostaglandin E2 positive macrophages via 5-hydroxytryptamine (serotonin) receptor 2A/3A. Prostaglandin E2 then promotes Wnt/β-catenin signaling in ISCs through prostaglandin E2 receptor 1/4 receptors[100]. This study reveals complex interactions among the microbiota and their metabolites, enteric neurons, intestinal immune cells, and ISCs, uncovering a new layer of ISC regulation by niche cells and microbial signals.
The inflammatory microenvironment in IBD plays a crucial role in the pathogenesis of both CD and UC, the two main types of IBD. This microenvironment is characterized by chronic inflammation, immune dysregulation, and interactions between immune cells, cytokines, and intestinal epithelial cells. First, In IBD, the persistent inflammation damages the intestinal epithelium, disrupts mucosal integrity, and drives disease progression[6]. The inflammatory environment directly harms intestinal epithelial cells, crucial for gut barrier formation. This damage results in ulcerations, crypt abscesses, and loss of the epithelial lining, intensifying the immune response and worsening gut inflammation. Secondly, IBD is marked by an aberrant immune response to normal gut flora, triggering immune activation against commensal bacteria. This dysregulation involves both innate and adaptive immune cells. Intestinal innate immunity is mediated by neutrophils, monocytes, macrophages, dendritic cells, ILCs, and natural killer cells, which are characterized by their ability to mount a rapid, nonspecific response as the first line of defense[101]. Compared to innate immune cells, adaptive immune cells exhibit high specificity and immunological memory, complementing each other to eliminate invading pathogens. In CD, Th1 and Th17 cells drive inflammation by secreting pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-6, IL-17, IFN-γ, which recruit immune cells and worsen tissue damage[102]. Whereas Th2 cells predominantly drive the inflammatory response in UC, secreting IL-4, IL-5, and IL-13, which contribute to tissue damage and mucus production[102]. In addition, dysbiosis or imbalance in the gut microbiota results in reduced microbial diversity and an overgrowth of pro-inflammatory bacteria[6]. In the IBD inflammatory microenvironment, immune cell-derived cytokines damage intestinal epithelial cells, compromising the epithelial barrier and allowing pathogens and luminal antigens to worsen inflammation. Furthermore, chronic inflammation in IBD activates fibroblasts and promotes the deposition of ECM proteins, such as collagen. This leads to intestinal fibrosis, which can cause strictures and impair the function of the affected region[103].
Epithelial barrier dysfunction and crypt destruction are hallmarks of IBD. The homeostatic repair and regeneration of the intestinal epithelium are controlled by ISCs located at the base of the crypts, which promote rapid turnover and generate various epithelial cell types. They differentiate into various cell types, including enterocytes (absorptive cells), goblet cells (mucus-secreting cells), and PCs (which support stem cell function and secrete antimicrobial peptides). The remaining stem cells following intestinal mucosal injury rapidly divide, replenish the stem cell population, proliferate, differentiate into mature intestinal epithelial cells, and repair the damaged mucosa.
In IBD, chronic inflammation impairs ISC function, resulting in compromised epithelial regeneration. Dysfunctional ISC activity results in decreased expression of epithelial tight junction proteins, which increases intestinal permeability and facilitates the translocation of harmful substances into the underlying tissue, thereby triggering an immune response[104]. In addition, Wnt and Notch signaling pathways are the most influential in regulating ISC function, especially in IBD. Studies have shown that in IBD patients, especially those with UC, increased Notch signaling and Wnt suppression lead to PC depletion, impairing the mucus barrier and ultimately compromising the intestinal barrier integrity[105,106]. Interesting, the intestinal epithelium’s response to inflammation involves not only stem and progenitor cells but also fully differentiated, post-mitotic PCs. Inflammation-induced stem cell factor secretion activates c-Kit signaling, triggering a cascade that leads to glycogen synthase kinase 3β inhibition and Wnt activation in PCs[106]. Multiple studies have shown that inhibiting Notch signaling and promoting goblet cell differentiation can repair the mucosal barrier and alleviate colitis[107-109]. In recent years, growing evidence has highlighted the essential role of ISCs in maintaining epithelial barrier function. Such as DEAH-box helicase 9 deficiency in ISCs or PCs leads to R-loop accumulation, genomic inst
The senescence and apoptosis of ISCs are pivotal mechanisms underlying the disruption of the intestinal epithelial barrier and disease progression in IBD. These processes compromise the gut’s regenerative capacity and self-repair ability, while further perpetuating chronic inflammation. In senescence elevates the risk of IBD and colon cancer. The persistent lesions in the same intestinal region in CD may result from aged stem cells’ inability to proliferate and generate new intestinal cells[113]. Mechanically, mTORC1 activation increases mitogen-activated protein kinases kinases 6 protein synthesis and enhances the activation of the p38 mitogen-activated protein kinases-p53 pathway, leading to a reduction in the number and activity of ISCs, as well as a decrease in villus size and density[114]. Furthermore, the gut microbiota can also influence the senescence of ISCs. Heat-inactivated Bifidobacterium adolescentis may improve colon senescence by enhancing the regeneration of ISCs both in vivo and in vitro, via the Wnt/β-catenin signaling pathway[115]. Meanwhile, exposure of colonic epithelial organoids to dextran sulfate sodium, oxazolone, or 2,4,6-trinitrobenzenesulfonic acid directly induced increased apoptosis and a depletion of Lgr5+ cells[116]. Depletion of fucosyltransferase 2 in ISCs exacerbates endoplasmic reticulum stress and apoptosis, whereas fucosylated hypoxia up-regulated 1 enhances stem cell resistance to pro-apo
Intestinal fibrosis is a significant complication of IBD, occurring in both UC and CD, although it is more commonly seen in CD[119]. As fibrosis progresses, specific areas of the intestine become narrowed, leading to substantial damage to its structure and function, which significantly impairs patients’ quality of life. Intestinal fibrosis is characterized by the excessive deposition of ECM components by activated cells derived from the mesenchyme in ISC niche. As an important regulatory component of the ISC niche, myofibroblasts play a key role in intestinal fibrosis. Following prolonged expo
Mucosal healing in inflamed tissues could represent a promising target for improving clinical outcomes, reducing disease recurrence, and enhancing resection-free survival in patients with IBD[16,17]. Several existing treatments may exert protective or regenerative effects on the damaged epithelium, thereby facilitating mucosal healing. TNF-α inhibitors (biological agents) have induced mucosal healing in a subset of IBD patients, representing a substantial advancement in IBD therapy[127]. A significant number of patients do not respond to these biologic agents, highlighting the need for alternative therapies in IBD. As basic biological research and clinical trials advance, stem cell therapy is expected to expand the therapeutic options for IBD (Table 1).
| Characteristic | HSCT | MSCT | Organoid therapy |
| Main source | Bone marrow, peripheral blood, cord blood | Bone marrow, adipose tissue, muscle connective tissue, periosteum, perichondrium | Adult stem cells (e.g., intestinal stem cells) |
| Primary mechanism | Reconstitution of the immune system, suppress abnormal immune responses | Anti-inflammatory, immunomodulatory, tissue repair | Replace damaged epithelium, reconstruct intestinal barrier |
| Clinical stage | Numerous clinical trials; used in refractory severe IBD cases | Active clinical trials; some products commercially available | Mostly in preclinical and early-stage research |
| Indications | Severe IBD unresponsive to conventional therapy | Moderate-to-severe IBD, especially perianal fistulizing Crohn’s disease | Severe epithelial damage |
| Adverse effects | Infections, high treatment risk | Rare; mild fever or local reactions occasionally reported | Safety still under investigation |
Currently, clinical research on IBD primarily involves the use of HSCs or MSCs. HSCs can be extracted from peripheral blood, bone marrow, and umbilical cord blood, migrating directly to damaged tissues or differentiating into epithelial and immunomodulatory cells to restore normal mucosal structures[128]. In 2010, Burt et al[129] investigated the impact of autologous HSC transplantation (HSCT) on disease-free survival in patients with severe anti-TNFα refractory CD. Following treatment, all patients achieved a Crohn’s Disease Activity Index of less than 150. The proportion of CD patients remaining relapse-free after transplantation was 91% at 1 year, 63% at 2 years, 57% at 3 years, 39% at 4 years, and 19% at 5 years. Although HSCT still holds clinical research value, studies on autologous HSCT in patients with refractory CD have indicated that while the procedure cannot address the high relapse rates associated with genetic susceptibility, it is also linked to a considerable frequency of serious adverse events[130-133]. As a result, the safety of HSCT in the tre
In 2009, Sato et al[26] cultivated the first intestinal organoid using adult stem cells derived from mouse intestines, marking the beginning of the organoid research era. This study has demonstrated that Lgr5+ stem cells are both necessary and sufficient for the initiation and sustained growth of crypt-villus organoids[26]. Currently, there are two main approaches to establishing intestinal organoids: One involves isolating intestinal crypts directly from the donor for culture, while the other relies on in vitro differentiation of embryonic stem cells or induced pluripotent stem cells (iPSCs)[143]. Yui et al[144] showed that mouse colonic epithelial cells can be cultured as spherical aggregates in a 3D culture system, where they are embedded in a type I collagen gel, in the presence of specific cytokines. Furthermore, these spheres can be transplanted into sites of colonic epithelial damage in a UC model mouse. This study provides the first evidence that ex vivo cultured ISCs can be transplanted in situ, facilitating the regeneration of damaged mucosal tissue. Further studies revealed that organoids derived from fetal or adult small intestine can engraft onto damaged colonic epithelium, with differences in their adaptability to the surrounding environment due to cell plasticity[145]. Human intestinal organoids can also reconstruct the damaged mucosa in immunodeficient mice[146]. Although the progenitor cells within organoids are sufficient to drive proliferation and differentiation, their limited plasticity and stemness ultimately constrain the diversity of cell types within the organoids. Recently, Yang et al[147] utilize a combination of small molecule pathway modulators to boost the stemness of organoid stem cells, thereby enhancing their differentiation capacity and increasing cellular diversity within human intestinal organoids. On the other hand, although stem cell-derived organoids are powerful models for epithelial function, they lack tissue-resident immune cells, which are crucial for capturing organ-level processes. Nikolche and his team successfully developed human intestinal immune organoids that incorporate autologous tissue-resident memory T cells, thereby modeling the interactions between intestinal epithelial cells and immune cells. Tissue-resident memory T cells actively infiltrate the organoids and integrate into the epithelial barrier, forming intestinal immune organoids that effectively recapitulate the immune microenvironment of the intestinal tissue in vitro[148].
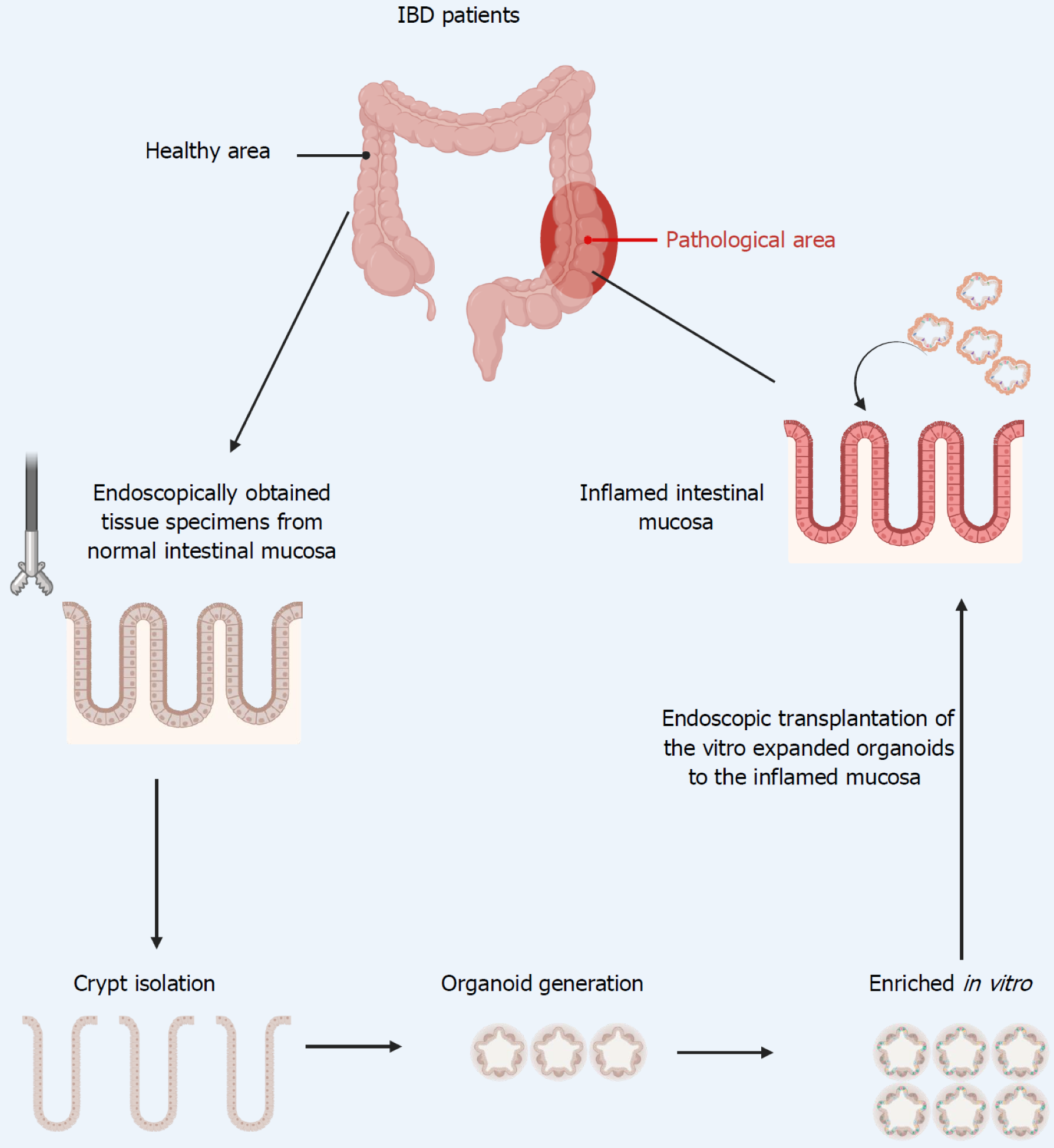
Building on these previous studies, intestinal organoids can now be considered as one of the potential sources for repairing ulcers in patients with refractory IBD. We propose collecting ISCs from the patient’s lesions via endoscopic biopsy, followed by ex vivo expansion using established organoid culture techniques. Once the required cell quantity is reached, they can be transplanted to the target site using an endoscopic delivery approach (Figure 3). However, many challenges remain in establishing organoid transplantation, including the development of an endoscopic cell delivery system, the quality of cultured ISCs, the identification of optimal indices to evaluate the clinical efficacy, and the tumorigenicity of donor organoids, among others[149]. Therefore, intestinal organoid transplantation for the treatment of IBD requires extensive clinical trials in the future for evaluation.
Meanwhile, advances in gene editing, stem cell engineering, and artificial intelligence (AI) are reshaping ISC research, creating new avenues for disease modeling and therapeutic innovation through their interdisciplinary integration. For instance, the combination of human iPSC and CRISPR-Cas9 technology offers a versatile and powerful platform for gene editing, enabling the investigation of disease mechanisms and the discovery of therapeutic targets. Using a lentiviral vector expressing single-guide RNA and CRISPR-Cas9, Sens et al[150] generated iPSC lines with targeted knockouts of IL-10RA, IL-10RB, and the downstream signaling molecules STAT1 and STAT3. In parallel, AI is emerging as a powerful tool in the diagnosis, monitoring, and management of IBD. Deep learning algorithms applied to endoscopic images and histopathological slides have demonstrated excellent performance in lesion detection, disease classification, and treatment response prediction[151,152]. Therefore, we propose that integrating AI with ISC-based therapies can further enhance clinical outcomes by enabling dynamic monitoring of epithelial regeneration and early relapse detection. Additionally, AI has the potential to accelerate research by analyzing complex data from ISC-derived organoids, revealing novel phenotypes and identifying therapeutic targets with greater speed and accuracy than traditional methods.
Initial studies suggested CBCs as progenitors of the intestinal epithelium, and subsequent lineage-tracing confirmed Lgr5+ CBCs as ISCs capable of self-renewal and multilineage differentiation. Additional markers, including CD133, Olfm4, achaete-scute homolog 2, and SRY-related high-mobility group box 9, have refined CBC identification. Quiescent +4 cells, located above PCs, serve as reserve ISCs with high radiation resistance, replenishing CBCs when needed. Markers like Bmi1, Hopx, telomerase reverse transcriptase, and leucine-rich repeats and immunoglobulin-like domains containing protein 1 are associated with +4 cells. Recent studies challenge the exclusive role of Lgr5+ CBCs in rege
The treatment of IBD has predominantly focused on immunosuppression. Many of these medications, especially steroids and immunomodulators, exert widespread immunosuppressive effects, which can result in an increased risk of infections and neoplastic complications. Stem cell-based therapies are emerging as promising treatments for IBD. HSCs and MSCs are the primary candidates, with HSCT showing potential but also high relapse rates and safety concerns. MSCs, through cytokine secretion and exosome-based mechanisms, promote tissue regeneration and modulate inflammation, demonstrating efficacy in IBD treatment. Additionally, intestinal organoids, derived from Lgr5+ stem cells, offer significant potential for mucosal repair. Recent innovations in organoid culture, including enhanced stemness and immune cell integration, bolster their therapeutic potential for refractory IBD. Despite these advances, challenges remain in optimizing cell delivery systems, evaluating clinical efficacy, and addressing tumorigenicity. Extensive clinical trials are necessary to fully assess the viability of organoid-based therapies for IBD. We suppose that ISC transplantation into the inflamed mucosa through endoscopic delivery approach will provide a new therapeutic approach to reconstruct the epithelial barrier in IBD.
| 1. | Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 934] [Reference Citation Analysis (104)] |
| 2. | Dolinger M, Torres J, Vermeire S. Crohn's disease. Lancet. 2024;403:1177-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 298] [Article Influence: 149.0] [Reference Citation Analysis (104)] |
| 3. | Aniwan S, Santiago P, Loftus EV Jr, Park SH. The epidemiology of inflammatory bowel disease in Asia and Asian immigrants to Western countries. United European Gastroenterol J. 2022;10:1063-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology. 2021;161:1118-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 574] [Article Influence: 114.8] [Reference Citation Analysis (1)] |
| 5. | Chang JT. Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med. 2020;383:2652-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 892] [Article Influence: 148.7] [Reference Citation Analysis (0)] |
| 6. | Gilliland A, Chan JJ, De Wolfe TJ, Yang H, Vallance BA. Pathobionts in Inflammatory Bowel Disease: Origins, Underlying Mechanisms, and Implications for Clinical Care. Gastroenterology. 2024;166:44-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 137] [Article Influence: 68.5] [Reference Citation Analysis (1)] |
| 7. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 676] [Article Influence: 48.3] [Reference Citation Analysis (1)] |
| 8. | Parikh K, Antanaviciute A, Fawkner-Corbett D, Jagielowicz M, Aulicino A, Lagerholm C, Davis S, Kinchen J, Chen HH, Alham NK, Ashley N, Johnson E, Hublitz P, Bao L, Lukomska J, Andev RS, Björklund E, Kessler BM, Fischer R, Goldin R, Koohy H, Simmons A. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 623] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 9. | Rieder F, Karrasch T, Ben-Horin S, Schirbel A, Ehehalt R, Wehkamp J, de Haar C, Velin D, Latella G, Scaldaferri F, Rogler G, Higgins P, Sans M. Results of the 2nd scientific workshop of the ECCO (III): basic mechanisms of intestinal healing. J Crohns Colitis. 2012;6:373-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1379] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 11. | Williams JM, Duckworth CA, Burkitt MD, Watson AJ, Campbell BJ, Pritchard DM. Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet Pathol. 2015;52:445-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 12. | Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 723] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 13. | Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 995] [Article Influence: 76.5] [Reference Citation Analysis (2)] |
| 14. | Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1429] [Cited by in RCA: 1290] [Article Influence: 143.3] [Reference Citation Analysis (1)] |
| 15. | Beumer J, Clevers H. Cell fate specification and differentiation in the adult mammalian intestine. Nat Rev Mol Cell Biol. 2021;22:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 463] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 16. | Colombel JF, Narula N, Peyrin-Biroulet L. Management Strategies to Improve Outcomes of Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:351-361.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (1)] |
| 17. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1953] [Article Influence: 390.6] [Reference Citation Analysis (1)] |
| 18. | Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S; AGA Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology. 2020;158:1450-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 527] [Article Influence: 87.8] [Reference Citation Analysis (3)] |
| 19. | Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, Warusavitarne J, Agrawal M, Allocca M, Atreya R, Battat R, Bettenworth D, Bislenghi G, Brown SR, Burisch J, Casanova MJ, Czuber-Dochan W, de Groof J, El-Hussuna A, Ellul P, Fidalgo C, Fiorino G, Gisbert JP, Sabino JG, Hanzel J, Holubar S, Iacucci M, Iqbal N, Kapizioni C, Karmiris K, Kobayashi T, Kotze PG, Luglio G, Maaser C, Moran G, Noor N, Papamichael K, Peros G, Reenaers C, Sica G, Sigall-Boneh R, Vavricka SR, Yanai H, Myrelid P, Adamina M, Raine T. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2024;18:1531-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 187] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 20. | Baumgart DC, Le Berre C. Newer Biologic and Small-Molecule Therapies for Inflammatory Bowel Disease. N Engl J Med. 2021;385:1302-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 251] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 21. | Villablanca EJ, Selin K, Hedin CRH. Mechanisms of mucosal healing: treating inflammatory bowel disease without immunosuppression? Nat Rev Gastroenterol Hepatol. 2022;19:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 22. | Zhang HM, Yuan S, Meng H, Hou XT, Li J, Xue JC, Li Y, Wang Q, Nan JX, Jin XJ, Zhang QG. Stem Cell-Based Therapies for Inflammatory Bowel Disease. Int J Mol Sci. 2022;23:8494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Wang R, Yao Q, Chen W, Gao F, Li P, Wu J, Yu J, Cao H. Stem cell therapy for Crohn's disease: systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther. 2021;12:463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 24. | Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1050] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 25. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4488] [Article Influence: 236.2] [Reference Citation Analysis (0)] |
| 26. | Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4394] [Cited by in RCA: 5463] [Article Influence: 321.4] [Reference Citation Analysis (0)] |
| 27. | Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N, Clevers H. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 2009;136:2187-2194.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 440] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 29. | van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 574] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 30. | Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H, Magness ST. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology. 2015;149:1553-1563.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 336] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 226] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 975] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 34. | Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 621] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 35. | Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 36. | Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 582] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 37. | Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, van Oudenaarden A. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol. 2011;14:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 38. | Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011;30:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 39. | Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJ, Clevers H. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent '+4' cell markers. EMBO J. 2012;31:3079-3091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 635] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 40. | Tetteh PW, Farin HF, Clevers H. Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol. 2015;25:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 41. | Vanuytsel T, Senger S, Fasano A, Shea-Donohue T. Major signaling pathways in intestinal stem cells. Biochim Biophys Acta. 2013;1830:2410-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Malagola E, Vasciaveo A, Ochiai Y, Kim W, Zheng B, Zanella L, Wang ALE, Middelhoff M, Nienhüser H, Deng L, Wu F, Waterbury QT, Belin B, LaBella J, Zamechek LB, Wong MH, Li L, Guha C, Cheng CW, Yan KS, Califano A, Wang TC. Isthmus progenitor cells contribute to homeostatic cellular turnover and support regeneration following intestinal injury. Cell. 2024;187:3056-3071.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 43. | Capdevila C, Miller J, Cheng L, Kornberg A, George JJ, Lee H, Botella T, Moon CS, Murray JW, Lam S, Calderon RI, Malagola E, Whelan G, Lin CS, Han A, Wang TC, Sims PA, Yan KS. Time-resolved fate mapping identifies the intestinal upper crypt zone as an origin of Lgr5+ crypt base columnar cells. Cell. 2024;187:3039-3055.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 44. | Mehra L, Bhowmik S, Makharia GK, Das P. Intestinal stem cell niche: An upcoming area of immense importance in gastrointestinal disorders. Indian J Gastroenterol. 2025;44:8-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2119] [Cited by in RCA: 1994] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 46. | Cray P, Sheahan BJ, Dekaney CM. Secretory Sorcery: Paneth Cell Control of Intestinal Repair and Homeostasis. Cell Mol Gastroenterol Hepatol. 2021;12:1239-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 47. | Sei Y, Feng J, Samsel L, White A, Zhao X, Yun S, Citrin D, McCoy JP, Sundaresan S, Hayes MM, Merchant JL, Leiter A, Wank SA. Mature enteroendocrine cells contribute to basal and pathological stem cell dynamics in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2018;315:G495-G510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J, Wu Y, Bunte R, Williams BO, Rossant J, Virshup DM. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 49. | Xiang J, Guo J, Zhang S, Wu H, Chen YG, Wang J, Li B, Liu H. A stromal lineage maintains crypt structure and villus homeostasis in the intestinal stem cell niche. BMC Biol. 2023;21:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Viola MF, Boeckxstaens G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut. 2021;70:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 51. | Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, Ashenberg O, Su CW, Smillie C, Shekhar K, Chen Z, Wu C, Ordovas-Montanes J, Alvarez D, Herbst RH, Zhang M, Tirosh I, Dionne D, Nguyen LT, Xifaras ME, Shalek AK, von Andrian UH, Graham DB, Rozenblatt-Rosen O, Shi HN, Kuchroo V, Yilmaz OH, Regev A, Xavier RJ. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell. 2018;175:1307-1320.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 459] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 52. | Qin K, Yu M, Fan J, Wang H, Zhao P, Zhao G, Zeng W, Chen C, Wang Y, Wang A, Schwartz Z, Hong J, Song L, Wagstaff W, Haydon RC, Luu HH, Ho SH, Strelzow J, Reid RR, He TC, Shi LL. Canonical and noncanonical Wnt signaling: Multilayered mediators, signaling mechanisms and major signaling crosstalk. Genes Dis. 2024;11:103-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 131] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 53. | Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 1057] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 54. | Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2031] [Cited by in RCA: 3346] [Article Influence: 371.8] [Reference Citation Analysis (0)] |
| 55. | Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1218] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 56. | van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, Clevers H. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol. 2012;32:1918-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 57. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6857] [Article Influence: 489.8] [Reference Citation Analysis (10)] |
| 58. | van Neerven SM, de Groot NE, Nijman LE, Scicluna BP, van Driel MS, Lecca MC, Warmerdam DO, Kakkar V, Moreno LF, Vieira Braga FA, Sanches DR, Ramesh P, Ten Hoorn S, Aelvoet AS, van Boxel MF, Koens L, Krawczyk PM, Koster J, Dekker E, Medema JP, Winton DJ, Bijlsma MF, Morrissey E, Léveillé N, Vermeulen L. Apc-mutant cells act as supercompetitors in intestinal tumour initiation. Nature. 2021;594:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 59. | Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, de Punder K, Angers S, Peters PJ, Maurice MM, Clevers H. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 419] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 60. | Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, Ootani A, Roelf K, Lee M, Yuan J, Li X, Bolen CR, Wilhelmy J, Davies PS, Ueno H, von Furstenberg RJ, Belgrader P, Ziraldo SB, Ordonez H, Henning SJ, Wong MH, Snyder MP, Weissman IL, Hsueh AJ, Mikkelsen TS, Garcia KC, Kuo CJ. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature. 2017;545:238-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 61. | de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 1042] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 62. | de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28:305-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 518] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 63. | Storm EE, Durinck S, de Sousa e Melo F, Tremayne J, Kljavin N, Tan C, Ye X, Chiu C, Pham T, Hongo JA, Bainbridge T, Firestein R, Blackwood E, Metcalfe C, Stawiski EW, Yauch RL, Wu Y, de Sauvage FJ. Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature. 2016;529:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 64. | Goto N, Goto S, Imada S, Hosseini S, Deshpande V, Yilmaz ÖH. Lymphatics and fibroblasts support intestinal stem cells in homeostasis and injury. Cell Stem Cell. 2022;29:1246-1261.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 65. | Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, Clevers H. Induced Quiescence of Lgr5+ Stem Cells in Intestinal Organoids Enables Differentiation of Hormone-Producing Enteroendocrine Cells. Cell Stem Cell. 2017;20:177-190.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 66. | Calafiore M, Fu YY, Vinci P, Arnhold V, Chang WY, Jansen SA, Egorova A, Takashima S, Kuttiyara J, Ito T, Serody J, Nakae S, Turnquist H, van Es J, Clevers H, Lindemans CA, Blazar BR, Hanash AM. A tissue-intrinsic IL-33/EGF circuit promotes epithelial regeneration after intestinal injury. Nat Commun. 2023;14:5411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 67. | Zhang C, Jin Y, Marchetti M, Lewis MR, Hammouda OT, Edgar BA. EGFR signaling activates intestinal stem cells by promoting mitochondrial biogenesis and β-oxidation. Curr Biol. 2022;32:3704-3719.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 68. | Jardé T, Chan WH, Rossello FJ, Kaur Kahlon T, Theocharous M, Kurian Arackal T, Flores T, Giraud M, Richards E, Chan E, Kerr G, Engel RM, Prasko M, Donoghue JF, Abe SI, Phesse TJ, Nefzger CM, McMurrick PJ, Powell DR, Daly RJ, Polo JM, Abud HE. Mesenchymal Niche-Derived Neuregulin-1 Drives Intestinal Stem Cell Proliferation and Regeneration of Damaged Epithelium. Cell Stem Cell. 2020;27:646-662.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 69. | Zhou B, Lin W, Long Y, Yang Y, Zhang H, Wu K, Chu Q. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 717] [Article Influence: 179.3] [Reference Citation Analysis (1)] |
| 70. | Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol. 2016;594:4791-4803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 71. | VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 72. | Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230-1240.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 338] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 73. | Kim TH, Li F, Ferreiro-Neira I, Ho LL, Luyten A, Nalapareddy K, Long H, Verzi M, Shivdasani RA. Broadly permissive intestinal chromatin underlies lateral inhibition and cell plasticity. Nature. 2014;506:511-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 74. | Ueo T, Imayoshi I, Kobayashi T, Ohtsuka T, Seno H, Nakase H, Chiba T, Kageyama R. The role of Hes genes in intestinal development, homeostasis and tumor formation. Development. 2012;139:1071-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 728] [Article Influence: 29.1] [Reference Citation Analysis (7)] |
| 76. | Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 77. | Ludikhuize MC, Meerlo M, Gallego MP, Xanthakis D, Burgaya Julià M, Nguyen NTB, Brombacher EC, Liv N, Maurice MM, Paik JH, Burgering BMT, Rodriguez Colman MJ. Mitochondria Define Intestinal Stem Cell Differentiation Downstream of a FOXO/Notch Axis. Cell Metab. 2020;32:889-900.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 78. | Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3797] [Cited by in RCA: 4397] [Article Influence: 191.2] [Reference Citation Analysis (8)] |
| 79. | Zhang Y, Que J. BMP Signaling in Development, Stem Cells, and Diseases of the Gastrointestinal Tract. Annu Rev Physiol. 2020;82:251-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 80. | McCarthy N, Manieri E, Storm EE, Saadatpour A, Luoma AM, Kapoor VN, Madha S, Gaynor LT, Cox C, Keerthivasan S, Wucherpfennig K, Yuan GC, de Sauvage FJ, Turley SJ, Shivdasani RA. Distinct Mesenchymal Cell Populations Generate the Essential Intestinal BMP Signaling Gradient. Cell Stem Cell. 2020;26:391-402.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 81. | Wang Y, Lou R, Zhang Z, Xiao C, Yu S, Wei S, Liu Y, Fu W, Li B, Chen YG. Stromal BMP signaling regulates mucin production in the large intestine via interleukin-1/17. Sci Adv. 2023;9:eadi1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 82. | Kraiczy J, McCarthy N, Malagola E, Tie G, Madha S, Boffelli D, Wagner DE, Wang TC, Shivdasani RA. Graded BMP signaling within intestinal crypt architecture directs self-organization of the Wnt-secreting stem cell niche. Cell Stem Cell. 2023;30:433-449.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 83. | Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 746] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 84. | Takashima S, Martin ML, Jansen SA, Fu Y, Bos J, Chandra D, O'Connor MH, Mertelsmann AM, Vinci P, Kuttiyara J, Devlin SM, Middendorp S, Calafiore M, Egorova A, Kleppe M, Lo Y, Shroyer NF, Cheng EH, Levine RL, Liu C, Kolesnick R, Lindemans CA, Hanash AM. T cell-derived interferon-γ programs stem cell death in immune-mediated intestinal damage. Sci Immunol. 2019;4:eaay8556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 85. | Chen S, Zheng Y, Ran X, Du H, Feng H, Yang L, Wen Y, Lin C, Wang S, Huang M, Yan Z, Wu D, Wang H, Ge G, Zeng A, Zeng YA, Chen J. Integrin αEβ7(+) T cells direct intestinal stem cell fate decisions via adhesion signaling. Cell Res. 2021;31:1291-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Gronke K, Hernández PP, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, Witkowski M, Tizian C, Amann L, Schumacher F, Glatt H, Triantafyllopoulou A, Diefenbach A. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature. 2019;566:249-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 294] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 87. | Romera-Hernández M, Aparicio-Domingo P, Papazian N, Karrich JJ, Cornelissen F, Hoogenboezem RM, Samsom JN, Cupedo T. Yap1-Driven Intestinal Repair Is Controlled by Group 3 Innate Lymphoid Cells. Cell Rep. 2020;30:37-45.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 88. | Zhu P, Zhu X, Wu J, He L, Lu T, Wang Y, Liu B, Ye B, Sun L, Fan D, Wang J, Yang L, Qin X, Du Y, Li C, He L, Ren W, Wu X, Tian Y, Fan Z. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat Immunol. 2019;20:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 89. | Jowett GM, Norman MDA, Yu TTL, Rosell Arévalo P, Hoogland D, Lust ST, Read E, Hamrud E, Walters NJ, Niazi U, Chung MWH, Marciano D, Omer OS, Zabinski T, Danovi D, Lord GM, Hilborn J, Evans ND, Dreiss CA, Bozec L, Oommen OP, Lorenz CD, da Silva RMP, Neves JF, Gentleman E. ILC1 drive intestinal epithelial and matrix remodelling. Nat Mater. 2021;20:250-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 90. | Stojanović O, Miguel-Aliaga I, Trajkovski M. Intestinal plasticity and metabolism as regulators of organismal energy homeostasis. Nat Metab. 2022;4:1444-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 91. | Tinkum KL, Stemler KM, White LS, Loza AJ, Jeter-Jones S, Michalski BM, Kuzmicki C, Pless R, Stappenbeck TS, Piwnica-Worms D, Piwnica-Worms H. Fasting protects mice from lethal DNA damage by promoting small intestinal epithelial stem cell survival. Proc Natl Acad Sci U S A. 2015;112:E7148-E7154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 92. | Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AK, Deshpande V, Sabatini DM. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 609] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 93. | Imada S, Khawaled S, Shin H, Meckelmann SW, Whittaker CA, Corrêa RO, Alquati C, Lu Y, Tie G, Pradhan D, Calibasi-Kocal G, Nascentes Melo LM, Allies G, Rösler J, Wittenhofer P, Krystkiewicz J, Schmitz OJ, Roper J, Vinolo MAR, Ricciardiello L, Lien EC, Vander Heiden MG, Shivdasani RA, Cheng CW, Tasdogan A, Yilmaz ÖH. Short-term post-fast refeeding enhances intestinal stemness via polyamines. Nature. 2024;633:895-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 94. | Mattila J, Viitanen A, Fabris G, Strutynska T, Korzelius J, Hietakangas V. Stem cell mTOR signaling directs region-specific cell fate decisions during intestinal nutrient adaptation. Sci Adv. 2024;10:eadi2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 95. | Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan GC, Orkin SH, Sabatini DM, Yilmaz ÖH. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 647] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 96. | Burr AHP, Ji J, Ozler K, Mentrup HL, Eskiocak O, Yueh B, Cumberland R, Menk AV, Rittenhouse N, Marshall CW, Chiaranunt P, Zhang X, Mullinax L, Overacre-Delgoffe A, Cooper VS, Poholek AC, Delgoffe GM, Mollen KP, Beyaz S, Hand TW. Excess Dietary Sugar Alters Colonocyte Metabolism and Impairs the Proliferative Response to Damage. Cell Mol Gastroenterol Hepatol. 2023;16:287-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 97. | Calibasi-Kocal G, Mashinchian O, Basbinar Y, Ellidokuz E, Cheng CW, Yilmaz ÖH. Nutritional Control of Intestinal Stem Cells in Homeostasis and Tumorigenesis. Trends Endocrinol Metab. 2021;32:20-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 98. | Ma N, Chen X, Johnston LJ, Ma X. Gut microbiota-stem cell niche crosstalk: A new territory for maintaining intestinal homeostasis. Imeta. 2022;1:e54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 99. | Wu H, Mu C, Xu L, Yu K, Shen L, Zhu W. Host-microbiota interaction in intestinal stem cell homeostasis. Gut Microbes. 2024;16:2353399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 100. | Zhu P, Lu T, Wu J, Fan D, Liu B, Zhu X, Guo H, Du Y, Liu F, Tian Y, Fan Z. Gut microbiota drives macrophage-dependent self-renewal of intestinal stem cells via niche enteric serotonergic neurons. Cell Res. 2022;32:555-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 101. | Saez A, Herrero-Fernandez B, Gomez-Bris R, Sánchez-Martinez H, Gonzalez-Granado JM. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int J Mol Sci. 2023;24:1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 326] [Reference Citation Analysis (0)] |
| 102. | Lu Q, Yang MF, Liang YJ, Xu J, Xu HM, Nie YQ, Wang LS, Yao J, Li DF. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J Inflamm Res. 2022;15:1825-1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 103. | Rieder F, Mukherjee PK, Massey WJ, Wang Y, Fiocchi C. Fibrosis in IBD: from pathogenesis to therapeutic targets. Gut. 2024;73:854-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 104] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 104. | Chittimalli K, Jahan J, Sakamuri A, McAdams ZL, Ericsson AC, Jarajapu YPR. Restoration of the gut barrier integrity and restructuring of the gut microbiome in aging by angiotensin-(1-7). Clin Sci (Lond). 2023;137:913-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 105. | Gersemann M, Stange EF, Wehkamp J. From intestinal stem cells to inflammatory bowel diseases. World J Gastroenterol. 2011;17:3198-3203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 106. | Schmitt M, Schewe M, Sacchetti A, Feijtel D, van de Geer WS, Teeuwssen M, Sleddens HF, Joosten R, van Royen ME, van de Werken HJG, van Es J, Clevers H, Fodde R. Paneth Cells Respond to Inflammation and Contribute to Tissue Regeneration by Acquiring Stem-like Features through SCF/c-Kit Signaling. Cell Rep. 2018;24:2312-2328.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 107. | Wang X, Kong X, Qin Y, Zhu X, Liu W, Han J. Milk phospholipids ameliorate mouse colitis associated with colonic goblet cell depletion via the Notch pathway. Food Funct. 2019;10:4608-4619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Shinoda M, Shin-Ya M, Naito Y, Kishida T, Ito R, Suzuki N, Yasuda H, Sakagami J, Imanishi J, Kataoka K, Mazda O, Yoshikawa T. Early-stage blocking of Notch signaling inhibits the depletion of goblet cells in dextran sodium sulfate-induced colitis in mice. J Gastroenterol. 2010;45:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 109. | Zhao Y, Luan H, Gao H, Wu X, Zhang Y, Li R. Gegen Qinlian decoction maintains colonic mucosal homeostasis in acute/chronic ulcerative colitis via bidirectionally modulating dysregulated Notch signaling. Phytomedicine. 2020;68:153182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 110. | Ren X, Liu Q, Zhou P, Zhou T, Wang D, Mei Q, Flavell RA, Liu Z, Li M, Pan W, Zhu S. DHX9 maintains epithelial homeostasis by restraining R-loop-mediated genomic instability in intestinal stem cells. Nat Commun. 2024;15:3080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 111. | Buttó LF, Pelletier A, More SK, Zhao N, Osme A, Hager CL, Ghannoum MA, Sekaly RP, Cominelli F, Dave M. Intestinal Stem Cell Niche Defects Result in Impaired 3D Organoid Formation in Mouse Models of Crohn's Disease-like Ileitis. Stem Cell Reports. 2020;15:389-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Bao W, You Y, Ni J, Hou H, Lyu J, Feng G, Wang Y, You K, Zhang S, Zhang L, Cao X, Wang X, Li H, Li H, Xu J, Liu C, Luo X, Du P, Chen D, Shen X. Inhibiting sorting nexin 10 promotes mucosal healing through SREBP2-mediated stemness restoration of intestinal stem cells. Sci Adv. 2023;9:eadh5016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 113. | Wang X, Bootsma H, Kroese F, Dijkstra G, Pringle S. Senescent Stem and Transient Amplifying Cells in Crohn's Disease Intestine. Inflamm Bowel Dis. 2020;26:e8-e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | He D, Wu H, Xiang J, Ruan X, Peng P, Ruan Y, Chen YG, Wang Y, Yu Q, Zhang H, Habib SL, De Pinho RA, Liu H, Li B. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat Commun. 2020;11:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 115. | Qi Y, He J, Zhang Y, Ge Q, Wang Q, Chen L, Xu J, Wang L, Chen X, Jia D, Lin Y, Xu C, Zhang Y, Hou T, Si J, Chen S, Wang L. Heat-inactivated Bifidobacterium adolescentis ameliorates colon senescence through Paneth-like-cell-mediated stem cell activation. Nat Commun. 2023;14:6121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 116. | Girish N, Liu CY, Gadeock S, Gomez ML, Huang Y, Sharifkhodaei Z, Washington MK, Polk DB. Persistence of Lgr5+ colonic epithelial stem cells in mouse models of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2021;321:G308-G324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 117. | Wang Z, Tan C, Duan C, Wu J, Zhou D, Hou L, Qian W, Han C, Hou X. FUT2-dependent fucosylation of HYOU1 protects intestinal stem cells against inflammatory injury by regulating unfolded protein response. Redox Biol. 2023;60:102618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 118. | Zhang T, Ding C, Chen H, Zhao J, Chen Z, Chen B, Mao K, Hao Y, Roulis M, Xu H, Kluger Y, Zou Q, Ye Y, Zhan M, Flavell RA, Li HB. m(6)A mRNA modification maintains colonic epithelial cell homeostasis via NF-κB-mediated antiapoptotic pathway. Sci Adv. 2022;8:eabl5723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 119. | Bernstein CN, Loftus EV Jr, Ng SC, Lakatos PL, Moum B; Epidemiology and Natural History Task Force of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD). Hospitalisations and surgery in Crohn's disease. Gut. 2012;61:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 120. | Leeb SN, Vogl D, Grossmann J, Falk W, Schölmerich J, Rogler G, Gelbmann CM. Autocrine fibronectin-induced migration of human colonic fibroblasts. Am J Gastroenterol. 2004;99:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 121. | Lovisa S, Genovese G, Danese S. Role of Epithelial-to-Mesenchymal Transition in Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 122. | D'Alessio S, Ungaro F, Noviello D, Lovisa S, Peyrin-Biroulet L, Danese S. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastroenterol Hepatol. 2022;19:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 123. | Manetti M, Rosa I, Messerini L, Ibba-Manneschi L. Telocytes are reduced during fibrotic remodelling of the colonic wall in ulcerative colitis. J Cell Mol Med. 2015;19:62-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 124. | Ibba-Manneschi L, Rosa I, Manetti M. Telocytes in Chronic Inflammatory and Fibrotic Diseases. Adv Exp Med Biol. 2016;913:51-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 125. | Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, Larsen HL, Guiu J, Alves MRP, Rundsten CF, Johansen JV, Li Y, Madsen CD, Nakamura T, Watanabe M, Nielsen OH, Schweiger PJ, Piccolo S, Jensen KB. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell. 2018;22:35-49.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 513] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 126. | He S, Lei P, Kang W, Cheung P, Xu T, Mana M, Park CY, Wang H, Imada S, Russell JO, Wang J, Wang R, Zhou Z, Chetal K, Stas E, Mohad V, Bruun-Rasmussen P, Sadreyev RI, Hodin RA, Zhang Y, Breault DT, Camargo FD, Yilmaz ÖH, Fredberg JJ, Saeidi N. Stiffness Restricts the Stemness of the Intestinal Stem Cells and Skews Their Differentiation Toward Goblet Cells. Gastroenterology. 2023;164:1137-1151.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 127. | Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 128. | Salem GA, Selby GB. Stem cell transplant in inflammatory bowel disease: a promising modality of treatment for a complicated disease course. Stem Cell Investig. 2017;4:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 129. | Burt RK, Craig RM, Milanetti F, Quigley K, Gozdziak P, Bucha J, Testori A, Halverson A, Verda L, de Villiers WJ, Jovanovic B, Oyama Y. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood. 2010;116:6123-6132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 130. | Hawkey CJ, Allez M, Clark MM, Labopin M, Lindsay JO, Ricart E, Rogler G, Rovira M, Satsangi J, Danese S, Russell N, Gribben J, Johnson P, Larghero J, Thieblemont C, Ardizzone S, Dierickx D, Ibatici A, Littlewood T, Onida F, Schanz U, Vermeire S, Colombel JF, Jouet JP, Clark E, Saccardi R, Tyndall A, Travis S, Farge D. Autologous Hematopoetic Stem Cell Transplantation for Refractory Crohn Disease: A Randomized Clinical Trial. JAMA. 2015;314:2524-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 131. | Jauregui-Amezaga A, Rovira M, Marín P, Salas A, Pinó-Donnay S, Feu F, Elizalde JI, Fernández-Avilés F, Martínez C, Gutiérrez G, Rosiñol L, Carreras E, Urbano A, Lozano M, Cid J, Suárez-Lledó M, Mensa J, Rimola J, Rodríguez S, Masamunt MC, Comas D, Ruíz I, Ramírez-Morros A, Gallego M, Ordás I, Panés J, Ricart E. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn's disease. Gut. 2016;65:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 132. | López-García A, Rovira M, Jauregui-Amezaga A, Marín P, Barastegui R, Salas A, Ribas V, Feu F, Elizalde JI, Fernández-Avilés F, Martínez C, Gutiérrez G, Rosiñol L, Carreras E, Urbano A, Lozano M, Cid J, Suárez-Lledó M, Masamunt MC, Comas D, Giner A, Gallego M, Alfaro I, Ordás I, Panés J, Ricart E. Autologous Haematopoietic Stem Cell Transplantation for Refractory Crohn's Disease: Efficacy in a Single-Centre Cohort. J Crohns Colitis. 2017;11:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 133. | Lashner BA. Autologous hematopoietic stem cell transplantation for Crohn's disease: high risk for a high reward. Inflamm Bowel Dis. 2005;11:778-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 134. | Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 1190] [Article Influence: 99.2] [Reference Citation Analysis (1)] |
| 135. | Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 2011;2011:207326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 136. | Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, Liu B, Sun L. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012;61:468-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 137. | Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, Dionigi P, Perotti C, Locatelli F, Corazza GR. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 421] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 138. | Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim DS, Jung SH, Kim M, Yoo HW, Kim I, Ha H, Yu CS. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells. 2013;31:2575-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 139. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 777] [Article Influence: 77.7] [Reference Citation Analysis (1)] |
| 140. | Dietz AB, Dozois EJ, Fletcher JG, Butler GW, Radel D, Lightner AL, Dave M, Friton J, Nair A, Camilleri ET, Dudakovic A, van Wijnen AJ, Faubion WA. Autologous Mesenchymal Stem Cells, Applied in a Bioabsorbable Matrix, for Treatment of Perianal Fistulas in Patients With Crohn's Disease. Gastroenterology. 2017;153:59-62.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 141. | Yang S, Liang X, Song J, Li C, Liu A, Luo Y, Ma H, Tan Y, Zhang X. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 142. | Liu H, Liang Z, Wang F, Zhou C, Zheng X, Hu T, He X, Wu X, Lan P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019;4:e131273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 143. | Dedhia PH, Bertaux-Skeirik N, Zavros Y, Spence JR. Organoid Models of Human Gastrointestinal Development and Disease. Gastroenterology. 2016;150:1098-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 193] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 144. | Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5⁺ stem cell. Nat Med. 2012;18:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 145. | Fukuda M, Mizutani T, Mochizuki W, Matsumoto T, Nozaki K, Sakamaki Y, Ichinose S, Okada Y, Tanaka T, Watanabe M, Nakamura T. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28:1752-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 146. | Sugimoto S, Ohta Y, Fujii M, Matano M, Shimokawa M, Nanki K, Date S, Nishikori S, Nakazato Y, Nakamura T, Kanai T, Sato T. Reconstruction of the Human Colon Epithelium In Vivo. Cell Stem Cell. 2018;22:171-176.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 147. | Yang L, Wang X, Zhou X, Chen H, Song S, Deng L, Yao Y, Yin X. A tunable human intestinal organoid system achieves controlled balance between self-renewal and differentiation. Nat Commun. 2025;16:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 148. | Recaldin T, Steinacher L, Gjeta B, Harter MF, Adam L, Kromer K, Mendes MP, Bellavista M, Nikolaev M, Lazzaroni G, Krese R, Kilik U, Popovic D, Stoll B, Gerard R, Bscheider M, Bickle M, Cabon L, Camp JG, Gjorevski N. Human organoids with an autologous tissue-resident immune compartment. Nature. 2024;633:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 149. | Okamoto R, Shimizu H, Suzuki K, Kawamoto A, Takahashi J, Kawai M, Nagata S, Hiraguri Y, Takeoka S, Sugihara HY, Yui S, Watanabe M. Organoid-based regenerative medicine for inflammatory bowel disease. Regen Ther. 2020;13:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 150. | Sens J, Hoffmann D, Lange L, Vollmer Barbosa P, Morgan M, Falk CS, Schambach A. Knockout-Induced Pluripotent Stem Cells for Disease and Therapy Modeling of IL-10-Associated Primary Immunodeficiencies. Hum Gene Ther. 2021;32:77-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 151. | Stidham RW, Takenaka K. Artificial Intelligence for Disease Assessment in Inflammatory Bowel Disease: How Will it Change Our Practice? Gastroenterology. 2022;162:1493-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 152. | Iacucci M, Santacroce G, Zammarchi I, Maeda Y, Del Amor R, Meseguer P, Kolawole BB, Chaudhari U, Di Sabatino A, Danese S, Mori Y, Grisan E, Naranjo V, Ghosh S. Artificial intelligence and endo-histo-omics: new dimensions of precision endoscopy and histology in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2024;9:758-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/