Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.107480
Revised: April 12, 2025
Accepted: July 10, 2025
Published online: August 26, 2025
Processing time: 149 Days and 22.2 Hours
Mesenchymal stem cells (MSCs) derived from bone marrow or adipose tissue have promising potential in regenerative medicine. The regenerative capacity of MSCs depends on their successful migration and engraftment at the injured site. Several preclinical and clinical studies have reported that MSCs effectively treat cardiac dysfunction. However, significant obstacles to MSC homing include peri
Core Tip: In clinical and experimental research, mesenchymal stem cells (MSCs) are thought to hold promising therapeutic potential. Globally, cardiovascular diseases remain the leading cause of mortality. Thus, studying the specific molecular pathways by which MSCs mitigate cardiac injuries is essential to improving their therapeutic applications in the future. Through their immunoregulatory, anti-apoptotic, antifibrotic, antioxidant, and angiogenic activities, MSCs can influence the course of cardiovascular diseases. Successful homing and engraftment are necessary for the regenerative capacity of MSCs. Meanwhile, this review also focuses on the underlying mechanisms of MSC homing and the challenges that may hinder their effects.
- Citation: ShamsEldeen AM, Hosny SA, Maghib K, Ashour H. Current perspectives on regenerative potential of mesenchymal stem cells in alleviating cardiac injuries: Molecular pathways and therapeutic enhancement. World J Stem Cells 2025; 17(8): 107480
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/107480.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.107480
Stem cells are undifferentiated and unspecialized cells present in embryos and adult tissues[1]. They can extensively proliferate (self-renew) and differentiate into other cell types[2]. Stem cells can be classified as totipotent, pluripotent, multipotent, or unipotent cells depending on their differentiation ability[3]. Stem cells can be classified based on the location in which they reside; for example, the stem cells that present in an adult are called adult stem cells, stem cells that are located in an embryo are called embryonic stem cells, and stem cells present in the umbilical cord are called cord blood stem cells[4]. Additionally, stem cells that are derived from tissues are called tissue-derived stem cells[5]. Thus, mesenchymal stem cells (MSCs) are multipotent stem cells found in the medullary stroma of bone marrow surrounding the sinusoidal vessels of the bone marrow niche[6], craniofacial tissues originating from the neural crest and mesoderm during development[7], and white and brown adipose tissues, with the primary distinction being their differing characteristics[6].
Additionally, MSCs can be extracted from placental tissues (placenta-extracted MSCs); they exhibit superior immu
Substantial evidence suggests that stem cells promote regeneration by releasing extracellular vesicles (EVs). Stem cell-derived EVs have the ability to restore various tissues, offering therapeutic benefits for numerous pathophysiological disorders[8]. However, EVs cannot self-replicate, thus mitigating any concern about uncontrolled cell division[10]. EVs are membrane-enclosed vesicular structures that transport various cellular cargo, mediating their paracrine effects. This cargo includes lipids, nucleic acids, and proteins[11]. Additionally, EVs can be isolated in vitro from stem cell culture media. Their small size facilitates sterilization and enhances their ability to cross biological barriers[10]. Furthermore, use of EVs avoids stem cell-related complications, such as peripheral entrapment in lung microvasculature and immune rejection[12,13].
MSCs release two main types of EVs: Microvesicles (MVs) and exosomes[14], which differ in both size and biogenesis[15]. MVs range from approximately 100 to 1000 nm in diameter, whereas exosomes measure 30-150 nm[14]. Exosomes originate from the endosomal compartment, whereas MVs bud from the plasma membrane. Exosomes contain diverse transcription receptors, enzymes, transcription factors, extracellular matrix (ECM) proteins, and nucleic acids[16]. MVs facilitate cellular communication and carry numerous microRNAs (miRNAs), mRNAs, cellular organelles, membrane proteins, cytoskeletal proteins, heat shock proteins, integrins, and bioactive lipids[17].
Both local and systemic administration are important methods for MSC transplantation. Intravenous (IV) infusion is the standard method of administration due to its relative ease and minimally invasive procedure[18]. However, MSCs can undergo a process known as “lung entrapment”, which is cellular distribution in the lungs instead of homing to the target tissues[19]. Lung entrapment is also considered to be a primary effect in the early stages (30 minutes) after IV infusion; then, MSCs start homing to other tissues and organs over time[20]. Therefore, the ability of MSCs to reach the injured tissues through IV infusion remains controversial[19].
Clinical applications of MSCs are reliant on their ability to migrate and reach the desired tissues. Unfortunately, MSC homing is inefficient, with only a small percentage of cells reaching the target tissues[21]. A variety of strategies have been employed in the hope of improving this process[21]. The molecular mechanisms of MSC homing are based on a multistep model, including: (1) Initial tethering by selectins; (2) Activation by cytokines; (3) Arrest by integrins; (4) Diapedesis or transmigration using matrix remodelers; and (5) Extravascular migration toward chemokine gradients[21]. The main problem after MSC administration is that MSCs do not reach the diseased tissues at the therapeutic dose, thus limiting their potential effects[22].
Recent studies have focused on topical administration, involving direct MSC injection into the target tissue. While systemic and local administration contribute to therapeutic effects, their mechanisms may differ[23]. Identifying a simple and effective method for cell delivery to various organs remains a challenge, particularly for treating brain lesions[24]. Intracoronary and intramyocardial MSC therapy has been explored in cardiac disorders. Human UC-MSCs (hUC-MSCs) improved angiogenesis and alleviated cardiac fibrosis and hypertrophy in a rat model of myocardial infarction (MI) mediated by left coronary artery ligation[25]. In a study conducted by Attar et al[26] in 2023, as documented by echocardiography, the percutaneous intracoronary infusion of Wharton’s jelly-derived MSCs following MI improved left ventricular ejection fraction (LVEF).
In regenerative medicine, MSCs are among the most widely used stem cells. Numerous studies have demonstrated their potential in treating various cardiovascular diseases. Cardiovascular disorders remain the leading cause of mortality worldwide[27]. The following section discusses the advantages of MSC-based therapies for different cardiovascular conditions.
Previous studies reported inhibition of aortic aneurysm (AA) development and growth associated with elastin degradation and downregulation of many pro-inflammatory cytokines such as interleukins (IL-1β and IL-6) 4 weeks after bone marrow-derived MSC (BM-MSC) transplantation[28,29]. A meta-analysis of previous preclinical studies that investigated the therapeutic efficacy of MSCs in AA showed that MSC intervention suppressed aneurysm enlargement and reduced maximum aortic diameter in animals[30]. EVs mediated their therapeutic effects in AA by suppressing neu
Atherosclerosis is linked in its pathophysiology to the presence of different risk factors such as hypertension, dyslipidemia, diabetes, obesity, and smoking. The accumulation of subendothelial lipoprotein causes endothelial dysfunction and subsequent inflammatory cellular infiltration[32]. MSCs can modulate inflammation and improve the damaged endothelium reactivity[33]. In a study conducted by Egea et al[34] in 2023, human MSCs were able to differentiate into smooth muscle cell-like cells and confer a stabilizing effect in vulnerable plaques. Moreover, BM-MSC transplantation in an animal model of atherosclerosis enhanced anti-inflammatory cytokine production and suppressed pro-inflammatory cell activation, such as natural killer cells[34,35]. MSCs restore endothelial function by promoting the production of endothelial nitric oxide, angiogenesis, and vascular repair, reducing hyperlipidemia and differentiating into endothelial-like cells[36].
Meta-analyses of animal studies have confirmed the safety and efficacy of MSC therapy in treating ischemic heart disease. MSCs improve LVEF by repairing damaged myocardium, promoting myocardial regeneration, and restoring normal cardiac function[27,37]. Most studies suggest that MSCs exert their therapeutic benefits primarily through paracrine mechanisms, which enhance tissue perfusion, reduce scar size, and limit collagen deposition[38]. Furthermore, MSCs promote tissue repair by stimulating endogenous cardiac stem cells (CSCs)[39], enhancing neovascularization, preserving coronary flow, and supporting myocardial regeneration[40,41]. Additionally, the immunomodulatory effects of MSCs have been investigated in acute MI (AMI) therapy[42]. In this context, Jun Hong et al[41] reported that MSC tran
MSC-based cell therapy has shown promising results in improving LVEF in heart failure (HF). A meta-analysis of six trials using BM-MSCs reported a 6.37% improvement in LVEF[43], while a larger meta-analysis of 14 trials showed a 3.35% improvement[44]. Excessive collagen deposition after MI leads to myocardial fibrosis, resulting in ventricular stiffness and impaired diastolic and systolic function. This process contributes to ventricular remodeling, arrhythmias, and, in severe cases, mortality, when fibroblasts replace necrotic myocardium[45]. MSCs have been found to suppress fibroblast activation and reduce ECM deposition, mitigating fibrosis[46]. A clinical trial on ischemic cardiomyopathy patients demonstrated that younger individuals receiving trans-endocardial MSC injections reduced MI size compared to older patients, along with improved 6-minute walk distance and quality of life, as assessed by the MN (United States) living with HF questionnaire score[47]. Intracoronary infusion of BM-MSCs in ischemic heart disease significantly reduced infarct size and improved LVEF up to 1-year post-treatment. However, 3- and 5-year follow-ups revealed no significant differences in infarct size and LVEF compared to control groups[48]. BM-MSCs promote angiogenesis, increase anti-apoptotic cytokine production, stimulate local CSC proliferation, and contribute to myocardial repair and regeneration[31].
Diabetic cardiomyopathy (DCM) is one of the leading causes of morbidity and mortality among diabetics[49]. It is characterized by myocardial fibrosis and chronic low-grade inflammation. MSCs have demonstrated cardioprotective effects against DCM, exerting their influence through direct cellular mechanisms or cytokine secretion[50]. A single dose of MSCs failed to restore the normal glycemic state in type 2 diabetic rats induced by a combined intake of a high-fat diet and streptozotocin[51]. However, MSC infusion reduces blood glucose and insulin resistance and improves the prognosis of type 2 diabetes and its complication DCM. Therefore, MSCs improved dilated ventricular chambers and cardiac function in a transgenic mice model of dilated cardiomyopathy[52,53].
Cardiac fibrosis is one of the hallmarks of DCM. In this context, co-culturing MSCs with cardiac fibroblasts in high-glucose media led to decreased fibroblast proliferation and collagen secretion[54]. In an in vivo study conducted by Jin et al[49] in 2020, MSC infusion decreased myocardial fibrosis and collagen I and collagen III deposition in diabetic hearts. Increased ROS and reactive nitrogen species production can induce cardiomyocyte apoptosis and impair CSC proliferation, leading to cardiomyopathy[55]. BM-MSCs, with or without resveratrol conditioning, have demonstrated anti
A clinical study conducted by Marketou et al[59] in 2014 reported that patients suffering from essential hypertension have increased circulating MSCs compared to normotensive patients. MSCs can exert blood pressure-lowering effects, supposedly due to activation of the carotid baroreflex[60]. Moreover, MSCs prevent vascular remodeling in pulmonary hypertension by secreting tumor necrosis factor-alpha (TNF-α), which inhibits pulmonary artery smooth muscle cell pro
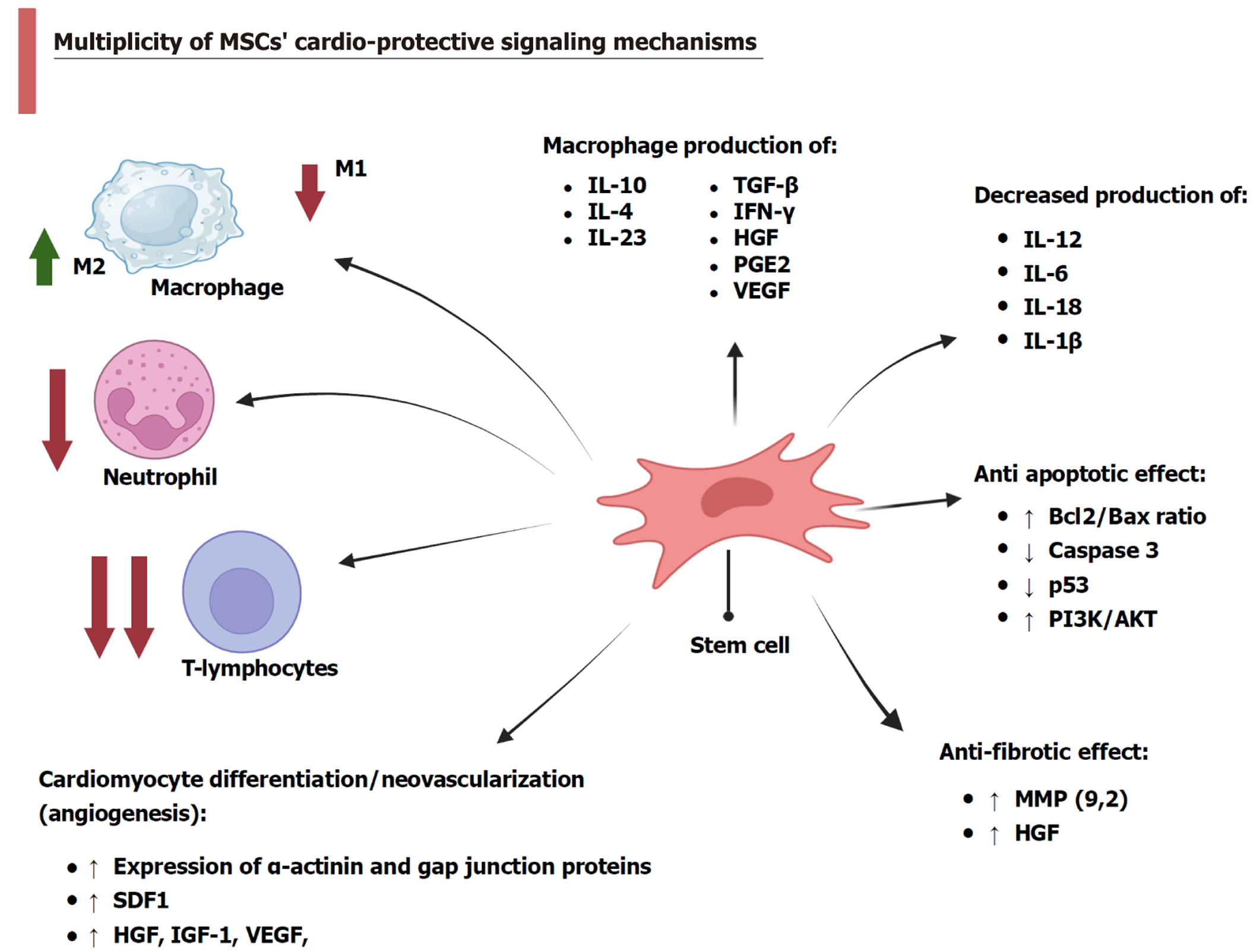
MSCs are multipotent, highly renewable cells currently regarded as a promising therapeutic strategy in clinical and experimental studies, as shown in Figure 1. MSCs can modulate disease progression through their immunoregulatory, antiapoptotic, antifibrotic, antioxidant, and angiogenic mechanisms.
The immunomodulatory capacity of MSCs has been extensively studied in cardiovascular diseases, where they regulate innate and adaptive immune responses. MSCs attenuate monocyte responses following MI and induce macrophages to produce the anti-inflammatory cytokine IL-10[66]. Also, MSCs reduced TNF-α and IL-12m production and increased transforming growth factor-beta (TGF-β), IL-10, and IL-23 Levels. The latter are anti-inflammatory cytokines that can suppress T-cell proliferation[35].
Atherosclerosis is driven by an inflammatory cellular response. However, MSCs modulate the immunoinflammatory environment to inhibit atherogenesis by reducing circulating low-density lipoprotein levels and suppressing TNF-α production while upregulating IL-10 expression[67]. Wharton’s jelly-derived MSCs have low immunogenicity, unlike BM-MSCs. They can produce large amounts of IL-10 and TGF-β, express vascular endothelial growth factor (VEGF), inhibit monocyte maturation and the formation of dendritic cells[68]. Philipp et al[69] found that BM-MSCs secreted interferon (IFN)-γ and IL-1β and increased VEGF. Generally, MSCs can secrete soluble factors, such as hepatocyte growth factor (HGF) and prostaglandin E2[68], which may inhibit B-lymphocyte proliferation and differentiation[70]. MSCs restored cardiac functions in rats subjected to MI by promoting the shift of immune response towards an increasing number of M2 phenotypic markers (IL-10, IL-4), reducing M1 phenotypic markers (IL-6, IL-1β), and inhibiting neutrophil infiltration[27]. Moreover, IV administration of UC-MSCs modulated adaptive immunity and myocardial remodeling in HF by releasing HGF, inhibiting T-cell proliferation, and reducing T-helper 1, T-helper 2, and cytotoxic T-cell activation[71]. Adipose-derived MSCs (AD-MSCs) reduced the expression of cell adhesion molecules by inhibiting the mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) pathways[72]. In addition, MSC-derived exosomes have multiple cardioprotective effects. Huang et al[73] found that the combined intake of MSCs and MSCs-extracted exosomes exerted more efficient anti-inflammatory actions by decreasing IL-6 and TNF-α levels in a rat model of MI. Additionally, exosomes extracted from cardiac AD-MSCs decrease the local infiltration of macrophage and T lym
Apoptosis is programmed cell death, and it involves many molecular pathways. MSCs have a dual mechanism and can inhibit or promote apoptosis through multiple signaling pathways[75]. In this regard, MSCs protect cardiomyocytes from MI-induced apoptosis by increasing the Bcl-2 to Bax ratio and inhibiting caspase-3 activation[76]. MSCs decrease mito
In addition, MSCs exert anti-apoptotic effects in ischemic conditions by transferring exosomal miR-22, which inhibits the p53-upregulated modulator of apoptosis[79]. Additionally, MSC-derived exosomes and conditioned media serve as novel therapeutic agents by supplying ischemic cardiomyocytes with miRNAs such as miR-486-5p, which activates the phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) signaling pathway, further enhancing cell survival[63].
Cardiac fibrosis often develops because of cardiomyocyte apoptosis following myocardial ischemia[80]. Therefore, MSCs’ anti-apoptotic properties indirectly reduce fibrosis. Moreover, MSCs directly prevent fibrosis by releasing matrix metalloproteinase (MMP)-9, decreasing collagen production and deposition[81]. In models of dilated cardiomyopathy, the intramyocardial injection of BM-MSCs reduced MMP-2 and MMP-9 protein levels, as well as fibrosis. Similarly, hUC-MSCs administered intramyocardially prevented tissue fibrosis and cardiomyocyte death[80]. Comparable results were observed following IV infusion of hUC-MSCs in a dilated cardiomyopathy model and after recurrent intramuscular injections of hUC-MSCs in an animal model of doxorubicin-induced dilated cardiomyopathy. These effects were mediated through regulation of the TGF-β/extracellular signal-regulated kinase (ERK) pathway, which inhibited fibroblast activation and interstitial fibrosis formation[82]. Besides, MSCs balance pro- and antifibrotic factors by producing tissue inhibitors of metalloproteinases-1 (TIMP-1) and IL-10[79]. Moreover, BM-MSCs overexpressing adiponectin suppressed the TGF-β1/Smad 2/3 signaling pathway, exhibiting superior antifibrotic effects compared to unmodified cells[80]. Resveratrol-preconditioned BM-MSCs enhanced antifibrotic effects by attenuating the pro-fibrotic secreted Frizzled-related protein/Wnt/β-catenin pathway[56]. Additionally, MSCs secrete HGF, a potent antifibrotic agent that prevents cardiac fibrosis after MI by inhibiting miR-155-mediated profibrotic signaling, while enhancing insulin-like growth factor 1 (IGF-1) secretion and miR-133 overexpression[46,55].
MSCs can undergo cardiomyogenic differentiation when exposed to DNA methyltransferase inhibitor 5-azacytidine (5-Aza), fibroblast growth factor (FGF)-4, bone morphogenetic protein-2, insulin, steroids, and antioxidants[83]. BM-MSC differentiation was confirmed by α-actinin and gap junction expression[84]. MSCs have paracrine-secreting ability, and they can secrete different factors such as VEGF, stromal cell-derived factor (SDF)-1, IGF-1, and HGF[85] in response to hypoxia, thus increasing vasculogenesis[79]. CD146+ MSCs have potential differentiation ability to form vascular smooth muscle cells. In this context, researchers successfully regenerated all three vessel wall layers from MSCs 90 days after aortic implantation[83,86].
An increasing body of research has validated the antioxidant properties of MSCs in various animal models of cardiovascular disease. MSCs transfer mitochondria to damaged tissues through tunneling nanotubes, mitigating oxidative stress and aiding cellular recovery in MI models[78]. Engrafted MSCs at infarction sites upregulate heme oxygenase-1 (HO-1), enhance mitochondrial biogenesis, and facilitate cardiac repair[87]. Both low and high doses of human BM-MSCs improved irradiation-induced aortic damage by increasing HO-1 and catalase enzyme expression[88].
MSC-derived exosomes play a critical role in cardioprotection. In a murine model of myocardial ischemia followed by 30 minutes of reperfusion injury, MSC-derived exosomes reduced infarct size, preserved left ventricular geometry, and maintained contractile function. These exosomes lowered oxidative stress and increased ATP and NADH levels during reperfusion injury[89]. Additionally, MSC-extracted exosomes enhanced the glycolytic potential of oligomycin-treated H9C2 cardiomyocytes by upregulating the expression of phosphoglucose kinase, a rate-limiting glycolytic enzyme, thereby improving ATP production[90]. In DCM models, MSC therapy significantly reduced fibrosis, oxidative stress, and inflammasome-related protein expression. This effect was confirmed by a marked decrease in thiobarbituric acid reactive substances, a key marker of lipid peroxidation[91], as summarized in Table 1[28,29,31,35,37,38,40-42,52,54,56,58,62,92-101].
| Type of mesenchymal stem cells | Human/animal study | Therapeutic role of the used stem cells | Mechanism of activation/enhancing stem cell action | Ref. |
| Bone marrow mesenchymal stem cells | Mice model | Paracrine secretion of pigment epithelium-derived factor | Using younger mesenchymal stem cells | [92] |
| Cord blood-derived human mesenchymal stem cell | Rat model | Formation of cardiomyocyte-like cells | Induction of differentiation into cardiomyocyte-like cells | [38] |
| Human mesenchymal stem cells | Canine animal model | Formation of three-dimensional spheroids | Induction of differentiation and formation of three-dimensional spheroids | [37] |
| Bone marrow mesenchymal stem cells | Yorkshire swine animal model | Cardiogenesis | Not activated | [93] |
| Adult human bone marrow mesenchymal stem cells | Human study | Reducing arrhythmia, promoting angiogenesis and tissue perfusion | Not activated | [40] |
| Human adipose tissue derived mesenchymal stem cells | Yorkshire cross domestic pigs | Immunomodulation and promoting angiogenic | Not activated | [41] |
| Rat bone marrow mesenchymal stem cells | Rat model | Immunomodulation | Cyclooxygenase-2 overexpressing cells | [42] |
| Rat and human bone marrow mesenchymal stem cells | Rat model | Immunomodulation | Sug1 knockdown MSCs | [94] |
| Mice bone marrow mesenchymal stem cells | Mice model and in vitro study | Immunomodulation | Not activated | [28,29] |
| Human mesenchymal stem cells | Rat model and in vitro study | Immunomodulation and differentiation into smooth muscle-like cells | LL-37-activated | [35] |
| Human mesenchymal stem cells | C57BL/6J mice | Inhibition of pyroptosis and immunomodulation | Not activated | [54] |
| Human umbilical cord blood-derived mesenchymal stem cells | cTnT (R141W) transgenic mouse and in vitro study | Cardiac regeneration, pro-angiogenesis, antifibrosis, and anti-apoptotic | In vivo (unconditioned); in vitro (hypoxic conditioning) | [52] |
| Rat bone marrow mesenchymal stem cells | Rat study | Angiogenesis and muscle regeneration | Not activated | [58] |
| Human umbilical cord blood-derived mesenchymal stem cells | Mice study | Attenuating pulmonary artery remodeling | Not activated | [62] |
| Wharton's jelly-derived mesenchymal stem cells | Human study (patients with ST elevation myocardial infarction) | Cardiogenesis | Not activated | [95] |
| Bone marrow mesenchymal stem cells | Human study (patients with acute myocardial infarction) | Cardiogenesis, immunomodulation, and pro-angiogenesis | Autologous cells, not activated | [96] |
| Adipose tissue derived mesenchymal stem cells | Rat study | Antioxidant and anti-apoptotic effect | Resveratrol activated | [97] |
| Umbilical cord-derived mesenchymal stem cells | Human study | Immunomodulatory effects | Not activated | [31] |
| Autologous mesenchymal stem cells or bone marrow mononuclear cells | Human study | Cardiogensis and antifibrotic | Not activated | [98] |
| Bone marrow mesenchymal stem cells | Rat study (both in vivo and in vitro) | Differentiation into functional beta-cells, restoration of glucose homeostasis, and antioxidant effect | Resveratrol-activated | [99] |
| Bone marrow mesenchymal stem cells | Rat study | Pro-angiogenesis, gap junction formation, and improving survival | Ang II-activated | [100] |
| Bone marrow mesenchymal stem cells | Rat study | Antiapoptotic effects, pro-angiogenesis, and anti-fibrotic effects | Not-activated and resveratrol-activated | [56] |
| Mesenchymal stem cells from various sources | Human and animal (diabetic rat models) | Antiapoptotic effects and improving proliferation and cell survival | Resveratrol-activated | [101] |
Stem cell-based therapy is the most promising strategy for preventing myocardial remodeling and damage. The regenerative potential of MSCs depends on their successful homing and engraftment in injured cardiac tissues, where they contribute to cell-based cardiac regeneration. Homing encompasses the biological processes facilitating MSC recruitment to the injury site[21]. MSCs can be delivered systemically, requiring migration through the bloodstream to reach target tissues, or directly applied to the affected area. Once localized, MSCs establish a pro-regenerative microenvironment. Their cardiac regenerative capacity primarily relies on: (1) Secretion of paracrine and autocrine factors, including growth factors, within the injured microenvironment; (2) Stimulation of differentiation and proliferation of endogenous cardiac progenitor cells; and (3) Direct differentiation into new cardiomyocytes and induction of neovascularization. However, microenvironmental alterations following myocardial injury may hinder this promising stem cell approach[102].
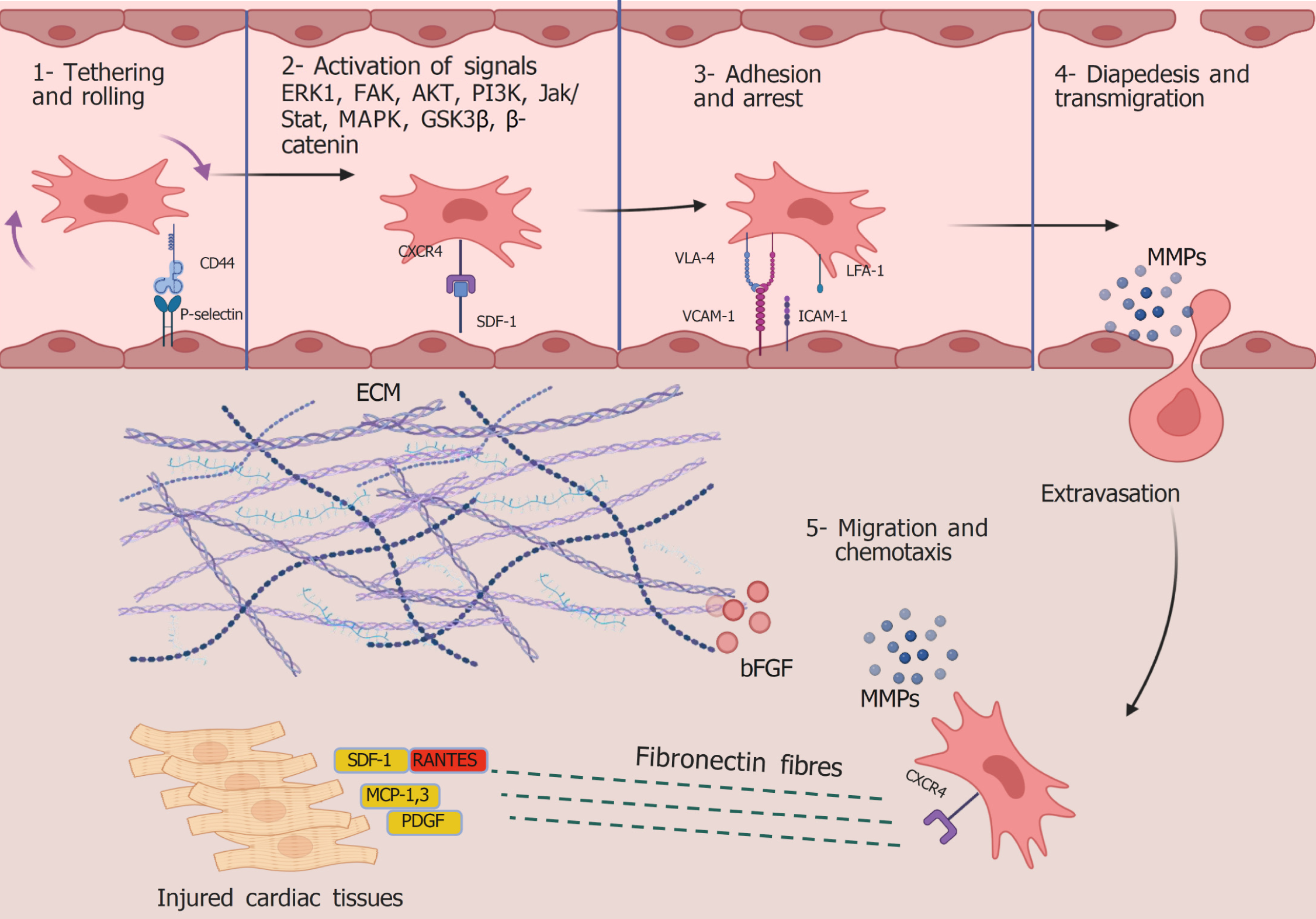
The homing mechanism of MSCs has not yet been thoroughly explained. However, successive research has identified MSC adhesion molecules, the potential mechanisms of vascular wall migration and adhesion, and the regulatory function of chemokines in directing MSCs to specific tissues. As demonstrated in Figure 2, sequential phases are required to guide stem cells from the bloodstream to the injured myocardium and can be categorized as follows: (1) Tethering and rolling; (2) Activation; (3) Arrest; (4) Diapedesis; and (5) Migration. This section discusses the molecular underpinnings of stem cell homing stages.
The interaction between circulating stem cells and vascular endothelial cells (VECs) guides the initial homing phase, as VECs express selectins. Selectins facilitate MSC attachment, slow their movement, and induce rolling and crawling on the VEC surface. Binding between MSCs and VECs requires surface marker expression on both cell types[103]. Rüster et al[104] investigated the role of P-selectin in MSC tethering. They developed a plate flow chamber model simulating cellular circulation to study the synchronized adhesion sequence using human MSCs and VECs. Their findings demonstrated that MSCs exposed to a plate containing P-selectin began to roll, whereas anti-P-selectin antibodies inhibited MSC attachment to VECs. Because VECs express selectin ligands, including the hematopoietic cell E-/L-selectin ligand and P-selectin glycoprotein ligand-1, hematopoietic cells initiate extravasation by binding to VECs. Although MSCs lack P-selectin glycoprotein ligand-1 and hematopoietic cell E-/L-selectin ligand expression, they adhere to VEC selectins via CD44 and begin rolling on the vascular surface[105].
SDF-1, a chemokine (C-X-C motif) ligand-12, is expressed on VECs and plays a key role in the activation stage of tethered MSCs. The ligand for SDF-1 is the C-X-C chemokine receptor 4 (CXCR4) or CXCR7, which are highly expressed by MSCs and exhibit a strong affinity for SDF-1. CXCR4 is essential for MSC migration, whereas CXCR7 is involved in MSC survival[106]. SDF-1/CXCR binding transduces multiple signaling pathways regulating several biological functions, including migration, proliferation, differentiation, chemotaxis, cell death, and survival. SDF-1α/CXCR4 signaling activates the ERK1/2/PI3K/Akt pathways, enhancing stem cell homing and mobilization from the bone marrow into peripheral circulation[107]. In contrast, the CXCR4 blocker AMD3100 downregulates activated focal adhesion kinases and other signaling molecules, including p-PI3K, p-focal adhesion kinase, p-Akt, β-catenin, and p-glycogen synthase kinase-3 beta, thereby inhibiting MSC migration induced by the SDF-1α/CXCR4 axis[108]. Additionally, MAPK, stimulated by ERK1/2 and Janus kinase/signal transducer and activator of transcription, mediates chemotaxis driven by SDF-1/CXCR4 signaling[109].
CXCR4 and SDF-1 are significantly overexpressed in ischemic and cardiomyopathic hearts[110,111]. However, SDF-1 upregulation is time-dependent, peaking 1 week after AMI and gradually declining thereafter. Thus, SDF-1 delivery could enhance stem cell homing, promote angiogenesis, and improve left ventricular function[112]. Despite its critical role, SDF-1 has a very short plasma half-life due to rapid cleavage by dipeptidyl peptidase-4[113,114]. Therefore, amplifying SDF-1/CXCR4 expression and targeting dipeptidyl peptidase-4 in injured cardiac tissue could potentially improve MSC transplantation efficacy.
During the activation phase, MSCs are prepared for the next step of adhesion and arrest, which is mediated by integrin expression. This process involves conformational modifications of integrin extracellular domains. Integrins play a crucial role in cell-cell and cell-matrix interactions[115]. Very late activation antigen-4 (VLA-4), the specific ligand for vascular cell adhesion molecule-1 (VCAM-1), which is expressed by activated endothelial cells, is the key adhesion molecule. SDF-1 enhances MSC attachment to the endothelium by activating VLA-4 and lymphocyte function-associated antigen-1 on MSCs[116]. VCAM-1 and intercellular adhesion molecule-1 (ICAM-1), expressed by activated endothelial cells, bind to VLA-4 and lymphocyte function-associated antigen-1, respectively, leading to MSC arrest. Notably, VLA-4 overexpression has been shown to enhance MSC homing to bone marrow[104]. Interestingly, researchers confirmed that activated MSCs can express the adhesion molecules that are produced by VECs, including VCAM-1 and ICAM-1[117]. This allows for strong attachment and the formation of docking structures between MSCs and the endothelium, guarding against drifting MSCs by the bloodstream.
Efficient lytic enzymes are required for MSCs to traverse the intact capillary wall, penetrating the endothelial cell layer and the underlying basement membrane[118]. In particular, MMP-2 and MMP-9 are essential in degrading collagen and gelatin, the primary components that maintain basement membrane integrity. MMPs are secreted as proenzymes. ProMMP-2 is activated through interactions with membrane type 1 MMP and TIMP-2, and TIMP-1 inhibits it. The molecular mechanisms underlying MSC extravasation were confirmed by Ries et al[119] in 2007. Using a synthetic model of human basement membranes, they demonstrated that MMP-2, membrane type 1 MMP, or TIMP-2 ablation reduced MSC invasion, whereas TIMP-1 knockdown enhanced MSC invasion capacity.
In the final step, the extravasated MSCs are directed to the injury site through the interstitium, aided by MMPs. Fibronectin has an important role in attaching ECM components collagen, fibrin, and heparan sulfate proteoglycans[120] and is important for stem cell migration. It acts as a framework to facilitate the mechanical transport of MSCs[121]. In addition, fibronectin promotes the differentiation of circulating endothelial progenitor cells into endothelial cells[122]. SDF-1 is normally expressed constitutively in a broad range of tissues[123]. SDF-1 expression is upregulated in injured hearts through proinflammatory stimuli such as TNF-α and IL-1[124]. SDF-1/CXCR4 expression increases immediately following AMI and then markedly decreases at day 3[125]. SDF-1 plays a critical role in effective MSC engraftment into the damaged myocardium. According to Sordi et al[126], MSCs exhibit significant migration in response to SDF-1 and the chemokine C-X3-C motif chemokine ligand, mediated by CXCR4 and C-X3-C motif receptor 1 receptors, respectively. Only 3.9% of CXCR4 is localized to the MSC membrane, while 83%-98% remains intracellular. CXCR4 translocation to the membrane occurs in a dose-dependent manner upon SDF-1 exposure[127]. Other chemokines also play vital roles in MSC migration and homing. “Monocyte chemoattractant protein 1” is upregulated in injured mouse hearts and recruits MSCs expressing CC chemokine receptor type 2 (CCR2)[128]. Chemotactic signals released in response to tissue damage guide MSCs toward the injured sites, with MSCs responding to chemokines such as macrophage-derived chemokines CCR2, CCR3, CCR4, and regulated upon activation normal T cell expressed and presumably secreted[129]. Additionally, MSC migration is influenced by growth factor receptor expression. HGF and platelet-derived growth factor (PDGF)-AB enhance MSC migration[129]. Basic FGF (bFGF) in the ECM facilitates MSC migration by activating the Akt pathway. However, its effects are concentration-dependent, with low bFGF concentrations promoting MSC attraction and higher concentrations inhibiting migration efficiency[130].
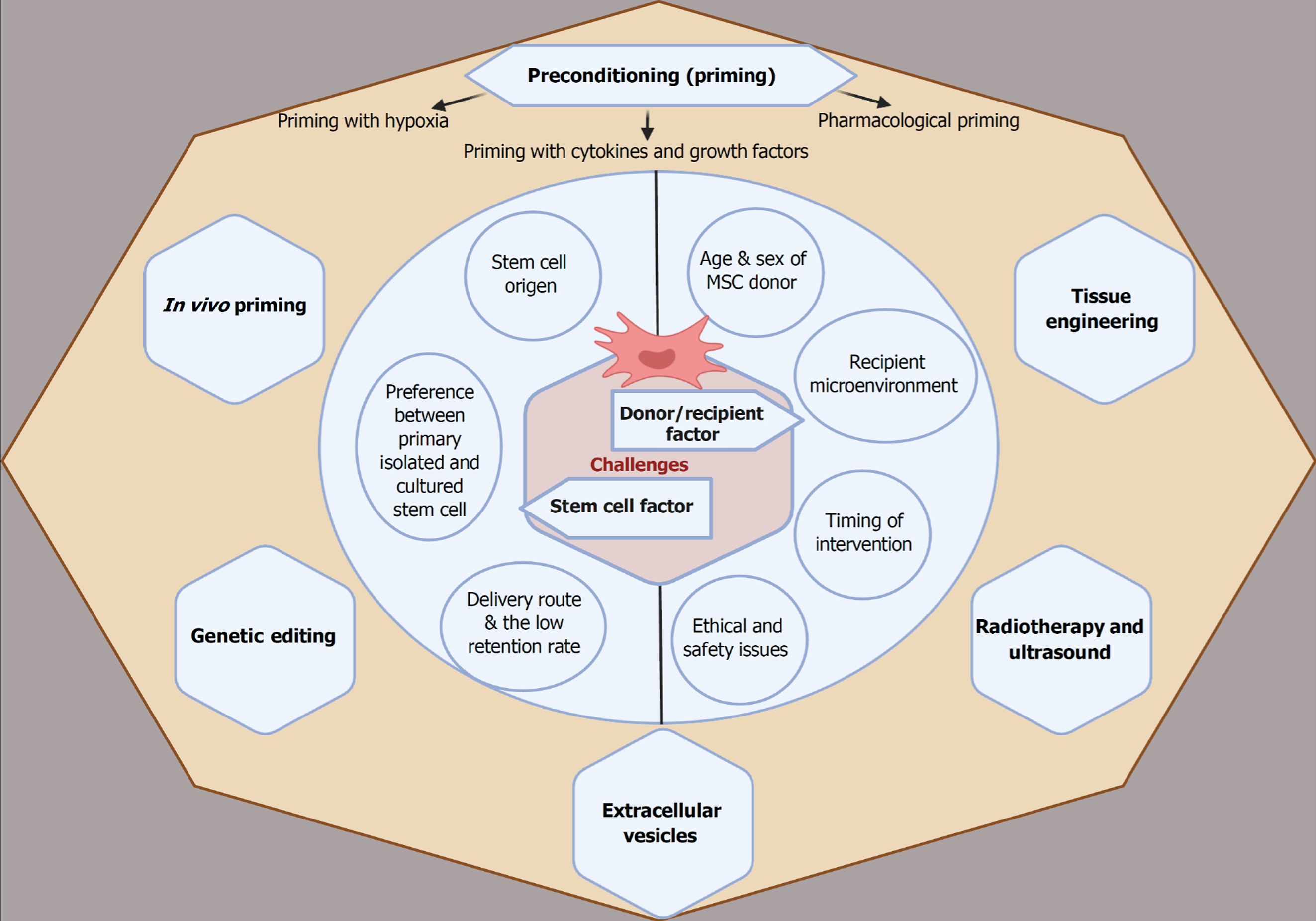
The therapeutic application of MSC treatments has significantly advanced over the past two decades, progressing from preclinical studies to clinical trials. However, clinical studies on cardiac ischemic patients treated with MSCs have yielded conflicting results. Unfortunately, several reports indicated that only a few MSCs successfully engraft into the target tissue, posing a significant challenge to MSC therapy despite its promising potential[79]. The limited homing efficiency of MSCs is attributed to multiple factors, which can be categorized into stem cell-related and patient-related factors, as illustrated in Figure 3. This section discusses the challenges associated with MSC therapy and explores potential strategies to enhance its therapeutic efficacy.
Stem cell origin: MSC populations derived from different tissues exhibit significant variations in proliferation, differentiation, and phenotypic characteristics. These differences in regenerative behavior are partially attributed to the distinct origins of stem cells. BM-MSCs are widely used, but they are distinct from MSCs obtained from perinatal tissues. A notable study compared UC-MSC adhesion characteristics to BM-MSCs, which may influence preferences for systemic injections. Compared to BM-MSCs, UC-MSCs exhibited superior adhesion to ECM proteins such as collagen and fibronectin[131]. Another study further supports the differences in MSC origin, demonstrating variations in adhesion and migration properties[132]. UC-MSCs showed the highest adherence under flow conditions despite increased wall shear stress (UC-MSCs > BM-MSCs), followed by rapid migration, which suggests more effective delivery from circulation into injured tissue[132]. These variations may be explained by the native microenvironment from which the MSCs are isolated and the differential expression of homing molecules. As previously mentioned, MSC homing, from initial endothelial attachment to successful engraftment in injured tissues, relies on the expression of specific homing molecules[56]. Table 2 illustrates the differential expression of MSC surface markers related to tissue origin, particularly among commonly used MSCs and adipose-derived stem cells[133-135]. Goessler et al[115] did not identify any significant differences in integrin expression between freshly isolated BM-MSCs and AD-MSCs. However, they observed progressive expression of the fibronectin receptor (integrin α5β1) and vitronectin/osteopontin receptors (αvβ5) during chondrogenic differentiation of both MSC types. Discrepancies in marker expression across studies may be attributed to cell culture variations and passage number variations.
| Adhesion molecules and growth factors | Differential expression | Ref. |
| CD11c (lymphocyte function-associated antigen 1) | Similar expression in both adipose tissue-derived mesenchymal stem cells and bone marrow mesenchymal stem cells | [133] |
| CD31 (platelet endothelial cell adhesion molecule) | Similar expression in both adipose tissue-derived mesenchymal stem cells and bone marrow mesenchymal stem cells | [133] |
| Not detected in either adipose tissue-stromal or bone marrow mesenchymal stem cells | [134] | |
| CD34 | Bone marrow mesenchymal stem cells were negative, while adipose tissue-stromal cells were positive | [134] |
| Low expression by adipose tissue-derived mesenchymal stem cells and no detection by bone marrow mesenchymal stem cells | [133] | |
| CD44 | Similar expression in both adipose tissue-derived mesenchymal stem cells and bone marrow mesenchymal stem cells | [134] |
| CD49d (integrin α4) | Found in adipose tissue-derived mesenchymal stem cells, while bone marrow mesenchymal stem cells did not | [133] |
| CD54 (intercellular adhesion molecule 1) | Adipose tissue-derived mesenchymal stem cells expressed high levels, while bone marrow mesenchymal stem cells had minimal expression | [134] |
| CD106 (vascular cell adhesion protein 1) | Bone marrow mesenchymal stem cells were positive, while adipose tissue-derived mesenchymal stem cells were negative | [134] |
| CD191 (C-C chemokine receptor type 1) | Expressed in human adipose tissue-derived mesenchymal stem cells more than bone marrow mesenchymal stem cells | [135] |
| Chemokines receptor types 1, 4, 6, and CX3C motif chemokine receptor 1 | Not detected in bone marrow mesenchymal stem cells compared to relevant expression in adipose tissue-derived mesenchymal stem cells | [135] |
Preference between primary isolated and cultured stem cells: Although researchers commonly use extended culturing techniques to expand MSC populations, high-passage MSCs exhibit diminished homing efficiency. Rombouts and Ploemacher[136] conducted a comparative study to examine the homing behavior of freshly isolated vs cultured MSCs. They assessed homing efficiency in the bone marrow and spleens of irradiated mice, reporting superior engraftment of primary uncultured MSCs. Twenty-four hours post-injection, approximately 55%-65% of primary MSCs were detected in the bone marrow and 3.5%-7% in the spleen. However, when MSCs were cultured for 24 hours, their homing capacity dramatically declined to approximately 10%, and after 48 hours of culturing, it was nearly undetectable[136].
Similarly, Kyriakou et al[137] investigated the impact of passage number (6-15) on the homing efficiency of (1.5-2.0) × 106 human MSCs in a xenogeneic mouse model. Mice receiving human MSCs from passages > 12 exhibited significantly fewer labeled cells in the spleen and bone marrow compared to those receiving MSCs from earlier passages < 8 (P < 0.01). Additionally, higher passage numbers were associated with decreased adipogenic differentiation. Lo Surdo and Bauer[138] observed a marked reduction in adipocyte differentiation by passage 7, likely due to defective expression of biological molecules essential for homing, which deteriorates as MSCs age in vitro. MSCs in passages 4 and 6 expressed different types and quantities of adhesion molecules[138]. While CD49b expression increased linearly, CD44 expression declined in passage 6. These molecular changes, altered morphology, and reduced proliferation rates indicate passage-dependent modifications. Moreover, passaging led to decreased CD34 expression[134]. However, some reports suggest no significant differences in CD73, CD90, and CD105 expression between passages 3 and 7[139]. Thus, MSC origin, culture conditions, and expansion methods should be carefully considered when evaluating their adhesive properties and therapeutic potential.
Delivery route and low retention rate: The efficacy and functionality of MSCs following delivery depend on the administration method. To achieve the maximal benefit of MSC engraftment, we should consider the process of MSC administration to potentiate homing to the desired destination. As mentioned earlier, cardiac regeneration using MSCs can be achieved through several injection routes, including IV, intra-arterial, intraperitoneal, intramyocardial, intracoronary, and retrograde coronary venous injection. Nevertheless, the fraction of MSCs retained in the damaged myocardium is a common obstacle faced by all existing routes of administration and delivery[140].
IV infusion of MSCs is the most common route of administration[141]. However, the major drawback of this method is the trapping into capillary beds of various tissues, particularly the lungs[142]. Although intra-arterial injection benefits from bypassing the lungs, there was a higher risk of microvascular accumulation and occlusions, a state known as passive entrapment[143]. An interesting study evaluated the three routes, IV, intra-aortic, and intramyocardial injection of BM-stromal cells, in the treatment of ischemic rat hearts. Direct intramyocardial injection gave the best cell retention and survival after 48 hours[142]. Therefore, local administration is preferable to avoid extravasation and cell arrest in the lung capillaries.
Other studies have shown comparable therapeutic effects between IV and intramyocardial MSC therapy in improving heart function and reducing fibrosis in a HF rat model[144]. However, human CSCs could not be detected in left ventricular samples of healthy pigs 30 days after delivery[145]. Moreover, locally injected cells may die due to a limited supply of oxygen and nutrients and local inflammation in injured tissues[146]. Hence, intracardiac MSCs injected into patients suffering from cardiomyopathy did not improve 10-year survival rates[147].
The age and gender of MSC donors: The donor’s age influences MSC proliferative capacity. When BM-MSCs were extracted from mice aged 6 days, 6 weeks, or 1 year, the cells from younger donors exhibited superior adhesion to tissue culture surfaces and proliferated more efficiently than those from older donors. Furthermore, chondrogenic, adipogenic, and osteogenic differentiation potential declines with age[148]. Similarly, the cardiac regenerative capacity of human MSCs significantly decreased by advanced age when comparing donors aged 1-5 years with those of 50-70 years[149]. A study involving human donors aged 13 to 80 years demonstrated that BM-MSCs from younger donors exhibited enhanced expression of adhesion molecules and growth factors, including: Activated leukocyte cell adhesion molecule, PDGF receptor β, melanoma cell adhesion molecule, VCAM-1, Thy1, programmed cell death ligand-1, and CD71, and displayed anti-inflammatory potential by reducing IL-6 production from activated T cells[150]. Similarly, homing was more effective when MSCs were retrieved from younger (< 10 weeks) mice than those from older ones[137].
In addition to age, donor sex is an important factor affecting MSC heterogeneity and therapeutic efficiency. Ock et al[151] found that AD-MSCs from young porcine females are more resistant to senescence and telomere length shortening. Female adipose stem cells also promoted the downregulation of IL-2 receptor gene expression, showing higher immunomodulatory effects than male cells, suggesting that female BM-MSCs are preferred in treating autoimmune diseases[151]. Human BM-MSCs retrieved from female donors were better at suppressing T cell proliferation. BN-MSCs possessed immunosuppressive properties over male cells. Indoleamine 2,3-dioxygenase, IFN-γ receptor 1, and IL-6β mRNA were more highly expressed in female than male BM-MSCs. Moreover, the female cells divided more rapidly. Female adipose stem cells produced higher levels of the anti-inflammatory mediators IL-1Ra, prostaglandin E2, and indoleamine 2,3-dioxygenase than those of males. In addition, female AD-MSCs showed prolonged expression of the adhesive molecule VCAM-1 when compared to male AD-MSCs[150]. Female BM-MSCs are recommended for cardiac regeneration over male BM-MSCs, as the Bcl-2/Bax ratio was significantly potentiated after treatment with female BM-MSCs compared to male-derived cells[152].
However, Zhang et al[153] did not identify any sex-related differences in immunosuppressive properties of UC-MSCs obtained from twins of different sexes. The authors documented similar differentiation potential but higher proliferation in male-derived cells. Additionally, male cells expressed more inflammatory cytokines in response to lipopolysaccharide exposure[153]. Researchers have attributed these sex-related differences in MSC regenerative potential to intrinsic biological factors, likely influenced by sex hormones. Estrogen exhibited stronger mitogenic effects in female BM-MSCs, while male BM-MSC proliferation was enhanced by preconditioning with estrogen and dexamethasone[154]. However, unlike their female counterparts, male muscle-derived cells did not exhibit improved skeletal muscle regeneration following estrogen treatment[155]. Thus, donor sex is an important consideration when using MSCs for myocardial regenerative therapy.
Immediately after AMI, the stressed cells from ischemia and/or reperfusion potentiate ROS and reactive nitrogen species generation due to disturbed mitochondrial machinery[156], which activate and promote cell death pathways. This triggers a massive loss of cardiomyocytes and microvessel destruction[102]. Additionally, secreted chemokines enhance the migration of T and B lymphocytes and innate immune cells, including neutrophils and macrophages. The recruited leukocytes, polarized macrophages, and damaged cells collectively produce a strong inflammatory response by expressing pro-inflammatory cytokines[157]. The highly inflammatory microenvironment of AMI negatively impacts MSC regenerative capacity. Pro-inflammatory cytokines such as NF-κB, TNF-α, and IL-1 inhibit MSC proliferation and differentiation, significantly contributing to the failure of stem cell therapy[158]. Given the weak regenerative capacity of cardiomyocytes, lost cardiomyocytes are replaced by fibrotic repair to maintain tissue integrity. Recruited macrophages and lymphocytes secrete bioactive pro-fibrotic mediators, particularly TGF-β1, which induces fibroblast transdifferentiation into active myofibroblasts. These myofibroblasts deposit ECM, remodel cardiac muscle, impair contractility, and progressively lead to HF[159].
Cardiac cell survival following AMI depends on an intact vasculature that delivers oxygen, nutrients, and growth factors. However, VECs are highly vulnerable to ischemic damage, which increases capillary permeability. Moreover, the basement membrane loses integrity, and vascular smooth muscle cells undergo apoptosis or dysfunction, exacerbating fluid and macromolecule leakage. This process accelerates fibrosis, tissue remodeling, and contractile dysfunction[160,161].
An intact vasculature is essential for stem cell survival, effective homing, and regenerative initiation. Myocardial hypoxia following AMI induces neovascularization through endothelial cell proliferation, migration, and tube formation[162]. While crucial for cellular nourishment, this process is constrained by the fibrogenic microenvironment. To overcome this limitation, stem cells promote angiogenesis by secreting paracrine factors, including angiopoietin-1, SDF-1, VEGF, IGF-1, HGF, bFGF, and activated eNOS[163,164] Some authors have implicated mesenchymal- endothelial-transition[165]. Hypoxia directs MSC differentiation toward VECs and smooth muscle cell generation[166,167]. However, their contribution to regeneration remains limited.
Because AMI is a critical emergency, clinical practice emphasizes early intervention to preserve cardiac muscle, hence the phrase “time is muscle”[168]. However, stem cell therapy was preferred to be postponed, ensuring thrombolysis and reperfusion[169]. In general, the efficacy of stem cells’ regenerative potential partially depends on the pathological condition of the host tissue microenvironment. MSC homing is positively affected by chemoattractants secreted by the ischemic and the inflammatory cells. The immune response starts immediately after ischemic injury and typically peaks at 4-7 days, then gradually regresses to a stable state over time[170]. In the same context, injection of MSCs within 1 week for AMI after percutaneous coronary intervention improved left ventricular systolic function[171]. Others prefer multiple injections of MSCs at the mid-term stage of AMI on days 7, 11, and 14[124].
Ethical and political concerns exist regarding the use of human embryonic stem cells. These cells are extracted from embryos at the blastocyst stage of preimplantation, which inevitably leads to the embryo’s destruction[172,173]. As an alternative, somatic undifferentiated pluripotent stem cells exhibit embryonic-like properties and possess strong cardiac regenerative potential. These cells are characterized by the distinct expression of cardiac markers and their differentiation into cardiomyocytes and VECs. However, challenges related to their isolation present a major limitation[174]. Compared to human embryonic stem cells, MSCs are less ethically controversial because their isolation does not directly threaten human life. However, ethical concerns may arise if MSCs undergo genetic modification. Additionally, their integration into recipient tissues carries the risk of genomic instability, potentially leading to malignancies[175]. For instance, in breast cancer mouse models, human MSCs have been shown to localize at tumor sites and promote tumor growth through immunomodulatory T-helper cytokine secretion, which stimulates CD4+ Foxp3+ regulatory T cells[176]. Another critical issue is the unwanted differentiation into unintended tissues like cartilage and bone rather than cardiomyocytes. In a mouse model of MI treated with MSCs, the researchers noticed abnormal encapsulated structures with calcifications or ossifications embedded in the infarcted area, which may be arrhythmogenic. This supports the fact that unwanted differentiation could be achieved[177]. Therefore, extensive preclinical research should be performed to avoid risks and validate the safety of MSCs in cardiac regenerative therapy.
A comprehensive understanding of the molecular mechanisms underlying MSC homing provides multiple strategies to enhance their regenerative efficacy. The objective is to optimize MSCs’ immunomodulatory, anti-inflammatory, antiapoptotic, and antifibrotic properties while improving their homing efficiency and proangiogenic effects. Several strategies, summarized in Figure 3, aim to enhance MSC survival and homing mechanisms to improve cardiac function.
Priming with hypoxia exposure: Oxygen is one of the principal factors for cell survival. Absolute or relative oxygen deficiency is a stressful condition that may progress to severe pathologies or death. MSCs showed diminished viability and proliferation when exposed to low-oxygen media[178]. However, researchers suggested that exposure to successive hypoxia and reoxygenation cycles could be effective in stimulating the expression of several pro-survival genes to enable MSCs to withstand the critical microenvironment[179]. Therefore, when MSCs were exposed to 1% oxygen media, the authors documented improved cell survival and enhanced angiogenesis properties associated with increased expression of VEGF and FGF2[180]. Moreover, hypoxia facilitates homing by inducing MMP expression by MSCs, resulting in enhanced migration[181].
Preimplantation priming of MSCs increases the expression of pro-angiogenic, pro-survival, and pro-differentiation factors, including VEGF, angiopoietin-1, erythropoietin, and hypoxia-inducible factor-1 (HIF-1), while downregulating pro-apoptotic proteins such as caspase-3[182]. Additionally, BM-MSC differentiation can be stimulated in vitro by conditioned medium containing hypoxia-induced cardiomyocyte differentiation factors, making it a viable strategy for cardiac regeneration[183].
Hypoxia influences differentiation, survival factor expression, and homing molecule expression. MSCs cultured under hypoxic conditions (1%-3% O2) exhibited increased migration and engraftment in ischemic regions, upregulating cMet, a key HGF receptor[184]. Culturing cells under 2% oxygen improved cell motility by inducing potassium channel and focal adhesion kinase expression. Moreover, hypoxia promotes cellular proliferation by elevating prion protein expression, which activates the Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway and inhibits apoptosis via caspase-3 suppression[185].
Minimizing MSC tumorigenicity in vivo is a critical clinical goal. Exposure to low oxygen tension (3% O2) reduced oxidative stress, prolonged cell lifespan by limiting telomere shortening, and protected against DNA damage and chromosomal aberrations. Thus, hypoxia preconditioning significantly contributes to MSC genetic stability[186]. How
Priming with cytokines and growth factors: Incubating MSCs with various cytokines subjects them to a stressful environment, enhancing their viability, stability, and homing capabilities. For instance, exposure to inflammatory cytokines such as IL-1, TGF-β1, or TNF-α enhances MSC migration by upregulating MMP expression[119]. These proinflammatory mediators like IL-1 can induce MSCs to release anti-inflammatory molecules, thus augmenting the immunosuppressive function of MSCs[187]. MSC migration in response to chemokines can be amplified by enriching the culture media with TNF-α; this promotes the upregulation of CCR2, CCR3, and CCR4 receptors[129]. MSC secretion of vascular regenerative factors like VEGF can be increased by preincubation with IL-8[188].
Preconditioned MSCs with IFN-γ and TNF-α have a significant immunosuppressive effect as they can inhibit T cell proliferation. Primed cells can direct monocyte differentiation into IL-10-secreting M2 cells[189]. In the same context, MSCs promoted by IFN-γ can suppress T cells via inhibiting T-helper 1 cytokine production and T cell degranulation. TNF-α and IL-1 have also been used to potentiate the immunomodulatory effects of IFN-γ on MSCs and inhibit complement activation[190]. Remarkably, the primed medium of MSCs induced by IL-1 has stronger immunomodulation than priming with IFN-γ or TNF-α. MSCs preconditioned with IL-1α or IL-1β secrete high levels of granulocyte colony-stimulating factor, IL-10, and other trophic factors compared to MSCs primed by IFN-γ or TNF-α[187]. Cytokine-preconditioned MSCs provide a future clinical insight for effective stem cell therapy. However, extensive research is still needed to determine the proper cytokine and dose.
Furthermore, various growth factors have been tested to determine whether they can improve MSC performance. Specifically, preconditioned MSCs with FGF-2 intensify MSC-induced vascularization by potentiating VEGF and HGF secretion[191]. The application of SDF-1 significantly promoted MSC survival and proliferation. This was achieved through upregulation of the Akt and ERK signaling pathways to increase survival, in addition to VEGF to enhance angiogenesis[192]. In the same context, the MSCs pretreated with TGF-α can increase angiogenesis through VEGF upregulation, which was achieved by activation of the p38 MAPK-dependent pathway[193].
Pharmacological enhancement: Preconditioning MSCs with various drugs, hormones, vitamins, and natural compounds has been shown to modify intracellular machinery, enhancing their therapeutic potential. The underlying mechanism of these substances generally involves modulating intracellular pathways to improve MSC efficacy. Several pharmacological agents, including anti-arrhythmic and anti-ischemic drugs, have been investigated for their ability to regulate MSC signaling.
Nicorandil, a known anti-arrhythmic drug, is an ATP-sensitive potassium channel opener. It has been shown to achieve protective effects against ischemic heart diseases. In a dose-dependent manner, Zhang et al[178] showed that nicorandil protected MSCs from hypoxia and serum deprivation-provoked apoptosis. Nicorandil preferentially diminished ROS production and increased stabilized mitochondrial membrane potential. It maintained the Bcl-2/Bax ratio and inhibited caspase-3 cleavage and activation. Nicorandil’s protective effects on stressed MSCs were mediated through PI3K signaling[178]. In another study by ShamsEldeen et al[194] in 2022, primed MSC cells with nicorandil upregulated the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway. The authors showed that nicorandil enhanced MSC proliferation, diminished apoptosis, and improved cell survival and homing efficacy.
Pretreatment with the anti-ischemic trimetazidine was effective in ischemic heart regeneration. Trimetazidine incubation with MSCs protected against oxidative stress-induced cell death through the stimulation of prosurvival factors HIF-1α, Akt, survivin, and Bcl-2[195]. In addition, preconditioned MSCs with atorvastatin maintained cell survival post-transplantation. Atorvastatin primed cardiac tissue repair by stimulating eNOS[196]. Further studies have demonstrated that angiotensin receptor blockers can improve cardiomyogenic differentiation efficiency[197].
Dexamethasone is a powerful anti-inflammatory glucocorticoid widely used in clinical practice. There was serious concern about the use of dexamethasone in MSC priming, as it may suppress the immunomodulatory effect of MSCs. However, Rawat et al[198] documented improved human MSCs immunomodulatory property, proliferation, and therapeutic efficacy in BM-MSCs, AD-MSCs, UC-MSCs, and dental pulp-derived MSC in a dose-dependent manner. Preincubated MSCs with hormones can also potentiate their survival and efficacy. The hormone possesses significant oxidative-scavenging properties. Melatonin upregulated the autophagy protein beclin-1 and superoxide dismutase, and it mitigated MSC proliferation and survival[199]. The YAP gene is a critical transcription factor downstream of Hippo, and it regulates cell proliferation, differentiation, growth, and apoptosis. Melatonin-primed cells conserve the YAP gene and cell viability[200]. Melatonin promotes MSC survival by modulating other specific pathways. It has emerged as an MSC signal modulator of the Wnt/β-catenin, MAPKs, and TGF-β pathways. These specific signals are crucial for controlling cell proliferation and apoptosis[201].
Oxytocin has been implicated in MSC modulation. Under hypoxic stress and serum deprivation, oxytocin-primed MSCs exhibited improved survival. Additionally, oxytocin promoted MSC migration and proliferation by inducing Akt and ERK1/2 protein expression[202]. Several natural compounds possess anti-inflammatory antioxidants and anti-apoptotic potentials. Priming with natural compounds could potentiate the regenerative power of MSCs. MSCs treatment with thymoquinone a nigella sativa extract increased total antioxidant capacity and potentiated migration efficiency based on the dose and time of exposure. The underlying mechanism is that thymoquinone induced c-Met and CXCR4 expression and further improved MSC immunogenicity mediated by modulating RORgammat and promyelocytic zinc finger protein expression[203].
The polyphenolic compound resveratrol ameliorates MSC survival and proliferation. Resveratrol suppresses apoptosis by regulating the Bcl-2/Bax ratio[204]. MSCs preconditioned with resveratrol mitigated DCM through the attenuation of secreted Frizzled-related proteins-mediated fibrosis and the downstream Wnt/β-catenin signals[56]. In addition, the bioactive compounds quercetin and rutin improved hUC-MSC viability, enhanced survival and homing[205]. Moreover, apple extract can prime MSCs and promote their proliferation via ERK phosphorylation and induction of VEGF and IL-6 expression[206].
Vitamins also participate in MSC priming to aid engraftment. Vitamin E increases MSC viability. The cells can survive despite hydrogen peroxide-induced oxidative stress[207]. Vitamin E potentiated the anti-inflammatory and immunomodulatory features of MSCs. Vitamin E increased the expression of cyclooxygenase-2, TNF-inducible gene 6 protein, and IL-1β genes while significantly decreasing cellular IL-6 and TGF-β expression[208]. Vitamin D3 application might also improve the cellular regenerative potential of MSCs therapy. Following a 5-day treatment, vitamin D3 stimulated proliferation and expression of pluripotency markers (NANOG, SOX2, and Oct4) and decreased senescence. The beneficial effects of vitamin D3 may be mediated through selective internal radiation therapy signals[209].
The primary biological function of platelets is to adhere to injured vascular endothelial and sub-endothelial structures, aggregate, and form a platelet plug for hemostasis. However, platelets have been implicated in the effective recruitment of MSCs. Direct observation of microvascular endothelial cell adhesion has revealed a complex interaction among MSCs, platelets, and leukocytes. In an interesting study, successful MSC homing to an inflamed ear were impaired if platelets were depleted from the blood. This suggests that platelets mediate MSC homing[210]. Increased recruitment of MSCs to human arterial endothelial cells was established when the endothelial cells were preincubated with platelets compared to endothelial cells primed with IL-1β. It was also shown that αvβ3-integrin blockade resulted in a significant reduction in the attachment between platelets and MSCs[211]. An in vitro flow assay further highlighted the critical role of platelet GpIIb/IIa and P-selectin in MSC adhesion. Blocking these molecules impaired MSC adhesion following injection for pulmonary arterial hypertension treatment[165]. These findings suggest that MSCs interact with blood platelets, which may influence MSC signaling, adhesion, and homing.
The effects of the in vitro priming strategy for cell transplantation into the heart may not last long. Therefore, research has focused on supporting in vivo priming approaches to extend the duration of in vitro preconditioning. The challenge of IV MSC injection is cell entrapment in the pulmonary microvasculature. An acceptable strategy is to precondition the host with a vasodilator such as sodium nitroprusside to reduce trapped cells[212].
From the recent advances to achieve in vivo priming, Park et al[213] rebuilt the myocardial microenvironment by priming heart tissues with loaded hMSCs with genetically engineered HGF implanted in the epicardium. The patch was designed for continuous HGF secretion into a three-dimensional-printed patch. The rationale behind this procedure was to prime BM-MSCs in vivo with HGF to promote cell survival, achieve efficient engraftment and cardiac regeneration, and restore cardiac functions.
Over the past decades, the innovations of biomaterial-based implants have extensively attracted special clinical interest in organ damage or failure management. Recent technologies in tissue engineering provide promising strategies for different cardiac injuries including acute and chronic ischemia, arrhythmias, and valvular dysfunctions. Tissue engi
It is preferred in cardiac tissue engineering to focus on the three-dimensional biomaterial scaffolds loaded with cells and growth factors to maximize the benefits of cardiac repair and regeneration[215]. With advances in tissue engineering, it is suspected to provide MSCs with long-term survival and effective cardiac regenerative potential if supported by a suitable scaffold biomaterial, microcapsule containing agarose, collagen, fibrin, and dextran sulfate. The microcapsule permits optimal oxygen and growth factor translocation and nutrient exchange[216].
One biomaterial that can support ECM proteins and enhance cell adhesion and proliferation is graphene oxide (GO). GO epicardial implantation can improve cardiac tissue’s mechanical and electrical properties[214]. Additionally, reduced GO has been found to enhance the paracrine activity of MSCs due to its strong affinity for ECM fibronectin. Preconditioning MSCs with reduced GO before cardiac implantation promotes cell-ECM interactions, induces the expression of angiogenic growth mediators, and upregulates the gap junction protein connexin 43, thereby improving MSC-mediated heart regeneration[217].
The current approach to MSC genome reprogramming is to up or down-regulate specific genes through non-viral or viral vectors. Genetic modifications are directed to modulate the expression of MSCs receptors and immunomodulators, induce secretion of paracrine mediators necessary for better survival, migration, adhesion, and angiogenesis, and improve cardiac-specific differentiation[218]. One of the most important drawbacks of MSCs in cardiac regeneration is the low retention and survival rates. The pro-survival strategy primarily targets the cell death machinery by knocking down molecules that contribute to the apoptotic signal. The other approach is to potentiate the expression of pro-survival genes and growth factors to develop super stem cells[200]. Cytoprotection was attained by overexpressing the pro-survival Akt gene in human MSCs. Ischemic hearts treated with those genetically modulated MSCs showed marked improvement in cardiac functions associated with inhibiting unfavorable remodeling[219,220]. Additionally, the dual expression of Akt and angiopoietin 1 in MSCs can result in prolonged survival, advanced angiogenesis, and cardiac function recovery[221].
Upregulating the pro-survival gene Bcl-2 remarkably inhibited apoptosis and amplified the secretion of the angiogenic factor VEGF. The transplanted cells in ischemic hearts revealed reduced infarct size along with higher capillary density[222]. HO-1 is a known cytoprotective agent transcribed during cellular stress to ensure cell survival against mediators such as high mobility group box 1[223]. HO-1 modified MSCs showed a 5-fold increase in survival compared to the non-modified cells. HO-1 transfected MSCs have improved the peri-infarction vascular density in a model of MI[224]. Additionally, overexpressing the immunosuppressive factor cyclooxygenase 2 can potentiate their survival rate in ischemic rat heart management[42]. Additionally, lentiviral transfection overexpressing IGF-1 in AD-MSCs promoted VEGF and HGF secretion and restored cardiac functions compared to the native MSCs[225]. Also, genetic modulation for IGF-1 upregulation combined with 5-Aza potentiated cardiogenic differentiation to a higher extent compared to 5-Aza alone[226].
The different homing phases can be facilitated by interfering with gene expression. Overexpressed genes encode molecules such as SDF-1, which preferentially enhance cell migration[227]. Similarly, upregulating CXCR or CCR receptors in MSCs facilitated cell migration, along with enhancing angiogenesis, and ultimately restored cardiac functions following MI[110]. Integrin-linked kinase has been overexpressed to help MSC adhesion. Transplanted integrin-linked kinase + MSCs diminished infarct size and cardiac fibrosis, increased vasculature, and supported cardiac recovery following injury[228]. Although preclinical studies and theoretical perspectives highlight the potential of genetic modification, long-term consequences remain uncertain. Safety remains a critical concern since integration into the host genome may increase tumorigenicity risks. Further research is necessary to accurately track the fate of gene-modified MSCs and evaluate potential safety risks.
Sublethal irradiation directed to specific organs triggers intended tissue injury[229]. This form of injury is responsible for the increased local secretion of cytokines and chemokines. Therefore, injected MSCs overexpressing CXCR4 by lentiviral transduction enhanced bone marrow homing into animals preconditioned with irradiation[137]. Although radiotherapeutic priming is an attractive concept, it may not be valid in clinical applications because of human safety issues. As an alternative, ultrasound has gained attention for its therapeutic applications beyond diagnostics. Ultrasound waves exert mechanical pressure on tissues, triggering various biological responses[230]. Notably, ultrasound-mediated techniques enhance MSC homing and improve post-ischemic cardiac recovery. Ultrasound exposure increased the expression of VCAM-1 and ICAM-1 while upregulating myocardial VEGF and bFGF levels[231].
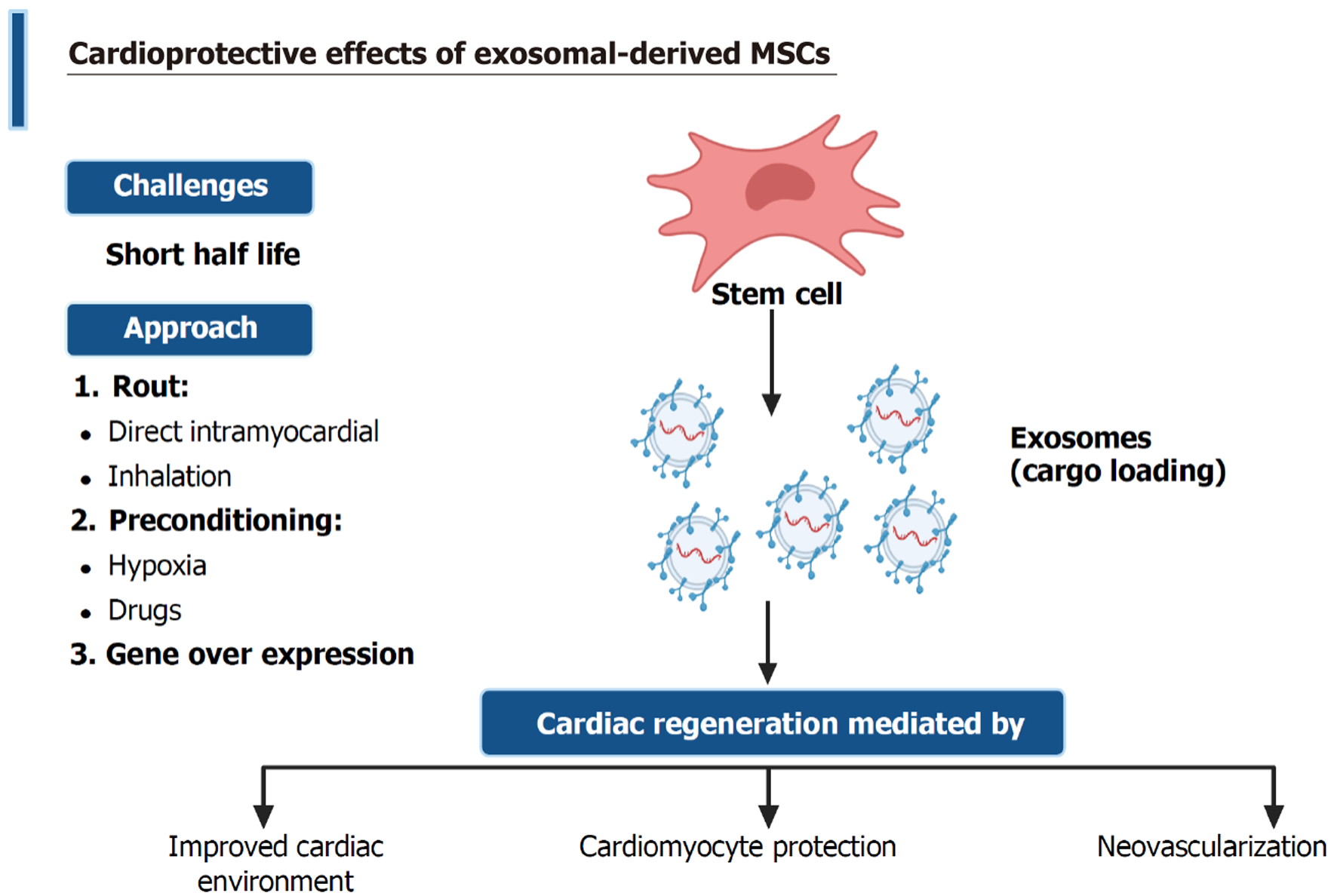
The recognition of small EVs has introduced a promising approach to overcoming the limitations of stem cell therapy. Unlike MSCs, EVs derived from stem cells do not exhibit the immunogenicity associated with MSC surface markers[232]. They have lower tumorigenic risk, higher immunological tolerance, and superior stability than their parent stem cells. Exosomes are the smallest form of EVs; thus, the therapeutic potential of MSC-derived exosomes has been investigated for use in cardiac repair and regeneration[233], as shown in Figure 4.
The efficacy of MSC-exosome treatments in several preclinical models of cardiovascular disease offers a promising perspective in modulating a wide range of cellular signals within cardiac tissues. However, as shown in Table 3, the defective standardization of exosome isolation, purification, and characterization may affect the potency and dose required to achieve a compelling target. A significant challenge is the deficient knowledge regarding the specific molecular composition of EVs[234].
| Cardiac injury | Isolation method | Dose | Number of injections/timing of injections | Route | Ref. |
| Ischemia/reperfusion | High-performance liquid chromatography | 0.4-0.8 μg | Single dose/5 minutes prior to reperfusion | In the perfusion fluid ex-vivo | [89] |
| Ischemia/reperfusion | High-performance liquid chromatography | 0.4 μg | Single/5 minutes prior to reperfusion | Intravenous injection | [235] |
| Myocardial infarction | Purification from conditioned media | 2 × 1011 particles | Single/tail-vein 3 hours after reperfusion | Intravenous injection | [254] |
| Myocardial infarction | ExoQuick-TC system | Harvested from 4 × 106 MSCs | Single/following left anterior descending artery ligation | Intramyocardial injection | [258] |
| Myocardial infarction | Exosome isolation reagent (#4478359, Thermo Scientific, San Jose, CA, United States) | 5 μg | Single/injected 30 minutes after left anterior descending artery ligation (at three sites of the border zone of the infarcted area) | Intramyocardial injection | [259] |
| Myocardial infarction | ExoQuick-TC system | 80 μg | Single/injected 60 minutes after left anterior descending artery ligation (at four sites of the border zone of the infarcted area) | Intramyocardial injection | [236] |
| Myocardial infarction | Ultrafiltration method | 1 × 1011 particles/kg body weight | Multiple/7 consecutive days after myocardial infarction | Inhalation | [235] |
| Doxorubicin/trastuzumab-induced cardiac toxicity | Purified exosomes filtered through a 0.2 mm membrane NTA technology | 3 × 1010 particles | Multiple/at days 5, 11, and 19 of the experiment | Intravenous injection | [260] |
Recently, researchers have made significant efforts to explore the specific molecular targets of EVs in mitigating cardiac dysfunction. Arslan et al[89] investigated MSC-exosomes in myocardial remodeling following ischemia/reperfusion injury. They documented diminished oxidative stress and promotion of the cardiac PI3K/Akt pathway[89]. Exosomes downregulated CD36 in VECs. CD36 is a fatty acid transporter, and its inhibition favors cardiac glucose utilization and enhances cardiac contractility[235]. Exosomes injected directly into the myocardium reduced infarct size and preserved cardiac performance. This effect was achieved through improved perfusion mediated by neovascularization in a rat MI model[236].
MSC-derived exosomes can reduce ECM deposition and cardiac apoptosis and increase HIF-1α and VEGF levels[43], as well as myocardial SDF-1 expression levels[237]. Additionally, EVs can be engineered to deliver specific therapeutic cargo to targeted tissues or organs[238]. MSC-derived EVs contain various bioactive molecules, including DNA, RNA, miRNA, cytokines, ILs, chemokines, and growth factors[239]. MSC-derived exosomes may act as carriers, facilitating the systemic delivery of different miRNAs such as miR-126, miR-144, miR-125b-5p, and miR-29b, which can mediate cardioprotection against hypoxic conditions both in vivo by preventing cardiac fibrosis and reducing infarction size and in vitro by decreasing cardiomyocyte apoptosis[240-243]. In addition, MSC-derived exosomes can regulate cell proliferation and autophagy following accumulation in ischemic myocardial tissue. ADSC-derived exosomes containing miR-93-5p can suppress autophagy and inflammation, thus reducing infarction-mediated myocardial damage[244].
In a study by Li et al[245] in 2019, the injection of bone marrow-derived exosomes containing miR-301 resulted in improved cardiac function, suppressed autophagy, decreased the number of autophagosomes, and ultimately reduced the light chain 3-II/Light chain 3-I ratio in rats subjected to MI. However, other studies have documented autophagy induction as one of the mechanisms underlying MSC-derived exosome-mediated effects. Liu et al[246] demonstrated reduced cardiac apoptosis by inducing cardiomyocyte autophagy via the AMP-activated protein kinase/mTOR and Akt/mTOR pathways. Therefore, the induction or suppression of autophagy may depend on the mechanism of action of exosomes and the contents of exosomal miRNA.
Cardiac repair is notably enhanced by EVs extracted from preconditioned stem cells and enriched with miRNAs. Zhang et al[247] demonstrated that hypoxia-preconditioned MSC-derived exosomes contained high levels of miR-24, which protected rat cardiomyocytes from senescence upon co-incubation. Similarly, exosomes extracted from MSCs subjected to hypoxic priming exhibited increased HIF-1α expression, leading to miR-210 enrichment. These exosomes effectively promoted cardiomyocyte survival and reduced cardiac scarring following ischemic injury[248]. Furthermore, MSC-derived exosomes from cells exposed to hypoxia/reoxygenation expressed high levels of miR-486-5p. This miRNA activated the PI3K/Akt pathway, preventing cardiomyocyte apoptosis[249]. In a doxorubicin-induced cardiac injury model, miR-96-enriched exosomes promoted cardiac recovery by reducing free radicals, inhibiting the Rac1/NF-κB pathway, and preventing fibrosis accumulation[250]. In another study, Chinnici et al[251] found that EVs expressing miRNA subtypes (27b-3p, 146a-5p, 125a-5p, 137, and 126-3p) stimulated the endogenous angiogenic factor VEGF-A. Ma et al[252] previously documented the delivery of protein by MSC-derived exosomes. They noticed better cardioprotective effects of exosomes derived from Akt gene-modified human umbilical cord MSCs and increased expression of PDGF-D in Akt-exosomes compared to exosomes extracted from non-genetically modified stem cells.
CSCs primed with MSC-derived exosomes exhibited significantly better survival, neovascular capillary formation, reduced fibrosis, and preserved myocardial contractility. MiRNA profiling identified a notable increase in miR-147 and decrease in miR-328 and 326[253]. Moreover, overexpression of CXCR4 in exosomes improves their cardioprotective efficacy. Thus, the IV injection of exosome-CXCR4 significantly diminished infarct size and restored left ventricular contractility 4 weeks after MI[254]. Preconditioning exosomes with drugs is an effective approach to validating exosomal efficacy. Intramyocardial injection of MSC-exosomes combined with IV infusion of atorvastatin-pretreated MSCs 30 minutes after MI in rat hearts improved the microenvironment, decreased infarction, and promoted neovascularization[73]. Similarly, priming with adiponectin stimulated exosome biogenesis and secretion. Adiponectin enhanced the therapeutic efficacy of MSCs via binding to T-cadherin on MSCs[255].
The pharmacological preconditioning of MSCs with a sodium-glucose cotransporter 2 inhibitor (specifically, empagliflozin) was previously investigated. The authors studied the cardioprotective effect of small EVs extracted from MSCs preconditioned with empagliflozin vs those from non-conditioned MSCs and found that empagliflozin-EVs provided stronger cardioprotection against MI than EVs derived from non-conditioned MSCs[256]. Although the preclinical studies are promising, there remains a significant gap between experimental findings and the clinical application of MSC-exosomes in myocardial injury. Moreover, the comparison between MSC-derived exosomes and pharmacological treatments in cardiac repair is still underexplored. Thus, future studies are needed to investigate and address these challenges, particularly in the context of cardiac injuries.
The estimated half-life of exosomes is as low as 2-30 minutes, with approximately 90% of exosomes removed within 5 minutes after IV injection. Circulating exosomes are primarily sequestered by liver macrophages[257]. Therefore, site-specific delivery of EVs could enhance their engraftment and improve cardioprotective effects. Alternating the route of administration may help to overcome the intrinsic challenges of exosomes’ short half-life. Direct application in the myocardium has successfully improved myocardial properties following ischemic injury[236,254,258,259]. Interestingly, inhalation of exosomes appears to be an effective method, particularly when administered in repeated doses[235]. Supporting the importance of repeated dosing, three IV injections of cardiac progenitor cell-derived exosomes on days 5, 11, and 19 of the experiment were effective in a doxorubicin/trastuzumab-induced cardiac toxicity rat model[260].
The underlying mechanism of MSCs’ actions and roles in combating cardiovascular diseases have been extensively investigated. The ameliorative effect of MSCs in mitigating cardiac injuries is mediated through their immunoregulatory, anti-apoptotic, antifibrotic, antioxidant, cardiogenesis, and angiogenic activities. In vascular disorders such as AA and atherosclerosis, MSCs suppress the production of pro-inflammatory cytokines and improve endothelial reactivity. In cardiomyopathies, MSCs improved LVEF by promoting local myocardial repair and regeneration. However, the primary challenge facing the regenerative capacity of stem cells is their homing potential. We have identified factors related to the stem cells, as well as the donor and recipient. It is crucial to select the stem cell origin and delivery route. Additionally, the high proliferation capacity of MSCs is influenced by the donor’s age. Moreover, in AMI, an absolute emergency, timely intervention is the main factor that is highly required to support MSC angiogenesis potential. The in vitro exposure to successive cycles of hypoxia and reoxygenation has been shown to stimulate the expression of MSC pro-survival genes. Preconditioning with cytokines and growth factors promotes and enhances their immunomodulatory properties, while pharmacological preconditioning modifies their intracellular machinery toward better homing. In recent decades, tissue engineering has provided promising strategies for enhancing the therapeutic potential of MSCs by offering suitable biomaterials that support stem cell viability and function or genetic editing to optimize cardiac repair and regeneration. Finally, future studies are highly recommended to explore more challenging factors and strategies to master them.
| 1. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 1052] [Article Influence: 150.3] [Reference Citation Analysis (35)] |
| 2. | Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 709] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 4. | Shah AA, Khan FA. Types and Classification of Stem Cells. In: Khan FA. Advances in Application of Stem Cells: From Bench to Clinics. Stem Cell Biology and Regenerative Medicine. Cham: Springer. [DOI] [Full Text] |
| 5. | Gucciardo L, Lories R, Ochsenbein-Kölble N, Done' E, Zwijsen A, Deprest J. Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG. 2009;116:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Casteilla L, Dani C. Adipose tissue-derived cells: from physiology to regenerative medicine. Diabetes Metab. 2006;32:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Xu X, Chen C, Akiyama K, Chai Y, Le AD, Wang Z, Shi S. Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J Dent Res. 2013;92:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Fuloria S, Subramaniyan V, Dahiya R, Dahiya S, Sudhakar K, Kumari U, Sathasivam K, Meenakshi DU, Wu YS, Sekar M, Malviya R, Singh A, Fuloria NK. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges. Biology (Basel). 2021;10:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Li Q, Sun W, Wang X, Zhang K, Xi W, Gao P. Skin-Derived Mesenchymal Stem Cells Alleviate Atherosclerosis via Modulating Macrophage Function. Stem Cells Transl Med. 2015;4:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front Cell Dev Biol. 2020;8:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 11. | Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1394] [Cited by in RCA: 2366] [Article Influence: 338.0] [Reference Citation Analysis (35)] |
| 12. | Fennema EM, Tchang LAH, Yuan H, van Blitterswijk CA, Martin I, Scherberich A, de Boer J. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J Tissue Eng Regen Med. 2018;12:e150-e158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Wang S, Guo L, Ge J, Yu L, Cai T, Tian R, Jiang Y, Zhao RCh, Wu Y. Excess Integrins Cause Lung Entrapment of Mesenchymal Stem Cells. Stem Cells. 2015;33:3315-3326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2699] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 15. | Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3959] [Cited by in RCA: 4429] [Article Influence: 402.6] [Reference Citation Analysis (0)] |
| 16. | Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 17. | Sędzik M, Rakoczy K, Sleziak J, Kisiel M, Kraska K, Rubin J, Łuniewska W, Choromańska A. Comparative Analysis of Exosomes and Extracellular Microvesicles in Healing Pathways: Insights for Advancing Regenerative Therapies. Molecules. 2024;29:3681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1109] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 19. | Spriet M, Hunt GB, Walker NJ, Borjesson DL. Scintigraphic tracking of mesenchymal stem cells after portal, systemic intravenous and splenic administration in healthy beagle dogs. Vet Radiol Ultrasound. 2015;56:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Salvadori M, Cesari N, Murgia A, Puccini P, Riccardi B, Dominici M. Dissecting the Pharmacodynamics and Pharmacokinetics of MSCs to Overcome Limitations in Their Clinical Translation. Mol Ther Methods Clin Dev. 2019;14:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 406] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 22. | Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1304] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 23. | Yan J, Liang J, Cao Y, El Akkawi MM, Liao X, Chen X, Li C, Li K, Xie G, Liu H. Efficacy of topical and systemic transplantation of mesenchymal stem cells in a rat model of diabetic ischemic wounds. Stem Cell Res Ther. 2021;12:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Achón Buil B, Tackenberg C, Rust R. Editing a gateway for cell therapy across the blood-brain barrier. Brain. 2023;146:823-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 25. | Liu J, Liang X, Li M, Lin F, Ma X, Xin Y, Meng Q, Zhuang R, Zhang Q, Han W, Gao L, He Z, Zhou X, Liu Z. Intramyocardial injected human umbilical cord-derived mesenchymal stem cells (HucMSCs) contribute to the recovery of cardiac function and the migration of CD4(+) T cells into the infarcted heart via CCL5/CCR5 signaling. Stem Cell Res Ther. 2022;13:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Attar A, Farjoud Kouhanjani M, Hessami K, Vosough M, Kojuri J, Ramzi M, Hosseini SA, Faghih M, Monabati A. Effect of once versus twice intracoronary injection of allogeneic-derived mesenchymal stromal cells after acute myocardial infarction: BOOSTER-TAHA7 randomized clinical trial. Stem Cell Res Ther. 2023;14:264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Qi Z, Yan Z, Ji N, Yang X, Gao D, Hu L, Lv H, Zhang J, Li M. Mesenchymal Stem Cell Immunomodulation: A Novel Intervention Mechanism in Cardiovascular Disease. Front Cell Dev Biol. 2021;9:742088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Hashizume R, Yamawaki-Ogata A, Ueda Y, Wagner WR, Narita Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J Vasc Surg. 2011;54:1743-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Fu XM, Yamawaki-Ogata A, Oshima H, Ueda Y, Usui A, Narita Y. Intravenous administration of mesenchymal stem cells prevents angiotensin II-induced aortic aneurysm formation in apolipoprotein E-deficient mouse. J Transl Med. 2013;11:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Li X, Wen H, Lv J, Luan B, Meng J, Gong S, Wen J, Xin S. Therapeutic efficacy of mesenchymal stem cells for abdominal aortic aneurysm: a meta-analysis of preclinical studies. Stem Cell Res Ther. 2022;13:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 31. | Chen Z, Xia X, Yao M, Yang Y, Ao X, Zhang Z, Guo L, Xu X. The dual role of mesenchymal stem cells in apoptosis regulation. Cell Death Dis. 2024;15:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 32. | Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1404] [Cited by in RCA: 1684] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 33. | Mahdavi Gorabi A, Banach M, Reiner Ž, Pirro M, Hajighasemi S, Johnston TP, Sahebkar A. The Role of Mesenchymal Stem Cells in Atherosclerosis: Prospects for Therapy via the Modulation of Inflammatory Milieu. J Clin Med. 2019;8:1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Egea V, Megens RTA, Santovito D, Wantha S, Brandl R, Siess W, Khani S, Soehnlein O, Bartelt A, Weber C, Ries C. Properties and fate of human mesenchymal stem cells upon miRNA let-7f-promoted recruitment to atherosclerotic plaques. Cardiovasc Res. 2023;119:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Frodermann V, van Duijn J, van Pel M, van Santbrink PJ, Bot I, Kuiper J, de Jager SC. Mesenchymal Stem Cells Reduce Murine Atherosclerosis Development. Sci Rep. 2015;5:15559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Kirwin T, Gomes A, Amin R, Sufi A, Goswami S, Wang B. Mechanisms underlying the therapeutic potential of mesenchymal stem cells in atherosclerosis. Regen Med. 2021;16:669-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Potapova IA, Doronin SV, Kelly DJ, Rosen AB, Schuldt AJ, Lu Z, Kochupura PV, Robinson RB, Rosen MR, Brink PR, Gaudette GR, Cohen IS. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Am J Physiol Heart Circ Physiol. 2008;295:H2257-H2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Chang SA, Lee EJ, Kang HJ, Zhang SY, Kim JH, Li L, Youn SW, Lee CS, Kim KH, Won JY, Sohn JW, Park KW, Cho HJ, Yang SE, Oh WI, Yang YS, Ho WK, Park YB, Kim HS. Impact of myocardial infarct proteins and oscillating pressure on the differentiation of mesenchymal stem cells: effect of acute myocardial infarction on stem cell differentiation. Stem Cells. 2008;26:1901-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 40. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 1015] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 41. | Jun Hong S, Rogers PI, Kihlken J, Warfel J, Bull C, Deuter-Reinhard M, Feng D, Xie J, Kyle A, Merfeld-Clauss S, Johnstone BH, Traktuev DO, Chen PS, Lindner JR, March KL. Intravenous xenogeneic transplantation of human adipose-derived stem cells improves left ventricular function and microvascular integrity in swine myocardial infarction model. Catheter Cardiovasc Interv. 2015;86:E38-E48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Sareen N, Abu-El-Rub E, Ammar HI, Yan W, Sequiera GL, ShamsEldeen AM, Moudgil M, Dhingra R, Shokry HS, Rashed LA, Kirshenbaum LA, Dhingra S. Hypoxia-induced downregulation of cyclooxygenase 2 leads to the loss of immunoprivilege of allogeneic mesenchymal stem cells. FASEB J. 2020;34:15236-15251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Kalou Y, Al-Khani AM, Haider KH. Bone Marrow Mesenchymal Stem Cells for Heart Failure Treatment: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2023;32:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Krishna Mohan GV, Tirumandyam G, Vemulapalli HS, Vajje J, Asif H, Saleem F. Mesenchymal Stem Cell Therapy for a Better Prognosis of Heart Failure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cureus. 2023;15:e43037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 45. | Li X, Zhao H, Qi C, Zeng Y, Xu F, Du Y. Direct intercellular communications dominate the interaction between adipose-derived MSCs and myofibroblasts against cardiac fibrosis. Protein Cell. 2015;6:735-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 47. | Golpanian S, El-Khorazaty J, Mendizabal A, DiFede DL, Suncion VY, Karantalis V, Fishman JE, Ghersin E, Balkan W, Hare JM. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol. 2015;65:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Tsai IT, Sun CK. Stem Cell Therapy against Ischemic Heart Disease. Int J Mol Sci. 2024;25:3778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 49. | Jin L, Zhang J, Deng Z, Liu J, Han W, Chen G, Si Y, Ye P. Mesenchymal stem cells ameliorate myocardial fibrosis in diabetic cardiomyopathy via the secretion of prostaglandin E2. Stem Cell Res Ther. 2020;11:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y, Si Y, Guo Y, Zang L, Mu Y, Han W. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016;34:627-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 51. | Hao H, Liu J, Shen J, Zhao Y, Liu H, Hou Q, Tong C, Ti D, Dong L, Cheng Y, Mu Y, Liu J, Fu X, Han W. Multiple intravenous infusions of bone marrow mesenchymal stem cells reverse hyperglycemia in experimental type 2 diabetes rats. Biochem Biophys Res Commun. 2013;436:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 52. | Gong X, Wang P, Wu Q, Wang S, Yu L, Wang G. Human umbilical cord blood derived mesenchymal stem cells improve cardiac function in cTnT(R141W) transgenic mouse of dilated cardiomyopathy. Eur J Cell Biol. 2016;95:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y, Shen J, Cheng Y, Fu X, Han W. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes. 2012;61:1616-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 54. | Yang Q, Chen Q, Li S, Luo J. Mesenchymal stem cells ameliorate inflammation and pyroptosis in diabetic cardiomyopathy via the miRNA-223-3p/NLRP3 pathway. Diabetol Metab Syndr. 2024;16:146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 55. | da Silva JS, Gonçalves RGJ, Vasques JF, Rocha BS, Nascimento-Carlos B, Montagnoli TL, Mendez-Otero R, de Sá MPL, Zapata-Sudo G. Mesenchymal Stem Cell Therapy in Diabetic Cardiomyopathy. Cells. 2022;11:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | ShamsEldeen AM, Ashour H, Shoukry HS, Fadel M, Kamar SS, Aabdelbaset M, Rashed LA, Ammar HI. Combined treatment with systemic resveratrol and resveratrol preconditioned mesenchymal stem cells, maximizes antifibrotic action in diabetic cardiomyopathy. J Cell Physiol. 2019;234:10942-10963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Nakazaki M, Oka S, Sasaki M, Kataoka-Sasaki Y, Nagahama H, Hashi K, Kocsis JD, Honmou O. Prolonged lifespan in a spontaneously hypertensive rat (stroke prone) model following intravenous infusion of mesenchymal stem cells. Heliyon. 2020;6:e05833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Khater NA, Selim SA, Abd El-Baset SA, Abd El Hameed SH. Therapeutic effect of mesenchymal stem cells on experimentally induced hypertensive cardiomyopathy in adult albino rats. Ultrastruct Pathol. 2017;41:36-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Marketou ME, Parthenakis FI, Kalyva A, Pontikoglou C, Maragkoudakis S, Kontaraki JE, Zacharis EA, Chlouverakis G, Patrianakos A, Papadaki HA, Vardas PE. Increased mobilization of mesenchymal stem cells in patients with essential hypertension: the effect of left ventricular hypertrophy. J Clin Hypertens (Greenwich). 2014;16:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Li R, Dai Z, Ye R, Liu X, Xia Z, Xu G. Magnetic stimulation of carotid sinus as a treatment for hypertension. J Clin Hypertens (Greenwich). 2019;21:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Liu J, Li J, Xie C, Xuan L, Tang B. MSCs attenuate hypoxia induced pulmonary hypertension by activating P53 and NF-kB signaling pathway through TNFα secretion. Biochem Biophys Res Commun. 2020;532:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Luo L, Chen Q, Yang L, Zhang Z, Xu J, Gou D. MSCs Therapy Reverse the Gut Microbiota in Hypoxia-Induced Pulmonary Hypertension Mice. Front Physiol. 2021;12:712139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Sun QW, Sun Z. Stem cell therapy for pulmonary arterial hypertension: An update. J Heart Lung Transplant. 2022;41:692-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 64. | Hansmann G, Chouvarine P, Diekmann F, Giera M, Ralser M, Mülleder M, von Kaisenberg C, Bertram H, Legchenko E, Hass R. Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension. Nat Cardiovasc Res. 2022;1:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Li X, Sun W, Xi W, Shen W, Wei T, Chen W, Gao P, Li Q. Transplantation of skin mesenchymal stem cells attenuated AngII-induced hypertension and vascular injury. Biochem Biophys Res Commun. 2018;497:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Melief SM, Geutskens SB, Fibbe WE, Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica. 2013;98:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 67. | Shi H, Liang M, Chen W, Sun X, Wang X, Li C, Yang Y, Yang Z, Zeng W. Human induced pluripotent stem cellderived mesenchymal stem cells alleviate atherosclerosis by modulating inflammatory responses. Mol Med Rep. 2018;17:1461-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Marino L, Castaldi MA, Rosamilio R, Ragni E, Vitolo R, Fulgione C, Castaldi SG, Serio B, Bianco R, Guida M, Selleri C. Mesenchymal Stem Cells from the Wharton's Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential. Int J Stem Cells. 2019;12:218-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 69. | Philipp D, Holthaus M, Basoah V, Pfannkuche K, Suhr L, Wahlers T, Paunel-Görgülü A. VEGF Contributes to Mesenchymal Stem Cell-Mediated Reversion of Nor1-Dependent Hypertrophy in iPS Cell-Derived Cardiomyocytes. Stem Cells Int. 2021;2021:8888575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Che N, Li X, Zhou S, Liu R, Shi D, Lu L, Sun L. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol. 2012;274:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 357] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 72. | Takafuji Y, Hori M, Mizuno T, Harada-Shiba M. Humoral factors secreted from adipose tissue-derived mesenchymal stem cells ameliorate atherosclerosis in Ldlr-/- mice. Cardiovasc Res. 2019;115:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 73. | Huang P, Wang L, Li Q, Xu J, Xu J, Xiong Y, Chen G, Qian H, Jin C, Yu Y, Liu J, Qian L, Yang Y. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res Ther. 2019;10:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 74. | Monguió-Tortajada M, Prat-Vidal C, Moron-Font M, Clos-Sansalvador M, Calle A, Gastelurrutia P, Cserkoova A, Morancho A, Ramírez MÁ, Rosell A, Bayes-Genis A, Gálvez-Montón C, Borràs FE, Roura S. Local administration of porcine immunomodulatory, chemotactic and angiogenic extracellular vesicles using engineered cardiac scaffolds for myocardial infarction. Bioact Mater. 2021;6:3314-3327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 75. | Niu J, Yu F, Luo X, Chen S. Human Umbilical Cord Mesenchymal Stem Cells Improve Premature Ovarian Failure through Cell Apoptosis of miR-100-5p/NOX4/NLRP3. Biomed Res Int. 2022;2022:3862122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 76. | He A, Jiang Y, Gui C, Sun Y, Li J, Wang JA. The antiapoptotic effect of mesenchymal stem cell transplantation on ischemic myocardium is enhanced by anoxic preconditioning. Can J Cardiol. 2009;25:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, Yan C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 78. | Zhang L, Liu Q, Hu H, Zhao L, Zhu K. Progress in mesenchymal stem cell mitochondria transfer for the repair of tissue injury and treatment of disease. Biomed Pharmacother. 2022;153:113482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 79. | Seow KS, Ling APK. Mesenchymal stem cells as future treatment for cardiovascular regeneration and its challenges. Ann Transl Med. 2024;12:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 80. | Gubert F, da Silva JS, Vasques JF, de Jesus Gonçalves RG, Martins RS, de Sá MPL, Mendez-Otero R, Zapata-Sudo G. Mesenchymal Stem Cells Therapies on Fibrotic Heart Diseases. Int J Mol Sci. 2021;22:7447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Huang W, Wang T, Zhang D, Zhao T, Dai B, Ashraf A, Wang X, Xu M, Millard RW, Fan GC, Ashraf M, Yu XY, Wang Y. Mesenchymal stem cells overexpressing CXCR4 attenuate remodeling of postmyocardial infarction by releasing matrix metalloproteinase-9. Stem Cells Dev. 2012;21:778-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Zhang C, Zhou Y, Lai X, Zhou G, Wang H, Feng X, Chen Y, Wu Y, Wang T, Ma L. Human Umbilical Cord Mesenchymal Stem Cells Alleviate Myocardial Endothelial-Mesenchymal Transition in a Rat Dilated Cardiomyopathy Model. Transplant Proc. 2019;51:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol Rev. 2016;96:1127-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 84. | Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658-2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 85. | Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 491] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 86. | Belmokhtar K, Bourguignon T, Worou ME, Khamis G, Bonnet P, Domenech J, Eder V. Regeneration of three layers vascular wall by using BMP2-treated MSC involving HIF-1α and Id1 expressions through JAK/STAT pathways. Stem Cell Rev Rep. 2011;7:847-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Shen Y, Jiang X, Meng L, Xia C, Zhang L, Xin Y. Transplantation of Bone Marrow Mesenchymal Stem Cells Prevents Radiation-Induced Artery Injury by Suppressing Oxidative Stress and Inflammation. Oxid Med Cell Longev. 2018;2018:5942916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Mahrouf-Yorgov M, Augeul L, Da Silva CC, Jourdan M, Rigolet M, Manin S, Ferrera R, Ovize M, Henry A, Guguin A, Meningaud JP, Dubois-Randé JL, Motterlini R, Foresti R, Rodriguez AM. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24:1224-1238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 89. | Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 883] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 90. | Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 757] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 91. | Hu WS, Chen TS, Cheang KH, Liao WY, Chang CH. Mesenchymal Stem Cell Transplantation Increases Antioxidant Protein Expression and Ameliorates GP91/ROS/Inflammasome Signals in Diabetic Cardiomyopathy. J Cardiovasc Dev Dis. 2022;9:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 92. | Liang H, Hou H, Yi W, Yang G, Gu C, Lau WB, Gao E, Ma X, Lu Z, Wei X, Pei J, Yi D. Increased expression of pigment epithelium-derived factor in aged mesenchymal stem cells impairs their therapeutic efficacy for attenuating myocardial infarction injury. Eur Heart J. 2013;34:1681-1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 93. | Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 544] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 94. | Abu-El-Rub E, Sareen N, Lester Sequiera G, Ammar HI, Yan W, ShamsEldeen AM, Rubinchik I, Moudgil M, Shokry HS, Rashed LA, Dhingra S. Hypoxia-induced increase in Sug1 leads to poor post-transplantation survival of allogeneic mesenchymal stem cells. FASEB J. 2020;34:12860-12876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Gao LR, Pei XT, Ding QA, Chen Y, Zhang NK, Chen HY, Wang ZG, Wang YF, Zhu ZM, Li TC, Liu HL, Tong ZC, Yang Y, Nan X, Guo F, Shen JL, Shen YH, Zhang JJ, Fei YX, Xu HT, Wang LH, Tian HT, Liu DQ, Yang Y. A critical challenge: dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int J Cardiol. 2013;168:3191-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 96. | Kim SH, Cho JH, Lee YH, Lee JH, Kim SS, Kim MY, Lee MG, Kang WY, Lee KS, Ahn YK, Jeong MH, Kim HS. Improvement in Left Ventricular Function with Intracoronary Mesenchymal Stem Cell Therapy in a Patient with Anterior Wall ST-Segment Elevation Myocardial Infarction. Cardiovasc Drugs Ther. 2018;32:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 97. | Chen TS, Ju DT, Day CH, Yeh YL, Chen RJ, Viswanadha VP, Chang RL, Lin YC, Yao CH, Huang CY. Protective effect of autologous transplantation of resveratrol preconditioned adipose-derived stem cells in the treatment of diabetic liver dysfunction in rat model. J Tissue Eng Regen Med. 2019;13:1629-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 442] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 99. | Khalil SG, Younis NN, Shaheen MA, Hammad SK, Elswefy SE. Evaluation of in vivo and ex vivo pre-treated bone marrow-derived mesenchymal stem cells with resveratrol in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol. 2023;75:1186-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 100. | Liu C, Fan Y, Zhou L, Zhu HY, Song YC, Hu L, Wang Y, Li QP. Pretreatment of mesenchymal stem cells with angiotensin II enhances paracrine effects, angiogenesis, gap junction formation and therapeutic efficacy for myocardial infarction. Int J Cardiol. 2015;188:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 101. | Yang Y, Lei T, Bi W, Xiao Z, Zhang X, Du H. The combined therapy of mesenchymal stem cell transplantation and resveratrol for diabetes: Future applications and challenges. Life Sci. 2022;301:120563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 102. | Khodayari S, Khodayari H, Amiri AZ, Eslami M, Farhud D, Hescheler J, Nayernia K. Inflammatory Microenvironment of Acute Myocardial Infarction Prevents Regeneration of Heart with Stem Cells Therapy. Cell Physiol Biochem. 2019;53:887-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 103. | Bailey AM, Lawrence MB, Shang H, Katz AJ, Peirce SM. Agent-based model of therapeutic adipose-derived stromal cell trafficking during ischemia predicts ability to roll on P-selectin. PLoS Comput Biol. 2009;5:e1000294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 104. | Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 105. | Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 478] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 106. | Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res. 2012;94:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 107. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 730] [Article Influence: 104.3] [Reference Citation Analysis (36)] |
| 108. | Li M, Sun X, Ma L, Jin L, Zhang W, Xiao M, Yu Q. SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3β/β-catenin pathways. Sci Rep. 2017;7:40161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 109. | Popielarczyk TL, Huckle WR, Barrett JG. Human Bone Marrow-Derived Mesenchymal Stem Cells Home via the PI3K-Akt, MAPK, and Jak/Stat Signaling Pathways in Response to Platelet-Derived Growth Factor. Stem Cells Dev. 2019;28:1191-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 111. | Ammar HI, Shamseldeen AM, Shoukry HS, Ashour H, Kamar SS, Rashed LA, Fadel M, Srivastava A, Dhingra S. Metformin impairs homing ability and efficacy of mesenchymal stem cells for cardiac repair in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol Heart Circ Physiol. 2021;320:H1290-H1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 112. | Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 951] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 113. | Anderluh M, Kocic G, Tomovic K, Kocic R, Deljanin-Ilic M, Smelcerovic A. Cross-talk between the dipeptidyl peptidase-4 and stromal cell-derived factor-1 in stem cell homing and myocardial repair: Potential impact of dipeptidyl peptidase-4 inhibitors. Pharmacol Ther. 2016;167:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 114. | Jiang Q, Huang K, Lu F, Deng S, Yang Z, Hu S. Modifying strategies for SDF-1/CXCR4 interaction during mesenchymal stem cell transplantation. Gen Thorac Cardiovasc Surg. 2022;70:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 115. | Goessler UR, Bugert P, Bieback K, Stern-Straeter J, Bran G, Hörmann K, Riedel F. Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. Int J Mol Med. 2008;21:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, Zipori D, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289-3296. [PubMed] |
| 117. | Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 509] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 118. | Basu P, Sen U, Tyagi N, Tyagi SC. Blood flow interplays with elastin: collagen and MMP: TIMP ratios to maintain healthy vascular structure and function. Vasc Health Risk Manag. 2010;6:215-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055-4063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 417] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 120. | Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 121. | Longstreth JH, Wang K. The role of fibronectin in mediating cell migration. Am J Physiol Cell Physiol. 2024;326:C1212-C1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 122. | Wijelath ES, Rahman S, Murray J, Patel Y, Savidge G, Sobel M. Fibronectin promotes VEGF-induced CD34 cell differentiation into endothelial cells. J Vasc Surg. 2004;39:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 123. | Ling L, Hou J, Liu D, Tang D, Zhang Y, Zeng Q, Pan H, Fan L. Important role of the SDF-1/CXCR4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (hAD-MSCs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (POI). Stem Cell Res Ther. 2022;13:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 124. | Xu J, Xiong YY, Li Q, Hu MJ, Huang PS, Xu JY, Tian XQ, Jin C, Liu JD, Qian L, Yang YJ. Optimization of Timing and Times for Administration of Atorvastatin-Pretreated Mesenchymal Stem Cells in a Preclinical Model of Acute Myocardial Infarction. Stem Cells Transl Med. 2019;8:1068-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 125. | Mayorga ME, Kiedrowski M, McCallinhart P, Forudi F, Ockunzzi J, Weber K, Chilian W, Penn MS, Dong F. Role of SDF-1:CXCR4 in Impaired Post-Myocardial Infarction Cardiac Repair in Diabetes. Stem Cells Transl Med. 2018;7:115-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 126. | Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, Zerbini G, Allavena P, Bonifacio E, Piemonti L. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 444] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 127. | Wang J, Guan E, Roderiquez G, Calvert V, Alvarez R, Norcross MA. Role of tyrosine phosphorylation in ligand-independent sequestration of CXCR4 in human primary monocytes-macrophages. J Biol Chem. 2001;276:49236-49243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Guo J, Zhang H, Xiao J, Wu J, Ye Y, Li Z, Zou Y, Li X. Monocyte chemotactic protein-1 promotes the myocardial homing of mesenchymal stem cells in dilated cardiomyopathy. Int J Mol Sci. 2013;14:8164-8178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 129. | Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 730] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 130. | Schmidt A, Ladage D, Schinköthe T, Klausmann U, Ulrichs C, Klinz FJ, Brixius K, Arnhold S, Desai B, Mehlhorn U, Schwinger RH, Staib P, Addicks K, Bloch W. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24:1750-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 131. | Sheriff L, Alanazi A, Ward LSC, Ward C, Munir H, Rayes J, Alassiri M, Watson SP, Newsome PN, Rainger GE, Kalia N, Frampton J, McGettrick HM, Nash GB. Origin-Specific Adhesive Interactions of Mesenchymal Stem Cells with Platelets Influence Their Behavior After Infusion. Stem Cells. 2018;36:1062-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Alanazi A, Munir H, Alassiri M, Ward LSC, McGettrick HM, Nash GB. Comparative adhesive and migratory properties of mesenchymal stem cells from different tissues. Biorheology. 2019;56:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 133. | De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, Benhaim P, Hedrick MH, Fraser JK. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 327] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 134. | Noël D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, Jorgensen C, Cousin B. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 135. | Ahmadian Kia N, Bahrami AR, Ebrahimi M, Matin MM, Neshati Z, Almohaddesin MR, Aghdami N, Bidkhori HR. Comparative analysis of chemokine receptor's expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J Mol Neurosci. 2011;44:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 136. | Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 429] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 137. | Kyriakou C, Rabin N, Pizzey A, Nathwani A, Yong K. Factors that influence short-term homing of human bone marrow-derived mesenchymal stem cells in a xenogeneic animal model. Haematologica. 2008;93:1457-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 138. | Aldridge V, Garg A, Davies N, Bartlett DC, Youster J, Beard H, Kavanagh DP, Kalia N, Frampton J, Lalor PF, Newsome PN. Human mesenchymal stem cells are recruited to injured liver in a β1-integrin and CD44 dependent manner. Hepatology. 2012;56:1063-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 139. | Lo Surdo J, Bauer SR. Quantitative approaches to detect donor and passage differences in adipogenic potential and clonogenicity in human bone marrow-derived mesenchymal stem cells. Tissue Eng Part C Methods. 2012;18:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 140. | Li J, Hu S, Zhu D, Huang K, Mei X, López de Juan Abad B, Cheng K. All Roads Lead to Rome (the Heart): Cell Retention and Outcomes From Various Delivery Routes of Cell Therapy Products to the Heart. J Am Heart Assoc. 2021;10:e020402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 141. | Sajjad U, Ahmed M, Iqbal MZ, Riaz M, Mustafa M, Biedermann T, Klar AS. Exploring mesenchymal stem cells homing mechanisms and improvement strategies. Stem Cells Transl Med. 2024;13:1161-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 142. | Li SH, Lai TY, Sun Z, Han M, Moriyama E, Wilson B, Fazel S, Weisel RD, Yau T, Wu JC, Li RK. Tracking cardiac engraftment and distribution of implanted bone marrow cells: Comparing intra-aortic, intravenous, and intramyocardial delivery. J Thorac Cardiovasc Surg. 2009;137:1225-33.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 143. | Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 144. | Mokhtari B, Aboutaleb N, Nazarinia D, Nikougoftar M, Razavi Tousi SMT, Molazem M, Azadi MR. Comparison of the effects of intramyocardial and intravenous injections of human mesenchymal stem cells on cardiac regeneration after heart failure. Iran J Basic Med Sci. 2020;23:879-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 145. | Kim H, Kim SW, Nam D, Kim S, Yoon YS. Cell therapy with bone marrow cells for myocardial regeneration. Antioxid Redox Signal. 2009;11:1897-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 146. | Szydlak R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J Stem Cells. 2021;13:619-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 147. | Yagyu T, Yasuda S, Nagaya N, Doi K, Nakatani T, Satomi K, Shimizu W, Kusano K, Anzai T, Noguchi T, Ohgushi H, Kitamura S, Kangawa K, Ogawa H. Long-Term Results of Intracardiac Mesenchymal Stem Cell Transplantation in Patients With Cardiomyopathy. Circ J. 2019;83:1590-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 148. | Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 149. | Fan M, Chen W, Liu W, Du GQ, Jiang SL, Tian WC, Sun L, Li RK, Tian H. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010;13:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 150. | Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 381] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 151. | Ock SA, Lee YM, Park JS, Shivakumar SB, Moon SW, Sung NJ, Lee WJ, Jang SJ, Park JM, Lee SC, Lee SL, Rho GJ. Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source. J Vet Med Sci. 2016;78:987-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 152. | Manukyan MC, Weil BR, Wang Y, Abarbanell AM, Herrmann JL, Poynter JA, Brewster BD, Meldrum DR. Female stem cells are superior to males in preserving myocardial function following endotoxemia. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1506-R1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 153. | Zhang Y, Lv P, Li Y, Zhang Y, Cheng C, Hao H, Yue H. Comparison of the biological characteristics of umbilical cord mesenchymal stem cells derived from the human heterosexual twins. Differentiation. 2020;114:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 154. | Hong L, Sultana H, Paulius K, Zhang G. Steroid regulation of proliferation and osteogenic differentiation of bone marrow stromal cells: a gender difference. J Steroid Biochem Mol Biol. 2009;114:180-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 155. | Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, Rubin RT, Huard J. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 156. | Bagheri F, Khori V, Alizadeh AM, Khalighfard S, Khodayari S, Khodayari H. Reactive oxygen species-mediated cardiac-reperfusion injury: Mechanisms and therapies. Life Sci. 2016;165:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 157. | Shao L, Shen Y, Ren C, Kobayashi S, Asahara T, Yang J. Inflammation in myocardial infarction: roles of mesenchymal stem cells and their secretome. Cell Death Discov. 2022;8:452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 158. | Sitcheran R, Cogswell PC, Baldwin AS Jr. NF-kappaB mediates inhibition of mesenchymal cell differentiation through a posttranscriptional gene silencing mechanism. Genes Dev. 2003;17:2368-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 159. | Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119:91-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 1729] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 160. | Chu Q, Song X, Xiao Y, Kang YJ. Alteration of endothelial permeability ensures cardiomyocyte survival from ischemic insult in the subendocardium of the heart. Exp Biol Med (Maywood). 2023;248:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 161. | Chu Q, Jiang X, Xiao Y. Rebuilding the myocardial microenvironment to enhance mesenchymal stem cells-mediated regeneration in ischemic heart disease. Front Bioeng Biotechnol. 2024;12:1468833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 162. | Nofi C, Bogatyryov Y, Dedkov EI. Preservation of Functional Microvascular Bed Is Vital for Long-Term Survival of Cardiac Myocytes Within Large Transmural Post-Myocardial Infarction Scar. J Histochem Cytochem. 2018;66:99-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 163. | Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 164. | Korf-Klingebiel M, Kempf T, Sauer T, Brinkmann E, Fischer P, Meyer GP, Ganser A, Drexler H, Wollert KC. Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction. Eur Heart J. 2008;29:2851-2858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 165. | Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 302] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 166. | Lee SW, Jeong HK, Lee JY, Yang J, Lee EJ, Kim SY, Youn SW, Lee J, Kim WJ, Kim KW, Lim JM, Park JW, Park YB, Kim HS. Hypoxic priming of mESCs accelerates vascular-lineage differentiation through HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol Med. 2012;4:924-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 167. | Lin J, Zhu Q, Huang J, Cai R, Kuang Y. Hypoxia Promotes Vascular Smooth Muscle Cell (VSMC) Differentiation of Adipose-Derived Stem Cell (ADSC) by Regulating Mettl3 and Paracrine Factors. Stem Cells Int. 2020;2020:2830565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 168. | Barrère-Lemaire S, Vincent A, Jorgensen C, Piot C, Nargeot J, Djouad F. Mesenchymal stromal cells for improvement of cardiac function following acute myocardial infarction: a matter of timing. Physiol Rev. 2024;104:659-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 169. | Miettinen JA, Ylitalo K, Hedberg P, Jokelainen J, Kervinen K, Niemelä M, Säily M, Koistinen P, Savolainen ER, Ukkonen H, Pietilä M, Airaksinen KE, Knuuti J, Vuolteenaho O, Mäkikallio TH, Huikuri HV. Determinants of functional recovery after myocardial infarction of patients treated with bone marrow-derived stem cells after thrombolytic therapy. Heart. 2010;96:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 170. | Sun K, Li YY, Jin J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct Target Ther. 2021;6:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 171. | Wang Z, Wang L, Su X, Pu J, Jiang M, He B. Rational transplant timing and dose of mesenchymal stromal cells in patients with acute myocardial infarction: a meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2017;8:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 172. | Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 302] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 173. | Park SJ, Kim YY, Han JY, Kim SW, Kim H, Ku SY. Advancements in Human Embryonic Stem Cell Research: Clinical Applications and Ethical Issues. Tissue Eng Regen Med. 2024;21:379-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 174. | Amosse J, Martinez MC, Le Lay S. Extracellular vesicles and cardiovascular disease therapy. Stem Cell Investig. 2017;4:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 175. | Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 580] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 176. | Ljujic B, Milovanovic M, Volarevic V, Murray B, Bugarski D, Przyborski S, Arsenijevic N, Lukic ML, Stojkovic M. Human mesenchymal stem cells creating an immunosuppressive environment and promote breast cancer in mice. Sci Rep. 2013;3:2298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 177. | Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 470] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 178. | Zhang F, Cui J, Lv B, Yu B. Nicorandil protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Int J Mol Med. 2015;36:415-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 179. | Wei ZZ, Zhu YB, Zhang JY, McCrary MR, Wang S, Zhang YB, Yu SP, Wei L. Priming of the Cells: Hypoxic Preconditioning for Stem Cell Therapy. Chin Med J (Engl). 2017;130:2361-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 180. | Liu L, Gao J, Yuan Y, Chang Q, Liao Y, Lu F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int. 2013;37:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 181. | Choi JH, Lee YB, Jung J, Hwang SG, Oh IH, Kim GJ. Hypoxia Inducible Factor-1α Regulates the Migration of Bone Marrow Mesenchymal Stem Cells via Integrin α 4. Stem Cells Int. 2016;2016:7932185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 182. | Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 478] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 183. | Choi JW, Kim KE, Lee CY, Lee J, Seo HH, Lim KH, Choi E, Lim S, Lee S, Kim SW, Hwang KC. Alterations in Cardiomyocyte Differentiation-Related Proteins in Rat Mesenchymal Stem Cells Exposed to Hypoxia. Cell Physiol Biochem. 2016;39:1595-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 184. | Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 545] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 185. | Han YS, Lee JH, Yoon YM, Yun CW, Noh H, Lee SH. Hypoxia-induced expression of cellular prion protein improves the therapeutic potential of mesenchymal stem cells. Cell Death Dis. 2016;7:e2395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 186. | Estrada JC, Albo C, Benguría A, Dopazo A, López-Romero P, Carrera-Quintanar L, Roche E, Clemente EP, Enríquez JA, Bernad A, Samper E. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19:743-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 187. | Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 188. | Hou Y, Ryu CH, Jun JA, Kim SM, Jeong CH, Jeun SS. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int. 2014;38:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 189. | François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 552] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 190. | Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J Immunol. 2014;192:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 191. | Gorin C, Rochefort GY, Bascetin R, Ying H, Lesieur J, Sadoine J, Beckouche N, Berndt S, Novais A, Lesage M, Hosten B, Vercellino L, Merlet P, Le-Denmat D, Marchiol C, Letourneur D, Nicoletti A, Vital SO, Poliard A, Salmon B, Muller L, Chaussain C, Germain S. Priming Dental Pulp Stem Cells With Fibroblast Growth Factor-2 Increases Angiogenesis of Implanted Tissue-Engineered Constructs Through Hepatocyte Growth Factor and Vascular Endothelial Growth Factor Secretion. Stem Cells Transl Med. 2016;5:392-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 192. | Liu X, Duan B, Cheng Z, Jia X, Mao L, Fu H, Che Y, Ou L, Liu L, Kong D. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 193. | Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 194. | ShamsEldeen AM, El-Aal SAA, Aboulhoda BE, AbdAllah H, Gamal SM, Hassan FE, Mehesen MN, Rashed LA, Mostafa A, Sadek NB. Combined Systemic Intake of K-ATP Opener (Nicorandil) and Mesenchymal Stem Cells Preconditioned With Nicorandil Alleviates Pancreatic Insufficiency in a Model of Bilateral Renal Ischemia/Reperfusion Injury. Front Physiol. 2022;13:934597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 195. | Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther. 2009;329:543-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 196. | Song L, Yang YJ, Dong QT, Qian HY, Gao RL, Qiao SB, Shen R, He ZX, Lu MJ, Zhao SH, Geng YJ, Gersh BJ. Atorvastatin enhance efficacy of mesenchymal stem cells treatment for swine myocardial infarction via activation of nitric oxide synthase. PLoS One. 2013;8:e65702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 197. | Numasawa Y, Kimura T, Miyoshi S, Nishiyama N, Hida N, Tsuji H, Tsuruta H, Segawa K, Ogawa S, Umezawa A. Treatment of human mesenchymal stem cells with angiotensin receptor blocker improved efficiency of cardiomyogenic transdifferentiation and improved cardiac function via angiogenesis. Stem Cells. 2011;29:1405-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 198. | Rawat S, Dadhwal V, Mohanty S. Dexamethasone priming enhances stemness and immunomodulatory property of tissue-specific human mesenchymal stem cells. BMC Dev Biol. 2021;21:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 199. | Rashed LA, Elattar S, Eltablawy N, Ashour H, Mahmoud LM, El-Esawy Y. Mesenchymal stem cells pretreated with melatonin ameliorate kidney functions in a rat model of diabetic nephropathy. Biochem Cell Biol. 2018;96:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 200. | Haider KH. Melatonin-based priming of stem cells to alleviate oxidative stress. World J Stem Cells. 2024;16:985-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 201. | Luchetti F, Canonico B, Bartolini D, Arcangeletti M, Ciffolilli S, Murdolo G, Piroddi M, Papa S, Reiter RJ, Galli F. Melatonin regulates mesenchymal stem cell differentiation: a review. J Pineal Res. 2014;56:382-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 202. | Noiseux N, Borie M, Desnoyers A, Menaouar A, Stevens LM, Mansour S, Danalache BA, Roy DC, Jankowski M, Gutkowska J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology. 2012;153:5361-5372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 203. | Rezaei N, Sardarzadeh T, Sisakhtnezhad S. Thymoquinone promotes mouse mesenchymal stem cells migration in vitro and induces their immunogenicity in vivo. Toxicol Appl Pharmacol. 2020;387:114851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 204. | AboZaid O, Hussein SA, El-Sonbaty SM, Afifi MW. Mesenchymal stem cells proliferation augmentation by preconditioning with resveratrol (In vitro Study). Benha Vet Med J. 2021;41:169-172. [DOI] [Full Text] |
| 205. | Irfan F, Jameel F, Khan I, Aslam R, Faizi S, Salim A. Role of quercetin and rutin in enhancing the therapeutic potential of mesenchymal stem cells for cold induced burn wound. Regen Ther. 2022;21:225-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 206. | Lee J, Shin MS, Kim MO, Jang S, Oh SW, Kang M, Jung K, Park YS, Lee J. Apple ethanol extract promotes proliferation of human adult stem cells, which involves the regenerative potential of stem cells. Nutr Res. 2016;36:925-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 207. | Bhatti FU, Mehmood A, Latief N, Zahra S, Cho H, Khan SN, Riazuddin S. Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide-induced oxidative stress in vitro and improves their therapeutic potential in surgically-induced rat model of osteoarthritis. Osteoarthritis Cartilage. 2017;25:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 208. | Mehrzad S, sadat Hosseini S, Momeni-Moghaddam M, Farshchian M, Hassanzadeh H, Mirahmadi M, Sadeghifar F, Reza Bidkhori H. Vitamin E Pretreatment of Mesenchymal Stem Cells: The Interplay of Oxidative Stress and Inflammation. J Cell Mol Res. 2020;11:99-107. [DOI] [Full Text] |
| 209. | Borojević A, Jauković A, Kukolj T, Mojsilović S, Obradović H, Trivanović D, Živanović M, Zečević Ž, Simić M, Gobeljić B, Vujić D, Bugarski D. Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, Partly through SIRT1 Signaling. Biomolecules. 2022;12:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 210. | Teo GS, Yang Z, Carman CV, Karp JM, Lin CP. Intravital imaging of mesenchymal stem cell trafficking and association with platelets and neutrophils. Stem Cells. 2015;33:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 211. | Langer HF, Stellos K, Steingen C, Froihofer A, Schönberger T, Krämer B, Bigalke B, May AE, Seizer P, Müller I, Gieseke F, Siegel-Axel D, Meuth SG, Schmidt A, Wendel HP, Müller I, Bloch W, Gawaz M. Platelet derived bFGF mediates vascular integrative mechanisms of mesenchymal stem cells in vitro. J Mol Cell Cardiol. 2009;47:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 212. | Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 505] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 213. | Park BW, Jung SH, Das S, Lee SM, Park JH, Kim H, Hwang JW, Lee S, Kim HJ, Kim HY, Jung S, Cho DW, Jang J, Ban K, Park HJ. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv. 2020;6:eaay6994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 214. | Saravanan S, Sareen N, Abu-El-Rub E, Ashour H, Sequiera GL, Ammar HI, Gopinath V, Shamaa AA, Sayed SSE, Moudgil M, Vadivelu J, Dhingra S. Graphene Oxide-Gold Nanosheets Containing Chitosan Scaffold Improves Ventricular Contractility and Function After Implantation into Infarcted Heart. Sci Rep. 2018;8:15069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 215. | Augustine R, Dan P, Hasan A, Khalaf IM, Prasad P, Ghosal K, Gentile C, McClements L, Maureira P. Stem cell-based approaches in cardiac tissue engineering: controlling the microenvironment for autologous cells. Biomed Pharmacother. 2021;138:111425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 216. | Zoneff E, Wang Y, Jackson C, Smith O, Duchi S, Onofrillo C, Farrugia B, Moulton SE, Williams R, Parish C, Nisbet DR, Caballero-Aguilar LM. Controlled oxygen delivery to power tissue regeneration. Nat Commun. 2024;15:4361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 217. | Park J, Kim YS, Ryu S, Kang WS, Park S, Han J, Jeong HC, Hong BH, Ahn Y, Kim BS. Graphene Potentiates the Myocardial Repair Efficacy of Mesenchymal Stem Cells by Stimulating the Expression of Angiogenic Growth Factors and Gap Junction Protein. Adv Funct Mater. 2015;25:2590-2600. [DOI] [Full Text] |
| 218. | Damasceno PKF, de Santana TA, Santos GC, Orge ID, Silva DN, Albuquerque JF, Golinelli G, Grisendi G, Pinelli M, Ribeiro Dos Santos R, Dominici M, Soares MBP. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Front Cell Dev Biol. 2020;8:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 219. | Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 509] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 220. | Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1200] [Cited by in RCA: 1147] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 221. | Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008;77:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 222. | Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lützow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 338] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 223. | Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol Pharmacol. 2009;76:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 224. | Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 225. | Bagno LL, Carvalho D, Mesquita F, Louzada RA, Andrade B, Kasai-Brunswick TH, Lago VM, Suhet G, Cipitelli D, Werneck-de-Castro JP, Campos-de-Carvalho AC. Sustained IGF-1 Secretion by Adipose-Derived Stem Cells Improves Infarcted Heart Function. Cell Transplant. 2016;25:1609-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 226. | Farag A, Koung Ngeun S, Kaneda M, Aboubakr M, Tanaka R. Optimizing Cardiomyocyte Differentiation: Comparative Analysis of Bone Marrow and Adipose-Derived Mesenchymal Stem Cells in Rats Using 5-Azacytidine and Low-Dose FGF and IGF Treatment. Biomedicines. 2024;12:1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 227. | Hwang JH, Kim SW, Park SE, Yun HJ, Lee Y, Kim S, Jo DY. Overexpression of stromal cell-derived factor-1 enhances endothelium-supported transmigration, maintenance, and proliferation of hematopoietic progenitor cells. Stem Cells Dev. 2006;15:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 228. | Song SW, Chang W, Song BW, Song H, Lim S, Kim HJ, Cha MJ, Choi E, Im SH, Chang BC, Chung N, Jang Y, Hwang KC. Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 2009;27:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 229. | François S, Bensidhoum M, Mouiseddine M, Mazurier C, Allenet B, Semont A, Frick J, Saché A, Bouchet S, Thierry D, Gourmelon P, Gorin NC, Chapel A. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 230. | Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR; Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31:623-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 231. | Zen K, Okigaki M, Hosokawa Y, Adachi Y, Nozawa Y, Takamiya M, Tatsumi T, Urao N, Tateishi K, Takahashi T, Matsubara H. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J Mol Cell Cardiol. 2006;40:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 232. | Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J. Exosomes: new molecular targets of diseases. Acta Pharmacol Sin. 2018;39:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 339] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 233. | Hu J, Chen X, Li P, Lu X, Yan J, Tan H, Zhang C. Exosomes derived from human amniotic fluid mesenchymal stem cells alleviate cardiac fibrosis via enhancing angiogenesis in vivo and in vitro. Cardiovasc Diagn Ther. 2021;11:348-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 234. | Willis GR, Kourembanas S, Mitsialis SA. Toward Exosome-Based Therapeutics: Isolation, Heterogeneity, and Fit-for-Purpose Potency. Front Cardiovasc Med. 2017;4:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 235. | Li J, Sun S, Zhu D, Mei X, Lyu Y, Huang K, Li Y, Liu S, Wang Z, Hu S, Lutz HJ, Popowski KD, Dinh PC, Butte AJ, Cheng K. Inhalable Stem Cell Exosomes Promote Heart Repair After Myocardial Infarction. Circulation. 2024;150:710-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 236. | Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell Physiol Biochem. 2015;37:2415-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 237. | Ahmed ZT, Alkahlot MH, Haider KH. MSC-Derived Exosomes: Advances in Cell-Free Therapy. In: Haider KH. Handbook of Stem Cell Applications. Singapore: Springer, 2024. [DOI] [Full Text] |
| 238. | Ashour H, Rashed L, Elkordy MA, Abdelwahed OM. Remote liver injury following acute renal ischaemia-reperfusion: involvement of circulating exosomal miR-687 and regulation by thymoquinone. Exp Physiol. 2021;106:2262-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 239. | Sareen N, Srivastava A, Alagarsamy KN, Lionetti V, Dhingra S. Stem cells derived exosomes and biomaterials to modulate autophagy and mend broken hearts. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 240. | Yuan J, Yang H, Liu C, Shao L, Zhang H, Lu K, Wang J, Wang Y, Yu Q, Zhang Y, Yu Y, Shen Z. Microneedle Patch Loaded with Exosomes Containing MicroRNA-29b Prevents Cardiac Fibrosis after Myocardial Infarction. Adv Healthc Mater. 2023;12:e2202959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 241. | Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, Wang KK, Shen H, Zhang GG, Bai YP. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163-6177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 435] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 242. | Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D, Wang J. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 243. | Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell Physiol Biochem. 2017;44:2105-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 244. | Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, Gong P, Shen X, Ruan H, Jin M, Wang H. miR-93-5p-Containing Exosomes Treatment Attenuates Acute Myocardial Infarction-Induced Myocardial Damage. Mol Ther Nucleic Acids. 2018;11:103-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 245. | Li Y, Yang R, Guo B, Zhang H, Zhang H, Liu S, Li Y. Exosomal miR-301 derived from mesenchymal stem cells protects myocardial infarction by inhibiting myocardial autophagy. Biochem Biophys Res Commun. 2019;514:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 246. | Liu L, Jin X, Hu CF, Li R, Zhou Z, Shen CX. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell Physiol Biochem. 2017;43:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 247. | Zhang CS, Shao K, Liu CW, Li CJ, Yu BT. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur Rev Med Pharmacol Sci. 2019;23:6691-6699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 248. | Cheng H, Chang S, Xu R, Chen L, Song X, Wu J, Qian J, Zou Y, Ma J. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther. 2020;11:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 249. | Sun XH, Wang X, Zhang Y, Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 250. | Lei B, Wu X, Xia K, Sun H, Wang J. Exosomal Micro-RNA-96 Derived From Bone Marrow Mesenchymal Stem Cells Inhibits Doxorubicin-Induced Myocardial Toxicity by Inhibiting the Rac1/Nuclear Factor-κB Signaling Pathway. J Am Heart Assoc. 2021;10:e020589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 251. | Chinnici CM, Iannolo G, Cittadini E, Carreca AP, Nascari D, Timoneri F, Bella MD, Cuscino N, Amico G, Carcione C, Conaldi PG. Extracellular Vesicle-Derived microRNAs of Human Wharton's Jelly Mesenchymal Stromal Cells May Activate Endogenous VEGF-A to Promote Angiogenesis. Int J Mol Sci. 2021;22:2045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 252. | Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W. Exosomes Derived from Akt-Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration and Promote Angiogenesis via Activating Platelet-Derived Growth Factor D. Stem Cells Transl Med. 2017;6:51-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (5)] |
| 253. | Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J Am Heart Assoc. 2016;5:e002856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 254. | Ciullo A, Biemmi V, Milano G, Bolis S, Cervio E, Fertig ET, Gherghiceanu M, Moccetti T, Camici GG, Vassalli G, Barile L. Exosomal Expression of CXCR4 Targets Cardioprotective Vesicles to Myocardial Infarction and Improves Outcome after Systemic Administration. Int J Mol Sci. 2019;20:468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 255. | Nakamura Y, Kita S, Tanaka Y, Fukuda S, Obata Y, Okita T, Nishida H, Takahashi Y, Kawachi Y, Tsugawa-Shimizu Y, Fujishima Y, Nishizawa H, Takakura Y, Miyagawa S, Sawa Y, Maeda N, Shimomura I. Adiponectin Stimulates Exosome Release to Enhance Mesenchymal Stem-Cell-Driven Therapy of Heart Failure in Mice. Mol Ther. 2020;28:2203-2219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 256. | Chi B, Zou A, Mao L, Cai D, Xiao T, Wang Y, Wang Q, Ji Y, Sun L. Empagliflozin-Pretreated Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Attenuated Heart Injury. Oxid Med Cell Longev. 2023;2023:7747727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 257. | Morishita M, Takahashi Y, Nishikawa M, Takakura Y. Pharmacokinetics of Exosomes-An Important Factor for Elucidating the Biological Roles of Exosomes and for the Development of Exosome-Based Therapeutics. J Pharm Sci. 2017;106:2265-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 258. | Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 386] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 259. | Peng Y, Zhao JL, Peng ZY, Xu WF, Yu GL. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 2020;11:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 260. | Milano G, Biemmi V, Lazzarini E, Balbi C, Ciullo A, Bolis S, Ameri P, Di Silvestre D, Mauri P, Barile L, Vassalli G. Intravenous administration of cardiac progenitor cell-derived exosomes protects against doxorubicin/trastuzumab-induced cardiac toxicity. Cardiovasc Res. 2020;116:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/