Published online Nov 26, 2025. doi: 10.4252/wjsc.v17.i11.112476
Revised: September 16, 2025
Accepted: October 20, 2025
Published online: November 26, 2025
Processing time: 120 Days and 22.3 Hours
This article comments on the study by Fang, which demonstrates that reduced nuclear factor erythroid-derived 2 (NRF2) activity promotes endoplasmic re
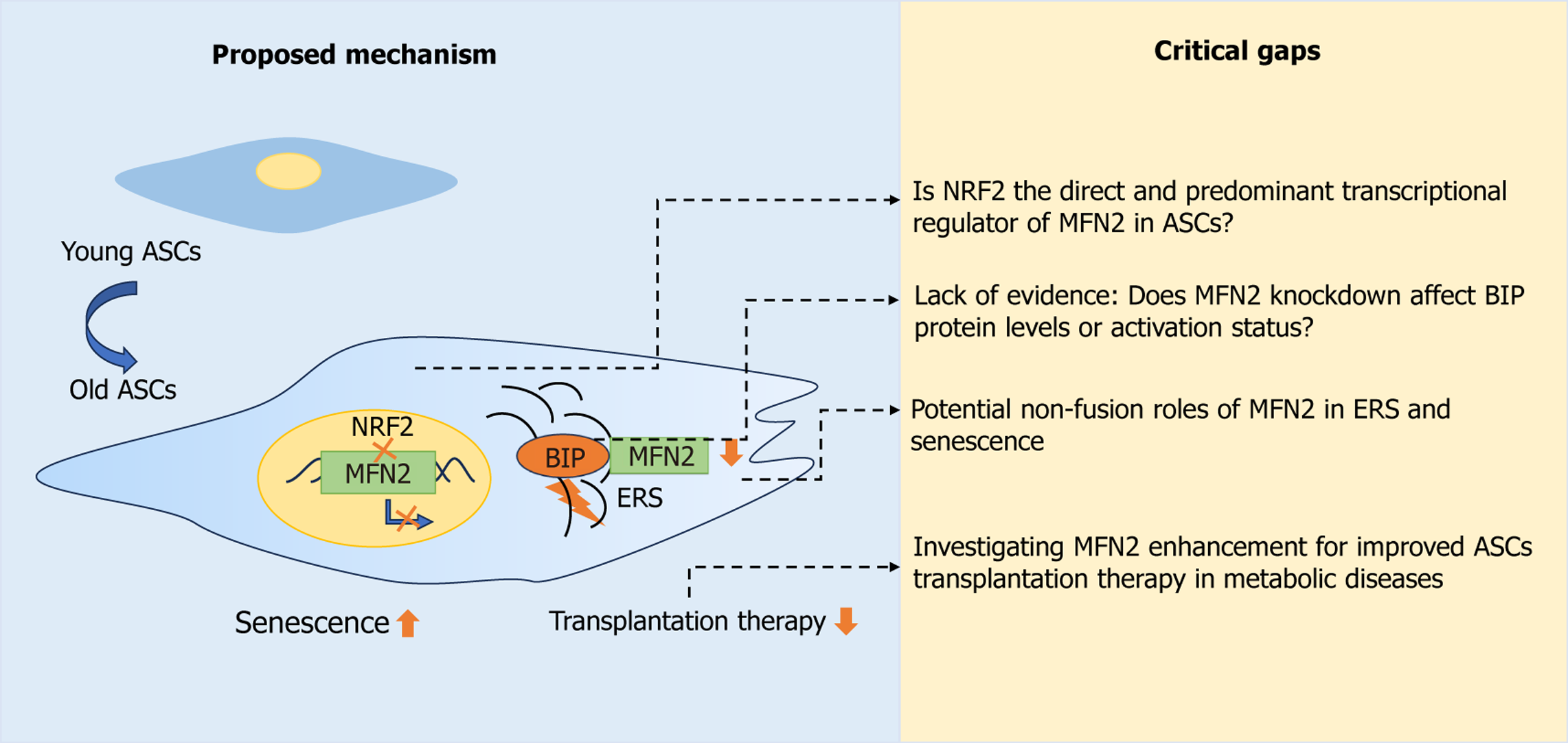
Core Tip: Fang’s study links the nuclear factor erythroid-derived 2-mitofusin-2 (MFN2) axis to endoplasmic reticulum stress and senescence in adipose-derived mesenchymal stem cells from obese mice. While the evidence for reduced nuclear factor erythroid-derived 2 and MFN2 suppression is compelling, key issues remain. The specific function of MFN2 in aging adipose-derived mesenchymal stem cells, its potential interaction with binding immunoglobulin protein, and its influence on endoplasmic reticulum stress remain unresolved. Future studies should elucidate the role of MFN2, establish direct biochemical evidence, and assess the therapeutic relevance of targeting this axis in insulin resistance using diet-induced obesity models.
- Citation: Lin F, Ma KX, Liang XT. Insights into mitofusin-2 and endoplasmic reticulum stress regulation in adipose-derived mesenchymal stem cells senescence. World J Stem Cells 2025; 17(11): 112476
- URL: https://www.wjgnet.com/1948-0210/full/v17/i11/112476.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i11.112476
We read with great interest the article by Fang[1], which reported reduced nuclear factor erythroid-derived 2 (NRF2) activity and expression in adipose-derived mesenchymal stem cells (ASCs) from obese mice. This reduction promoted ASC senescence by increasing endoplasmic reticulum stress (ERS). The authors further demonstrated that NRF2 recognizes the promoter region of mitofusin-2 (Mfn2), thereby influencing its transcription. This work establishes a connection between metabolic disease-associated obesity and ASC senescence, linking them to ERS and mitochondrial homeostasis. It also highlights a potential interrelationship between NRF2, MFN2, and binding immunoglobulin protein (BIP). These findings, which offer significant scientific merit and potential clinical translational value, contribute to the exploitation of the relationship between ERS, mitochondrial dynamics, and senescence for the treatment of metabolic diseases.
Building upon prior research that demonstrated reduced NRF2 expression in ASCs from hypertrophic obese mice, this study systematically validated a cascade of events: Decreased NRF2 activity, downregulated NRF2 expression, increased intracellular ERS, and impaired adipogenic differentiation capacity. This work thereby establishes a link between ERS and cellular senescence. Furthermore, the authors observed decreased MFN2 expression in ASCs from hypertrophic obese mice and in NRF2-knockdown ASCs.
Given the presence of an NRF2-binding antioxidant response element within the MFN2 promoter region, the authors used dual-luciferase reporter assays, chromatin immunoprecipitation quantitative polymerase chain reaction, and immunofluorescence to confirm that NRF2 serves as a transcriptional regulator of MFN2 and modulates its expression. The authors demonstrated that NRF2 knockdown triggers mitochondrial stress in ASCs and promotes ASC senescence. Conversely, concurrent overexpression of MFN2 ameliorated both mitochondrial stress and the extent of cellular senescence, without altering NRF2 protein levels. Collectively, these data definitively demonstrate that within ASCs derived from hypertrophic obese mice: (1) ERS and senescence are increased while stemness is impaired; (2) NRF2 expression is reduced and regulates MFN2 expression; and (3) A mechanistic link exists connecting ERS, cellular senescence, and mitochondrial function. However, several critical considerations warrant discussion. Firstly, MFN2 transcription is subject to regulation by multiple transcription factors, including the sirtuin 1/peroxisome proliferator-activated receptor gamma co-activator 1 alpha/peroxisome proliferator-activated receptor gamma pathway[2], Krüppel-like factor 9[3], signal transducer and activator of transcription 3[4], and E2F transcription factor 1[5]. This suggests that MFN2 expression is controlled by a broader regulatory network, and whether NRF2 serves as the predominant regulator under obesity-related oxidative stress requires further investigation. Secondly, NRF2, ERS, and senescence engage in intricate and interdependent interactions, forming a critical regulatory network that influences cellular homeostasis, damage repair, and senescence. Under basal conditions, NRF2 is maintained at low levels within the cell through its binding to the inhibitory protein Kelch-like ECH-associated protein 1, leading to ubiquitination and proteasomal degradation. Upon stimulation (e.g., by oxidative stress), Kelch-like ECH-associated protein 1 is inactivated, allowing NRF2 to be stabilized and subsequently translocated to the nucleus[6]. As a key stress-responsive transcription factor, NRF2 binds to the antioxidant response element to activate the expression of a wide range of antioxidant and cytoprotective genes. It promotes the transcription of enzymes such as glutathione peroxidase, heme oxygenase-1, NAD(P)H quinone dehydrogenase 1, superoxide dismutase, as well as multiple enzymes involved in glutathione biosynthesis, including glutamate-cysteine ligase catalytic and modifier subunits, and glutathione S-transferases[7]. Impaired NRF2-mediated antioxidant response is a critical contributor to aging and neurodegenerative diseases[8]. This intricate regulatory network raises an important question: Given the further reduction of NRF2 levels observed in ASCs from hypertrophic obese mice, could the downregulation of MFN2 be primarily a downstream effect of diminished NRF2 activity? Thirdly, due to its established function in mitochondrial dynamics, MFN2 is critically involved in morphological changes. In light of these considerations, although the presented immunofluorescence data suggest the altered mitochondrial density, this finding is not sufficiently conclusive. A definitive assessment requires transmission electron microscopy to quantify the mitochondrial number, size, and, crucially, the distance between mitochondria and the endo
Another issue pertains to the absence of conclusive evidence establishing that MFN2 regulates cellular senescence through its interaction with BIP. Although the authors identified BIP as a potential MFN2-binding partner via immunoprecipitation-mass spectrometry and suggested a potential interaction using methods such as co-immunoprecipitation and immunofluorescence co-localization, the molecular mechanism by which MFN2, via BIP, modulates ERS and senescence remains incompletely characterized. MFN2 is critically involved in mitochondria-associated ER membranes (MAMs). Song et al[9] found that the reduced MFN2 level augmented MAM biogenesis and increased mitochondria-MAM tethering. Disruption of MFN2 impairs MAMs and can induce ERS. Another study suggested that MFN2 interacts with DIAPH1 to regulate the distance between mitochondria and the ER/sarcoplasmic reticulum. Increasing the mito-sarcoplasmic reticulum/ER distance reduces mitochondrial Ca2+ overload, attenuates oxidative stress, and inhibits the opening of the mitochondrial permeability transition pore, thereby conferring cellular protection against superimposed stresses such as hypoxia and ischemia[10]. In light of these findings, it is recommended that the following experimental strategies to further validate and expand this study: First, an in vitro cross-linking reaction using purified MFN2 and BIP proteins, combined with cross-linking mass spectrometry, could be employed to directly confirm their interaction and accurately identify the binding sites. Second, isolating MAM fractions followed by cross-linking mass spectrometry analysis could systematically elucidate MFN2’s positioning within the MAM protein interaction network and reveal its potential binding partners.
In vivo studies utilizing an ASC transplantation model demonstrated that knockdown of either NRF2 or MFN2 impaired the therapeutic efficacy of ASCs, thereby establishing a link between NRF2/MFN2 modulation and insulin resistance. This bridges the gap between the fundamental findings and potential therapeutic applications for obesity-related metabolic disorders.
To fully elucidate the therapeutic potential of the NRF2/MFN2 axis, it would be valuable to complement these findings with additional in vivo studies examining whether enhanced expression of NRF2 or MFN2 improves the therapeutic efficacy of ASCs. To investigate this, a diet-induced obesity mouse model could be utilized, in which lentiviral vector-mediated gene delivery is used to specifically introduce Mfn2 into ASCs. For instance, injection of lentiviruses encoding an ASC-specific promoter-driven Mfn2 overexpression construct would enable examination of how Mfn2 upregulation affects ASC senescence in obese mice. Histological assessments should also be performed to evaluate morphological alterations in transplanted adipose tissue, such as adipocyte diameter and markers of adipose browning. Additionally, a systematic evaluation of metabolic improvements following this intervention is essential. Key endpoints should include attenuation of adipocyte hypertrophy, reduced adipose tissue inflammation, lowered fasting and postprandial blood glucose, decreased glycated hemoglobin, reduced plasma free fatty acids and triglycerides, and improved insulin resistance. Such integrative assessments would provide a thorough evaluation of the therapeutic potential of the NRF2/MFN2 axis in obesity.
The study proposes an interaction between NRF2, MFN2, and BIP within ASCs, exploring the connection between ERS, cellular senescence, and mitochondrial homeostasis. This conceptual framework is logically presented and commendable. The methodologies employed for assessing ERS and senescence are robust and reproducible. However, given that BIP acts as a key ERS sensor and MFN2 is proposed to influence its function, the mechanistic description of how NRF2/MFN2 regulates ASC senescence and ERS would be strengthened by examining downstream effectors of BIP. Investigating these downstream components would provide a more comprehensive theoretical basis for the proposed pathway. We summarize the proposed mechanism and critical evidential gaps in Figure 1.
To fully elucidate the role of the NRF2/MFN2 axis in regulating ERS and senescence, several follow-up studies are warranted: (1) Validation of direct MFN2-BIP binding in ASCs (e.g., via protein structure modeling, protein purification, and binding site mutagenesis); (2) Loss-of-function studies of BIP effectors in ASCs: Investigate alterations in key downstream effectors of the BIP pathway within ASCs; and (3) Evaluation of therapeutic potential via MFN2-overexpressing ASCs in insulin-resistant hypertrophic obese mice.
This study identifies a positive correlation between reduced NRF2 levels and diminished MFN2 expression in ASCs. It demonstrates NRF2-dependent transcriptional regulation of MFN2 and further reveals impaired ASC stemness. Crucially, NRF2 knockdown induces ASC senescence, establishing a mechanistic link between ERS and senescence in obesity-related metabolic disease. In vivo validation remains essential to advance this conceptual framework into a clinically relevant therapeutic strategy.
| 1. | Fang J. Reduced NRF2/Mfn2 activity promotes endoplasmic reticulum stress and senescence in adipose-derived mesenchymal stem cells in hypertrophic obese mice. World J Stem Cells. 2025;17:104367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Hu L, Guo Y, Song L, Wen H, Sun N, Wang Y, Qi B, Liang Q, Geng J, Liu X, Fu F, Li Y. Nicotinamide riboside promotes Mfn2-mediated mitochondrial fusion in diabetic hearts through the SIRT1-PGC1α-PPARα pathway. Free Radic Biol Med. 2022;183:75-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Zhang L, Zhang M, Huang J, Huang J, Zhang Y, Zhang Y, Chen H, Wang C, Xi X, Fan H, Wang J, Jiang D, Tian J, Zhang J, Chang Y. Klf9 is essential for cardiac mitochondrial homeostasis. Nat Cardiovasc Res. 2024;3:1318-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Ding M, Shi R, Fu F, Li M, De D, Du Y, Li Z. Paeonol protects against doxorubicin-induced cardiotoxicity by promoting Mfn2-mediated mitochondrial fusion through activating the PKCε-Stat3 pathway. J Adv Res. 2023;47:151-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 5. | Zhang WB, Feng SY, Xiao ZX, Qi YF, Zeng ZF, Chen H. Down-regulating of MFN2 promotes vascular calcification via regulating RAS-RAF-ERK1/2 pathway. Int J Cardiol. 2022;366:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Adinolfi S, Patinen T, Jawahar Deen A, Pitkänen S, Härkönen J, Kansanen E, Küblbeck J, Levonen AL. The KEAP1-NRF2 pathway: Targets for therapy and role in cancer. Redox Biol. 2023;63:102726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 187] [Reference Citation Analysis (0)] |
| 7. | Shakya A, McKee NW, Dodson M, Chapman E, Zhang DD. Anti-Ferroptotic Effects of Nrf2: Beyond the Antioxidant Response. Mol Cells. 2023;46:165-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 8. | George M, Tharakan M, Culberson J, Reddy AP, Reddy PH. Role of Nrf2 in aging, Alzheimer's and other neurodegenerative diseases. Ageing Res Rev. 2022;82:101756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 9. | Song Z, Song H, Liu D, Yan B, Wang D, Zhang Y, Zhao X, Tian X, Yan C, Han Y. Overexpression of MFN2 alleviates sorafenib-induced cardiomyocyte necroptosis via the MAM-CaMKIIδ pathway in vitro and in vivo. Theranostics. 2022;12:1267-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 10. | Yepuri G, Ramirez LM, Theophall GG, Reverdatto SV, Quadri N, Hasan SN, Bu L, Thiagarajan D, Wilson R, Díez RL, Gugger PF, Mangar K, Narula N, Katz SD, Zhou B, Li H, Stotland AB, Gottlieb RA, Schmidt AM, Shekhtman A, Ramasamy R. DIAPH1-MFN2 interaction regulates mitochondria-SR/ER contact and modulates ischemic/hypoxic stress. Nat Commun. 2023;14:6900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/