Published online Nov 26, 2025. doi: 10.4252/wjsc.v17.i11.112393
Revised: August 18, 2025
Accepted: November 3, 2025
Published online: November 26, 2025
Processing time: 119 Days and 3.7 Hours
The micro-injury of collagen fibers occurs as the tendon is stretched repeatedly between the strains of 4% and 8%, which results in the cumulative micro-damage in tendon. In prior studies, we have shown that micro-injured tendon slices with 6.4% strain promoted the chondrogenic differentiation of tendon-derived stem cells (TDSCs) through the activation of endoplasmic reticulum (ER) stress.
To investigate the potential of thymoquinone (TQ) to alleviate ER stress, and, consequently, to suppress the chondrogenic differentiation of TDSCs.
Decellularized tendon slices, subjected to micro-injury with 6.4% strain, were prepared for the culture of TDSCs. Additionally, a rat model of Achilles tendon injury via treadmill running was established. The expression levels of tenocyte and chondrocyte markers, along with ER stress-related factors, were examined in TDSCs cultured on micro-injured tendon slices, and in injured rat tendons, using reverse transcription-quantitative polymerase chain reaction, immunofluorescence staining, and western blot analysis. Furthermore, the inhibitory effects of TQ on ER stress, and the chondrogenic differentiation of TDSCs, were evaluated.
In both TDSCs on micro-injured tendon slices, and injured rat tendons, tenocyte-related markers were downregulated, whereas chondrocyte-related markers were upregulated. Treatment with TQ significantly reduced the expression of ER stress markers, including glucose-regulated protein 78 (3.59 ± 0.41 vs 1.18 ± 0.23, P < 0.001), activating transcription factor 4 (2.67 ± 0.26 vs 1.16 ± 0.13, P < 0.001), CCAAT/enhancer-binding protein homologous protein (2.90 ± 0.37 vs 1.24 ± 0.35, P < 0.001), as well as phosphorylated protein kinase RNA-like ER kinase, and phosphorylated eukaryotic initiation factor 2, thereby attenuating ER stress. Furthermore, TQ diminished the chondrogenic differentiation of TDSCs, as evidenced by decreased expression of collagen II (4.80 ± 0.47 vs 1.38 ± 0.28, P < 0.001), aggrecan (2.83 ± 0.26 vs 1.44 ± 0.19, P < 0.001), and SOX9 (4.13 ± 0.46 vs 1.26 ± 0.25, P < 0.001), effects comparable to those observed with 4-phenylbutyric acid.
These findings suggested that TQ inhibited the protein kinase RNA-like ER kinase/
Core Tip: In our previous investigation, we demonstrated that micro-injured tendon with 6.4% strain promoted the chondrogenic differentiation of tendon-derived stem cells, by activating endoplasmic reticulum (ER) stress. To further validate this phenomenon in the animal model, we established a rat Achilles tendon injury model via treadmill running. In the present study, tendon injury was found to activate ER stress by upregulating the protein kinase RNA-like ER kinase/eukaryotic initiation factor 2/activating transcription factor 4/CCAAT/enhancer-binding protein homologous protein pathway. And thymoquinone was shown to alleviate ER stress, while inhibiting the chondrogenic differentiation of tendon-derived stem cells in both in vitro and in vivo models. These findings suggest that thymoquinone may hold potential as a preventive and therapeutic agent for tendinopathy.
- Citation: Tu YJ, Liu YQ, Pan YY, Cai HY, Liu C. Thymoquinone inhibited the chondrogenic differentiation of tendon-derived stem cells caused by tendon injury. World J Stem Cells 2025; 17(11): 112393
- URL: https://www.wjgnet.com/1948-0210/full/v17/i11/112393.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i11.112393
Tendons connect muscles to bones, and enable force transfer between them. They are prone to acute and chronic injuries because of the repetitive mechanical loading. Micro-injury in tendon occurs as the tendon is stretched repeatedly within the strain range of 4% and 8%, which results in cumulative micro-damage to collagen fibers, and may lead to ten
Following the identification and characterization of the tendon-derived stem cells (TDSCs), the differentiation of TDSCs into non-tenocyte lineages, including adipocytes, chondrocytes, and osteocytes, has been implicated in the pathogenesis of tendinopathy[5,6]. It has been proposed that large mechanical stretching exceeding 4% facilitates the differentiation of TDSCs into adipogenic, chondrogenic, and osteogenic lineages[6]. However, it remains unclear whether micro-injuries to the tendon induce non-tendinous differentiation of TDSCs, thereby contributing to the development of tendinopathy.
Yuan et al[7] developed the tendinopathic animal models by inducing the chondrogenic differentiation of TDSCs using kartogenin, thereby demonstrating that the chondrogenic differentiation of TDSCs contributes to the pathogenesis of tendinopathy. Additionally, our previous research confirmed that disruption of tendon microstructure, resulting from micro-injury, promoted the chondrogenic differentiation of TDSCs through the activation of endoplasmic reticulum (ER) stress. This was evidenced by increased expression levels of protein kinase RNA-like ER kinase (PERK), activating transcription factor 4 (ATF4), and chondrogenic markers, including collagen II, aggrecan, and SOX9[8]. ER stress, which arises from the accumulation of unfolded or misfolded proteins within the ER, plays a critical role in stem cell differentiation process, particularly chondrogenic differentiation[9,10].
Thymoquinone (TQ, 2-isopropyl-5-methylbenzo-1,4-quinone), constitutes a principal bioactive component of the hydroalcoholic extract derived from Nigella sativa L. Previous studies have demonstrated that TQ exhibits antioxidant, anti-inflammatory, and anti-apoptotic properties, among other beneficial effects[11,12]. Notably, TQ has been reported to mitigate ER stress through the modulation of the ER chaperone glucose-regulated protein 78 (GRP78)[13-15]. In addition, TQ has shown efficacy in promoting the repair of injured rabbit Achilles tendons, as evidenced by enhancements in biomechanical parameters, increased hydroxyproline content, greater density of aligned collagen fibers, and elevated fibrocyte proliferation[16].
We hypothesized that TQ mitigates ER stress triggered by tendon injury, thereby inhibiting the chondrogenic differentiation of TDSCs. Accordingly, this study aimed to evaluate the effects of TQ on the suppression of ER stress, and chondrogenic differentiation of TDSCs cultured on micro-injured tendon slices, as well as in a rat model of Achilles tendon injury. The objective was to assess the therapeutic potential of TQ in prevention and treatment of tendinopathy.
All chemicals, including 4-phenylbutyric acid (4-PBA, P21005-25G), were purchased from Sigma-Aldrich (St. Louis, MO, United States), unless otherwise specified. Thapsigargin (HY-13433) and TQ (HY-D0803) were obtained from MedChemExpress (Monmouth Junction, NJ, United States).
Decellularized tendon slices were prepared and subjected to either 0% strain or 6.4% strain, following the protocols and stress-strain characteristics established in our previous investigation[8]. Briefly, the bovine Achilles tendons, 50 mm in length, were embedded in Tissue-Tek® O.C.T. Compound (Sakura Finetek, St. Torrance, CA, United States), and longitudinally sectioned into 100 μm-thick slices using a cryostat (CM1950, Leica, Wetzlar, Germany). Subsequently, each tendon slice was held in a tensile testing apparatus (PINDE, Nanjing, China), where either 0 mm displacement (control group) or 2.5 mm displacement (micro-injured group) was applied. The samples underwent ten cycles of stretching at a velocity of 20 mm/minute. Decellularization was achieved through repetitive freeze/thaw cycles, followed by treatment with 200 μg/mL of RNase and 400 μg/mL of DNase. Prior to experimental use, the tendon slices were sterilized via Cobalt-60 irradiation.
TDSCs were isolated from rat Achilles tendons, and characterized following the protocols established in our prior studies[8,17]. TDSCs were seeded at a density of 1000 cells/cm2, onto decellularized tendon slices experienced with 0% or 6.4% stain. The cells were then cultured in Dulbecco’s Modified Eagle Medium (Procell System, Wuhan, China) supplemented with 10% fetal bovine serum (Procell System, Wuhan, China) for 10 days.
TDSCs were inoculated into 96-well plate, with the density of 5000 cells/well, 2000 cells/well, 1000 cells/well, and 500 cells/well for 1 day, 4 days, 7 days, and 10 days of culture, respectively. After 6 hours of culture, different concentrations (0 μΜ, 5 μΜ, 10 μΜ, 20 μΜ, 50 μΜ, and 100 μΜ) of TQ were incubated with TDSCs. At the time point of day 1, day 4, day 7, and day 10, the cell viability was detected using a Cell Counting Kit-8 (Seven Biotech, Beijing, China) assay, according to the manufacturer’s instructions. Briefly, cells were incubated with culture medium plus 10% (v/v) Cell Counting Kit-8 at 37 °C for 2 hours, and then 200 μL supernatant was used to test the absorbance. The optical density at 450 nm was determined using a microplate reader (Infinite M Nano, Tecan, Männedorf, Switzerland). The culture medium with different concentrations of TQ was refreshed every other day.
Thirty Sprague-Dawley rats, half male and half female, approximately 8 weeks of age (250-300 g), were purchased from Beijing Vital River Laboratory Animal Technology Company. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 hours/12 hours light/dark, 50% humidity, ad libitum access to food and water), for 2 weeks prior to experimentation. The rats were divided into two groups, ten in control (cage activity) and twenty in tendon injury group (treadmill running 17 m/minute at 10° uphill incline, 2 hours/day, 5 days/week for 12 weeks). As for treadmill running, the rats were acclimated to treadmill running over an eight-day period prior to the start of the regime by increasing the running time by 15 minutes per day. 10 rats in the 20 tendon injury rats were injected with dimethyl sulfoxide (DMSO) (injury + DMSO group) or TQ (injury + TQ group), the other 10 rates were not given any treatment (injury group). The small-animal treadmill model XR-PT-10B was acquired from Shanghai Xinruan Information Technology Co., Ltd. The rats were anesthetized with pentobarbital sodium (30 mg/kg) and sacrificed by intravenous injection with 150 mg/kg of pentobarbital sodium. The Achilles tendons were dissected from animals immediately after sacrifice, and prepared for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis. The animal experiments were performed with the approval of the Ethic Committee of Central Hospital of Dalian University of Technology. The animal welfare procedures adhered to the General Rules for the Welfare of Laboratory Animals (No. GB_T 42011-2022).
Of 10 μM TQ was added into the culture medium of TDSCs cultured on decellularized tendon slices with 6.4% stain, the medium was changed every other day for 10 days. In the animal experiment, TQ was initially dissolved in DMSO to achieve a concentration of 100 mg/mL, and subsequently diluted with saline to obtain a final concentration of 2 mg/0.1 mL. In this study, the left hind limbs of 10 rats received peri-tendinous injections of 0.1 mL TQ, at the injury site through the skin, every 5 days over a 2-week period following treadmill exercise (injury + TQ group). Concurrently, the right hind limbs were administered peri-tendinous injections of an equivalent concentration of DMSO dissolved in saline (injury + DMSO group).
TDSCs were cultured on decellularized tendon slices with 0% stain. Upon reaching 60% confluence, 0.5 μmol/L of thapsigargin was administrated to the culture medium to induce ER stress. For the inhibition of ER stress, following 12 hours of TDSC culture on decellularized tendon slices with 6.4% stain, either 0.5 mmol/L of 4-PBA, or 10 μmol/L of TQ was added into the culture medium. The medium was changed every other day over a 10-day period, to maintain ER stress suppression.
Total RNA from TDSCs on the decellularized tendon slices, and from rat tendons, were isolated using the RNeasy Mini Kit (QIAGEN, Hilden, Germany). RT was performed using the PrimeScript RT Reagent Kit (Takara, Kyoto, Japan). RT-qPCR was carried out with SYBR Premix Ex Taq (Perfect Real Time) (Takara, Kyoto, Japan). PCR amplification and fluorescence detection were performed using a ViiA™ 7 Real-Time PCR System (Thermo Fisher Scientific, Pittsburgh, PA, United States). GAPDH was used as the internal control. The primers used in this study were listed in Supplementary Table 1. The results were presented as the calculated comparative expression ratios of the target sample to the control group for each sample, using the Ct method 2-ΔΔCt.
TDSCs cultured on the decellularized tendon slices, experienced with 0% or 6.4% strain were lysed using lysis buffer supplemented with protease and phosphatase inhibitors (Keygentec, Nanjing, China). Rat tendons from the control and the injury group were excised into small segments, and subsequently immersed in lysis buffer, followed by ultrasonic fragmentation (SCIENTZ, Ningbo, China). Protein concentration was quantified by BCA protein assay kit (Keygentec, Nanjing, China), and the samples were loaded onto polyacrylamide gels with equal protein amounts. Constant voltage electrophoresis was applied. Then the proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Merck, Darmstadt, Germany). The PVDF membranes were initially blocked using 3% bovine serum albumin (Sigma-Aldrich, MO, United States), and subsequently incubated overnight at 4 °C with primary antibodies targeting GRP78 (1:2000) (Proteintech, Wuhan, China), phospho-PERK (Thr980) (dilution 1:1000, Thermo Fisher Scientific, PA, United States), PERK (dilution 1:5000, Proteintech, Wuhan, China), phospho-eukaryotic initiation factor 2S1 (EIF2S1) (Ser51) (dilution 1:5000, Proteintech, Wuhan, China), EIF2S1 (dilution 1:5000, Proteintech, Wuhan, China), ATF4 (dilution 1:1000, Pro
TDSCs cultured on tendon slices experienced with 0% and 6.4% strain, were fixed with 40 g/L paraformaldehyde, and subsequently washed with phosphate-buffered saline. Following permeabilization with 0.05% Triton-X 100 (Sigma-Aldrich, MO, United States), the TDSCs were incubated overnight at 4 °C with either CoraLite® Plus 488-conjugated collagen type II polyclonal antibody (dilution 1:200, Proteintech, Wuhan, China), or aggrecan antibody (dilution 1:100, Proteintech, Wuhan, China) diluted in phosphate buffered saline containing 1% goat serum (Thermo Fisher Scientific, PA, United States). Subsequently, the cells were incubated at room temperature for 60 minutes with CoraLite 594-conjugated goat anti-rabbit IgG (H + L) secondary antibody (dilution 1:500, Proteintech, Wuhan, China). Negative control samples were prepared by omitting the primary antibodies. Nuclear staining was performed using Hoechst 33342 (1:1000, Thermo Fisher Scientific, PA, United States). The samples were observed using a laser scanning confocal microscope (STELLARIS 5, Leica).
Each experiment was performed at least three times. The data were shown as means ± SD. One-way analysis of variance (ANOVA) and Tukey’s test were used to examine the differences among groups. P < 0.05 was considered the statistical significance. The statistical methods of this research were reviewed by Xiu-Liang Guan, from Central Hospital of Dalian University of Technology.
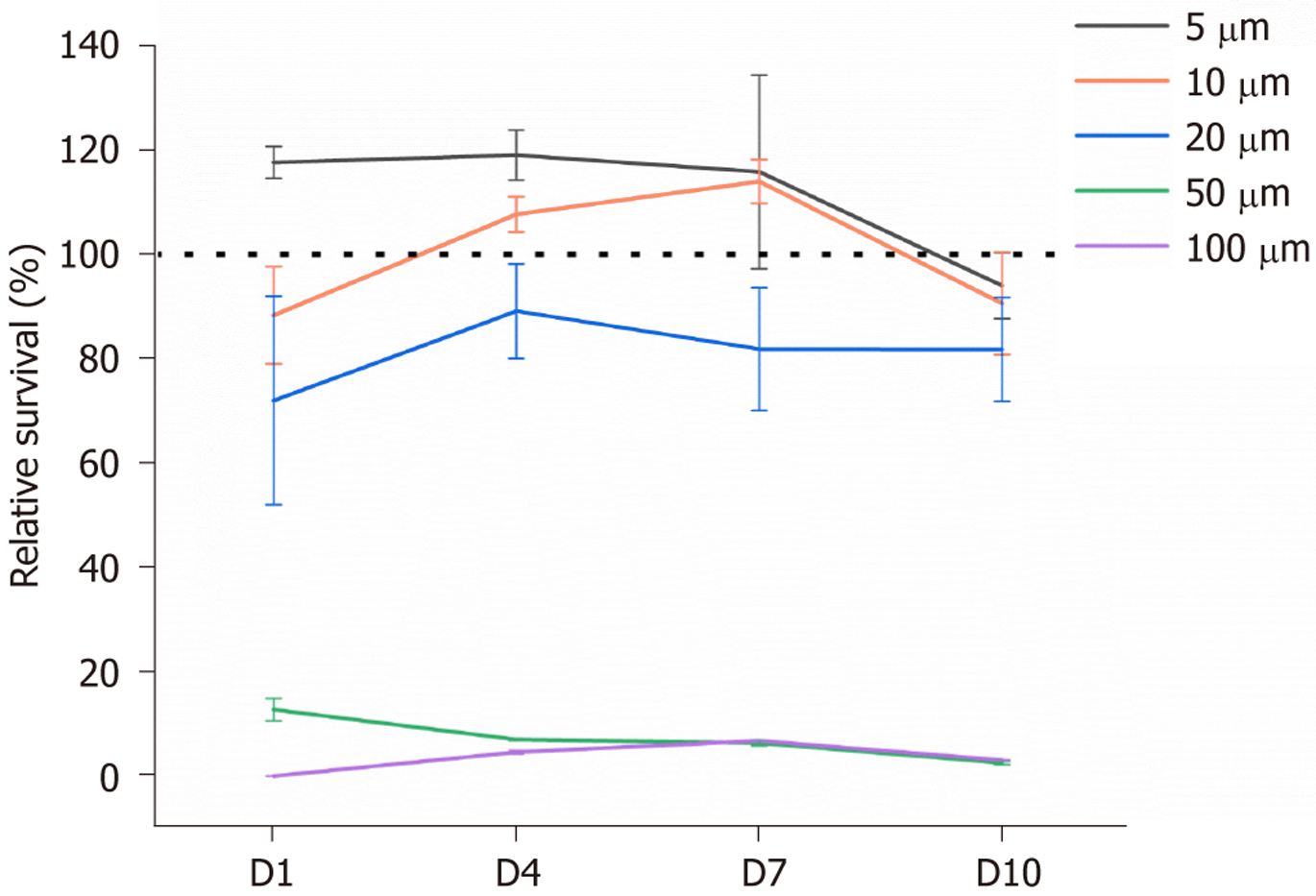
To determine the optimal concentration of TQ, TDSCs were cultured with various concentrations of TQ (0 μmol/L, 5 μmol/L, 10 μmol/L, 20 μmol/L, 50 μmol/L, 100 μmol/L) over a 10-day period. Results indicated that exposure to 50 μmol/L and 100 μmol/L TQ significantly reduced TDSC viability at all assessed time points, compared to the control group (0 μmol/L), with statistical significance observed on day 1 (P = 0.00261 and 0.00299), day 4 (P = 0.0084 and 0.00797), day 7 (P = 0.00575 and 0.00588), and day 10 (P = 0.00165 and 0.00171) (Figure 1). In contrast, treatment with 5 μmol/L, 10 μmol/L, and 20 μmol/L TQ did not result in statistically significant changes in cell viability after 10 days (day 1: P = 0.0688, 0.2551, 0.184; day 4: P = 0.1454, 0.4694, 0.371; day 7: P = 0.31465, 0.16586, 0.1691; day 10: P = 0.62093, 0.94606, 0.27529). However, 20 μmol/L of TQ decreased the cellular viability of TDSCs, the relative survival rate was approximately 80%. Based on these findings, a concentration of 10 μmol/L of TQ was selected for subsequent investigations into its effects on chondrogenic differentiation and ER stress in TDSCs.
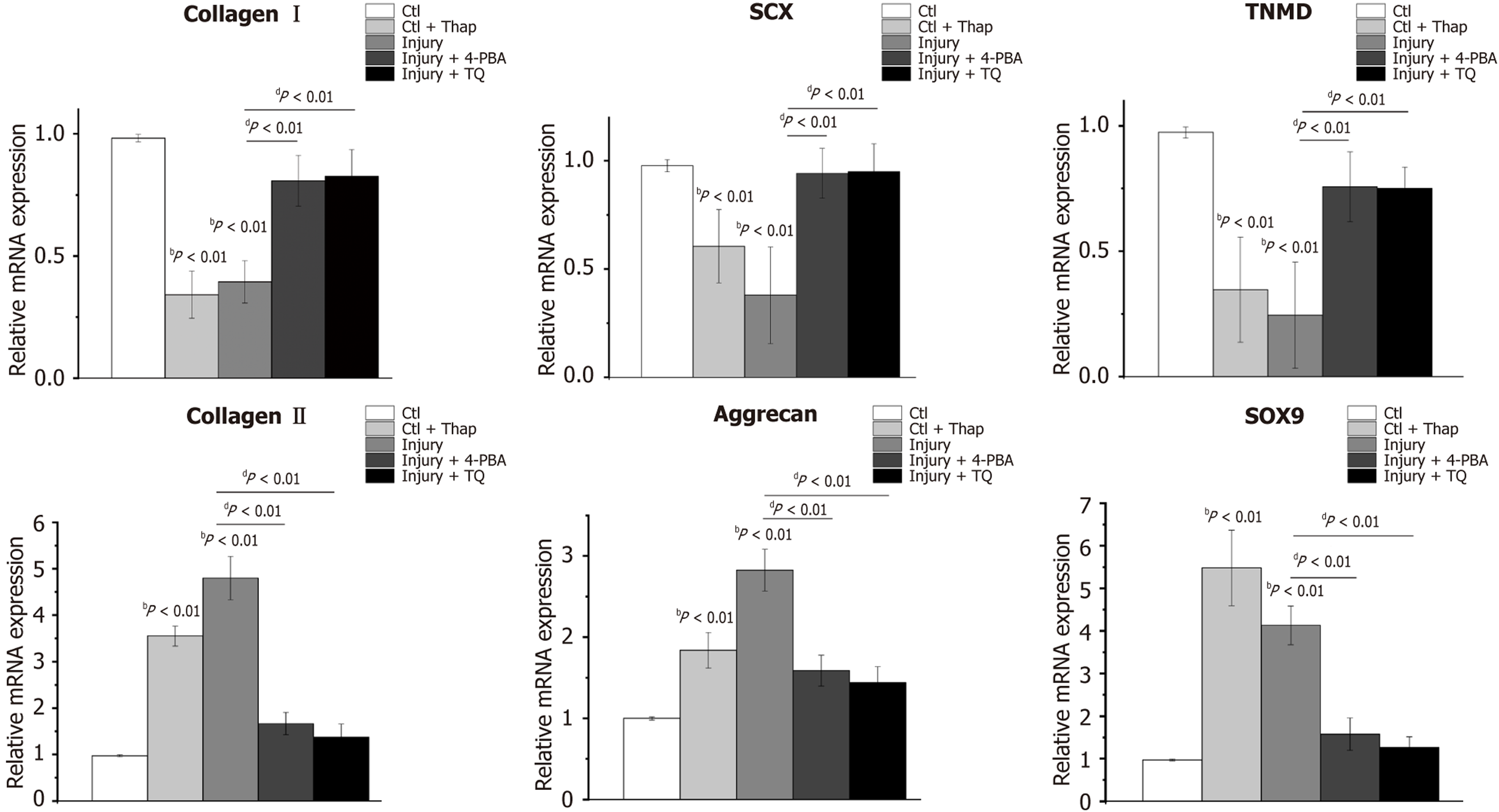
Thapsigargin, an ER stress inducer, and 4-PBA, an ER stress inhibitor, were employed to respectively induce and inhibit ER stress in TDSCs. Compared to the TDSCs cultured on tendon slices with 0% strain (control group), those treated with thapsigargin on 0% strain tendon slices (control + thapsigargin group), and TDSCs on tendon slices with 6.4% strain (injury group), exhibited a significant downregulation of tenocyte-associated markers, including collagen I, scleraxis (SCX), and tenomodulin (TNMD) (Figure 2). Concurrently, the expression of chondrogenic markers, specifically collagen II and aggrecan, was markedly upregulated. Notably, collagen II expression increased by 3.55 ± 0.21-fold and 4.80 ± 0.47-fold, in the control + thapsigargin and injury groups, respectively (Figure 2). Similarly, aggrecan expression rose by 1.84 ± 0.22-fold and 2.83 ± 0.26-fold in these groups (Figure 2). The cartilage-specific transcription factor SOX9 also de
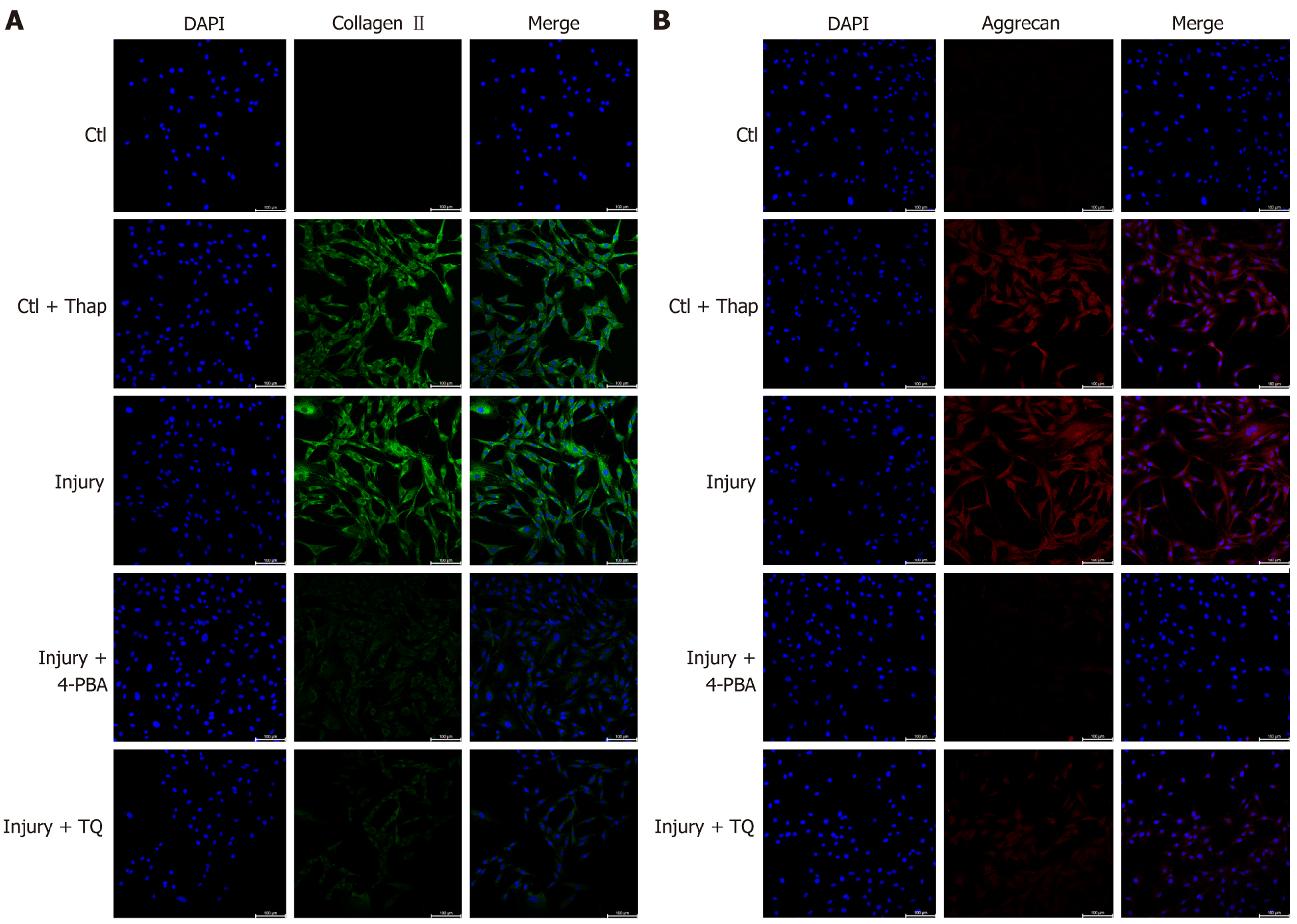
Furthermore, treatment of TDSCs in the injury group either with 4-PBA (injury + 4-PBA group) or TQ (injury + TQ group) resulted in a significant restoration of tenocyte-related gene expression, alongside a reduction in chondrogenic gene expression, compared to untreated TDSCs in the injury group (Figure 2). These results indicated that tendon micro-injury inhibited the tenogenic differentiation, while promoting the chondrogenic differentiation of TDSCs, and that TQ effectively suppressed chondrogenic gene expression, while restoring tenogenic gene expression in these cells. Complementary fluorescence imaging analyses of protein expression further corroborated these results. Specifically, the chondrogenic markers collagen II (Figure 3A) and aggrecan (Figure 3B), were elevated in TDSCs following tendon micro-injury, whereas treatment with TQ attenuated this increase, confirming its inhibitory effect on chondrogenic differentiation.
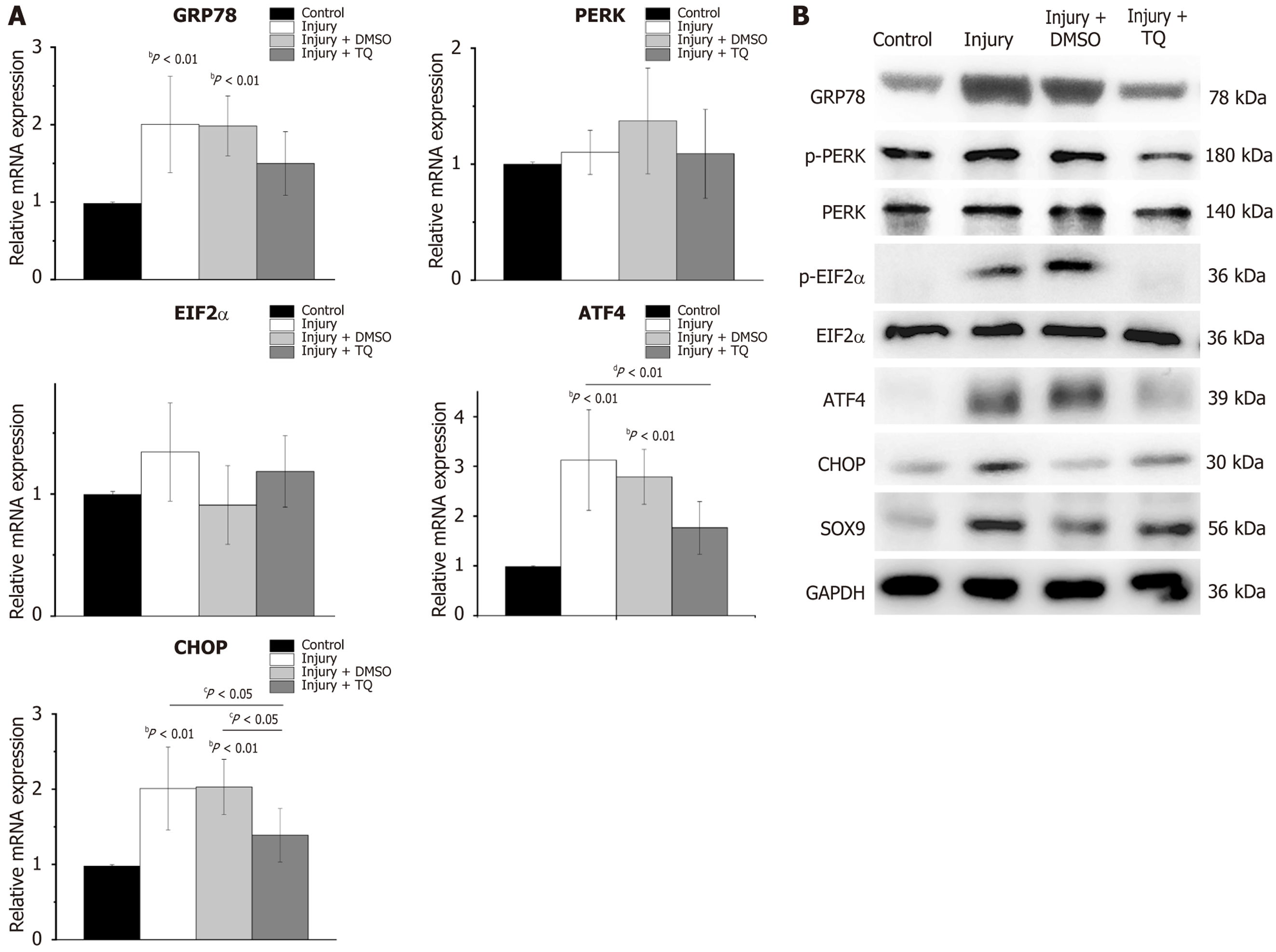
Thapsigargin treatment was observed to significantly increase the expression of the ER chaperone GRP78 by 2.41 ± 0.38-fold relative to the control group. Similarly, GRP78 expression in the injury group exhibited 3.59 ± 0.41-fold elevation compared to controls (Figure 4A). Furthermore, ER stress-related factors ATF4 and CHOP were markedly upregulated in thapsigargin-treated TDSCs, with 1.78 ± 0.24-fold and 1.78 ± 0.20-fold increase, respectively, compared to control (Figure 4A). In the injury group, their expressions were increased by 2.68 ± 0.26-fold and 2.90 ± 0.37-fold, respectively, compared to controls (Figure 4A). In addition, PERK expression was increased by 1.72 ± 0.34-fold in the injury group relative to control, whereas EIF2α mRNA level was not significantly different between the groups. These findings indicated that tendon micro-injury, analogous to thapsigargin treatment, activated the ER stress in TDSCs. Notably, treatment with TQ, which exhibited effects comparable to the ER stress inhibitor 4-PBA, significantly reduced the expression of GRP78, ATF4, and CHOP, relative to the injury group (Figure 4A). Consistent with these results, western blot analysis demonstrated elevated levels of ER stress-related markers including GRP78, phosphorylated PERK, phosphorylated EIF2α, ATF4, and CHOP, in TDSCs from injury group (Figure 4B and Supplementary Figure 1A). TQ effectively mitigated ER stress, and concomitantly decreased the expression of cartilage-specific transcription factor SOX9 (Figure 4B and Supplementary Figure 1A).
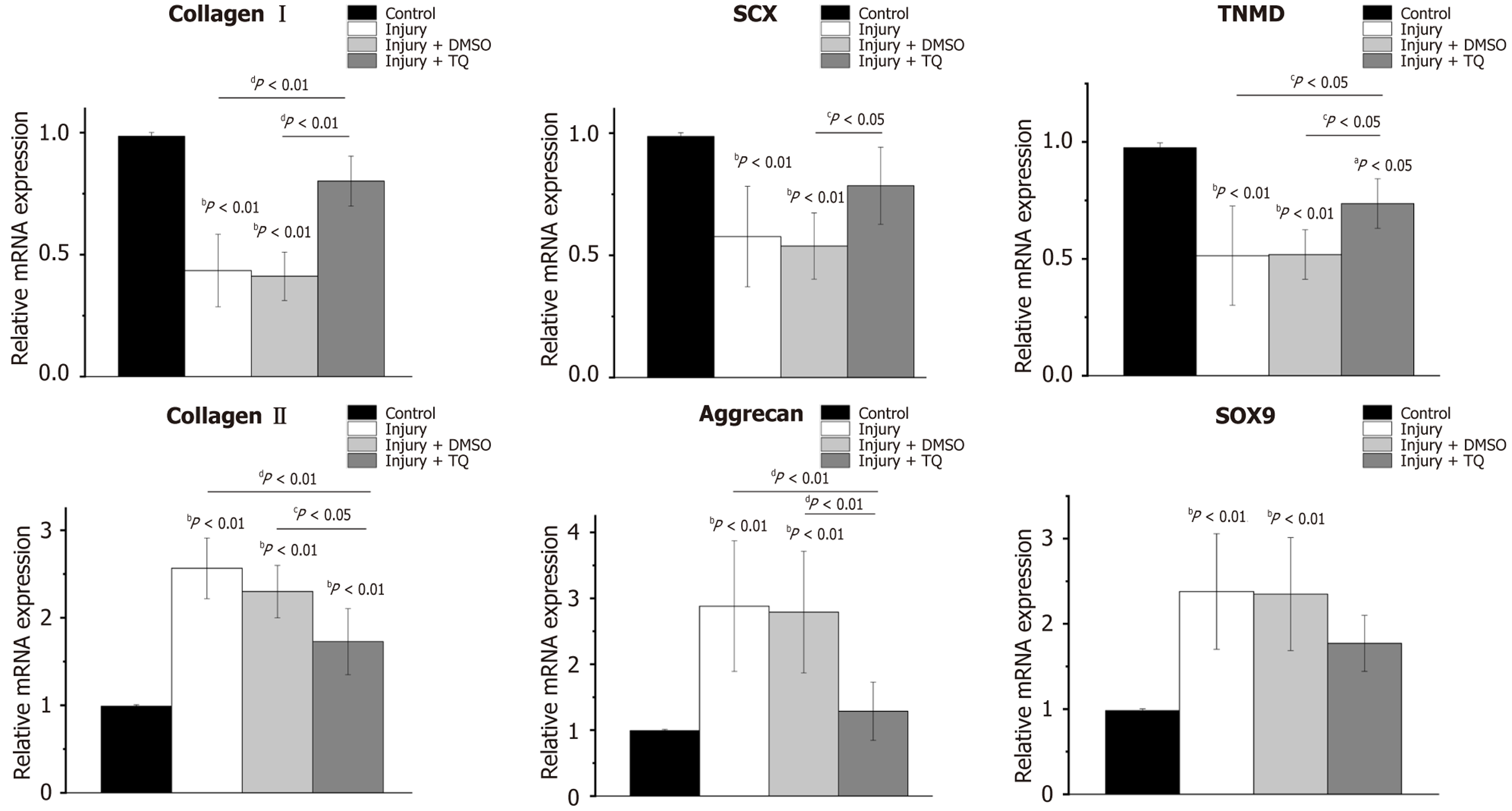
Treadmill running is widely recognized as an optimal approach for inducing tendon injury models due to it is physiological relevance, in the sense that it creates tendon injury that occurs naturally. In the present study, the Achilles tendon injuries were induced in rats via treadmill running, to further evaluate the protective effects of TQ on ER stress, and the erroneous differentiation of TDSCs in vivo. In the injured rat tendons (injury group), the expression levels of tenocyte-related markers including collagen I, SCX, and TNMD were decreased significantly. Conversely, chondrocyte-related markers including collagen II, aggrecan, and SOX9 exhibited significant upregulation, with fold increases of 2.57 ± 0.35, 2.88 ± 0.99, and 2.38 ± 0.68, respectively, relative to tendons from normal rats maintained under cage activity conditions (control group) (Figure 5). A comparable pattern was observed in the injury + DMSO group. However, following a two-week treatment with TQ (injury + TQ group), there was a notable upregulation of tenocyte-related markers. Specifically, the fold changes in collagen I and TNMD were significantly elevated compared to both the injury and injury + DMSO groups (Figure 5). Additionally, SCX expression was significantly increased relative to the injury + DMSO group (Figure 5). Concurrently, chondrocyte-related markers were downregulated in the injury + TQ group, with significant reductions in the fold changes of collagen II and aggrecan (Figure 5). Collectively, these findings substantiated that TQ effectively inhibited the erroneous differentiation of TDSCs in injured rat tendons.
ER chaperone GRP78 was markedly up-regulated in both the injury group and the injury + DMSO group. Expression levels of ATF4 and CHOP were increased by 3.13 ± 1.01-fold and 2.01 ± 1.55-fold, respectively, in the injury group, demonstrating a statistically significant difference compared to the control group (Figure 6A). A comparable pattern was observed in injury + DMSO group (Figure 6A). Following treatment with TQ, ATF4 and CHOP were significantly down regulated compared with those in the injury and the injury + DMSO group (Figure 6A). Additionally, western blot analysis revealed reductions in GRP78, phosphorylated PERK, phosphorylated EIF2α, and SOX9 protein levels in Figure 6B and Supplementary Figure 1B. Collectively, these results indicated that TQ mitigated ER stress in injured rat tendons by inhibiting the PERK/EIF2α/ATF4/CHOP signaling pathway.
The accumulation of unfolded or misfolded proteins within the lumen of the ER is referred to as ER stress. The regulation of ER stress plays a critical role in the process of chondrogenesis[18]. Specifically, the induction of ER stress has been shown to enhance the chondrogenic differentiation of bone marrow stem cells, whereas the inhibition of ER stress diminishes the chondrogenic potential of these cells[18]. GRP78 is recognized as a key regulator of ER function[19], primarily through its role in activating unfolded protein response. Unfolded protein response facilitates the activation of PERK. Upon activation, GRP78 dissociates from the lumeinal domain of PERK, triggering PERK oligomerization and subsequent trans-autophosphorylation, thereby converting PERK into its active kinase form. The phosphorylated PERK is capable of phosphorylating the α subunit of EIF2α, resulting in the elevated level of phosphorylated EIF2α. This pho
Oxidative stress induces a pro-inflammatory response, and can also impair ER function, resulting in ER stress[26]. TQ has been demonstrated to possess antioxidant and anti-inflammatory activities[11,12]. Furthermore, TQ has been shown to alleviate ER stress. Specifically, TQ suppressed ER stress by reducing the expression of ER chaperone GRP78[13-15,27,28], which served as the master regulator of ER stress. Yan et al[27] reported that administration of TQ inhibited ER stress by decreasing GRP78, ATF4, and CHOP expression, both at the mRNA and protein levels, in hepatic ischemia-reperfusion injury rats. Similarly, Bouhlel et al[28] confirmed that TQ attenuated ER stress by down-regulating GRP78, CHOP, and ATF4 expression in the liver-injured rats. In addition, another study from Bouhlel et al[15] corroborated the capacity of TQ to decrease the expression of ER stress parameters, including GRP78 and CHOP. Our findings align with previous research demonstrating that TQ can downregulate GRP78 and the PERK/EIF2α/ATF4/CHOP signaling pathway. Similarly, 4-PBA has also been shown to effectively alleviate ER stress by inhibiting the PERK/ATF4/CHOP pathway[29]. Our current investigation corroborated these effects and revealed that 10 μmol/L TQ exerted comparable efficacy to 0.5 mmol/L 4-PBA, thereby positioning TQ as a promising and cost-effective inhibitor of ER stress.
In recent years, TQ has demonstrated therapeutic potential in animal models of tendinopathy. Peri-tendinous administration of TQ has been reported to exert protective and regenerative effects on collagen synthesis[30]. Additionally, Soltanfar et al[16] confirmed that TQ treatment enhanced biomechanical properties, including increased breakpoint and yield points, elevated hydroxyproline content, and reduced edema and hemorrhage in trauma-induced tendon injuries. Furthermore, TQ promoted the proliferation of fibroblasts and fibrocytes, and improved fiber orientation following rabbit tendon injury[16]. In the present study, peri-tendinous injection of TQ in the injured rat tendons subjected to treadmill running, inhibited ER stress, and attenuated the chondrogenic differentiation of TDSCs. These findings suggested that TQ may hold potential as a preventive and therapeutic agent for tendinopathy.
Nevertheless, the study could not avoid a few limitations. Firstly, decellularized bovine tendon slices experienced with 6.4% strain were employed to stimulate the micro-injured tendons resulting from mechanical overloading. However, the TDSCs cultured on these tendon slices could not fully replicate the complex cellular and physiological processes that the TDSCs undergo within the native tendon environment in vivo. Secondly, although treadmill running induced tendon injury, the precise extent of tendon damage could not be precisely controlled, rendering the animal model an imperfect representation of the micro-injury induced by 6.4% strain. Thirdly, this investigation focused solely on the effects of TQ in inhibiting ER stress and chondrogenic differentiation of TDSCs, it did not assess the improvement of tendinopathy manifestations, such as collagen fiber orientation or overall tendon repair following the injury. Future research should therefore explore the protective effects and tendon repair capabilities of TQ using appropriate animal models.
This study demonstrated that tendon injury induced ER stress through the upregulation of the PERK/EIF2α/ATF4/ CHOP signaling pathway in TDSCs. Furthermore, TQ was shown to mitigate ER stress and inhibit the chondrogenic differentiation of TDSCs, in both in vitro and in vivo models. These findings implied that TQ might hold therapeutic potential for the prevention and treatment of tendinopathy.
The authors express their gratitude to professor Yu-Long Sun for his valuable contributions to the preparation of micro-injured tendon slices.
| 1. | Kannus P. Etiology and pathophysiology of chronic tendon disorders in sports. Scand J Med Sci Sports. 1997;7:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 626] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 3. | Stańczak M, Kacprzak B, Gawda P. Tendon Cell Biology: Effect of Mechanical Loading. Cell Physiol Biochem. 2024;58:677-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 4. | Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507-1525. [PubMed] |
| 5. | Jiang D, Wang JH. Tendinopathy and its treatment with platelet-rich plasma (PRP). Histol Histopathol. 2013;28:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Zhang J, Wang JH. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010;28:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Yuan T, Zhang J, Zhao G, Zhou Y, Zhang CQ, Wang JH. Creating an Animal Model of Tendinopathy by Inducing Chondrogenic Differentiation with Kartogenin. PLoS One. 2016;11:e0148557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Liu C, Li TY, Chen Y, Yang HH, Sun YL. Tendon microstructural disruption promotes tendon-derived stem cells to express chondrogenic genes by activating endoplasmic reticulum stress. J Orthop Res. 2023;41:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Shen C, Jiang T, Zhu B, Le Y, Liu J, Qin Z, Chen H, Zhong G, Zheng L, Zhao J, Zhang X. In vitro culture expansion impairs chondrogenic differentiation and the therapeutic effect of mesenchymal stem cells by regulating the unfolded protein response. J Biol Eng. 2018;12:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Xiong Z, Jiang R, Zhang P, Han X, Guo FJ. Transmission of ER stress response by ATF6 promotes endochondral bone growth. J Orthop Surg Res. 2015;10:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Samarghandian S, Farkhondeh T, Samini F. A Review on Possible Therapeutic Effect of Nigella sativa and Thymoquinone in Neurodegenerative Diseases. CNS Neurol Disord Drug Targets. 2018;17:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Ragheb A, Attia A, Eldin WS, Elbarbry F, Gazarin S, Shoker A. The protective effect of thymoquinone, an anti-oxidant and anti-inflammatory agent, against renal injury: a review. Saudi J Kidney Dis Transpl. 2009;20:741-752. [PubMed] |
| 13. | Landucci E, Mazzantini C, Buonvicino D, Pellegrini-Giampietro DE, Bergonzi MC. Neuroprotective Effects of Thymoquinone by the Modulation of ER Stress and Apoptotic Pathway in In Vitro Model of Excitotoxicity. Molecules. 2021;26:1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Farghaly ME, Khowailed AA, Aboulhoda BE, Rashed LA, Gaber SS, Ashour H. Thymoquinone Potentiated the Anticancer Effect of Cisplatin on Hepatic Tumorigenesis by Modulating Tissue Oxidative Stress and Endoplasmic GRP78/CHOP Signaling. Nutr Cancer. 2022;74:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Bouhlel A, Ben Mosbah I, Hadj Abdallah N, Ribault C, Viel R, Mannaï S, Corlu A, Ben Abdennebi H. Thymoquinone prevents endoplasmic reticulum stress and mitochondria-induced apoptosis in a rat model of partial hepatic warm ischemia reperfusion. Biomed Pharmacother. 2017;94:964-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Soltanfar A, Meimandi Parizi A, Foad-Noorbakhsh M, Sayyari M, Iraji A. The healing effects of thymoquinone on experimentally induced traumatic tendinopathy in rabbits. J Orthop Surg Res. 2023;18:233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Liu C, Luo JW, Zhang KK, Lin LX, Liang T, Luo ZP, Zhuang YQ, Sun YL. Tendon-Derived Stem Cell Differentiation in the Degenerative Tendon Microenvironment. Stem Cells Int. 2018;2018:2613821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Saito A, Hino S, Murakami T, Kanemoto S, Kondo S, Saitoh M, Nishimura R, Yoneda T, Furuichi T, Ikegawa S, Ikawa M, Okabe M, Imaizumi K. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat Cell Biol. 2009;11:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641-3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 621] [Cited by in RCA: 609] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 20. | Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr Mol Med. 2016;16:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 696] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 21. | B'chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41:7683-7699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 642] [Cited by in RCA: 920] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 22. | Wang C, Tan Z, Niu B, Tsang KY, Tai A, Chan WCW, Lo RLK, Leung KKH, Dung NWF, Itoh N, Zhang MQ, Chan D, Cheah KSE. Inhibiting the integrated stress response pathway prevents aberrant chondrocyte differentiation thereby alleviating chondrodysplasia. Elife. 2018;7:e37673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Guo H, Zhang J, Jiang Z, Zhu X, Yang J, Mu R, Du Y, Tian Y, Zhu P, Fan Z. Noncoding RNA circBtnl1 suppresses self-renewal of intestinal stem cells via disruption of Atf4 mRNA stability. EMBO J. 2023;42:e112039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 882] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 25. | Takigawa Y, Hata K, Muramatsu S, Amano K, Ono K, Wakabayashi M, Matsuda A, Takada K, Nishimura R, Yoneda T. The transcription factor Znf219 regulates chondrocyte differentiation by assembling a transcription factory with Sox9. J Cell Sci. 2010;123:3780-3788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 1083] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 27. | Yan L, Luo H, Li X, Li Y. d-Pinitol protects against endoplasmic reticulum stress and apoptosis in hepatic ischemia-reperfusion injury via modulation of AFT4-CHOP/GRP78 and caspase-3 signaling pathways. Int J Immunopathol Pharmacol. 2021;35:20587384211032098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Bouhlel A, Bejaoui M, Ben Mosbah I, Hadj Abdallah N, Ribault C, Viel R, Hentati H, Corlu A, Ben Abdennebi H. Thymoquinone protects rat liver after partial hepatectomy under ischaemia/reperfusion through oxidative stress and endoplasmic reticulum stress prevention. Clin Exp Pharmacol Physiol. 2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Li Y, Guo Y, Wu D, Ai L, Wu R, Ping Z, Zhu K. Phenylbutyric acid inhibits hypoxia-induced trophoblast apoptosis and autophagy in preeclampsia via the PERK/ATF-4/CHOP pathway. Mol Reprod Dev. 2024;91:e23742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 30. | Micheli L, Di Cesare Mannelli L, Mosti E, Ghelardini C, Bilia AR, Bergonzi MC. Antinociceptive Action of Thymoquinone-Loaded Liposomes in an In Vivo Model of Tendinopathy. Pharmaceutics. 2023;15:1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/