Published online Nov 26, 2025. doi: 10.4252/wjsc.v17.i11.112484
Revised: September 2, 2025
Accepted: October 9, 2025
Published online: November 26, 2025
Processing time: 120 Days and 19.2 Hours
Stem cell therapy has been recognized as a promising strategy for enhancing cardiac function after myocardial infarction. Nonetheless, its clinical benefits are frequently limited by the poor survival and differentiation rates of the trans
To clarify the role of hypoxia-inducible factor-1α (HIF-1α)/β-catenin in survival and angiogenesis of peripheral blood mesenchymal stem cells (PBMSCs).
PBMSCs were isolated from rat abdominal aorta blood and characterized by multipotent differentiation assays. Cells were cultured under hypoxic conditions, followed by either overexpression or silencing of HIF-1α/β-catenin. Proliferative capacity was evaluated via colony formation assays, while cellular senescence was assessed using β-galactosidase staining. The protein and/or mRNA expressions of HIF-1α, β-catenin, basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), survivin, Bcl2, Bax, cleaved caspase 3 were detected via western blotting and/or quantitative real-time polymerase chain reaction. PBMSCs co-expressing elevated HIF-1α and β-catenin levels were transplanted into infarcted myocardial tissue to evaluate their therapeutic potential in vivo.
HIF-1α or β-catenin overexpression enhanced self-renewal and inhibit apoptosis of PBMSCs by up-regulating Bcl2 and survivin, down-regulating Bax and cleaved-caspase 3. Besides, HIF-1α or β-catenin overexpression elevated angiogenesis via increasing bFGF and VEGF expressions. Silence of HIF-1α or β-catenin had opposite effect. Upregulation of HIF-1α increased β-catenin expression, whereas modifications in β-catenin did not influence HIF-1α expression. Chromatin immunoprecipitation assay verified that HIF-1α directly modulates β-catenin transcription. In vivo, HIF-1α overexpression significantly improved the retention of transplanted PBMSCs in infarcted myocardium and enhanced myocardial repair. Functional analysis further confirmed that HIF-1α operated through β-catenin, which directly modulated the expression of bFGF, VEGF, survivin, Bcl2, Bax and cleaved caspase 3, thereby coordinating the anti-apoptotic and pro-angiogenic functions of transplanted PBMSCs.
This study highlights the modulatory function of HIF-1α on PBMSCs via β-catenin-driven anti-apoptotic and angiogenic signaling cascade under hypoxia environment, offering a promising strategy for improving the therapeutic effectiveness PBMSCs-based transplantation after myocardial infarction.
Core Tip: The finding of this study indicates that hypoxia-inducible factor-1α (HIF-1α) and β-catenin facilitate peripheral blood mesenchymal stem cells (PBMSCs) self-renewal and inhibit PBMSCs apoptosis. In vivo, PBMSCs with upregulated HIF-1α achieved enhanced retention within infarcted myocardial and contributed markedly to myocardial regeneration. These observations highlight the modulatory role of HIF-1α in modulating PBMSCs function via β-catenin-dependent anti-apoptotic and pro-angiogenic pathway, thereby offering a potential strategy to improve the longevity and therapeutic efficacy of PBMSCs.
- Citation: Wang PZ, Wang AQ, Tian PG, Zhu PP, Yuan W, Wu J, Zhang R. HIF-1α modulates β-catenin pathway to enhance the survival and angiogenesis of PBMSCs under hypoxia environment. World J Stem Cells 2025; 17(11): 112484
- URL: https://www.wjgnet.com/1948-0210/full/v17/i11/112484.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i11.112484
Cardiovascular diseases are the leading cause of death worldwide[1], with myocardial infarction (MI) representing a major contributor to this burden[2]. There is an urgent need to intensify research on the pathophysiological mechanisms of MI and to develop effective therapeutic interventions. Stem cell transplantation has been identified as a promising approach for myocardial repair after MI, attracting increasing academic attention in recent years[3]. Peripheral blood mesenchymal stem cells (PBMSCs), known for their multipotent differentiation capacity, possess the capacity to secrete vascular growth factors, differentiate into vascular endothelial cells, and promote functional recovery of impaired myocardial tissue[4]. Due to its attributes such as accessibility, minimal ethical constraints, ease of isolation, low immunogenicity, and high proliferative potential, PBMSCs are regarded as a highly favorable source of seed cells for therapeutic application[5]. Nevertheless, considered that the poor survival, suboptimal engraftment, and low differentiation efficiency within the infarcted myocardial microenvironment, the clinical utility of PBMSCs remains constrained.
Previous study has indicated that culturing PBMSCs under hypoxic conditions (5% O2) markedly enhances their biological activity and elevates the both hypoxia-inducible factor-1α (HIF-1α) and β-catenin levels[6]. Against the backdrop of rising global prevalence of cardiovascular diseases, accumulating evidence supports the vital role of HIF-1α in promoting stem cell survival, neovascularization, and myocardial tissue regeneration after MI[7]. The HIF-α protein family, comprising hypoxia-inducible transcriptional regulators in mammalian cells, is rapid stabilized under hypoxia condition[8]. In oxygen-deprived environments, HIF-1α becomes stabilized and forms a functional complex with HIF-1β, thereby modulates expressions of numerous downstream target genes, encompassing vascular endothelial growth factor (VEGF)[9]. Besides, recent finding has emphasized the critical role of β-catenin, an essential downstream mediator of the Wnt signaling cascade, in modulating stem cell behavior and maintaining their long-term regenerative potential[10]. Notably, β-catenin signaling has been demonstrated to support the sustained survival of neural stem cells, implying that it may also play an essential role in mediating the persistence and biological function of PBMSC[11].
Moreover, β-catenin has been identified as a pivotal modulator of stem cell survival and neovascularization, contributing markedly to maintain tissue homeostasis and promote regeneration[12]. Functioning as a primary effector of the Wnt/β-catenin signaling pathway, it facilitates stem cell self-renewal and suppresses apoptosis by triggering downstream effectors such as cyclin D1 and survivin, thereby preserving the stem cell population[13]. In angiogenesis, β-catenin upregulates the expression of pro-angiogenic factors, like VEGF, and enhances endothelial cell proliferation, migration, and tubulogenesis, all of which are fundamental to the formation of new vasculature[14,15]. Nonetheless, the mechanistic interplay between β-catenin and HIF-1α remains inadequately defined. Although both pathways are known to jointly facilitate angiogenesis under hypoxic conditions, the precise molecular frameworks and regulatory dynamics linking HIF-1α and β-catenin collectively influence stem cell behavior and vascular formation are still not fully clarified. Further research is warranted to delineate the convergence and divergence of these signaling cascades in orchestrating cellular functions under both physiological and pathological conditions.
Besides, this study will evaluate the pro-angiogenic and anti-apoptotic effects of HIF-1α on PBMSCs mediated through β-catenin signaling cascade under hypoxic conditions. The anticipated findings are intended to provide a novel theoretical basis for enhancing the clinical application of stem cell-based interventions targeting MI. This research specifically aims to clarify the regulatory relationship between HIF-1α and β-catenin in PBMSCs and to define how this interaction modulates cell survival and angiogenic capacity under hypoxia environments. By focusing on these signaling mechanisms, this study is expected to advance the development of innovative therapeutic strategies to improve the efficacy of PBMSC-centered approaches for ischemic heart disease. Beyond deepening the understanding of the molecular mechanisms in stem cell therapy, this study is projected to offer valuable implications for optimizing treatment outcomes in patients with cardiovascular disorders.
Inbred Lewis male rats (250-300 g) were utilized as experimental subjects, and ethical approval for all procedures was granted by the Animal Care and Use Committee of Guangzhou Red Cross Hospital (No. 2023-027-1). The rats were maintained in specific pathogen-free facilities under a controlled 12-hour light/dark cycle at 22 ± 2 °C, with free access to food and water.
Rats were randomly divided into two groups: Control group and trauma group. Subjects in the trauma group (n = 5) were anesthetized with ketamine & diazepam at a dosage of 75 mg/kg & 5 mg/kg and underwent surgical intervention on the dorsal area, whereas those in the control group (n = 5) received no treatment. One week later, all rats were re-anesthetized using the same anesthetic regimen. No unintended fatalities occurred throughout the experimental period. Following anesthesia, 25 mL of blood was collected from the abdominal aorta using a heparinized fine needle. PBMSCs were then isolated from the blood samples and cultivated according to the adherent method outlined in the previous study[16]. Cells at the fourth passage (P4) were utilized for subsequent analyses.
Phenotypic characterization of PBMSCs was analyzed utilizing flow cytometry (Becton Dickinson, CA, United States) for assessing the expression of typical surface antigens associated with mesenchymal stem cells (MSCs), including CD44, CD71, CD90, CD73, and CD105. Additionally, hematopoietic stem cell (HSC) markers (CD45 and CD34) and endothelial progenitor cell (EPC) markers (CD133 and CD31) were also examined to confirm the purity and identity of the isolated cells.
PBMSCs were induced to differentiate into osteogenesis, chondrogenesis and adipogenesis using specific growth factors. Osteogenic differentiation was achieved using a commercially available osteogenic medium kit (HUXUB-90021, Cyagen, Suzhou, China), and mineral deposition was subsequently evaluated by Alizarin Red staining[17]. Chondrogenic differentiation was induced using chondrogenic differentiation medium (HUXUB-90041, Cyagen, Suzhou, China), followed by assessment through Alcian Blue staining[18]. For adipogenic differentiation, PBMSCs were exposed to adipogenic induction medium (HUXUB-90031, Cyagen, Suzhou, China), and lipid accumulation was examined via Oil Red O staining[19].
Lentiviral plasmid vectors pMXs, specifically constructed to overexpress HIF-1α or β-catenin, were utilized for cell transfection. These vectors, along with the pReceiver-LV233 lentiviral vector (GeneCopoeia, Rockville, MD, United States) were co-transfected into PBMSCs using Fugene HD reagent (Fugene, Middleton, WI, United States) per the supplier’s protocol. Additionally, the pSi-LVRU6GP vector harboring a puromycin resistance cassette (GeneCopoeia, Rockville, MD, United States) was applied for the delivery of short hairpin (sh) RNAs targeting HIF-1α or β-catenin.
To examine HIF-1α’s influence in regulating PBMSC proliferation and viability under hypoxic conditions, cells were randomly assigned into five experimental groups: Normoxia, hypoxia, hypoxia with HIF-1α overexpression (oeHIF-1α), hypoxia with HIF-1α knockdown (shHIF-1α), and hypoxia with concurrent HIF-1α overexpression and knockdown (oe + shHIF-1α). Similarly, to evaluate the impact of β-catenin on PBMSC proliferation and survival in hypoxia environments, cells were also divided into five groups: Normoxia, hypoxia, hypoxia with β-catenin overexpression (oeβ-catenin), hypoxia with β-catenin knockdown (shβ-catenin), and hypoxia with both overexpression and knockdown of β-catenin (oe + shβ-catenin).
Following transfected with the target genes, PBMSCs were subjected to colony formation assays. For each experimental group, 500 cells were plated per well in 6-well culture dishes. Aggregates comprising more than 500 cells were defined as colonies[20]. The total number of colonies was determined using a phase-contrast microscope (Olympus, Tokyo, Japan).
To evaluate cellular senescence, PBMSCs attached to glass slides were covered with parafilm and subsequently treated with β-galactosidase solution (Cell Signaling Technology, Danvers, MA, United States) for visualization. After incubation, senescent cells were identified by the development of blue staining. A phase-contrast microscope (Olympus, Tokyo, Japan) was utilized to distinguish stained cells. The proportion of β-galactosidase positive cells was calculated based on the ratio of bule-stained cells in the visual field.
Chromatin immunoprecipitation (ChIP) assays were carried out using approximately 2.0 × 106 PBMSCs per sample with ChIP assay kit (Merck Millipore, Billerica, MA, United States) according to the manufacturer’s instruction. Briefly, after fixed with 1% formaldehyde solution, PBMSCs were lysis, chromatin was fragmented by sonication into small pieces. Antibody specific against HIF-1α was used to immunoprecipitated the protein-DNA complexes. Following reversal of cross-links, the enriched DNA fragments are purified and analyzed by real-time polymerase chain reaction (PCR) to examine the occupancy of HIF-1α at β-catenin promoter. The primer sequences were listed in Table 1. Data analysis was conducted using dedicated software to assess statistical relevance among the experimental groups.
| Genes | Forward | Reverse |
| GAPDH | 5’-TGATGGGTGTGAACCACGAG-3’ | 5’-AGTGATGGCATGGACTGTGG-3’ |
| β-catenin | 5’-TTTGGTACCACACGGAGAGC-3’ | 5’-CCAAATCCCCAGCCCATCTT-3’ |
| HIF-1α | 5’-TTTTCTGGTCTGACCGACGG-3’ | 5’-GCTGTGACACGGGTACTTGA-3’ |
| bFGF | 5’-TCCATCAAGGGAGTGTGTGC-3’ | 5’-GGACTCCAGGCGTTCAAAGA-3’ |
| Survivin | 5’-GTTGTGCAAGGCCTTTCTGG-3’ | 5’-ACCCCATGGTAGGAGGACTC-3’ |
| Bcl2 | 5’-CTTCTCTCGTCGCTACCGTC-3’ | 5’-CAATCCTCCCCCAGTTCACC-3’ |
| Bax | 5’-CACCTGAGCTGACCTTGGAG-3’ | 5’-TCCTCTGCAGCTCCATGTTG-3’ |
Immunofluorescence staining was conducted on cultured PBMSCs or tissue sections. Samples were fixed with 4% paraformaldehyde solution (Sigma, Japan), and subsequently incubated with primary antibodies targeting HIF-1α, β-catenin, and CD31. 4’,6-diamidino-2-phenylindole was utilized for staining cell nucleus[21].
Total RNA was extracted from PBMSCs or tissues utilizing TRIzol reagent (Invitrogen, MA, United States) per the supplier’s protocols. The concentration and purity of RNA were determined through spectrophotometric analysis (NanoDrop 2000, Thermo Fisher Scientific, MA, United States). First-strand cDNA was synthesized from 1 μg of total RNA using PrimeScript RT reagent kit (Takara Bio, Japan) with oligo(dT) primers. Subsequently, quantitative real-time PCR was performed utilizing primers depicted in Table 1. The relative gene expression was calculated using 2-ΔΔCt formula, employing GAPDH as the internal reference gene[22].
An MI model was established in inbred Lewis rats through ligation of the left anterior descending coronary artery under general anesthesia with ketamine & diazepam at a dosage of 75 mg/kg & 5 mg/kg. Thirty adult male rats were randomly assigned into six groups: Sham-operated, MI, oeHIF-1α-PBMSCs, oeβ-catenin-PBMSCs, shHIF-1α + oeβ-catenin-PBMSCs, and oeHIF-1α + shβ-catenin-PBMSCs. At 24 hours post-transfection with oeHIF-1α, shHIF-1α, oeβ-catenin, or shβ-catenin, PBMSCs were co-transfected with a lentiviral vector encoding enhanced green fluorescent protein (EGFP) cDNA. The resulting dual-transfected PBMSCs, expressing both EGFP and target genes, were then intramyocardially injected into the four sites across with the infarct and peri-infarct zones at a total of 5.0 × 106 cells per rat. Postoperative analgesia was provided via topical application of bupivacaine and intramuscular administration of butorphanol hydrochloride. Surgical wounds were treated with triple antibiotic ointment. To minimize immune rejection, cyclosporine A was administered beginning on day 1 post-MI and continued until euthanasia on day 90. No unintentional deaths occurred during the study.
At the experimental endpoint (90 days following cell transplantation), rats were euthanized by intraperitoneal administration of 0.8% pentobarbital sodium (150 mg/kg). Subsequently, the hearts were perfused with 4% buffered formalin, surgically excised, and meticulously sectioned into three transverse slices. Myocardial samples from five randomly selected rats in each group were subjected to triphenyltetrazolium chloride staining for quantifying infarct size. The ratio of necrotic-to-total area within the left ventricle was assessed using Scion ImageJ software (NIH, Bethesda, MD, United States). Paraffin embedding and serial sectioning were conducted on peri-infarct regions obtained from both MI-induced rats and those treated with PBMSC-based therapies, followed by Masson trichrome, hematoxylin and eosin, and immunofluorescence staining. Imaging was accomplished using an inverted phase-contrast microscope (CKX41-A32PH, Olympus, Tokyo, Japan).
All data were denoted as the mean ± SEM, based on results derived from three independent experimental. Statistical comparisons between groups were executed utilizing one-way ANOVA in SPSS 17.0 software (IBM Corporation, NY, New York). P < 0.05 was interpreted as statistically significant, with further distinctions indicated for P < 0.01 or P < 0.001.
Following 24 hours of primary culture, mononuclear cells were observed to adhere the bottom of the culture dish. By day 14, cell colonies began to form, and by day 21, a confluent monolayer of PBMSCs was established. Upon subculturing, adherent cells at the P4 exhibited a stable homogeneous fibroblast-like morphology (Figure 1A), which were utilized for subsequent experiments. Compared to PBMSCs derived from the abdominal aorta of uninjured control rats, PBMSCs isolated from trauma-exposed rats displayed a markedly enhanced proliferation rate, implying a lower yield of MSCs in healthy peripheral blood. Therefore, PBMSCs used in this study were obtained from the injured rats. Flow cytometric analysis confirmed that P4 PBMSCs expressed high levels of MSC-specific markers, including CD44 (99.1%), CD105 (98.8%), CD90 (99.2%), CD73 (98.7%) and CD71 (95.9%). In contrast, minimal expression was detected for HSC markers CD34 (0.48%) and CD45 (0.48%), as well as EPC markers CD133 (0.76%) and CD31 (0.096%) (Figure 1B).
The multipotent differentiation potential of PBMSCs was further assessed via induction into adipocytes, chondrocytes, and osteoblasts with the application of targeted growth factors and cultivation on tailored extracellular matrices. Under adipogenic condition, cytoplasmic lipid droplets were observed in PBMSCs using light microscopy, and the accumulation of lipids was verified by Oil Red O staining (Figure 1C). Chondrogenic differentiation was indicated by proteoglycan deposition stained with Alcian Blue staining. On the 21st day of osteogenic induction, mineralized nodule-like clusters were observed and positively stained with Alizarin Red. Corresponding negative staining outcomes reflecting the differential lineage commitment of PBMSCs were also presented in Figure 1C.
To elucidate the influence of HIF-1α on PBMSCs proliferation under hypoxic exposure, cell colony formation assay was conducted. After 14 days incubation, the number of colonies in the hypoxia-Con group was approximately 2.0 times than that observed in the normoxia-Con group (Figure 2A and B). Compared to hypoxia-Con group, oeHIF-1α transfected PBMSCs exhibited a 1.6-fold increase in colony formation, while the shHIF-1α group displayed a 5.6-fold reduction. Notably, the dual-transfected oe + shHIF-1α group showed no statistically difference in colony counts compared to the hypoxia-Con condition. These results emphasize the key role of HIF-1α in promoting PBMSCs viability and sustained proliferation.
β-galactosidase staining was employed to determine the proportion of senescent cells in each group. As depicted in Figure 2C and D, a marked decline in the percentage of β-galactosidase positive cells was observed in the hypoxia-Con group, compared to the normoxia-Con group. Under hypoxic conditions, oeHIF-1α transfection further reduced the percentage of β-galactosidase positive cells, relative to the hypoxia-Con group, whereas shHIF-1α transfection markedly increased β-galactosidase positive cells ratio. No statistically significant variation was detected between the oe + shHIF-1α group and the hypoxia-Con group.
The regulatory effects of β-catenin on PBMSCs proliferation and senescence were further investigated. Following 14 days incubation, the number of colonies in hypoxia-Con group was approximately 2.0-fold greater than that observed in normoxia-Con group (Figure 3A and B). PBMSCs exposed to oeβ-catenin exhibited a 1.6-fold increase in colony formation, whereas those treated with shβ-catenin demonstrated a 7.7-fold reduction, relative to the hypoxia-Con group. Notably, no statistically significant variation was identified between the oe + shβ-catenin group and the hypoxia-Con group. These results emphasize the essential role of β-catenin in enhancing both the viability and sustained proliferation of PBMSCs.
β-galactosidase staining was used to test the proportion of senescent cells in each group. As demonstrated in Figure 3C and D, a marked reduction in the percentage of β-galactosidase positive cells was observed in the hypoxia-Con group, relative to the normoxia-Con group. Under hypoxic conditions, a markedly lower percentage of β-galactosidase positive cells was identified in the oeβ-catenin group, while an obviously higher percentage of β-galactosidase positive cells was observed in the shβ-catenin group, relative to the hypoxia-Con group. No statistically significant difference was detected between the oe + shβ-catenin group and the hypoxia-Con group.
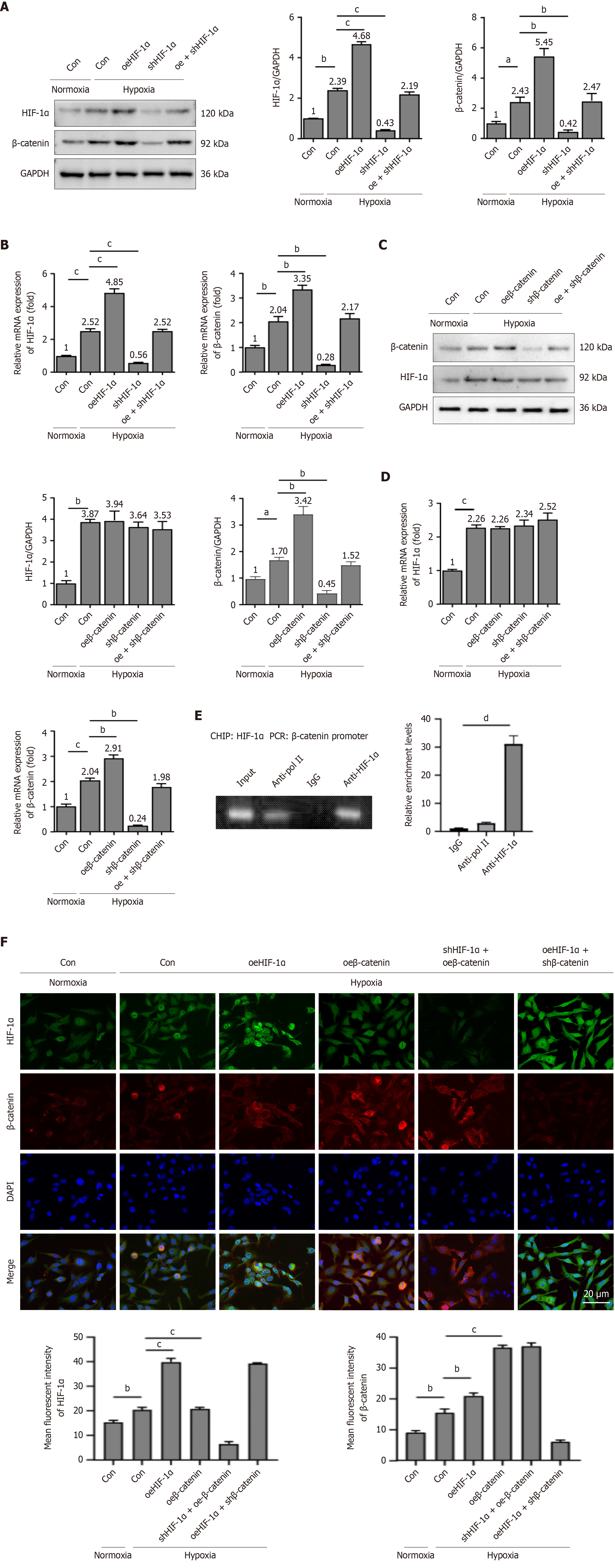
Subsequently, the regulatory relationship between HIF-1α and β-catenin was investigated. As depicted in Figure 4A and B, both mRNA and protein expression levels of HIF-1α and β-catenin were markedly upregulated in PBMSCs under hypoxic conditions, relative to the normoxia-Con group. Under hypoxia condition, HIF-1α overexpression resulted in a significant elevation of β-catenin expression, while HIF-1α silencing led to a pronounced reduction of β-catenin expression. No statistically alterations concerning the expression of either HIF-1α or β-catenin were observed between the oe + shHIF-1α group and the hypoxia-Con group.
Although either the overexpression or knockdown of HIF-1α resulted in respective increases or decreases in β-catenin mRNA and protein levels, no significant differences were identified in HIF-1α expression among the hypoxia-Con, oeβ-catenin, shβ-catenin, and oe + shβ-catenin groups (Figure 4C and D). To determine whether HIF-1α directly binds to the β-catenin promoter, ChIP assay was conducted. The result demonstrated that β-catenin promoter sequences were amplified exclusively from chromatin complexes immunoprecipitated with anti-HIF-1α antibodies (Figure 4E). Collectively, these results indicate that HIF-1α mediates the transcription of β-catenin in PBMSCs, with no evidence of a reciprocal regulatory influence of β-catenin on HIF-1α.
The immunofluorescence staining results of PBMSCs under normoxic and hypoxic conditions supported these findings. Relative to the normoxia-Con group, the hypoxia-Con group showed markedly elevated expression levels of both HIF-1α and β-catenin. Under hypoxic exposure, the oeHIF-1α group displayed enhanced fluorescence intensity of HIF-1α and β-catenin, in comparison to the hypoxia-Con group. Furthermore, overexpression of β-catenin resulted in a pronounced increase in its own expression, while HIF-1α expression remained unchanged (Figure 4F). Evaluation of HIF-1α and β-catenin levels in the shHIF-1α + oeβ-catenin and oeHIF-1α + shβ-catenin groups revealed that HIF-1α modulated β-catenin expression, whereas β-catenin did not reciprocally regulate HIF-1α. These observations further corroborate the regulatory capacity of HIF-1α in β-catenin transcription in PBMSCs.
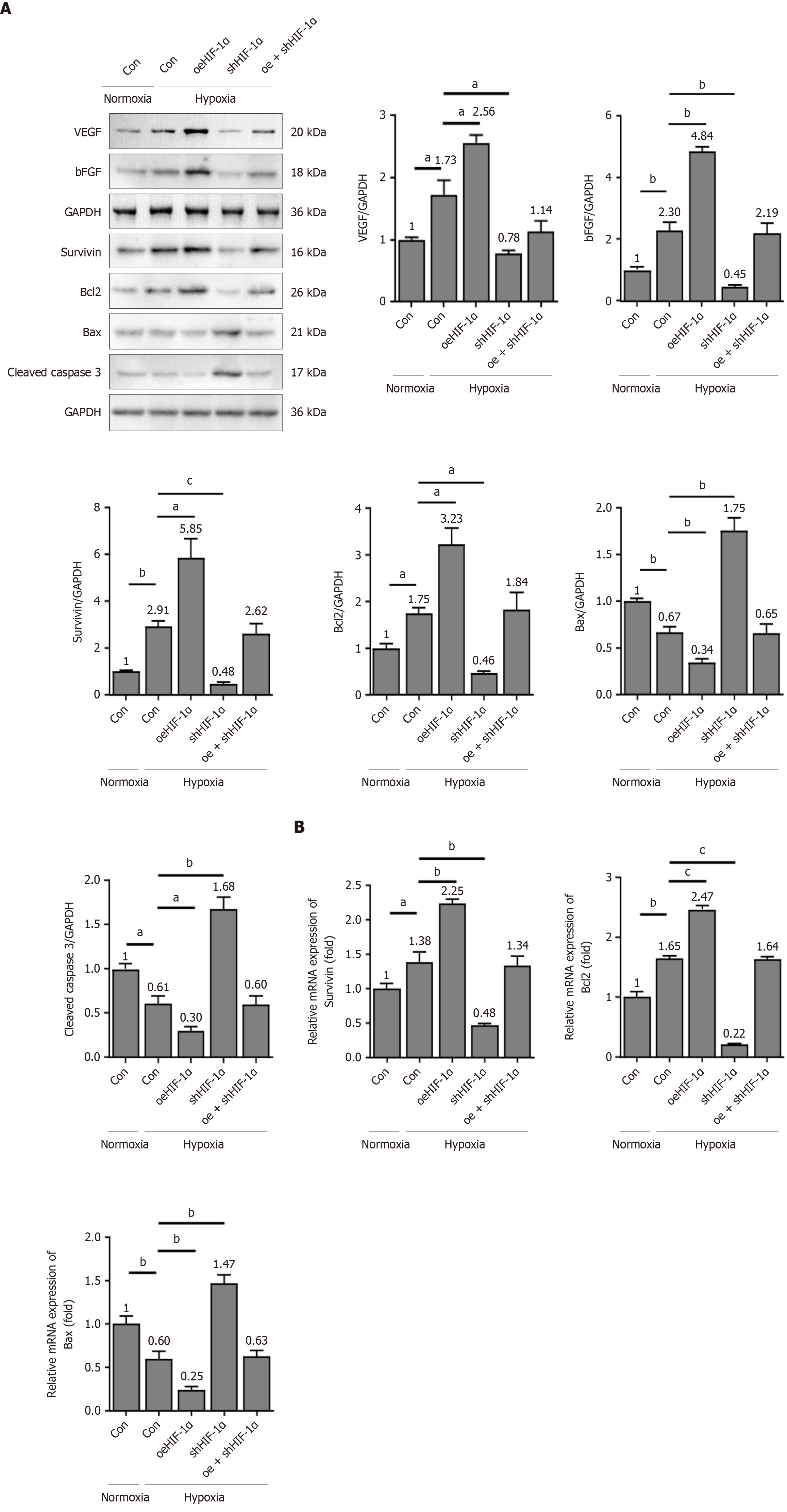
To elucidate the mechanism underlying the HIF-1α/β-catenin-mediated cytoprotective effects against apoptosis, direct gain- and loss-of-function experiments were subsequently employed. PBMSCs were transfected with either oeHIF-1α/oeβ-catenin or shHIF-1α/shβ-catenin. As illustrated in Figure 5A, the protein expressions of VEGF and basic fibroblast growth factor (bFGF) were increased in PBMSCs under hypoxia conditions, relative to the normoxia environment. Overexpression of HIF-1α further enhanced the VEGF and bFGF protein levels in PBMSCs, while silence of HIF-1α reduced the VEGF and bFGF protein levels, compared to hypoxia-Con group. No statistically significant differences were observed in the expressions of VEGF and bFGF between the oeHIF-1α + shHIF-1α group and the hypoxia-Con group.
In addition, the mRNA and protein expression levels of antiapoptotic markers survivin and Bcl2 were markedly elevated in PBMSCs, compared to normoxia condition (Figure 5A and B). Relative to the hypoxia-Con group, overexpression of HIF-1α led to a significant upregulation of survivin and Bcl2 at both mRNA and protein levels, whereas silencing of HIF-1α had opposite influences. Notably, no statistically significant alterations were observed in the ex
In addition, β-catenin overexpression markedly increased the expressions of survivin and Bcl2, reduced the expressions of Bax and cleaved caspase-3 under hypoxia environment (Figure 6A and B). shβ-catenin had opposite effects. No significant variations concerning the expressions of survivin, Bcl2, Bax and cleaved caspase-3 were observed between the oe + shβ-catenin group and the hypoxia-Con group. Collectively, these findings indicated that HIF-1α/β-catenin signaling axis governed the expressions of key apoptosis and angiogenic-associated genes, thereby contributing to the sustained survival of PBMSCs under hypoxic conditions.
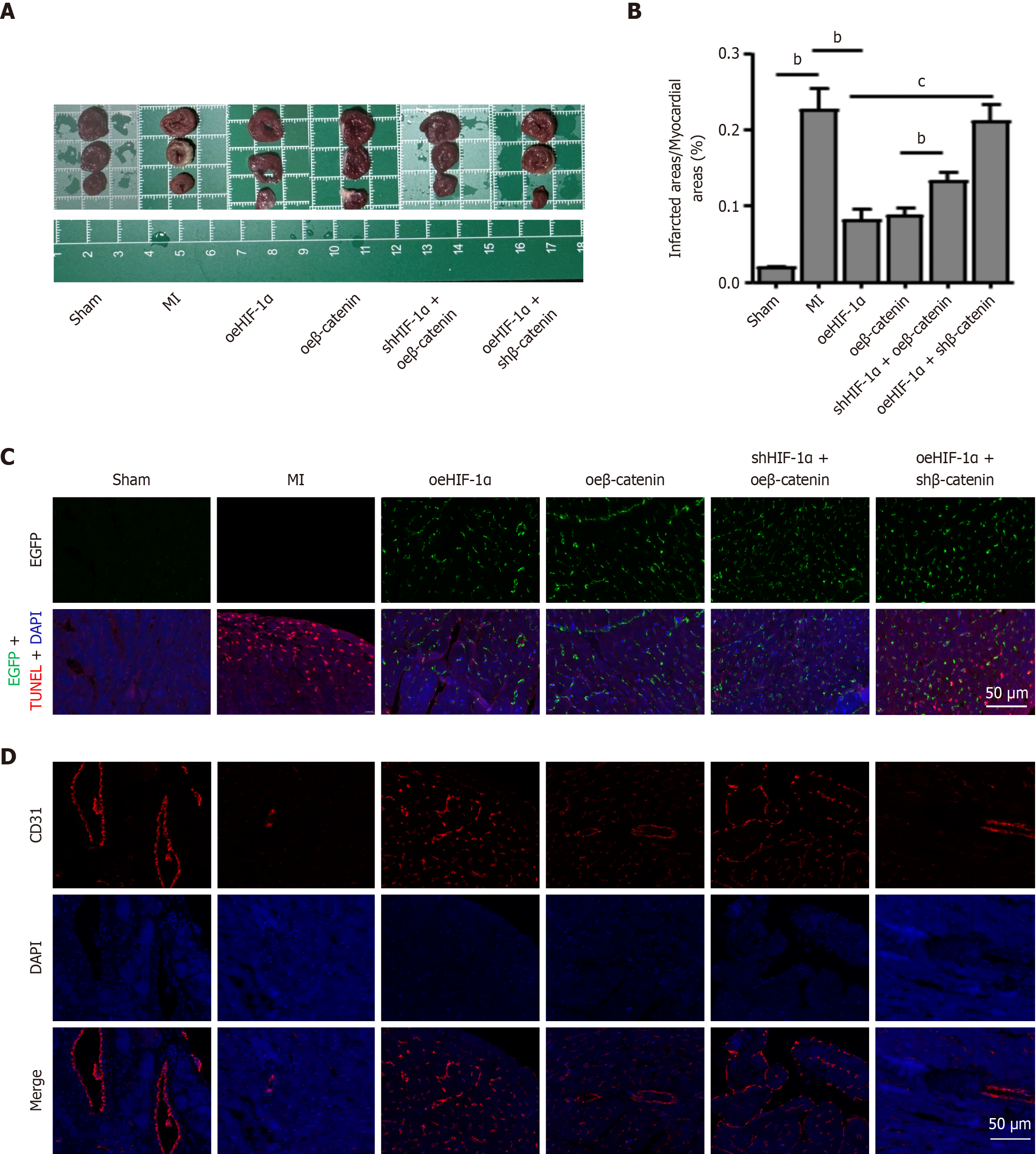
Subsequently, the potential roles of the HIF-1α/β-catenin signaling cascade in enhancing PBMSC engraftment, survival, and post-MI functional restoration were explored. An MI model was established in rats via ligation of the left anterior descending coronary artery. Following co-transfection with oeHIF-1α, oeβ-catenin, shHIF-1α + oeβ-catenin, or oeHIF-1α + shβ-catenin, the modified PBMSCs were intramyocardially transplanted into the infarcted cardiac tissue. Rats in the sham and MI groups did not receive cell transplantation, serving as negative controls. Morphometric evaluation based on triphenyltetrazolium chloride staining at 90 days post-MI revealed that the infarct size in the MI group was markedly greater than that in the sham group. Notably, both the oeHIF-1α and oeβ-catenin groups exhibited markedly diminished infarct areas. Furthermore, the infarct size was increased in the shHIF-1α + oeβ-catenin group when compared to the oeβ-catenin group. Besides, oeHIF-1α + shβ-catenin group also showed a significant increase in infarct size relative to the oeHIF-1α group (Figure 7A and B).
Figure 7C illustrated the long-term retention of PBMSCs within infarcted myocardium at 90 days post-transplantation. To enable visualization, PBMSCs overexpressing HIF-1α or β-catenin were labeled with EGFP (green fluorescence). TUNEL staining assays were utilized to evaluate the anti-apoptotic effects mediated by HIF-1α/β-catenin. The results demonstrated that HIF-1α or β-catenin overexpression markedly reduced PBMSC apoptosis relative to the MI group, whereas shβ-catenin effectively abrogated the anti-apoptotic protection conferred by oeHIF-1α. Collectively, these observations suggest that HIF-1α/β-catenin signaling facilitates PBMSC engraftment and mitigates cell loss during MI. Given the critical role of angiogenesis in MSC-mediated cardiac repair, further investigation was performed to assess the regulatory influence of HIF-1α/β-catenin signaling on angiogenic capacity of PBMSCs (Figure 7D). CD31, a vascular endothelial marker belonging to the immunoglobulin superfamily with a molecular weight of 130-140 kDa, was examined. Compared to the sham group, CD31 expression was markedly diminished in the MI group. Both oeHIF-1α and oeβ-catenin groups showed elevated CD31 levels relative to the MI group. Notably, CD31 expression was markedly reduced in the shHIF-1α + oeβ-catenin group compared to the oeβ-catenin group. Similarly, CD31 levels were reduced in the oeHIF-1α + shβ-catenin group relative to the oeHIF-1α group. These findings indicate that preconditioning PBMSCs with HIF-1α overexpression markedly enhances their angiogenic properties through β-catenin activation.
Cardiac tissue samples were additionally analyzed using Masson’s trichrome staining. As presented in Figure 8A, in comparison to the sham group, the MI group exhibited a substantial expansion in the blue-stained collagen fiber region along with a marked reduction in red-stained myocardial fibers. In contrast, transplantation of PBMSCs overexpressing HIF-1α or β-catenin led to a considerable decrease in collagen fiber accumulation across the myocardial tissue. A notable increase in collagen content was observed in the oeHIF-1α + shβ-catenin group relative to the oeHIF-1α group. Histological examination of hematoxylin and eosin-stained cardiac sections from rats in MI group revealed prominent inflammatory infiltration and fibrosis within the infarct zone. Nonetheless, administration of PBMSCs transfected with oeHIF-1α or oeβ-catenin markedly alleviated myocardial inflammation. Compared to the oeHIF-1α group, oeHIF-1α + shβ-catenin group exhibited enhanced fibrotic remodeling, increased inflammatory cell infiltration, and more severe interstitial edema. Collectively, these findings indicate that PBMSCs overexpressing HIF-1α/β-catenin markedly ameliorate infarction-induced myocardial damage.
Following isolation of rat cardiac tissues, whether activated HIF-1α/β-catenin signaling correlates with elevated expression of proteins related to angiogenesis and apoptosis was examined. As depicted in Figure 8B and C, the MI group showed markedly reduced protein expressions of HIF-1α, β-catenin, anti-apoptotic regulators (Bcl2 and survivin), and angiogenic markers (bFGF and VEGF), compared to the sham group. In contrast, PBMSCs overexpressing HIF-1α or β-catenin exhibited marked upregulation of these proteins relative to the MI group. Furthermore, oeHIF-1α + shβ-catenin group displayed notable decreases in the levels of HIF-1α, β-catenin, Bcl2, survivin, bFGF, and VEGF, compared to the oeHIF-1α group. Similarly, shHIF-1α + oeβ-catenin group showed significant decreases of these proteins, in comparison to the oeβ-catenin group.
The expressions of pro-apoptotic proteins (Bax and cleaved caspase 3) were also detected. Compared to the sham group, markedly elevated protein levels of Bax and cleaved caspase 3 were detected in the MI group (Figure 8C). However, PBMSCs overexpressing HIF-1α or β-catenin showed significant reductions of Bax and cleaved caspase 3 expressions, relative to the MI group. Notably, compared to the oeHIF-1α group, oeHIF-1α + shβ-catenin group exhibited increased expressions of Bax and cleaved caspase 3. Conversely, in comparison to the oeβ-catenin group, shHIF-1α + oeβ-catenin PBMSCs group showed reduced expressions of these pro-apoptotic proteins. Collectively, these results indicate that the overexpression of HIF-1α facilitates the sustained survival and engraftment of PBMSCs in ischemic myocardium by modulating β-catenin-mediated anti-apoptotic pathways.
The therapeutic application of PBMSCs in MI has garnered substantial attention owing to their regenerative potential[23]. Nonetheless, their clinical utility is often hindered by poor cell retention and inadequate integration within ischemic tissues[24]. Emerging evidence has highlighted the significance of hypoxic conditions in enhancing PBMSC function, with HIF-1α identified as a central mediator in hypoxia-driven cellular adaptations[25]. In this study, the regulatory axis between HIF-1α and β-catenin signaling pathway was delineated. Specifically, HIF-1α was found to promote β-catenin transcription, thereby strengthened the anti-apoptotic and angiogenic properties of PBMSCs (Figures 4 and 5). β-catenin has been considered as key factors for regulating Bcl2, survivin, and bFGF, which are instrumental in promoting cell survival and vascular formation. Importantly, the identification of a direct regulatory effect of HIF-1α on β-catenin reveals a previously unrecognized mechanism that may be leveraged to improve the therapeutic efficacy of PBMSC-based treatments. These findings advance the understanding of stem cell-mediated cardiac repair and pave the way for novel strategies to improve therapeutic outcomes in ischemic heart disease.
In this research, peripheral blood was validated as a readily accessible and minimally invasive source of MSCs in rat models. Initially, PBMSCs were effectively isolated from peripheral arterial blood, consistent with earlier studies that isolated MSCs from the peripheral venous system and injured coronary arteries in MI patients. Moreover, several protocols have been established for the isolation and expansion of PBMSCs from peripheral blood[26,27], reinforcing its feasibility as a practical MSCs source. Additionally, although MSCs do not express unique surface membrane markers and their immunophenotypic profile may vary depending on isolation and culture techniques, this study confirmed that PBMSCs expressed classical MSC markers (CD44, CD90, CD71, CD73, and CD105) and displayed minimal expressions of HSC markers and EPC markers (Figure 1B).
The vascular differentiation potential of PBMSCs, particularly their ability to promote vascularization, has been well established and is considered essential for facilitating angiogenesis in ischemic myocardium[28]. The reconstruction of vascular structures remains a key goal in developing effective therapies for cardiac repair. PBMSCs are regarded as highly promising candidates for myocardial regeneration. Their autologous origin offers practical advantages such as self-renewal capacity, operational feasibility, and immediate applicability in MI intervention. Nonetheless, the therapeutic application of PBMSCs is currently limited by their poor survival rates following transplantation, underscoring the need for further systematic exploration.
HIF-1α is broadly acknowledged as a central modulator of cellular adaptation to hypoxia. In PBMSCs, hypoxic exposure has been demonstrated to stabilize HIF-1α, thereby inducing transcriptional programs that promote cell survival and angiogenesis. In this study, we found that HIF-1α overexpression markedly enhances PBMSC proliferation, resulting in an increase in colony formation and a reduction in senescence-associated β-galactosidase activity under hypoxia (Figure 2). Mechanistically, HIF-1α upregulates glycolytic enzymes, such as glucose transporter type 1 and lactate dehydrogenase, ensuring sustained ATP generation during ischemic stress[29,30]. Previous study has reported that astragaloside IV-induced bone marrow-derived MSC-derived exosomes contribute to neovascularization and cardiac protection in MI mice model via miR-411/HIF-1α pathway[31], emphasizing the regulatory influence of HIF-1α in bone marrow-derived MSC-mediated therapeutic strategies for MI. Moreover, HIF-1α-enriched exosomes have been shown to restore angiogenic potential, migration, and proliferative capacity of hypoxia-treated human umbilical vein endothelial cells in vitro, while concurrently mediating cardioprotective effects by promoting the expression of proangiogenic molecules and new vessel formation[32]. Herein, we revealed that HIF-1α overexpression notably amplified the angiogenic activity of PBMSCs in rat MI models, whereas its inhibition markedly curtailed their proliferative function (Figure 8). These results align with previously published findings and collectively support the proposition that HIF-1α overexpression enhances the therapeutic efficacy of stem cells by improving their survival under ischemic environments.
Based on the anti-apoptotic and pro-angiogenic functions of HIF-1α, we observed that its activation elicited a stronger response to β-catenin overexpression compared with non-intervention conditions. The HIF-1α signaling cascade not only contributes to maintain PBMSCs proliferation but also directly modulates β-catenin gene expression. in PBMSCs, HIF-1α overexpression markedly increased β-catenin expression, accompanied by upregulation of downstream targets including VEGF, survivin, bFGF, and Bcl-2 expressions, thereby enhancing both angiogenesis and cell survival (Figures 4, 5 and 6). These findings suggest that β-catenin expression is specifically driven by HIF-1α activity. Notably, HIF-1α expression remained unchanged irrespective of alterations in β-catenin levels post-intervention. ChIP assays further confirmed that HIF-1α transcriptional regulated β-catenin via direct binding promoter in PBMSCs, solidifying β-catenin’s role as a downstream effector of HIF-1α signaling. Furthermore, study in Wnt-activated mouse embryonic stem cells had shown that β-catenin, functioning as a transcriptional co-activator, bound to T-cell factor/Lymphoid enhancer-binding factor family transcription factors and directly associates with the VEGF promoter to initiate its transcription[33].
β-catenin has been reported to initiate the transcription of multiple downstream genes associated with MSC proliferation and survival[34-36]. In this study, it was observed that β-catenin overexpression resulted in elevated levels of the anti-apoptotic proteins Bcl2 and survivin, accompanied by reductions in pro-apoptotic markers such as Bax and cleaved caspase 3 (Figure 6). Additionally, β-catenin overexpression was found to markedly enhance the proliferative potential of PBMSCs and markedly decreased cell senescence (Figure 3). Under conditions of HIF-1α overexpression, upregulation of β-catenin, Bcl2, and survivin was also evident (Figure 5), which may contribute to the sustained retention of PBMSCs in ischemic myocardium. Importantly, the detrimental effects associated with HIF-1α knockdown, such as impaired cellular implantation and elevated apoptosis, were mitigated by β-catenin overexpression. Collectively, these findings offer mechanistic insights into how activation of the HIF-1α/β-catenin axis facilitates PBMSC survival, reinforcing the importance of this pathway in enhancing PBMSC viability for cardiac regeneration after MI.
Based on in vitro experimental findings, the influence of HIF-1α/β-catenin signaling cascade on PBMSC-mediated myocardial repair was subsequently assessed. In patients with MI, ventricular remodeling is typically characterized by infarct expansion and left ventricular dilation[37,38]. Analogously, similar pathological features, including enlargement of the infarct zone, inflammatory infiltration, and extensive fibrosis, were observed in the MI rat model (Figures 7A and 8A). Transplantation of PBMSCs overexpressing HIF-1α or β-catenin markedly reduced infarct size at 90 days following administration, concurrently mitigating fibrosis, cardiomyocyte depletion, and inflammatory responses. Improved cardiac function was observed following the delivery of HIF-1α- or β-catenin-overexpressing PBMSCs, accompanied by enhanced neovascularization and reduced apoptosis. Correspondingly, elevated levels of HIF-1α and β-catenin were associated with increased expression of anti-apoptotic proteins (Figure 8C), suggesting a strong correlation between this signaling axis and the durable retention of PBMSCs in infarcted myocardium. Importantly, when β-catenin expression was silenced via β-catenin shRNA (oeHIF-1α + shβ-catenin group), the beneficial effects conferred by HIF-1α overexpression were entirely abrogated, as reflected by exacerbated cardiac dysfunction, increased fibrosis, larger infarct regions, greater cell loss, and diminished PBMSC engraftment. Conversely, despite the knockdown of HIF-1α, PBMSC proliferation and survival were still preserved in vivo under β-catenin overexpression. Collectively, these results emphasize the essential role of HIF-1α in supporting stem cell viability and functional cardiac recovery through β-catenin-dependent anti-apoptotic mechanisms.
Additionally, HIF-1α signaling axis has been widely implicated in sustaining cancer stem cell viability across diverse tissues via regulation of angiogenesis, epithelial-mesenchymal transition, and the modulation of the cellular microenvironment[39,40]. In ischemic myocardium, it was observed that elevated expression of HIF-1α led to increases of pro-angiogenic cytokine levels (Figure 8B), thereby facilitating the vascular differentiation of PBMSCs, highlighting the relevance of mesenchymal-endothelial transition during the angiogenic process. Rats receiving PBMSCs modified to overexpress HIF-1α, β-catenin levels were substantially elevated within ischemic cardiomyocytes, which in turn augmented the secretion of key pro-angiogenic cytokines such as angiopoietin-1, bFGF, HGF, and VEGF. This enhancement contributed to improved neovascularization, functional recovery, and attenuation of pathological myocardial remodeling. Moreover, overexpression of β-catenin was found to mitigate the adverse effects imposed on PBMSCs by HIF-1α silencing (Figure 8).
Conversely, complete ablation of β-catenin abolished the pro-angiogenic response in PBMSCs triggered by HIF-1α overexpression, as indicated by reduced vascular formation, decreased vessel density, and diminished secretion of HIF-1α-induced pro-angiogenic cytokines. Collectively, these observations suggest an alternative mechanistic pathway by which HIF-1α facilitates mesenchymal-endothelial transition through β-catenin-dependent signaling, thereby potentiating the angiogenic capacity of PBMSCs under ischemic conditions. This mechanism is anticipated to be a focus of future research. The present findings offer a conceptual framework supporting the advancement of gene-engineered stem cell therapies currently under clinical evaluation[41]. HIF-1α acts as a pivotal modulator of β-catenin signaling, contributing to the enhancement of PBMSC viability and angiogenic potential in hypoxic settings. The regulatory function of HIF-1α within the β-catenin network has been elucidated. Furthermore, β-catenin has been identified as a determinant factor in this signaling paradigm.
This study provides important insights into the regulatory influence of the HIF-1α/β-catenin signaling axis in enhancing PBMSC viability for myocardial tissue restoration. Nonetheless, several limitations should be acknowledged. Firstly, the reliance on rat model may limit the generalizability of the findings, as this model may not comprehensively mirror the complex pathophysiological of human MI. Secondly, we also did not detect the dwell time of exogenously delivered MSCs in rat model. The relatively limited duration of post-transplantation observation may fail to capture long-term PBMSC survival and functional integration. The effect of electrical stability of myocardium was also not tested. We used young healthy rats, not elderly with comorbidity in this research. Besides, elevated β-catenin expression is discovered to associate with tumor transformation. Further in-depth investigations are necessary to extend current knowledge and explore the long-term safety and effectiveness of this method in a clinical setting.
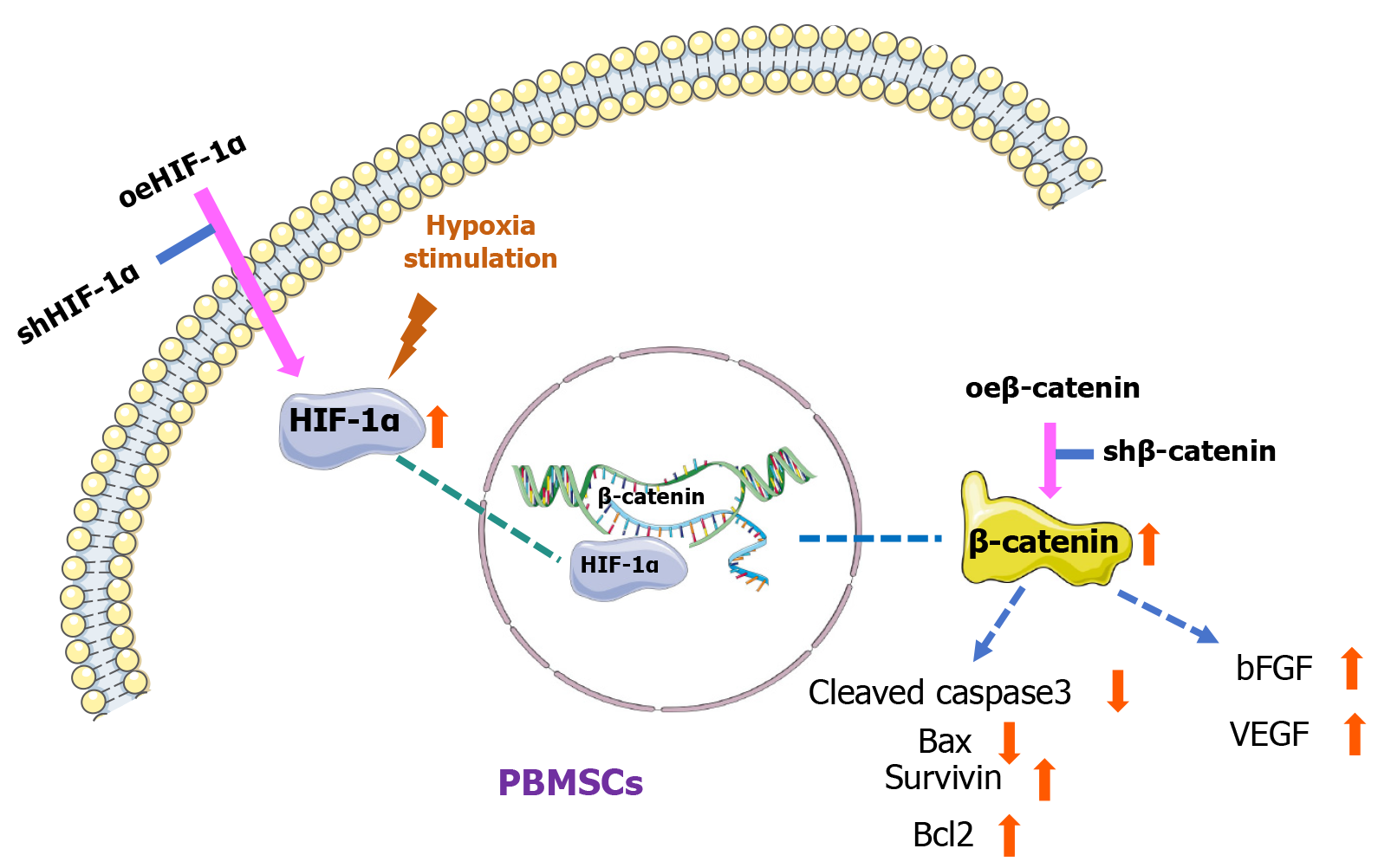
The therapeutic potential of PBMSCs in MI is further reinforced through their engagement with the HIF-1α and β-catenin signaling cascades. In this study, it was demonstrated that HIF-1α improved PBMSC viability by facilitating β-catenin-dependent induction of anti-apoptotic molecules, thereby supporting enhanced cellular survival and neovascularization. A novel mechanism has been revealed, wherein activation of the HIF-1α/β-catenin axis markedly augments PBMSC engraftment in ischemic myocardium, offering a viable approach for enhancing cardiac repair after infarction. These findings not only broaden the current understanding of stem cell regulatory mechanisms but also contribute to refining PBMSC-based therapeutic strategies for clinical applications (Figure 9).
| 1. | Wal P, Aziz N, Singh CP, Rasheed A, Tyagi LK, Agrawal A, Wal A. Current Landscape of Gene Therapy for the Treatment of Cardiovascular Disorders. Curr Gene Ther. 2024;24:356-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Zhu Y, Lai Y, Hu Y, Fu Y, Zhang Z, Lin N, Huang W, Zheng L. The mechanisms underlying acute myocardial infarction in chronic kidney disease patients undergoing hemodialysis. Biomed Pharmacother. 2024;177:117050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 3. | Yan W, Xia Y, Zhao H, Xu X, Ma X, Tao L. Stem cell-based therapy in cardiac repair after myocardial infarction: Promise, challenges, and future directions. J Mol Cell Cardiol. 2024;188:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 4. | Wang P, Deng Z, Li A, Li R, Huang W, Cui J, Chen S, Li B, Zhang S. β-Catenin promotes long-term survival and angiogenesis of peripheral blood mesenchymal stem cells via the Oct4 signaling pathway. Exp Mol Med. 2022;54:1434-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 5. | Pang QM, Qian NN, Zou WH, Yang YC, Chen H, Zhang M, Zhang Q, Ao J, Zhang T. PBMSCs transplantation facilitates functional recovery after spinal cord injury by regulating microglia/macrophages plasticity. Transpl Immunol. 2022;72:101592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 6. | Wang P, Zhu P, Yu C, Wu J. The Proliferation and Stemness of Peripheral Blood-Derived Mesenchymal Stromal Cells Were Enhanced by Hypoxia. Front Endocrinol (Lausanne). 2022;13:873662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Alanova P, Alan L, Opletalova B, Bohuslavova R, Abaffy P, Matejkova K, Holzerova K, Benak D, Kaludercic N, Menabo R, Di Lisa F, Ostadal B, Kolar F, Pavlinkova G. HIF-1α limits myocardial infarction by promoting mitophagy in mouse hearts adapted to chronic hypoxia. Acta Physiol (Oxf). 2024;240:e14202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Guan Z, Jin X, Guan Z, Liu S, Tao K, Luo L. The gut microbiota metabolite capsiate regulate SLC2A1 expression by targeting HIF-1α to inhibit knee osteoarthritis-induced ferroptosis. Aging Cell. 2023;22:e13807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 9. | Song S, Zhang G, Chen X, Zheng J, Liu X, Wang Y, Chen Z, Wang Y, Song Y, Zhou Q. HIF-1α increases the osteogenic capacity of ADSCs by coupling angiogenesis and osteogenesis via the HIF-1α/VEGF/AKT/mTOR signaling pathway. J Nanobiotechnology. 2023;21:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 10. | Hu X, Gan L, Tang Z, Lin R, Liang Z, Li F, Zhu C, Han X, Zheng R, Shen J, Yu J, Luo N, Peng W, Tan J, Li X, Fan J, Wen Q, Wang X, Li J, Zheng X, Liu Q, Guo J, Shi GP, Mao H, Chen W, Yin S, Zhou Y. A Natural Small Molecule Mitigates Kidney Fibrosis by Targeting Cdc42-mediated GSK-3β/β-catenin Signaling. Adv Sci (Weinh). 2024;11:e2307850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Xu J, Liu S, Xu L, Zhang Y, Jiang W, Chu L. Therapeutic Strategies for Ischemic Stroke: Modulating the Adult Neural Stem Cell Niche through the Wnt/β-catenin Pathway. J Integr Neurosci. 2024;23:131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3740] [Cited by in RCA: 4522] [Article Influence: 323.0] [Reference Citation Analysis (0)] |
| 13. | Tian L, Chen X, Cao L, Zhang L, Chen J. Effects of plant-based medicinal food on postoperative recurrence and lung metastasis of gastric cancer regulated by Wnt/β-catenin-EMT signaling pathway and VEGF-C/D-VEGFR-3 cascade in a mouse model. BMC Complement Med Ther. 2022;22:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 398] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 15. | Sui H, Zhao J, Zhou L, Wen H, Deng W, Li C, Ji Q, Liu X, Feng Y, Chai N, Zhang Q, Cai J, Li Q. Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 2017;403:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Fazeli Z, Rajabibazl M, Faramarzi S, Omrani MD, Ghaderian SMH, Safavi Naini N. Correlation of TCF4, GSK, TERT and TERC Expressions with Proliferation Potential of Early and Late Culture of Human Peripheral Blood Mesenchymal Stem Cells. Cell J. 2021;22:431-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Kim HY, Kim HS. Podoplanin depletion in tonsil-derived mesenchymal stem cells induces cellular senescence via regulation of the p16(Ink4a)/Rb pathway. Cell Commun Signal. 2024;22:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Khodabandehloo F, Aflatoonian R, Zandieh Z, Rajaei F, Sayahpour FA, Nassiri-Asl M, Baghaban Eslaminejad M. Functional differences of Toll-like receptor 4 in osteogenesis, adipogenesis and chondrogenesis in human bone marrow-derived mesenchymal stem cells. J Cell Mol Med. 2021;25:5138-5149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Wu M, Liu F, Yan L, Huang R, Hu R, Zhu J, Li S, Long C. MiR-145-5p restrains chondrogenic differentiation of synovium-derived mesenchymal stem cells by suppressing TLR4. Nucleosides Nucleotides Nucleic Acids. 2022;41:625-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Wang J, Sun L, Liu Y, Zhang Y. FIGNL1 Promotes Hepatocellular Carcinoma Formation via Remodeling ECM-receptor Interaction Pathway Mediated by HMMR. Curr Gene Ther. 2024;24:249-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 21. | Liang L, Li Y, Jiao Y, Zhang C, Shao M, Jiang H, Wu Z, Chen H, Guo J, Jia H, Zhao T. Maprotiline Prompts an Antitumour Effect by Inhibiting PD-L1 Expression in Mice with Melanoma. Curr Mol Pharmacol. 2024;17:e18761429259562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 139067] [Article Influence: 5562.7] [Reference Citation Analysis (3)] |
| 23. | Wang J, Chen Z, Dai Q, Zhao J, Wei Z, Hu J, Sun X, Xie J, Xu B. Intravenously delivered mesenchymal stem cells prevent microvascular obstruction formation after myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2020;115:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Zhang F, Hu G, Chen X, Zhang L, Guo L, Li C, Zhao H, Cui Z, Guo X, Sun F, Song D, Yan W, Xia Y, Wang S, Fan M, Tao L. Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in myocardial infarction. Signal Transduct Target Ther. 2022;7:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 25. | Zhou B, Ge T, Zhou L, Jiang L, Zhu L, Yao P, Yu Q. Dimethyloxalyl Glycine Regulates the HIF-1 Signaling Pathway in Mesenchymal Stem Cells. Stem Cell Rev Rep. 2020;16:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Sato K, Yamawaki-Ogata A, Kanemoto I, Usui A, Narita Y. Isolation and characterisation of peripheral blood-derived feline mesenchymal stem cells. Vet J. 2016;216:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Zhang K, Xu T, Xie H, Li J, Fu W. Donor-Matched Peripheral Blood-Derived Mesenchymal Stem Cells Combined With Platelet-Rich Plasma Synergistically Ameliorate Surgery-Induced Osteoarthritis in Rabbits: An In Vitro and In Vivo Study. Am J Sports Med. 2023;51:3008-3024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 28. | Bussche L, Van de Walle GR. Peripheral Blood-Derived Mesenchymal Stromal Cells Promote Angiogenesis via Paracrine Stimulation of Vascular Endothelial Growth Factor Secretion in the Equine Model. Stem Cells Transl Med. 2014;3:1514-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Selvaraju V, Parinandi NL, Adluri RS, Goldman JW, Hussain N, Sanchez JA, Maulik N. Molecular mechanisms of action and therapeutic uses of pharmacological inhibitors of HIF-prolyl 4-hydroxylases for treatment of ischemic diseases. Antioxid Redox Signal. 2014;20:2631-2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Fu ZJ, Wang ZY, Xu L, Chen XH, Li XX, Liao WT, Ma HK, Jiang MD, Xu TT, Xu J, Shen Y, Song B, Gao PJ, Han WQ, Zhang W. HIF-1α-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol. 2020;36:101671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 31. | Yang L, Liu N, Yang Y. Astragaloside IV-induced BMSC exosomes promote neovascularization and protect cardiac function in myocardial infarction mice via the miR-411/HIF-1α axis. J Liposome Res. 2024;34:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 32. | Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, Yang Z, Shen Z. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther. 2020;11:373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 33. | Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW. β-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Xiong K, Niu J, Zheng R, Liu Z, Song Y, Wang L, Zhu C, Fan L. The Role of β-Catenin in Th1 Immune Response against Tuberculosis and Profiles of Expression in Patients with Pulmonary Tuberculosis. J Immunol Res. 2021;2021:6625855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Chen H, Hu Y, Xu X, Dai Y, Qian H, Yang X, Liu J, He Q, Zhang H. DKK1 Activates the PI3K/AKT Pathway via CKAP4 to Balance the Inhibitory Effect on Wnt/β-Catenin Signaling and Regulates Wnt3a-Induced MSC Migration. Stem Cells. 2024;42:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Li X, Feng J, Cheng H, Jin N, Jin S, Liu Z, Xu J, Xie J. Human umbilical cord mesenchymal stem cells enhance liver regeneration and decrease collagen content in fibrosis mice after partial hepatectomy by activating Wnt/β-catenin signaling. Acta Biochim Biophys Sin (Shanghai). 2024;57:604-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Fayol A, Schiele F, Ferrières J, Puymirat E, Bataille V, Tea V, Chamandi C, Albert F, Lemesle G, Cayla G, Weizman O, Simon T, Danchin N; FAST-MI Investigators. Association of Use and Dose of Lipid-Lowering Therapy Post Acute Myocardial Infarction With 5-Year Survival in Older Adults. Circ Cardiovasc Qual Outcomes. 2024;17:e010685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Buda KG, Hryniewicz K, Eckman PM, Basir MB, Cowger JA, Alaswad K, Mukundan S, Sandoval Y, Elliott A, Brilakis ES, Megaly MS. Early vs. delayed mechanical circulatory support in patients with acute myocardial infarction and cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2024;13:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 39. | Zhang Q, Dunbar KB, Odze RD, Agoston AT, Wang X, Su T, Nguyen AD, Zhang X, Spechler SJ, Souza RF. Hypoxia-inducible factor-1α mediates reflux-induced epithelial-mesenchymal plasticity in Barrett's oesophagus patients. Gut. 2024;73:1269-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 40. | Yuan M, Wu Y, Zhou X, Cai Y, Li H, Xia A, Wang X, Wen J, Duan Q, Xu C, Cao H, Miao C. Clematichinenoside AR alleviates rheumatoid arthritis by inhibiting synovial angiogenesis through the HIF-1α/VEGFA/ANG2 axis. Phytomedicine. 2025;139:156552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 41. | Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 356] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/