Published online Nov 26, 2025. doi: 10.4252/wjsc.v17.i11.111162
Revised: August 11, 2025
Accepted: October 9, 2025
Published online: November 26, 2025
Processing time: 154 Days and 13.8 Hours
Type 2 diabetes mellitus, particularly when accompanied by obesity, has become a major global public health burden. Visceral adipose tissue accumulation contributes to insulin resistance, lipotoxicity, and chronic inflammation, thereby accelerating metabolic deterioration. Although pharmacological agents such as pioglitazone and metformin are effective in modulating fat distribution and improving metabolic parameters, their roles in adipose tissue remodeling remain insufficiently elucidated. Recent advances in regenerative medicine have high
Core Tip: This review explored how pharmacological agents (pioglitazone, metformin, glucagon-like peptide-1 receptor agonist, sodium-glucose cotransporter-2 inhibitors) and adipose-derived stem cells synergistically remodel dysfunctional adipose tissue in obesity-associated type 2 diabetes. It highlighted the roles of adipose browning, mito
- Citation: Luo C, Yu XM, Hua LY, Zeng MQ, Xu H, Duan CZ, Xu SY, Sun D, Ye LY, He DJ. Targeting adipose remodeling: Synergistic mechanisms of drugs and adipose-derived stem cells in obese type 2 diabetes mellitus. World J Stem Cells 2025; 17(11): 111162
- URL: https://www.wjgnet.com/1948-0210/full/v17/i11/111162.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i11.111162
Diabetes has become one of the most severe metabolic disorders worldwide with the incidence of type 2 diabetes mellitus (T2DM), especially when accompanied by obesity, and it is continuing to rise. According to the latest Diabetes Atlas published by the International Diabetes Federation in 2025, approximately 589 million adults aged 20-79 years were living with diabetes globally in 2024, a number projected to increase to 853 million by 2050. The associated healthcare burden is also intensifying with annual diabetes-related expenditures exceeding one trillion United States dollars[1]. A concurrent Lancet systematic analysis based on Global Burden of Disease 2021 data indicated that T2DM accounts for approximately 96% of all diabetes cases and has shown a steady increase from 1990 to 2021. If current trends persist, it is estimated that by 2050, one in ten people worldwide will have diabetes[2].
Compared to overall obesity, visceral adipose tissue (VAT) expansion plays a critical role in promoting insulin resistance, lipotoxicity, and chronic low-grade inflammation. Recent studies have shown that VAT has a high fatty acid mobilization rate, releasing large quantities of free fatty acids (FFAs) that reach the liver directly via the portal vein, thereby triggering hepatic glucose production, ectopic fat deposition, and systemic inflammatory amplification[3,4]. Moreover, VAT remodeling is characterized by adipocyte hypertrophy, hypoxia, and stromal fibrosis, all of which further impair insulin signaling pathways, ultimately accelerating T2DM progression and increasing the risk of cardiovascular complications.
The insulin sensitizer pioglitazone [a peroxisome proliferator-activated receptor gamma (PPARγ) agonist] has been shown to reduce epicardial and pericardial fat area by approximately 9% over 24 weeks in patients with T2DM along with simultaneous improvements in left ventricular diastolic function and systemic insulin sensitivity[5]. Metformin promotes thermogenesis in brown adipose tissue via the adenosine monophosphate-activated protein kinase (AMPK)/fibroblast growth factor 21 path
In recent years adipose-derived stem cells (ADSCs) have demonstrated distinct advantages in enhancing tissue insulin sensitivity and remodeling the adipose microenvironment due to their robust differentiation potential and immunomodulatory properties. Evidence suggests that the paracrine effects of ADSCs when combined with insulin-sensitizing agents such as pioglitazone and metformin may synergistically reduce VAT volume and inflammatory activity, enhance ther
Based on this rationale, the present review focused on the vertical biological axis of adipose tissue remodeling-insulin resistance-stem cell regulation. Within this context we systematically examined the complementary mechanisms and translational potential of pharmacological and ADSC-based therapies in T2DM and obesity, aiming to establish a solid theoretical foundation for the development of future combined and precision-targeted drug + cell intervention strategies.
In the context of obesity, VAT and subcutaneous adipose tissue (SAT) exhibit markedly different metabolic profiles. Studies have shown that VAT secretes significantly higher levels of proinflammatory factors compared with SAT. As reported by Kahn et al[9], conditioned media derived from VAT of individuals with obesity is enriched with proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) as well as matrix remodeling proteins. These secretions can impair insulin sensitivity in hepatocytes and skeletal muscle cells and activate inflammatory signaling pathways.
In contrast, adipocytes in SAT are smaller, more insulin-sensitive, and secrete greater amounts of protective adipokines, such as adiponectin. According to a review by Dhokte and Czaja[3], SAT plays a buffering role by absorbing postprandial circulating FFAs, whereas VAT adipocytes are hypertrophic, prone to insulin resistance, exhibit elevated basal lipolysis, and have a reduced capacity to take up FFAs. Additionally, VAT is more susceptible to hypoxia, mito
Under normal physiological conditions SAT is the primary source of adiponectin, a key adipokine that enhances insulin sensitivity. In contrast, VAT secretes higher levels of proinflammatory adipokines such as resistin and visfatin, and in the obese state VAT becomes a major source of TNF-α and IL-6, exacerbating systemic inflammation and insulin resistance[10,11]. Regarding insulin sensitivity, SAT adipocytes are smaller and highly responsive to insulin, efficiently absorbing and storing FFAs. VAT adipocytes, however, are larger and less insulin-sensitive, resulting in greater FFA flux into the portal circulation and contributing to insulin resistance in the liver and skeletal muscle[3].
Furthermore, VAT is more prone than SAT to chronic low-grade inflammation, characterized by abundant macrophage infiltration and the formation of crown-like structures and accompanied by sustained secretion of inflammatory mediators such as TNF-α and IL-6[9,10]. In contrast, SAT contains fewer inflammatory cells and exhibits a relatively mild inflammatory response. At the metabolic level expansion of VAT adipocytes is often associated with tissue hypoxia and mitochondrial dysfunction, leading to increased ROS production and activation of cellular stress responses. In contrast, mitochondrial function in SAT remains relatively intact, supporting superior metabolic regulation[3,10]. These structural and functional disparities underscore the pivotal role of VAT in obesity-associated T2DM pathogenesis, identifying it as a hidden culprit driving insulin resistance and metabolic dysregulation (Table 1).
| Dimension | VAT | SAT |
| Adipocyte size/insulin sensitivity | Large adipocytes, insulin-resistant | Small adipocytes, insulin-sensitive |
| Proinflammatory/anti-inflammatory profile | IL-6 (↑), TNF-α (↑), resistin (↑) | Adiponectin (↑) |
| Lipolysis/FFA output | High basal lipolysis, FFA enters portal circulation first | Lower lipolysis, buffers postprandial FFA |
| Hypoxia/ROS and mitochondrial function | Marked hypoxia, ROS (↑), mitochondrial dysfunction | Relatively preserved mitochondrial function |
| ECM remodeling and fibrosis | ECM deposition (↑), prone to fibrosis | Mild |
| Macrophage infiltration | Increased M1 macrophages, crown-like structures prominent | Lower inflammatory infiltration |
Obesity leads to the excessive secretion of proinflammatory adipokines from adipose tissue, triggering chronic low-grade systemic inflammation that interferes with insulin signaling pathways. In the obese state elevated levels of leptin can stimulate monocytes and macrophages to secrete proinflammatory cytokines such as TNF-α and IL-6 while also enhancing its own proinflammatory signaling[12]. This forms a positive feedback loop in which inflammatory mediators like TNF-α and IL-1β further stimulate adipocytes to produce more leptin, perpetuating the activation of inflammatory signaling pathways and sustaining chronic inflammation[12]. In contrast, adiponectin is a prototypical anti-inflammatory and insulin-sensitizing adipokine that under healthy conditions enhances insulin sensitivity in peripheral tissues by activating pathways such as AMPK and suppressing nuclear factor kappa B (NF-κB) signaling. However, in obesity adiponectin levels decline, diminishing its protective effects and thereby exacerbating defects in insulin signaling[10].
Moreover, obesity is often accompanied by elevated plasma levels of FFAs, which can activate toll-like receptor 4 (TLR4)/NF-κB and c-Jun N-terminal kinase (JNK) pathways in adipose tissue, liver, and skeletal muscle cells. This activation leads to serine phosphorylation of insulin receptor substrate 1 [IRS-1(Ser-P)], weakening downstream phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling and ultimately resulting in insulin resistance[3,13]. In summary leptin, adiponectin, FFAs, and proinflammatory cytokines such as TNF-α and IL-6 interact through multiple intersecting inflammatory pathways, collectively disrupting downstream insulin receptor signaling and contributing to systemic metabolic dysregulation[12,13].
Further studies have shown that not only do the levels of adipokines and inflammatory mediators change significantly in obesity, but their coordinated signaling interactions also play crucial roles in obesity-related insulin resistance. Elevated leptin levels can continuously stimulate macrophages to express activation markers such as cluster of differentiation (CD)25 and CD69 and to secrete TNF-α and IL-6 while these inflammatory cytokines in turn upregulate leptin expression, reinforcing a stable positive feedback loop within the leptin-inflammation axis and maintaining chronic inflammation in adipose tissue[12]. Meanwhile, the significant reduction of adiponectin in obesity weakens its original functions of regulating glucose and lipid metabolism via AMPK and PPAR-α activation and of suppressing NF-κB and JNK activity, thereby rendering insulin signaling more vulnerable to inflammatory inhibition[10].
Additionally, high FFA levels, a hallmark of obesity, can activate TLR4-NF-κB and JNK pathways in the liver and skeletal muscle, leading to increased activity of IκB kinase (IKK) and JNK. This results in IRS-1(Ser-P), thereby impairing PI3K/Akt-mediated insulin signaling[3,13]. Proinflammatory cytokines TNF-α and IL-6 are not only produced by hypertrophic adipocytes but also by infiltrating M1-polarized macrophages. TNF-α directly activates IKKβ/NF-κB and JNK signaling, promoting IRS-1 phosphorylation and weakening insulin receptor signaling capacity. IL-6 on the other hand indirectly suppresses insulin signaling by inducing suppressor of cytokine signaling 3 (SOCS3) expression[14,15]. These signaling pathways intersect and amplify one another in a cascade-like fashion, ultimately resulting in systemic impairment of insulin signaling, a molecular basis for obesity-associated insulin resistance.
Lipotoxicity refers to cellular dysfunction caused by the abnormal accumulation of fatty acids in non-adipose tissues[16]. In obesity impaired adipose tissue function leads to excessive release of FFAs, which undergo excessive β-oxidation in mitochondria, generating large amounts of ROS and metabolic intermediates that trigger endoplasmic reticulum (ER) stress, oxidative stress, and inflammatory responses[17,18]. For instance, the saturated fatty acid palmitic acid can induce ER stress and elevate ROS levels in pancreatic β cells, impairing mitochondrial function and ultimately triggering β cell apoptosis and disrupting insulin secretion[19].
Prolonged elevation of FFAs also leads to the accumulation of lipid metabolites such as long-chain acyl-CoA, diacylglycerol, and ceramide in hepatic and muscular tissues. These lipids activate protein kinase C and protein phosphatase 2A, thereby inhibiting the Akt/IRS signaling pathway and directly impairing insulin signaling[3,13]. In addition, excess lipids are ectopically deposited in the liver, skeletal muscle, and pancreatic β cells, causing organ damage: Lipid overload in the pancreas leads to β cell apoptosis and secretory dysfunction; intramuscular lipid accumulation reduces glucose uptake capacity; and hepatic steatosis induces cellular stress and disrupts glucose metabolism[12]. Together, these mechanisms contribute to a deleterious lipotoxic effect central to the development of obesity-associated T2DM[12].
Under high-fat conditions mitochondrial β-oxidation becomes overloaded, resulting in excessive ROS production that triggers oxidative stress, disrupting cellular structural integrity and functional homeostasis. ROS not only directly damage organelle membranes but also activate classical inflammatory pathways such as NF-κB and JNK, thereby enh
As adipose tissue gradually reaches its storage capacity, FFAs are transported and ectopically deposited in non-adipose tissues such as the liver, skeletal muscle, and pancreas, further increasing the metabolic burden. Lipid accumulation in the pancreas impairs β cell function and survival. Lipid buildup in skeletal muscle inhibits glucose uptake and oxidation. Hepatic steatosis enhances gluconeogenesis and may lead to hepatocellular necrosis[19]. These pathological processes collectively drive systemic insulin resistance and glucose metabolic disturbances, forming a key mechanistic foundation for obesity-related type 2 diabetes.
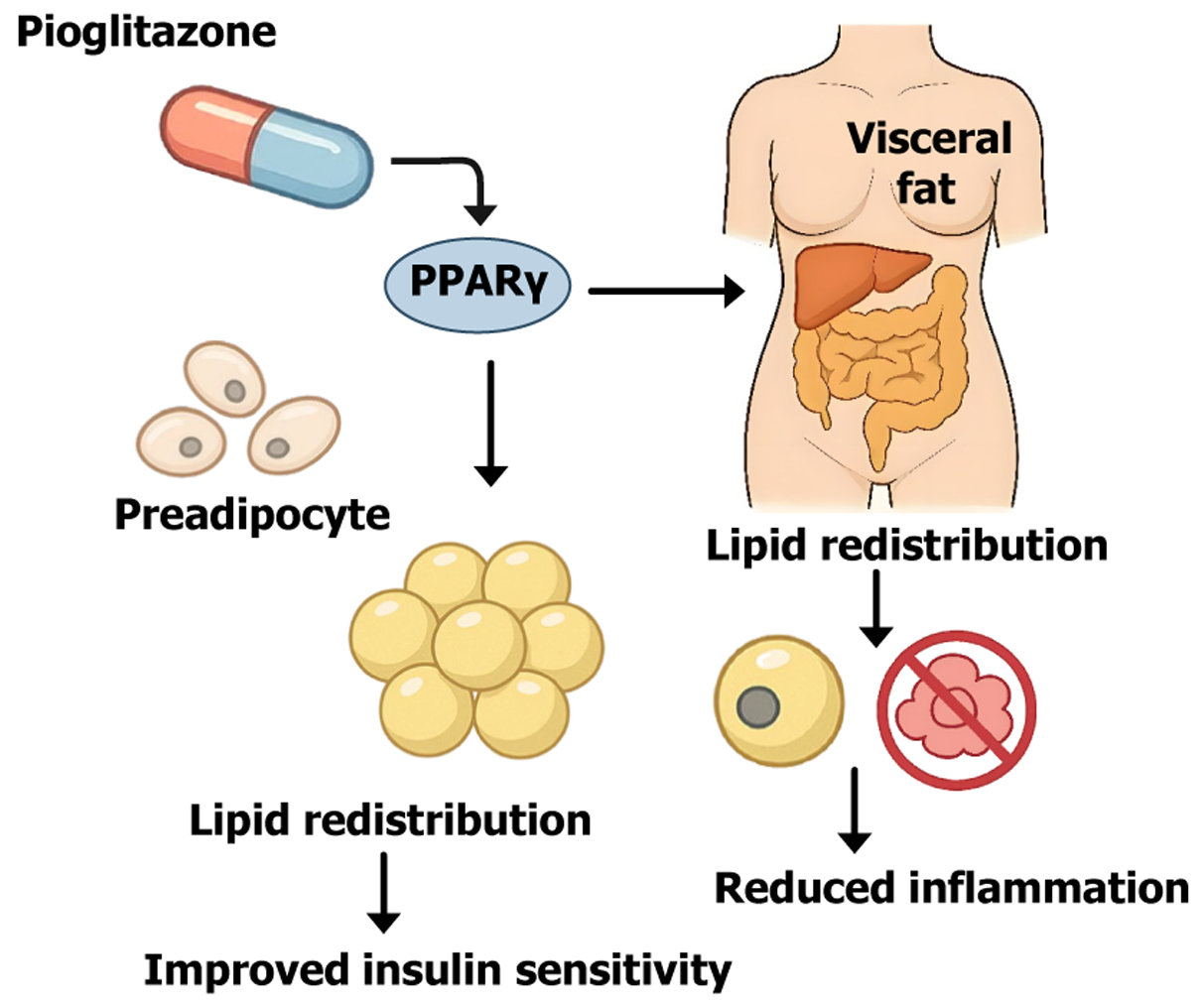
Pioglitazone is a thiazolidinedione (TZD) class insulin sensitizer, and its primary mechanism of action is the activation of PPARγ[23]. PPARγ is a master regulator of adipocyte differentiation. Upon activation by pioglitazone PPARγ promotes the differentiation of preadipocytes and increases the formation of new small adipocytes (adipocyte hyperplasia), particularly within SAT[24]. Although pioglitazone treatment may increase overall fat mass, this fat is preferentially redi
Through PPARγ-mediated adipose tissue remodeling, pioglitazone exerts multiple effects to enhance insulin sensitivity[26,28]. On one hand, the expansion of the subcutaneous fat depot increases the body’s capacity for safe lipid storage, lowering plasma FFAs levels and reducing ectopic fat accumulation, which alleviates insulin resistance in muscle and liver[25]. On the other hand, pioglitazone upregulates beneficial adipokines such as adiponectin while downregulating adverse ones like leptin[29]. Increased adiponectin enhances hepatic and muscular insulin responsiveness and exerts anti-inflammatory effects. In fact, TZD treatment has been shown to improve the antilipolytic effect of insulin on adipose tissue, significantly reducing excessive lipolysis and FFA release[29]. Furthermore, PPARγ activation modulates immune cell phenotypes; pioglitazone has been shown to activate anti-inflammatory pathways and suppress proinflammatory signals such as NF-κB, thereby attenuating obesity-related chronic adipose tissue inflammation[30].
In summary, pioglitazone reprograms adipose tissue by promoting adipocyte differentiation and redistributing fat, effectively transforming it into a metabolic buffer for insulin action, thereby improving the overall metabolic status of patients with T2DM[27]. Figure 1 illustrates the mechanism by which pioglitazone activates PPARγ, promotes prea
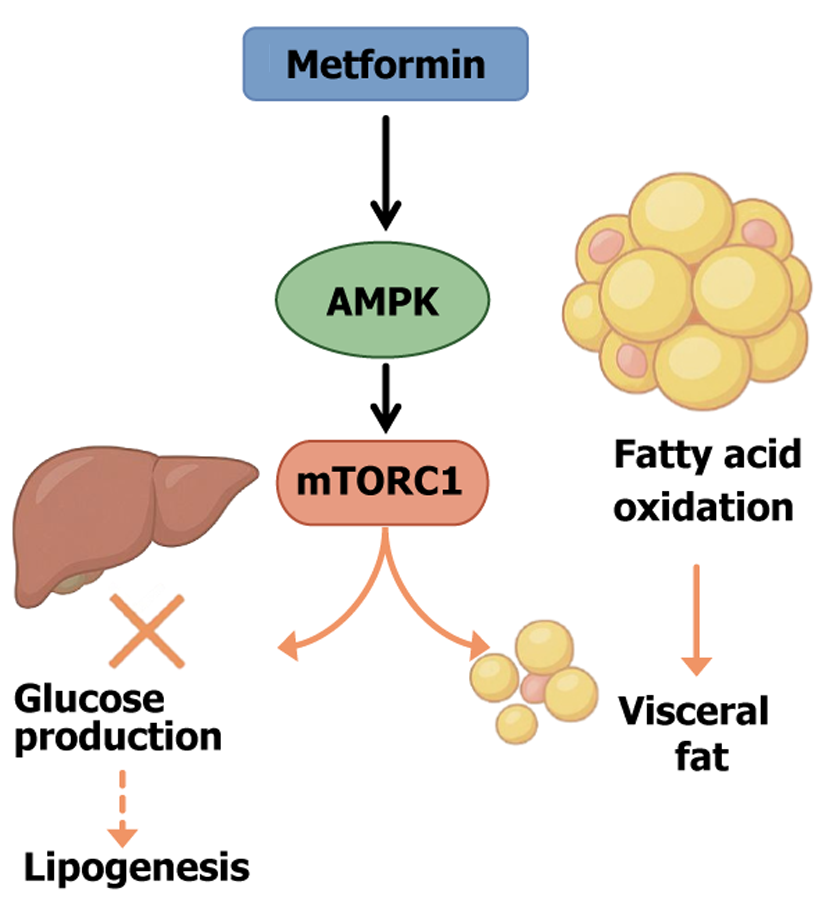
Metformin is a first-line treatment for T2DM known for its favorable safety profile and significant glucose-lowering effect. It is frequently associated with modest weight loss or at minimum weight neutrality[31]. The primary mechanism of metformin centers on the regulation of cellular energy metabolism, primarily through the activation of AMPK[32]. Activated AMPK functions as a cellular energy sensor, promoting catabolic pathways while inhibiting anabolic ones. Specifically, AMPK activation suppresses the mechanistic target of rapamycin complex 1 signaling pathway[33]. Mechanistic target of rapamycin is a critical regulator of cell growth and lipogenesis; inhibition by metformin reduces hepatic lipid synthesis and export while also suppressing gluconeogenesis, thereby improving hepatic glucose hom
Within adipose tissue AMPK activation inhibits lipogenic enzymes (such as fatty acid synthase) and promotes fatty acid oxidation. Several studies have shown that metformin can promote the browning of white adipose tissue (WAT) and enhance thermogenesis by upregulating the expression of uncoupling protein 1 (UCP1) and UCP3 (among other mechanisms), thereby increasing fatty acid combustion for heat production[25,34]. This effect is particularly significant in visceral fat and is believed to underlie the preferential reduction of visceral adiposity by metformin[25]. Notably, some reports indicate that metformin can substantially reduce hepatic fat content without significantly lowering overall body weight. This suggests that metformin can remodel the metabolism of visceral and ectopic fat, reducing its accumulation and thereby improving metabolic health even in the absence of major weight loss[35,36].
Beyond its impact on energy metabolism pathways, metformin also directly improves the inflammatory and endocrine function of adipose tissue. Chronic inflammation is a key contributor to insulin resistance in VAT. Metformin has been shown to suppress activation of the NOD-like receptor protein 3 (NLRP3) inflammasome in VAT, leading to reduced expression of downstream proinflammatory cytokines such as TNF-α, IL-6, IL-1β, and monocyte chemoattractant protein 1 (MCP-1)[37]. This underscores the significant anti-inflammatory effects of metformin and its capacity to improve the adipose microenvironment in obesity and diabetes. Additionally, metformin modulates lipid mobilization within adipose tissue. It partially inhibits catecholamine-induced lipolysis by reducing cyclic adenosine monophosphate (cAMP) production and protein kinase A (PKA) activity, which decreases hormone-sensitive lipase phosphorylation on lipid droplets, thereby suppressing excessive lipolysis[29]. This results in lower circulating FFA levels and helps mitigate FFA-induced disruption of insulin signaling (lipotoxicity). Equally important, metformin influences adipokine secretion profiles. For instance, it has been shown to reduce plasma levels of plasminogen activator inhibitor-1 and leptin. Although findings regarding its effect on adiponectin levels vary across studies, animal experiments have demonstrated that metformin upregulates adiponectin receptors AdipoR1 and AdipoR2[29,38].
Overall, metformin reprograms the metabolic functions of adipocytes and hepatocytes through AMPK activation, reducing lipid synthesis and accumulation, enhancing fatty acid oxidation, and alleviating inflammation in adipose tissue. These multitarget effects not only improve glycemic control but also enhance adipose tissue function and distribution in patients with obesity and T2DM, offering long-term benefits for insulin sensitivity and cardiometabolic risk reduction. Figure 2 illustrates the schematic model of the action of metformin on the AMPK/mechanistic target of rapamycin signaling pathway.
Since individual drugs target different aspects of metabolic dysfunction, combining agents with distinct mechanisms of action can yield synergistic effects by addressing adipose tissue pathology from multiple angles. This multipronged approach may more effectively improve metabolic outcomes in patients with obesity and T2DM[39]. Recent studies and clinical practice support the use of combination pharmacotherapy to promote fat redistribution and enhance insulin sen
Pioglitazone combined with metformin: Combining a PPARγ agonist (pioglitazone) with an AMPK activator (met
This combination improves insulin sensitivity through complementary mechanisms: Pioglitazone expands lipid storage capacity and reduces ectopic fat burden; and metformin decreases hepatic glucose output and promotes visceral fat oxidation. Together they contribute to improved glycemic and lipid profiles[27]. In clinical applications the weight-lowering tendency of metformin may partially counteract the weight gain associated with pioglitazone, enhancing tr
Glucagon-like peptide-1 receptor agonists combined with metformin: Glucagon-like peptide-1 (GLP-1) receptor agonists (e.g., liraglutide, semaglutide) improve glycemic control by stimulating insulin secretion, delaying gastric emptying, and suppressing appetite while also producing significant weight loss. Notably, GLP-1 receptor agonists (GLP-1RAs) have a preferential effect on reducing visceral fat. In patients with obesity and T2DM, GLP-1RAs reduce both sub
Combining GLP-1RAs with metformin is a widely adopted strategy in managing patients with obesity and T2DM, offering synergistic benefits for glycemic control, weight loss, and adipose tissue optimization. For example, in patients on background metformin therapy, the addition of liraglutide at 1.8 mg/day significantly reduced visceral fat volume[45]. A placebo-controlled trial in South Asian patients with T2DM found that liraglutide treatment led to a marked reduction in intra-abdominal fat, and the extent of visceral fat loss positively correlated with glycated hemoglobin (HbA1c) reduction, suggesting a direct link between fat loss and metabolic improvement[45].
Other studies have reported that adding semaglutide (a GLP-1RA) to oral antidiabetic therapy for 52 weeks led to significant reductions in both visceral and hepatic fat content. Similarly, oral semaglutide also demonstrated significant visceral fat reduction after 26 weeks of treatment[46]. Thus, GLP-1RA-based combination therapy not only enhances glycemic control but also contributes to cardiometabolic health by promoting weight loss and reducing visceral fat accumulation. Importantly, GLP-1RAs may also improve adipose tissue endocrine function. For instance, liraglutide added to metformin therapy has been shown to elevate levels of the anti-inflammatory adipokine omentin-1, which may help modulate the inflammatory microenvironment in adipose tissue. Given their superior glycemic and weight-lowering effects, clinical guidelines recommend GLP-1RAs in combination with metformin and/or other agents for patients with T2DM requiring weight management to achieve maximum therapeutic efficacy[47,48].
Combination of sodium-glucose co-transporter 2 inhibitors with metformin: Sodium-glucose co-transporter 2 (SGLT-2) inhibitors (e.g., dapagliflozin, empagliflozin) promote urinary glucose excretion by inhibiting renal glucose reabsorption, resulting in an average daily caloric loss of approximately 300 kcal and thereby contributing to sustained weight reduction[49]. SGLT-2 inhibitors alone can reduce body fat with studies showing that most of the weight loss is due to fat mass reduction, particularly targeting metabolically harmful VAT. For example, compared with metformin 100 mg canagliflozin daily for 12 weeks significantly reduced VAT volume in patients with T2DM[50].
Combining SGLT-2 inhibitors with other antidiabetic agents often enhances the beneficial effects on fat distribution. In an open-label controlled trial comparing the addition of dapagliflozin (10 mg/day) to metformin vs metformin mono
In addition, dapagliflozin combined with other oral agents has demonstrated advantages in fat redistribution. For instance, dapagliflozin plus saxagliptin combined with metformin significantly reduced hepatic steatosis and total body fat in patients with nonalcoholic fatty liver disease. In patients with T2DM with suboptimal glycemic control on met
SGLT-2 inhibitors also improve adipose tissue inflammation. In T2DM rat models empagliflozin significantly downregulated proinflammatory cytokines such as MCP-1, TNF-α, IL-1β, and IL-6 in VAT and reduced macrophage infiltration into adipose tissue. This anti-inflammatory remodeling further enhanced insulin sensitivity in adipose tissue. Thus, combining SGLT-2 inhibitors with other drugs (especially metformin or GLP-1RAs) can act via a dual mechanism (increasing energy expenditure and improving the inflammatory microenvironment) to more effectively reduce harmful fat accumulation and improve metabolism.
Multiagent combinations and translational prospects: With growing insight into the multifactorial pathogenesis of T2DM, early implementation of combination therapies targeting multiple pathways is increasingly advocated to correct metabolic derangements comprehensively[52]. The combination of GLP-1RAs and SGLT-2 inhibitors has shown additive and synergistic effects on lowering glucose and body weight and improving fat distribution, including greater reductions in visceral and hepatic fat as well as cardiovascular and renal benefits[53]. Based on these findings, some experts have proposed adding a PPARγ agonist (e.g., pioglitazone) to the GLP-1RA + SGLT-2 inhibitor regimen to create a triple the
A recent systematic review and meta-analysis (involving 19 studies and over 25000 patients in real-world settings) provided strong empirical support: Compared with using newer antidiabetic drugs alone, adding pioglitazone to either GLP-1RA or SGLT-2 inhibitor significantly further reduced HbA1c (by approximately 1.0% and 0.5%, respectively) and body weight without increasing hypoglycemia risk[56]. Notably, the pioglitazone + SGLT-2 inhibitor combination resulted in an additional mean weight loss of 2.3 kg compared with SGLT-2 inhibitor monotherapy[56]. More importantly, combination therapy has been associated with a reduced risk of adverse cardiovascular outcomes during follow-up. For example, real-world cohort data showed significantly reduced risks of cardiovascular events (adjusted hazard ratio = 0.76, 95% confidence interval: 0.66-0.88) and heart failure (HF) (adjusted hazard ratio = 0.67, 95% confidence interval: 0.55-0.82) with pioglitazone + SGLT-2 inhibitor therapy[55].
These findings highlight the significant clinical potential of combination therapies targeting fat distribution and metabolic pathways. In summary, the rational combination of agents acting on different targets, PPARγ, AMPK, GLP-1 receptors, and renal glucose reabsorption, can comprehensively remodel adipose tissue in patients with obesity-associated T2DM. This includes reducing harmful visceral and ectopic fat accumulation, expanding metabolically safe subcutaneous fat depots, and improving adipokine profiles and inflammation, thereby significantly enhancing insulin sensitivity and metabolic health[29]. This multitarget strategy offers promising translational potential in the treatment of obesity-associated T2DM. Table 2 summarizes the main targets, signaling pathways, adipose regions affected, weight change trends, and metabolic benefits of currently used monotherapies and combination regimens in clinical practice, showcasing their mechanistic differences, shared benefits, and synergistic potential in adipose remodeling and insulin sensitivity enhancement.
| Drug/regimen | Primary target | Key signaling pathway | Main affected fat depot | Body weight impact | Key metabolic benefits |
| Pioglitazone | PPARγ | PPARγ: Promotes adipocyte differentiation, increases adiponectin, anti-inflammatory | Subcutaneous fat (↑), visceral fat (↓) | Increased (mainly subcutaneous) | Improves insulin sensitivity, redistributes fat, alleviates lipotoxicity and inflammation |
| Metformin | AMPK | AMPK: Inhibits mTORC1, promotes FA oxidation, anti-inflammatory | Visceral and hepatic fat (↓) | Decreased or weight-neutral | Lowers glucose, anti-lipogenesis, promotes lipid oxidation, reduces inflammation, and improves adipokine profile |
| Pioglitazone + metformin | PPARγ + AMPK | Fat redistribution (PPARγ) + improved glucose metabolism (AMPK) | Subcutaneous fat (↑), visceral fat (↓) | Balanced | Complementary effects improve fat deposition and insulin resistance, broad metabolic improvement |
| GLP-1RA + metformin | GLP-1R + AMPK | Appetite suppression, insulin promotion (GLP-1R) + lipid oxidation (AMPK) | Visceral fat (↓) > subcutaneous fat (↓) | Decreased significantly | Antihyperglycemic + weight loss + visceral fat optimization, improved adipokines, anti-inflammatory |
| SGLT-2i + metformin | SGLT-2 + AMPK | Calorie loss via glycosuria (SGLT-2) + anti-lipogenesis, lipid oxidation (AMPK) | Abdominal, hepatic, and perirenal fat (↓) | Decreased (mainly fat reduction) | Glycemic control + fat loss + anti-inflammatory, reduces lipotoxicity, enhances insulin sensitivity |
| GLP-1RA + SGLT-2i | GLP-1R + SGLT-2 | Dual mechanism: Appetite suppression + urinary glucose excretion | Visceral and hepatic fat (marked) (↓) | Decreased (more pronounced) | Multitarget fat reduction, weight loss, metabolic enhancement, cardiovascular and renal benefits |
| Pioglitazone + GLP-1RA or SGLT-2i | PPARγ + GLP-1R/SGLT-2 | Fat redistribution reprogramming (PPARγ) + fat reduction/glycemic control (GLP-1RA/SGLT-2i) | Fat in multiple depots (↓), subcutaneous storage (↑) | Decreased (via GLP-1RA or SGLT-2i) | Multi-action: Glucose lowering, anti-inflammatory, improved adipokines/Lipotoxicity, better cardiovascular outcomes |
ADSCs are a subtype of mesenchymal stem cells (MSCs) that characteristically express high levels of MSC surface markers such as CD73, CD90, and CD105 while lacking or exhibiting low expression of hematopoietic markers such as CD34 and CD45[57]. Originating from the mesoderm, ADSCs possess multipotent differentiation potential, enabling them to differentiate into various cell lineages, including adipocytes, osteoblasts, chondrocytes, cardiomyocytes, myocytes, and even neurons and hepatocytes[58,59]. ADSCs derived from different adipose depots exhibit variations in phenotype and differentiation potential. For example, ADSCs derived from brown adipose tissue display a stronger proliferative capacity and higher differentiation potential into osteogenic, adipogenic, and myogenic lineages compared with those derived from WAT[60]. Additionally, ADSCs can give rise to white, beige, or brown adipocyte lineages, providing new therapeutic avenues for obesity-related disorders. The multipotent differentiation capacity and expression of MSC markers make ADSCs an ideal cell source for regenerative medicine[57,58]. Table 3 summarizes the phenotypic differences and major differentiation potentials of ADSCs from different origins.
| Source | Typical surface markers | Differentiation potential/characteristics |
| Subcutaneous fat | CD73+, CD90+, CD105+; CD34-/Low, CD45- | Strong adipogenic differentiation; moderate osteogenic/chondrogenic potential; suitable for tissue engineering |
| Visceral (intra-abdominal) fat | Same as subcutaneous; some studies report SC-ASCs (high CD10) vs VS-ASCs (high CD200) | Slight variation in adipogenic potential; possibly different impacts on metabolic regulation |
| Brown adipose tissue | Same as MSC markers; commonly express Sca-1, CD29, CD49 in experimental settings | Stronger differentiation capacity; high proliferation; excellent adipogenic, osteogenic, and myogenic potential |
| Others (e.g., retroperitoneal, periovarian fat) | Similar MSC markers; may include more vascular-related markers (e.g., CD146) | Broad differentiation spectrum; capable of differentiating into mitochondria-rich cell lineages; less studied |
ADSCs exert paracrine regulatory functions by secreting a variety of factors and extracellular vesicles (EVs), thereby improving inflammation and insulin signaling in adipose tissue, liver, muscle, and other insulin-sensitive tissues. It has been reported that ADSCs can secrete anti-inflammatory cytokines such as IL-10 as well as proangiogenic and tissue repair factors including transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), placental growth factor, and angiopoietin-1[58]. IL-10 suppresses inflammation via the Janus kinase 1/tyrosine kinase 2-signal transducer and activator of transcription 3 (STAT3) pathway[61,62]. TGF-β binds its receptor to activate mothers against decapentaplegic homolog (SMAD) signaling and regulate gene expression[63]. VEGF acts on endothelial cells to promote their proliferation and survival[64].
Moreover, ADSC-derived EVs are rich in microRNAs (miRNAs) and growth factors (such as VEGFA, fibroblast growth factor, insulin-like growth factor, and platelet-derived growth factor), which can be taken up by target cells (e.g., adi
Through the secretion of various paracrine factors and miRNA-enriched EVs, ADSCs exert broad metabolic regulatory effects across multiple tissues. Their anti-inflammatory cytokines (e.g., IL-10) inhibit inflammatory responses via STAT3 activation while growth and immunomodulatory factors such as TGF-β, VEGF, HGF, and MCP-1 participate in tissue repair and immune regulation[67-69]. Among the bioactive miRNAs in ADSC-derived EVs, miR-223-3p has been shown to downregulate the profibrotic gene E2F transcription factor 1 in hepatocytes, thereby attenuating hepatic lipid accumulation and fibrosis. Other miRNAs promote adipose tissue browning and adiponectin secretion by activating the AMPK/PPARγ coactivator 1-alpha (PGC-1α) pathway, thereby enhancing systemic metabolic function[66,70].
At the target tissue level, ADSC-derived immunomodulators can suppress macrophage infiltration in adipose tissue and enhance insulin signaling[71,72]. In the liver VEGF and angiopoietin-1 jointly promote angiogenesis and tissue regeneration while EV-derived anti-inflammatory components reduce inflammatory and oxidative stress[73]. In skeletal muscle IL-10 and related factors alleviate inflammation and improve glucose uptake[74]. Overall, ADSCs remodel the microenvironment of insulin-sensitive tissues through the secretion of a spectrum of bioactive molecules, thereby cooperatively enhancing metabolic function at multiple levels (Table 4).
| Category of secretion | Representative molecules/miRNAs | Main target tissues or cells | Typical receptors/signaling pathways | Expected metabolic and inflammatory effects |
| Cytokines | IL-10, TGF-β, VEGF, HGF | VAT, SAT, liver, skeletal muscle, macrophages | JAK1/Tyk2-STAT3; SMAD2/3; VEGFR2-PI3K/Akt | Inhibit NF-κB and JNK-mediated inflammation; promote angiogenesis and tissue repair; enhance insulin signaling throughput |
| Exosomal miRNAs | miR-223-3p, miR-29a, miR-155, miR-210 | Hepatocytes, adipocytes, macrophages | Target E2F1, SOCS1/STAT1, SHIP1, IRAK1/NF-κB | Alleviate hepatic steatosis and fibrosis; modulate macrophage polarization; promote adipose browning and adiponectin secretion |
| Growth/chemotactic factors | Ang-1, FGF, IGF-1, MCP-1 | Vascular endothelium, fibroblasts, immune cells | Tie2-Akt; FGFR-MAPK; CCR2 | Promote vascular remodeling; chemotactic regulation of immune cells; improve tissue microcirculation |
| Antioxidant regulatory axis | PGC-1α-induced upregulation of SOD2, UCP1 | White/brown adipocytes | PGC-1α-TFAM; UCP1-uncoupling | Enhance mitochondrial biogenesis and oxidative phosphorylation; reduce ROS load; increase energy expenditure |
Numerous animal studies have demonstrated that ADSC therapy can significantly improve obesity and metabolic disorder parameters. In high-fat diet-fed mice ADSC infusion led to a marked reduction in body fat percentage (from approximately 46.7% to 32.3%)[75] alongside improvements in blood glucose, glucose tolerance, and HbA1c levels, suggesting a decrease in Homeostasis Model Assessment of Insulin Resistance and enhanced insulin sensitivity[75]. In another study intravenous administration of ADSCs into type 2 diabetic rats significantly reduced fasting glucose and insulin levels, improved insulin resistance index, decreased liver enzymes (alanine aminotransferase, aspartate aminotransferase), and attenuated hepatic fibrosis[76]. In a nonalcoholic steatohepatitis model, ADSC-derived exosomes carrying miR-223-3p inhibited hepatic lipid accumulation and the expression of fibrotic markers, significantly lowering hepatic triglyceride/cholesterol content and alanine aminotransferase/aspartate aminotransferase activities[70]. In a polycystic ovary syndrome rat model, intraperitoneal injection of ADSC-conditioned medium led to reductions in body weight and blood glucose, decreased ovarian volume, and partial restoration of estrogen levels and normal estrous cycles, indicating improvements in both metabolic and reproductive functions[77].
Although limited in number, some clinical studies have also shown the potential benefits of ADSC therapy. Animal models and early-phase clinical trials suggest that ADSC transplantation may promote endogenous β cell regeneration, protect residual β cells, modulate immune responses, and enhance insulin sensitivity in peripheral tissues[70]. While phase II human studies remain scarce, reports have indicated that ADSC therapy can improve insulin resistance and glycemic control in patients with type 2 diabetes (e.g., reductions in Homeostasis Model Assessment of Insulin Resistance and increases in adiponectin levels). Overall, the current literature provides high-quality evidence supporting the therapeutic role of ADSCs in obesity, T2DM, nonalcoholic fatty liver disease, and polycystic ovary syndrome (diseases related to metabolic syndrome). The underlying mechanisms involve immunomodulation, inflammation suppression, and restoration of endocrine functions[70,75,76]. Future efforts should focus on large-scale clinical trials to validate the safety and long-term efficacy of ADSC therapy while exploring optimized delivery routes and preconditioning strategies to enhance its translational potential.
Adipose tissue beiging refers to the process whereby white adipocytes acquire characteristics of brown adipocytes, including the expression of mitochondrial UCPs such as UCP1, thereby enhancing energy expenditure. This process plays a critical role in combating obesity and improving glucose metabolism. ADSCs have a dual role in this transformation process[78]. On one hand, ADSCs inherently possess the potential to differentiate into beige or brown adipocytes. For instance, Silva et al[79] found that ADSCs isolated from mediastinal fat expressed brown adipocyte markers such as PR domain zinc finger protein 16 (PRDM16) and UCP1 during differentiation, ultimately becoming thermogenically active brown-like adipocytes. On the other hand, exogenous stimulation of ADSCs can induce their conversion into a brown-like phenotype[80]. For example, treatment of human ADSCs with a combination of metformin and vitamin D sig
These findings suggest that pharmacological or signaling pathway modulation of ADSCs can activate thermogenic transcriptional networks, including PGC-1α, PRDM16, and UCP1, thereby promoting differentiation or transdifferentiation of ADSCs into brown/beige adipocytes. Additionally, ADSCs exert regulatory effects on surrounding cells via the secretion of various cytokines or exosomes. Recent studies have shown that exosomes derived from healthy mouse ADSCs when injected into obese mice markedly promote the polarization of adipose tissue macrophages toward the anti-inflammatory M2 phenotype [characterized by high IL-10 and arginase-1 (ARG1) expression] and induce beiging of WAT, thereby contributing to metabolic improvement[82]. Mechanistically, ADSC-derived exosomes facilitate M2 macrophage polarization (IL-10/TNF-stimulated gene-6 ↑) and in vivo white-fat beiging, leading to reduced adipose inflammation and improved systemic insulin sensitivity. These effects have been demonstrated to attenuate obesity and drive UCP1+ remodeling in WAT[68,83].
Pharmacologic priming of human ADSCs with metformin plus vitamin D further enhances PGC-1α/PRDM16/UCP1 signaling programs and induces a brown-like phenotype[81]. Notably, in T2DM the beiging capacity of ADSC-derived beige adipocytes is impaired with reduced UCP1 expression and increased mitochondrial ROS, reinforcing the causal link between thermogenic failure and insulin resistance[84]. Collectively, these findings connect ADSC-mediated M2 polarization and beige induction with UCP1-mitochondrial activation, culminating in the alleviation of insulin resistance at both the tissue and whole-body levels. In summary, ADSCs and their secreted products play a key role in the browning of WAT by upregulating thermogenic regulators including PRDM16, PGC-1α, and UCP1 and activating mitochondrial biogenesis and thermogenic programs[81,85].
Obesity and diabetes are commonly accompanied by chronic inflammation of adipose tissue, which is closely associated with dysregulated adipokine secretion. In the obese state anti-inflammatory factors such as adiponectin are downregulated while proinflammatory cytokines including leptin, IL-6, and TNF-α are markedly elevated, resulting in a persistent low-grade inflammatory milieu within adipose tissue[86]. This proinflammatory environment alters ADSC function. ADSCs derived from individuals with obesity are often reprogrammed toward a proinflammatory phenotype characterized by a reduced capacity to suppress T cell proliferation and an increase in the secretion of inflammatory cytokines such as IL-6 and TNF-α[87]. These alterations not only impair the regenerative potential of ADSCs but also exacerbate local inflammation.
In contrast, ADSCs derived from healthy donors exhibit pronounced anti-inflammatory properties. Studies have shown that ADSCs from normal mice can modulate the immune microenvironment of adipose tissue by secreting anti-inflammatory factors such as IL-10 and releasing exosomes that promote macrophage polarization toward the M2 phenotype, marked by high expression of IL-10 and ARG1[88,89]. For example, injecting exosomes from healthy donor-derived ADSCs into obese mouse models significantly increased the proportion of M2 macrophages in adipose tissue and markedly improved insulin sensitivity. M2 macrophages possess anti-inflammatory functions, secreting cytokines such as IL-10 to suppress the expression of proinflammatory signals like IL-6 and TNF-α. This leads to elevated adiponectin levels, attenuation of chronic inflammation in adipose tissue, and normalization of lipid metabolism[68,71].
It is important to note that a bidirectional interaction between ADSCs and macrophages can trigger and sustain an inflammatory positive feedback loop during obesity progression. Specifically, hypertrophic adipocytes release exosomes enriched with proinflammatory mediators (e.g., MIF, RBP4) and several miRNAs (e.g., miR-29a, miR-155, miR-34a), which promote macrophage polarization toward the classically activated M1 phenotype. These M1 macrophages are characterized by elevated expression of inducible nitric oxide synthase and TNF-α, thereby intensifying tissue inflammation[90,91]. M1-derived cytokines such as IL-6 and TNF-α along with M1-secreted exosomes containing miR-155, miR-29a, and miR-210, inhibit the differentiation and regenerative capacity of ADSCs and impair their immunomodulatory function. This reciprocal amplification ultimately establishes a persistent inflammatory loop, aggravating the immunometabolic dysfunction of adipose tissue[82,92]. At the molecular level proinflammatory cues (e.g., TLR4 → IKKβ/NF-κB, JNK) drive IRS-1(Ser-P), weakening PI3K-Akt-glucose transporter type 4 (GLUT4) signaling and propagating adipose and systemic insulin receptor. NLRP3 inflammasome activation further amplifies IL-1β/IL-18 signaling in adipose immunometabolism[93-95]. Obesity-derived exosomal miR-29a/miR-155 from adipose macrophages directly induces the insulin receptor across adipocytes, myocytes, and hepatocytes, establishing an EV-mediated endocrine axis[83,96]. In contrast, ADSC-EVs dampen TLR-NF-κB/NLRP3 activity and restore adipokine balance (adiponectin ↑, IL-6/TNF-α ↓), thereby reducing
In summary, ADSCs, adipokines, and immune cells form a regulatory network. Healthy ADSCs secrete anti-inflammatory cytokines and ADSC-derived EVs that promote M2 macrophage polarization and suppress IL-6/TNF-α signaling while ADSCs in an obese environment tend to adopt a proinflammatory phenotype, favoring M1 polarization and enhanced cytokine production[82,87]. This regulatory mechanism highlights the central role of ADSCs in obesity-associated adipose tissue inflammation and provides insights into their therapeutic potential in metabolic disease mana
| Molecule | Category | Major source | Action on insulin signaling | Regulation trend (in obesity) | Regulation evidence by drugs/ADSCs |
| Leptin | Proinflammatory adipokine | VAT > SAT | Promotes IRS-1 Ser P, inhibits PI3K/Akt | ↑ | Downregulated by GLP-1RA; indirectly inhibited by ADSC EVs |
| Adiponectin | Anti-inflammatory/sensitizing | SAT | Activates AMPK/PPARα | ↓ | Upregulated by pioglitazone, SGLT-2 inhibitors |
| TNF-α | Classical proinflammatory cytokine | M1 macrophages, hypertrophic adipocytes | IKKβ/NF-κB AND JNK → promotes IRS-1 Ser P | ↑ | Downregulated by pioglitazone, metformin, ADSC EVs |
| IL-6 | Proinflammatory cytokine | Same as above | SOCS3 inhibits IRS-1 | ↑ | Suppressed via ADSC-derived IL-10 axis |
| IL-10 | Anti-inflammatory cytokine | M2 macrophages, ADSCs | Inhibits NF-κB/JNK, enhances insulin signaling | ↓ | Healthy ADSC EVs → promote M2 macrophage polarization↑ |
| miRNA | Major direct target genes/pathways | Target tissues/cells | Metabolic or inflammatory effects | Evidence type1 |
| miR-223-3p | E2F1 → Inhibits adipogenesis/fibrosis | Hepatocytes | Reduces hepatic lipid accumulation and fibrosis | HFD mice (in vivo)/HepG2 (in vitro) |
| miR-29a | SOCS1/STAT1 | Macrophages | Inhibits M1 polarization, decreases TNF-α/IL-6 | LPS-stimulated RAW264.7/mice |
| miR-155 | SHIP1/SOCS1 | Macrophages | Promotes M1 polarization, amplifies inflammation | Functional antagonism in vitro |
| miR-34a | SIRT1/FGF21 | Liver and adipose tissue | Inhibits browning, promotes adipogenesis | DIO mice (in vivo) |
| miR-210 | IRAK1/NF-κB | Liver, skeletal muscle | Reduces inflammation, improves insulin sensitivity | db/db mice (in vivo) |
| miR-126 | IRS-1/PI3K-Akt | Endothelial cells | Promotes angiogenesis, improves insulin signaling | STZ rats (in vivo)/HUVEC |
Mitochondrial dysfunction in adipocytes is characterized by reduced mitochondrial content, impaired oxidative phosphorylation, and excessive ROS accumulation. It is a key pathological basis of obesity and insulin resistance. ADSCs can improve mitochondrial biogenesis and function in adipocytes through multiple mechanisms[98,99].
On one hand, ADSCs secrete various factors, including growth factors and miRNAs carried by microvesicles or exosomes that activate the mitochondrial biogenesis regulator PGC-1α pathway in adipocytes. PGC-1α promotes mitochondrial biogenesis by activating nuclear respiratory factors [nuclear factor erythroid 2-related factor 1 (Nrf1) and Nrf2] and estrogen-related receptor alpha, which in turn induce transcription factor A, mitochondrial (TFAM), and DNA polymerase γ, facilitating mitochondrial DNA replication[100]. Activation of this pathway increases mitochondrial number, enhances expression of respiratory chain complexes, and boosts oxidative phosphorylation capacity. During MSC differentiation into adipocytes, upregulation of PGC-1α is accompanied by increased mitochondrial content and improved oxidative capacity, indicating that PGC-1α overexpression significantly enhances mitochondrial oxidative metabolism[101]. By secreting factors that upregulate PGC-1α, ADSCs can simulate this process, thereby increasing PGC-1α levels in target adipocytes and initiating TFAM-mediated mitochondrial DNA replication.
On the other hand, PGC-1α also induces the expression of mitochondrial antioxidant enzymes, thereby reducing ROS-induced mitochondrial damage[102,103]. For example, activation of PGC-1α upregulates superoxide dismutase 2 (SOD2) in mitochondria, scavenging free radicals and maintaining mitochondrial homeostasis. During MSC differentiation it has been confirmed that PGC-1α regulation of SOD2 expression helps suppress ROS accumulation, suggesting that ADSCs may similarly protect adipocyte mitochondria from oxidative stress through activation of this pathway[101].
Furthermore, during ADSC-induced beige/brown adipocyte differentiation, the upregulation of UCPs such as UCP1 can lower mitochondrial membrane potential, reduce ROS production, and increase energy expenditure. Experiments have shown that treating human ADSCs with a combination of metformin and vitamin D significantly upregulates UCP1 expression in differentiated adipocytes, indicating that ADSC-mediated mitochondrial improvements are associated with enhanced UCP1 expression[81]. Beyond biogenesis ADSC-EVs activate PGC-1α → Nrf1/TFAM and mitochondrial-fusion programs (OPA1/MFN1/MFN2), increasing mitochondrial DNA replication and respiratory capacity in metabolic injury models[100].
By enhancing fatty-acid oxidation and UCP1-linked uncoupling, ADSC interventions lower lipotoxic intermediates (e.g., ceramides), a causal brake on Akt signaling. Conversely, pharmacologic ceramide-synthesis inhibition prevents the lipid-induced insulin receptor in vivo, underscoring this mediator pathway[104,105]. Finally, UCP1 status and redox control are intertwined: Defective UCP1 programs associate with ROS accumulation in ADSC-derived beige adipocytes from T2DM; and thermogenic activation can rebalance mitochondrial ROS and insulin signaling[84,106]. These mechanisms provide testable mediators (PGC-1α/TFAM, UCP1, mt-fusion markers, ceramide panels) linking ADSC therapy to insulin-signaling rescue.
In summary, ADSCs enhance mitochondrial biogenesis and oxidative phosphorylation activity in adipocytes by upregulating the PGC-1α/TFAM pathway and reducing ROS-induced mitochondrial damage by inducing antioxidant enzymes such as SOD2. These effects contribute to improved mitochondrial function in adipocytes affected by obesity, thereby increasing energy metabolism and insulin sensitivity. This provides a solid theoretical basis for the therapeutic application of ADSCs in metabolic diseases.
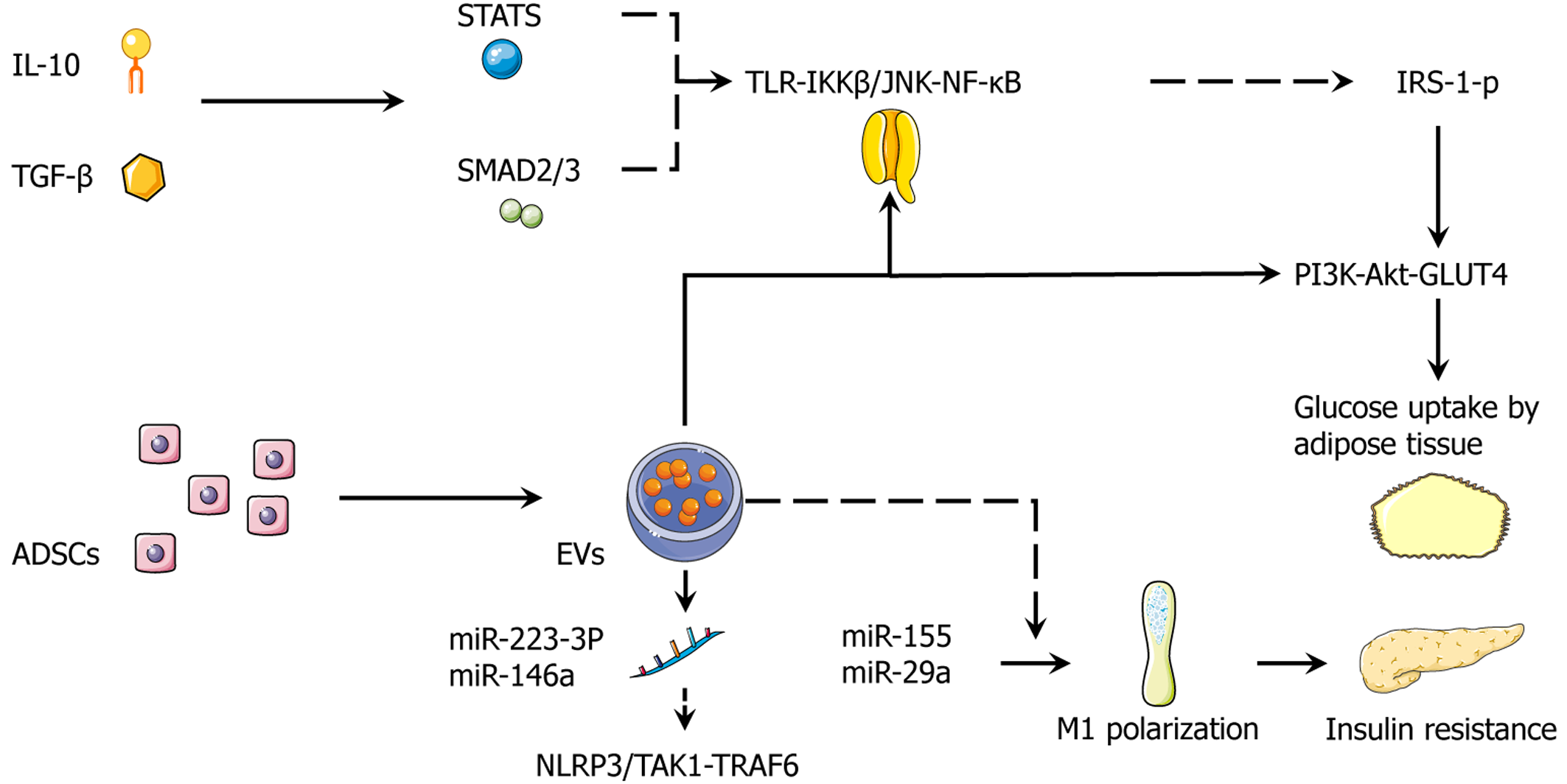
To clarify the cooperative roles of cytokines, growth factors, and exosomal miRNAs derived from ADSCs, we proposed an integrated secretome model in which metabolism, inflammation, and tissue regeneration are jointly regulated through a limited number of key crosstalk nodes. Anti-inflammatory cytokines such as IL-10 and TGF-β activate STAT3 and SMAD2/3 signaling, which suppresses the TLR-IKKβ/JNK-NF-κB pathway. This inhibition reduces IRS-1(Ser-P) and restores PI3K-Akt-GLUT4-mediated glucose uptake in adipocytes[107]. Simultaneously, proregenerative factors including insulin-like growth factor-1, HGF, and VEGF promote endothelial repair and increase capillary density, thereby reducing hypoxia-induced inflammation and fibrosis[108].
Exosomal miRNAs provide an additional, rapid regulatory layer. For example, miR-223-3p and miR-146a suppress NLRP3/TGF-β-activated kinase 1 (TAK1)-TNF receptor-associated factor 6 (TRAF6) activation, whereas obesogenic miRNAs such as miR-155 and miR-29a enhance M1 macrophage polarization and insulin resistance. ADSC-derived EVs shift this balance toward an M2 phenotype characterized by high ARG1 and IL-10 expression along with increased adiponectin, which collectively favor healthy hyperplastic expansion over maladaptive hypertrophy.
On the metabolic axis ADSCs activate the AMPK/SIRT1-PGC-1α-PRDM16-UCP1 pathway, increasing mitochondrial biogenesis and fatty acid oxidation while reducing ceramide and diacylglycerol accumulation[109]. This alleviates lipotoxic inhibition of Akt signaling. Together these actions of the ADSC secretome coordinate immune homeostasis, extracellular matrix softening and angiogenesis, and thermogenic remodeling. This integrated mechanism provides a coherent explanation for how ADSCs improve insulin sensitivity across adipose tissue, liver, and muscle (Figure 3).
The combined application of ADSCs with commonly used insulin-sensitizing agents, such as metformin, TZDs, GLP-1RAs, and SGLT-2 inhibitors may offer complementary benefits across multiple pathways. For example, an animal study demonstrated that simultaneous administration of ADSCs and the GLP-1 analog exenatide in diabetic rats significantly improved renal function and tissue structure with inflammation, fibrosis, and apoptosis markers further reduced compared to either monotherapy group[110]. Similarly, in a diabetic nephropathy model, repeated infusion of ADSCs combined with the SGLT-2 inhibitor empagliflozin resulted in more pronounced protection of renal function and ultrastructural integrity, whereas monotherapy showed inferior effects[111].
Additionally, coadministration of bone marrow MSCs with pioglitazone or GLP-1RA has been shown to significantly reduce insulin resistance and inflammation through various molecular mechanisms[7]. These findings suggest that insulin-sensitizing drugs may enhance ADSC viability, paracrine secretion, and regenerative capacity by improving the tissue metabolic microenvironment by reducing insulin resistance and modulating adipokine profiles, thereby achieving synergistic therapeutic effects. It is important to note that such synergism may vary depending on intervention timing and disease model. For example, in a murine model of radiation-induced skin fibrosis, preventive combination therapy did not yield additional benefit, suggesting that drug-ADSC interactions must be contextually assessed under different pathological conditions[112].
In summary, current experimental data support the promising potential of combining ADSCs with agents like GLP-1RA and SGLT-2 inhibitors[110,111] while the synergistic effects of metformin and other drugs remain less clear. This highlights the need for future studies to further elucidate the mechanisms and optimal timing of pharmacological-stem cell combination strategies.
Within the framework of precision medicine, transcriptomic, proteomic, and single-cell omics analyses of ADSC therapy can reveal individualized response markers and subtype differences[113,114]. For example, RNA sequencing comparing ADSCs from patients with diabetic nephropathy with those from healthy controls revealed significant differences in the expression of angiogenesis-related genes and miRNAs, suggesting that a hyperglycemic and uremic environment may alter ADSC function. However, functional assays indicated that their proangiogenic capacity remained intact, supporting the feasibility of autologous ADSC therapy with individualized transcriptomic-based adjustments[115].
Single-cell RNA sequencing provides even more refined insights into cellular lineage composition. A recent study performing single-cell RNA sequencing on clinical-grade ADRCs (adipose-derived stromal vascular fractions) found high consistency in cell composition across different donors with minimal influence of sex or body mass index on the pro
Genomic studies have also highlighted the role of a patient’s genetic background in shaping treatment response. For instance, a specific single nucleotide polymorphism identified in adipocyte-like cells derived from fat MSCs was found to affect the PPARγ binding site and the induction of ATP binding cassette transporter A1 expression by TZDs. This single nucleotide polymorphism also predicted an increased risk of cholesterol elevation following TZD therapy, implying that genotyping or transcriptomic analysis could be used to anticipate individual responses to drug or cell-based therapies[116].
In summary, integrating multiomics data, including single-cell transcriptomics, genomics, and proteomics, enables the identification of molecular markers and response subtypes associated with ADSC efficacy. These insights can guide optimal therapeutic timing and regimen selection. Although this research area is still in its early stages, existing evidence suggests that omics-based stratification based on metabolic status, genetic background, or ADSC subpopulation characteristics holds promise for the precision optimization of ADSC therapies (e.g., determining ideal administration windows or identifying suitable patient cohorts)[113,115].
Safety is a core consideration in the clinical application of ADSCs.
Immunogenicity: ADSCs are characterized by low immunogenicity, typically lacking expression of major histocompatibility complex class II costimulatory molecules. As such they elicit minimal immune rejection when used in allogeneic infusions[117]. Recent clinical trials (e.g., in patients with coronavirus disease 2019) have shown that repeated intravenous administration of allogeneic ADSCs did not result in treatment-related serious adverse events, indicating that short-term multiple dosing is safe and well-tolerated[117]. Nevertheless, rare infusion-related reactions or coagulation abnormalities should still be monitored.
Tumorigenic potential: ADSCs expanded in vitro are generally non-tumorigenic in vivo. Previous animal studies involving high-dose ADSC administration (with follow-up periods of up to 6 months) have not observed tumor for
Batch variability and donor heterogeneity: ADSCs derived from different donors or anatomical sites may exhibit interbatch variability. To reduce such inconsistencies researchers recommend the implementation of strict good manufacturing practice (GMP)-grade production and quality control systems. Standardized protocols including uniform lipo
GMP-compliant manufacturing and oversight: All therapeutic ADSCs must be produced under GMP guidelines to ensure sterility, consistency in cell quantity and viability, and full traceability throughout the manufacturing process[119]. Research protocols must be reviewed and approved by independent ethics committees and regulatory agencies to ensure compliance with ethical and safety standards.
Long-term follow-up: Although short-term safety appears favorable, patients receiving ADSC therapy require long-term monitoring to detect delayed adverse events (e.g., tumor development, immune reactions, or other complications) and to assess the durability of therapeutic effects. In conclusion, clinical translation of ADSCs must balance innovation with safety. While advancing efficacy validation, equal emphasis must be placed on rigorous quality control, ethical review, and long-term surveillance to ensure both patient safety and treatment reliability[118,119].
The burden of T2DM is driven primarily by macrovascular and microvascular complications, which frequently outweigh glycemic indices in determining morbidity and mortality[120]. Our unified framework of adipose tissue remodeling-insulin resistance-stem cell regulation complications can be viewed as downstream manifestations of chronic adipose inflammation, endothelial dysfunction, and fibrotic remodeling across organs. Here, we synthesized how combined pharmacologic-ADSC strategies may modify shared pathobiology and improve outcomes in cardiovascular disease, diabetic kidney disease (DKD), and neuropathy (Table 7).
| Combination | Dominant mechanism (within unified axis) | Target complication | Key endpoints | Notes |
| GLP-1RA + ADSC/EV | Reducing adipose inflammation → improved endothelial function; proangiogenic repair | ASCVD | MACE, FMD, hs-CRP | Weight loss enables ADSC efficacy |
| SGLT-2i + ADSC/EV | Hemodynamic unloading; anti-fibrosis + tubular regeneration | HF/CKD | HF hospitalization, eGFR slope, UACR | Monitor volume status |
| Metformin + ADSC/EV | AMPK activation; mitochondrial support + neurotrophic cues | Neuropathy | NCS, QST, CCM metrics | Consider B12 monitoring |
| Low-dose PPARγ + ADSC/EV | Adipose remodeling; anti-inflammation + anti-fibrosis | Multiorgan | Composite renal/cardiac endpoints | Consider edema risk |
Adipose inflammation and maladaptive adipokine signaling in T2DM drive atherogenesis and myocardial remodeling by impairing endothelial nitric oxide, amplifying oxidative stress, and promoting fibrosis, fitting our unified axis of adipose tissue remodeling-insulin resistance-stem cell regulation. Visceral/perivascular adipose dysfunction links directly to endothelial injury and plaque progression, underscoring therapy beyond glycemic targets[121,122]. GLP-1RAs reduce major adverse cardiovascular events independent of glucose lowering and are recommended by current American Diabetes Association Standards for patients with atherosclerotic cardiovascular disease risk[123-125].
SGLT-2 inhibitors consistently lower HF hospitalization and, in some trials cardiovascular death with benefits observed across the HF spectrum and supported by natriuresis improved energetics and anti-inflammatory/anti-fibrotic actions[126,127]. Complementarily, ADSCs and their EVs attenuate vascular inflammation, promote endothelial repair and angiogenesis, and limit adverse cardiac remodeling via paracrine cargo (including sphingosine-1-phosphate/sphingosine kinase-1/sphingosine-1-phosphate receptor-1 signaling and proreparative macrophage polarization), providing a biologic partner to GLP-1RAs/SGLT-2 inhibitors to stabilize plaque/microvascular dysfunction and restrain NLRP3/TGF-β-driven fibrosis[128-130].
For complication-focused studies endpoints should pair event reduction with remodeling mediation, including major adverse cardiovascular event and HF hospitalization alongside endothelial function measured by flow-mediated dilation, structural/matrix indices, and inflammatory biomarkers, anchored to American Diabetes Association guidance and cardiovascular outcome trial conventions[123].
Beyond simple additive effects ADSCs and conventional pharmacological agents appear to converge on shared molecular hubs, creating the potential for mechanistic synergy. GLP-1RAs via cAMP/PKA-cAMP response element-binding protein-PGC-1α signaling) and metformin (via AMPK activation) stimulate the PGC-1α-PRDM16-UCP1 thermogenic program. When combined with ADSC-mediated or EV-mediated induction of adipocyte beiging and mitochondrial repair, these effects may intensify thermogenic remodeling and attenuate the accumulation of lipotoxic intermediates. SGLT-2 inhibitors improve hemodynamic status and dampen systemic inflammatory tone. In parallel, ADSC-EV-derived miRNAs (e.g., miR-223-3p, miR-146a) inhibit NLRP3/TAK1-TRAF6 signaling, potentially enhancing anti-inflammatory reprogramming and limiting fibrosis through modulation of the TGF-β/SMAD pathway. At the level of insulin signaling, suppression of SOCS3 expression and IRS-1(Ser-P) by the ADSC secretome could augment GLP-1RA-induced improvements in Akt activation and GLUT4 translocation, an interaction amenable to experimental verification through paired adipose tissue biopsies and EV cargo profiling.
In T2DM ectopic lipid deposition and adipose-derived inflammation drive glomerular hyperfiltration, tubulointerstitial injury, and fibrosis, which are mechanisms aligned with our unified axis and with guideline emphasis on complication-focused care beyond glycemia[131]. Robust renal outcome trials show that SGLT-2 inhibitors slow estimated glomerular filtration rate decline and reduce kidney failure and cardiorenal events across broad chronic kidney disease (CKD) populations. The American Diabetes Association and Kidney Disease: Improving Global Outcomes recommend SGLT-2 inhibitors on top of renin-angiotensin-aldosterone system blockade with GLP-1RAs added for residual albuminuria and cardiometabolic risk, and the FLOW trial demonstrated renoprotection with semaglutide in T2DM with CKD[131-134].
Preclinical and early clinical data indicate ADSCs and their EVs mitigate renal inflammation/fibrosis, promote tubular repair and microvascular integrity (e.g., via VEGF/HGF, TGF-β modulation, Nrf2/Kelch-like ECH-associated protein 1), and are feasible in progressive DKD. Combining SGLT-2 inhibitors (hemodynamic/anti-fibrotic) with ADSC/EV therapy (pro-regenerative/anti-inflammatory) targets complementary nodes of remodeling while underscoring GMP standardization and potency assays for EV products[135-137]. Complication-oriented trials should pair event reduction with mechanistic mediation: EGFR slope; ≥ 40%-50% decline, kidney failure, or cardiorenal composites; urinary albumin-to-creatinine ratio; and imaging/biomarkers of perfusion/fibrosis (magnetic resonance imaging indices, neutrophil gelatinase-associated lipocalin, kidney injury molecule-1), consistent with the American Diabetes Association/Kidney Disease: Improving Global Outcomes conventions[131-133].
In T2DM lipotoxicity, mitochondrial dysfunction, and microvascular ischemia converge to cause axonal loss and demyelination. Current guidance emphasizes early, complication-focused screening and management of peripheral and autonomic neuropathies[138]. Emerging clinical evidence suggests GLP-1RA can improve structural and electrophysiological markers of diabetic peripheral neuropathy (e.g., reversal of nerve morphological abnormalities with semaglutide/dulaglutide in a prospective cohort) while broader reviews outline anti-inflammatory and mitochondrial mechanisms[139,140]. Metformin may confer ancillary neuroprotective effects but warrants vitamin B12 monitoring during long-term use per updated recommendations[141,142]. For SGLT-2 inhibitors small studies and reviews indicate potential benefit on autonomic indices (e.g., heart rate variability, skin sympathetic nerve activity), aligning with their favorable cardiorenal physiology although confirmatory trials are still needed[143].
Regenerative approaches add complementary biology. ADSCs/MSC-derived EVs dampen neuroinflammation, deliver neurotrophic cargo (e.g., nerve growth factor/brain-derived neurotrophic factor), and enhance endoneurial perfusion in preclinical models with early human evidence supporting feasibility and a shift toward standardized, cell-free EV products[144,145]. Complication-oriented studies should pair symptoms/events with mechanistic mediation using nerve conduction studies, quantitative sensory testing, corneal confocal microscopy for small-fiber integrity, and autonomic testing (e.g., sudomotor measures, heart rate variability/Ewing battery), aligning endpoints with guideline conventions[138,146].
A clinically pragmatic strategy would be to initiate treatment with pharmacological agents, such as GLP-1RAs, SGLT-2 inhibitors, and where appropriate metformin for an initial period of approximately 8-12 weeks[147,148]. This phase aims to reduce edema and systemic inflammation while improving endothelial function[149]. Once tissue hypoxia and fibrosis have sufficiently resolved, administration of ADSCs or their EVs can be introduced to promote tissue repair. Long-term maintenance would then be achieved through continued use of evidence-based, guideline-recommended therapies[65,150,151]. This sequential approach is designed to optimize both cell engraftment and paracrine activity while aligning with our proposed triad framework in which immune quiescence enables metabolic remodeling that supports sustained tissue regeneration.
Risk-stratified deployment should start from the complication profile. People with established or high-risk atherosclerotic cardiovascular disease favor GLP-1RAs with proven cardiovascular benefit. Those with HF or CKD should prioritize SGLT-2 inhibitors (on top of renin-angiotensin-aldosterone system blockade) while individuals with obesity-predominant insulin resistance benefit from weight-centric incretin therapy ideally integrated with ADSC/EV-based regeneration within our unified axis. These priorities align with the American Diabetes Association Standards of Care 2025 for cardiovascular risk and pharmacotherapy[123,152] and with the Kidney Disease: Improving Global Outcomes 2024 recommendations for CKD[131]. For HF the American Heart Association/American College of Cardiology/Heart Failure Society of America 2022 guidelines embed SGLT-2 inhibitors in guideline-directed medical therapy[153].
Safety monitoring should include volume status and periprocedural holding of SGLT-2 inhibitors (generally 3 days or 4 days for ertugliflozin) to mitigate euglycemic diabetic ketoacidosis risk[154]. For GLP-1RAs patients should be cou
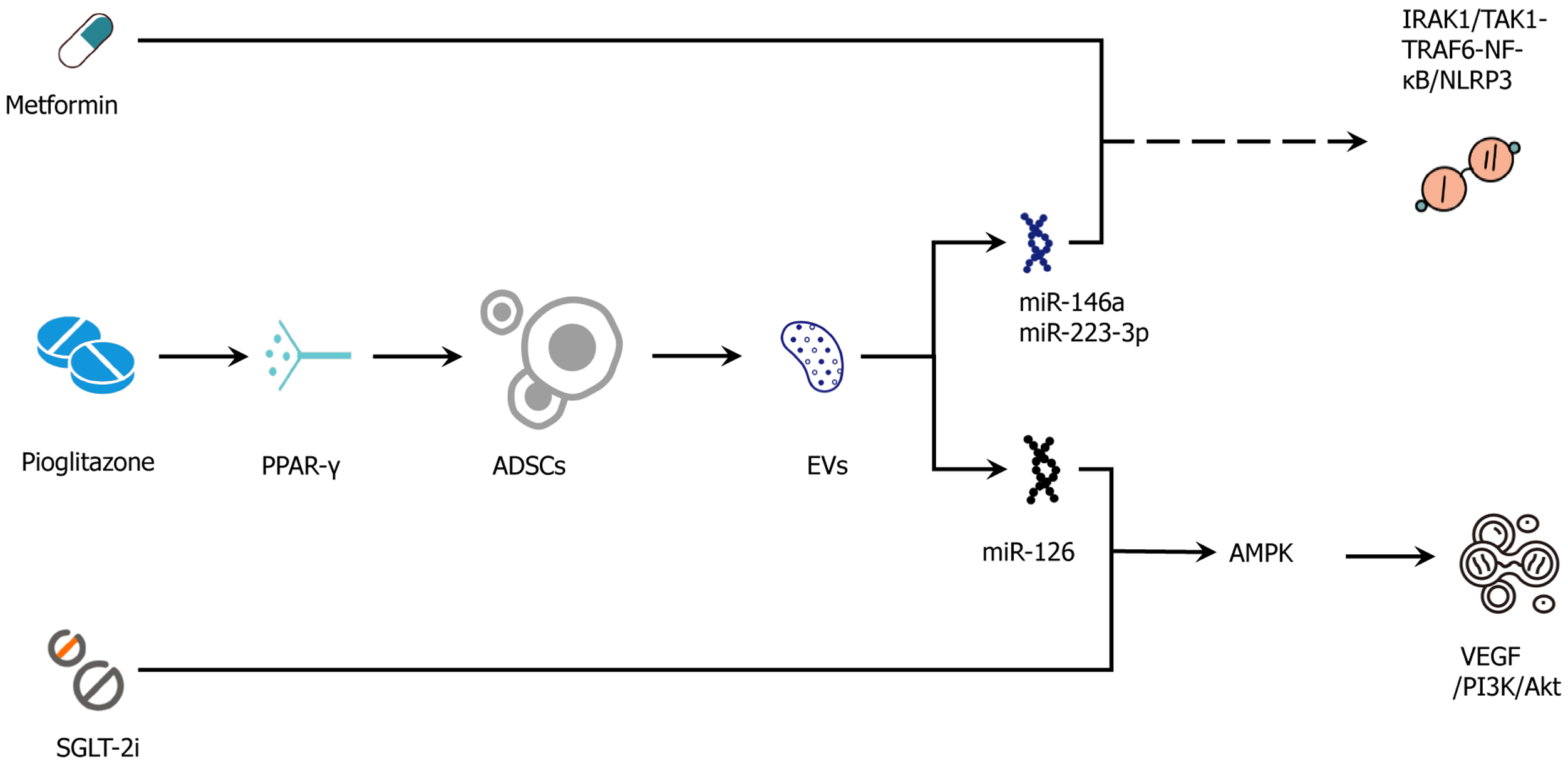
Beyond merely additive effects the ADSC secretome and its EVs intersect with the pharmacological mechanisms of conventional agents at several key nodes. Metformin through activation of AMPK acts synergistically with ADSC-EV-delivered miR-126, which promotes VEGF/PI3K-Akt/endothelial nitric oxide synthase signaling, thereby improving vascular endothelial function[161-164]. GLP-1RAs activate the cAMP/PKA-cAMP response element-binding protein pathway, aligning with ADSC-mediated AMPK/sirtuin 1-PGC-1α-PRDM16/UCP1 signaling to enhance thermogenesis and repair mitochondrial damage[165,166]. SGLT-2 inhibitors combine the reduction of circulating volume and cardiac preload/afterload with the anti-inflammatory effects of ADSC-EV-derived miR-146a and miR-223-3p, which inhibit IL-1 receptor-associated kinase 1/TAK1-TRAF6-NF-κB/NLRP3 signaling, thereby further reinforcing anti-fibrotic responses[167-169].
Pioglitazone via PPARγ activation increases ADSC survival, migration, and VEGF secretion, creating favorable microenvironmental conditions prior to cell or EV administration. At the insulin-signaling level ADSC-mediated do
The co-occurrence of obesity and T2DM is driven by underlying mechanisms including VAT accumulation, insulin resistance, lipotoxicity, chronic low-grade inflammation, and mitochondrial dysfunction. Existing pharmacological agents such as pioglitazone and metformin have demonstrated significant efficacy in improving fat distribution and metabolic profiles, particularly through mechanisms involving fat redistribution, adiponectin secretion, AMPK activation, and anti-inflammatory modulation. The emergence of novel therapies such as GLP-1RA and SGLT-2 inhibitors has further expanded therapeutic targets, offering promising avenues for multifaceted adipose tissue remodeling and systemic metabolic improvement. Simultaneously, ADSCs by virtue of their differentiation capacity, paracrine activity, and imm
Looking ahead, successful clinical translation will rely on individualized strategies informed by multiomics profiling, standardized ADSC manufacturing and quality-control protocols, and comprehensive long-term safety evaluation. To ensure conceptual coherence, we adopted a single precision-based, combined framework centered on adipose tissue remodeling-insulin resistance-stem cell regulation. Within this schema inflammation resolution is treated as a facet of adipose tissue remodeling, and tissue regeneration is encompassed by stem cell regulation. Grounded in this unified axis, targeted combinations hold the potential to deliver transformative prevention and management of obesity-associated T2DM.
| 1. | International Diabetes Federation. IDF Diabetes Atlas. 11th edition. [cited 15 March 2025]. Available from: https://diabetesatlas.org/media/uploads/sites/3/2025/04/IDF_Atlas_11th_Edition_2025-1.pdf. |
| 2. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2437] [Cited by in RCA: 2659] [Article Influence: 886.3] [Reference Citation Analysis (18)] |
| 3. | Dhokte S, Czaja K. Visceral Adipose Tissue: The Hidden Culprit for Type 2 Diabetes. Nutrients. 2024;16:1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 4. | Lee MJ, Kim J. The pathophysiology of visceral adipose tissues in cardiometabolic diseases. Biochem Pharmacol. 2024;222:116116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Moody AJ, Molina-Wilkins M, Clarke GD, Merovci A, Solis-Herrera C, Cersosimo E, Chilton RJ, Iozzo P, Gastaldelli A, Abdul-Ghani M, DeFronzo RA. Pioglitazone reduces epicardial fat and improves diastolic function in patients with type 2 diabetes. Diabetes Obes Metab. 2023;25:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 6. | Ziqubu K, Mazibuko-Mbeje SE, Mthembu SXH, Mabhida SE, Jack BU, Nyambuya TM, Nkambule BB, Basson AK, Tiano L, Dludla PV. Anti-Obesity Effects of Metformin: A Scoping Review Evaluating the Feasibility of Brown Adipose Tissue as a Therapeutic Target. Int J Mol Sci. 2023;24:2227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Mesbah Mohamed M, Ahmed Rashed L, Ahmed El-Boghdady N, Mohamed Said M. Bone Marrow-Derived Mesenchymal Stem Cells and Pioglitazone or Exendin-4 Synergistically Improve Insulin Resistance via Multiple Modulatory Mechanisms in High-Fat Diet/Streptozotocin-Induced Diabetes in Rats. Rep Biochem Mol Biol. 2023;12:42-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Chinnapaka S, Yang KS, Flowers Q, Faisal M, Nerone WV, Rubin JP, Ejaz A. Metformin Improves Stemness of Human Adipose-Derived Stem Cells by Downmodulation of Mechanistic Target of Rapamycin (mTOR) and Extracellular Signal-Regulated Kinase (ERK) Signaling. Biomedicines. 2021;9:1782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 9. | Kahn D, Macias E, Zarini S, Garfield A, Zemski Berry K, MacLean P, Gerszten RE, Libby A, Solt C, Schoen J, Bergman BC. Exploring Visceral and Subcutaneous Adipose Tissue Secretomes in Human Obesity: Implications for Metabolic Disease. Endocrinology. 2022;163:bqac140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (23)] |
| 10. | Dobre MZ, Virgolici B, Timnea O. Key Roles of Brown, Subcutaneous, and Visceral Adipose Tissues in Obesity and Insulin Resistance. Curr Issues Mol Biol. 2025;47:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Hildebrandt X, Ibrahim M, Peltzer N. Cell death and inflammation during obesity: "Know my methods, WAT(son)". Cell Death Differ. 2023;30:279-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 12. | Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, Gojobori T, Isenovic ER. Leptin and Obesity: Role and Clinical Implication. Front Endocrinol (Lausanne). 2021;12:585887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 662] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 13. | Elkanawati RY, Sumiwi SA, Levita J. Impact of Lipids on Insulin Resistance: Insights from Human and Animal Studies. Drug Des Devel Ther. 2024;18:3337-3360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 14. | Castelli V, Kacem H, Brandolini L, Giorgio C, Scenna MS, Allegretti M, Cimini A, d'Angelo M. TNFα-CXCR1/2 partners in crime in insulin resistance conditions. Cell Death Discov. 2024;10:486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Park JE, Han JS. Improving the Effect of Ferulic Acid on Inflammation and Insulin Resistance by Regulating the JNK/ERK and NF-κB Pathways in TNF-α-Treated 3T3-L1 Adipocytes. Nutrients. 2024;16:294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Engin AB. What Is Lipotoxicity? Adv Exp Med Biol. 2017;960:197-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 17. | Lipke K, Kubis-Kubiak A, Piwowar A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States-Current View of Knowledge. Cells. 2022;11:844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 18. | Khoi CS, Lin TY, Chiang CK. Targeting Insulin Resistance, Reactive Oxygen Species, Inflammation, Programmed Cell Death, ER Stress, and Mitochondrial Dysfunction for the Therapeutic Prevention of Free Fatty Acid-Induced Vascular Endothelial Lipotoxicity. Antioxidants (Basel). 2024;13:1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Chen B, Li T, Wu Y, Song L, Wang Y, Bian Y, Qiu Y, Yang Z. Lipotoxicity: A New Perspective in Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2025;18:1223-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Kasai S, Kokubu D, Mizukami H, Itoh K. Mitochondrial Reactive Oxygen Species, Insulin Resistance, and Nrf2-Mediated Oxidative Stress Response-Toward an Actionable Strategy for Anti-Aging. Biomolecules. 2023;13:1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Sharifi S, Yamamoto T, Zeug A, Elsner M, Avezov E, Mehmeti I. Non-esterified fatty acid palmitate facilitates oxidative endoplasmic reticulum stress and apoptosis of β-cells by upregulating ERO-1α expression. Redox Biol. 2024;73:103170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 22. | Chen CW, Guan BJ, Alzahrani MR, Gao Z, Gao L, Bracey S, Wu J, Mbow CA, Jobava R, Haataja L, Zalavadia AH, Schaffer AE, Lee H, LaFramboise T, Bederman I, Arvan P, Mathews CE, Gerling IC, Kaestner KH, Tirosh B, Engin F, Hatzoglou M. Adaptation to chronic ER stress enforces pancreatic β-cell plasticity. Nat Commun. 2022;13:4621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 23. | Teixeira C, Sousa AP, Santos I, Rocha AC, Alencastre I, Pereira AC, Martins-Mendes D, Barata P, Baylina P, Fernandes R. Enhanced 3T3-L1 Differentiation into Adipocytes by Pioglitazone Pharmacological Activation of Peroxisome Proliferator Activated Receptor-Gamma (PPAR-γ). Biology (Basel). 2022;11:806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Arbas R, Dayrit SA, Dimalanta A, Flores JA, Mañalac AR, Soriano DR, Vallo J, Tiongco RE, Pineda-Cortel MR. A meta-analysis of randomized clinical trials on the effect of metformin vs. pioglitazone monotherapy on plasma adiponectin levels among patients with diabetes mellitus. Egypt J Intern Med. 2024;36:5. [DOI] [Full Text] |
| 25. | Alser M, Elrayess MA. From an Apple to a Pear: Moving Fat around for Reversing Insulin Resistance. Int J Environ Res Public Health. 2022;19:14251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | White U, Fitch MD, Beyl RA, Hellerstein MK, Ravussin E. Adipose depot-specific effects of 16 weeks of pioglitazone on in vivo adipogenesis in women with obesity: a randomised controlled trial. Diabetologia. 2021;64:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Palavicini JP, Chavez-Velazquez A, Fourcaudot M, Tripathy D, Pan M, Norton L, DeFronzo RA, Shannon CE. The Insulin-Sensitizer Pioglitazone Remodels Adipose Tissue Phospholipids in Humans. Front Physiol. 2021;12:784391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Park JY, Lee J, Choi YH, Min KW, Han KA, Ahn KJ, Lim S, Kim YH, Ahn CW, Choi KM, Yoon KH. Efficacy and Safety of Alogliptin-Pioglitazone Combination for Type 2 Diabetes Mellitus Poorly Controlled with Metformin: A Multicenter, Double-Blind Randomized Trial. Diabetes Metab J. 2024;48:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Zhao Y, Yue R. White adipose tissue in type 2 diabetes and the effect of antidiabetic drugs. Diabetol Metab Syndr. 2025;17:116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Nascimento MT, Cordeiro RSO, Abreu C, Santos CP, Peixoto F, Duarte GA, Cardoso T, de Oliveira CI, Carvalho EM, Carvalho LP. Pioglitazone, a Peroxisome Proliferator-Activated Receptor-γ Agonist, Downregulates the Inflammatory Response in Cutaneous Leishmaniasis Patients Without Interfering in Leishmania braziliensis Killing by Monocytes. Front Cell Infect Microbiol. 2022;12:884237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Haber R, Zarzour F, Ghezzawi M, Saadeh N, Bacha DS, Al Jebbawi L, Chakhtoura M, Mantzoros CS. The impact of metformin on weight and metabolic parameters in patients with obesity: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2024;26:1850-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Mohammed I, Hollenberg MD, Ding H, Triggle CR. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front Endocrinol (Lausanne). 2021;12:718942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 33. | Van Nostrand JL, Hellberg K, Luo EC, Van Nostrand EL, Dayn A, Yu J, Shokhirev MN, Dayn Y, Yeo GW, Shaw RJ. AMPK regulation of Raptor and TSC2 mediate metformin effects on transcriptional control of anabolism and inflammation. Genes Dev. 2020;34:1330-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Kononova YA, Tuchina TP, Babenko AY. Brown and Beige Adipose Tissue: One or Different Targets for Treatment of Obesity and Obesity-Related Metabolic Disorders? Int J Mol Sci. 2024;25:13295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 35. | Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J, Guo X, Guo T, Botchlett R, Qi T, Pei Y, Zheng J, Xu Y, An X, Chen L, Chen L, Li Q, Xiao X, Huo Y, Wu C. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One. 2014;9:e91111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 36. | Pinyopornpanish K, Leerapun A, Pinyopornpanish K, Chattipakorn N. Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels. Gut Liver. 2021;15:827-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | Abad-Jiménez Z, López-Domènech S, Díaz-Rúa R, Iannantuoni F, Gómez-Abril SÁ, Periañez-Gómez D, Morillas C, Víctor VM, Rocha M. Systemic Oxidative Stress and Visceral Adipose Tissue Mediators of NLRP3 Inflammasome and Autophagy Are Reduced in Obese Type 2 Diabetic Patients Treated with Metformin. Antioxidants (Basel). 2020;9:892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Yu F, Xing C, Fan Y, Liu Y, Su P, Yang Q, Dong Y, Hou Y, Pan S. Aerobic exercise and metformin on intermuscular adipose tissue (IMAT): insights from multimodal MRI and histological changes in prediabetic rats. Diabetol Metab Syndr. 2023;15:221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 39. | Johansson L, Hockings PD, Johnsson E, Dronamraju N, Maaske J, Garcia-Sanchez R, Wilding JPH. Dapagliflozin plus saxagliptin add-on to metformin reduces liver fat and adipose tissue volume in patients with type 2 diabetes. Diabetes Obes Metab. 2020;22:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Hooshmand Gharabagh L, Shargh A, Mohammad Hosseini Azar MR, Esmaeili A. Comparison between the effect of Empagliflozin and Pioglitazone added to metformin in patients with type 2 diabetes and nonalcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. 2024;48:102279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Cuatrecasas G, De Cabo F, Coves MJ, Patrascioiu I, Aguilar G, Cuatrecasas G, March S, Calbo M, Rossell O, Balfegó M, Benito C, Di Gregorio S, Garcia Lorda P, Muñoz E. Dapagliflozin added to metformin reduces perirenal fat layer in type 2 diabetic patients with obesity. Sci Rep. 2024;14:10832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 42. | Heo JH, Han KA, Hong JH, Seo HA, Hong EG, Yu JM, Jung HS, Cha BS. Pioglitazone as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial. Diabetes Metab J. 2024;48:937-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 43. | Guo LX, Wang LW, Tian DZ, Xu FM, Huang W, Wu XH, Zhu W, Chen JQ, Zheng X, Zhou HY, Li HM, He ZC, Wang WB, Ma LZ, Duan JT. Efficacy and Safety of Pioglitazone/Metformin Fixed-Dose Combination Versus Uptitrated Metformin in Patients with Type 2 Diabetes without Adequate Glycemic Control: A Randomized Clinical Trial. Diabetes Ther. 2024;15:2351-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 44. | Liu J, Wang D, Xie Z, Ding L, Li S, Ma X, Liu J, Ren J, Xiao C, Yang C, Xiao X. Combination of Pioglitazone and Metformin Actions on Liver Lipid Metabolism in Obese Mice. Biomolecules. 2023;13:1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 45. | van Eyk HJ, Paiman EHM, Bizino MB, de Heer P, Geelhoed-Duijvestijn PH, Kharagjitsingh AV, Smit JWA, Lamb HJ, Rensen PCN, Jazet IM. A double-blind, placebo-controlled, randomised trial to assess the effect of liraglutide on ectopic fat accumulation in South Asian type 2 diabetes patients. Cardiovasc Diabetol. 2019;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 46. | Volpe S, Lisco G, Fanelli M, Racaniello D, Colaianni V, Triggiani D, Donghia R, Crudele L, Rinaldi R, Sabbà C, Triggiani V, De Pergola G, Piazzolla G. Once-Weekly Subcutaneous Semaglutide Improves Fatty Liver Disease in Patients with Type 2 Diabetes: A 52-Week Prospective Real-Life Study. Nutrients. 2022;14:4673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 47. | American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S167-S180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 48. | Yan P, Li L, Yang M, Liu D, Liu H, Boden G, Yang G. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on plasma omentin-1 levels in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;92:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Haas B, Eckstein N, Pfeifer V, Mayer P, Hass MD. Efficacy, safety and regulatory status of SGLT2 inhibitors: focus on canagliflozin. Nutr Diabetes. 2014;4:e143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Hao Z, Sun Y, Li G, Shen Y, Wen Y, Liu Y. Effects of canagliflozin and metformin on insulin resistance and visceral adipose tissue in people with newly-diagnosed type 2 diabetes. BMC Endocr Disord. 2022;22:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Okamoto A, Yokokawa H, Sanada H, Naito T. Changes in Levels of Biomarkers Associated with Adipocyte Function and Insulin and Glucagon Kinetics During Treatment with Dapagliflozin Among Obese Type 2 Diabetes Mellitus Patients. Drugs R D. 2016;16:255-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Kim JY, Kim NH. Initial Combination Therapy in Type 2 Diabetes. Endocrinol Metab (Seoul). 2024;39:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 53. | Gourdy P, Darmon P, Dievart F, Halimi JM, Guerci B. Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM). Cardiovasc Diabetol. 2023;22:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 54. | Lavynenko O, Abdul-Ghani M, Alatrach M, Puckett C, Adams J, Abdelgani S, Alkhouri N, Triplitt C, Clarke GD, Vasquez JA, Li J, Cersosimo E, Gastaldelli A, DeFronzo RA. Combination therapy with pioglitazone/exenatide/metformin reduces the prevalence of hepatic fibrosis and steatosis: The efficacy and durability of initial combination therapy for type 2 diabetes (EDICT). Diabetes Obes Metab. 2022;24:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 55. | Lo SC, Kornelius E, Liao PL, Huang JY, Yang YS, Huang CN. Pioglitazone, SGLT2 inhibitors and their combination for primary prevention of cardiovascular disease and heart failure in type 2 diabetes: Real-world evidence from a nationwide cohort database. Diabetes Res Clin Pract. 2023;200:110685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 56. | Anson M, Henney AE, Zhao SS, Ibarburu GH, Lip GYH, Cuthbertson DJ, Nabrdalik K, Alam U. Effect of combination pioglitazone with sodium-glucose cotransporter-2 inhibitors or glucagon-like peptide-1 receptor agonists on outcomes in type 2 diabetes: A systematic review, meta-analysis, and real-world study from an international federated database. Diabetes Obes Metab. 2024;26:2606-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Tao X, Wang J, Yan Y, Cheng P, Liu B, Du H, Niu B. Optimal Sca-1-based procedure for purifying mouse adipose-derived mesenchymal stem cells with enhanced proliferative and differentiation potential. Front Cell Dev Biol. 2025;13:1566670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 58. | Dong L, Li X, Leng W, Guo Z, Cai T, Ji X, Xu C, Zhu Z, Lin J. Adipose stem cells in tissue regeneration and repair: From bench to bedside. Regen Ther. 2023;24:547-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 59. | Suchanecka M, Grzelak J, Farzaneh M, Azizidoost S, Dari MAG, Józkowiak M, Data K, Domagała D, Niebora J, Kotrych K, Czerny B, Kamiński A, Torlińska-Walkowiak N, Bieniek A, Szepietowski J, Piotrowska-Kempisty H, Dzięgiel P, Mozdziak P, Kempisty B. Adipose derived stem cells - Sources, differentiation capacity and a new target for reconstructive and regenerative medicine. Biomed Pharmacother. 2025;186:118036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 60. | Yang YM, Dong XH, Ma WC, Guan LH, Wang YH, Huang XH, Chen JF, Zhao X. Proliferation, Differentiation and Immunoregulatory Capacities of Brown and White Adipose-Derived Stem Cells from Young and Aged Mice. Int J Stem Cells. 2020;13:246-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Kang S, Narazaki M, Metwally H, Kishimoto T. Historical overview of the interleukin-6 family cytokine. J Exp Med. 2020;217:e20190347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 62. | Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217:e20190418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 743] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 63. | Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol. 2016;8:a021873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 1035] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 64. | Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1864] [Cited by in RCA: 1985] [Article Influence: 283.6] [Reference Citation Analysis (0)] |
| 65. | Jia Q, Zhao H, Wang Y, Cen Y, Zhang Z. Mechanisms and applications of adipose-derived stem cell-extracellular vesicles in the inflammation of wound healing. Front Immunol. 2023;14:1214757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 66. | Kim B, Ronaldo R, Kweon BN, Yoon S, Park Y, Baek JH, Lee JM, Hyun CK. Mesenchymal Stem Cell-Derived Exosomes Attenuate Hepatic Steatosis and Insulin Resistance in Diet-Induced Obese Mice by Activating the FGF21-Adiponectin Axis. Int J Mol Sci. 2024;25:10447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 67. | Liu J, Qiu P, Qin J, Wu X, Wang X, Yang X, Li B, Zhang W, Ye K, Peng Z, Lu X. Allogeneic adipose-derived stem cells promote ischemic muscle repair by inducing M2 macrophage polarization via the HIF-1α/IL-10 pathway. Stem Cells. 2020;38:1307-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 68. | Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes. 2018;67:235-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 519] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 69. | Jiao Z, Ma Y, Liu X, Ge Y, Zhang Q, Liu B, Wang H. Adipose-Derived Stem Cell Transplantation Attenuates Inflammation and Promotes Liver Regeneration after Ischemia-Reperfusion and Hemihepatectomy in Swine. Stem Cells Int. 2019;2019:2489584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Niu Q, Wang T, Wang Z, Wang F, Huang D, Sun H, Liu H. Adipose-derived mesenchymal stem cell-secreted extracellular vesicles alleviate non-alcoholic fatty liver disease via delivering miR-223-3p. Adipocyte. 2022;11:572-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 71. | Wang B, Pi Q, Mohsin A, Gao WQ, Guo M, Xu H. Exosomal miR-1246 of adipose stem cells attenuates obesity by polarizing M2 macrophages, reducing fat mass, and beiging of white adipose tissue. J Adv Res. 2025;S2090-1232(25)00369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 72. | Lu X, Wang Y, Piao C, Li P, Cao L, Liu T, Ma Y, Wang H. Exosomes Derived from Adipose Mesenhymal Stem Cells Ameliorate Lipid Metabolism Disturbances Following Liver Ischemia-Reperfusion Injury in Miniature Swine. Int J Mol Sci. 2024;25:13069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Imamura H, Tomimaru Y, Kobayashi S, Harada A, Kita S, Sasaki K, Iwagami Y, Yamada D, Noda T, Takahashi H, Hokkoku D, Kado T, Toya K, Kodama T, Saito S, Shimomura I, Miyagawa S, Doki Y, Eguchi H. Adipose-derived stem cells using fibrin gel as a scaffold enhances post-hepatectomy liver regeneration. Sci Rep. 2025;15:6334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Zeinhom A, Fadallah SA, Mahmoud M. Human mesenchymal stem/stromal cell based-therapy in diabetes mellitus: experimental and clinical perspectives. Stem Cell Res Ther. 2024;15:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Jaber H, Issa K, Eid A, Saleh FA. The therapeutic effects of adipose-derived mesenchymal stem cells on obesity and its associated diseases in diet-induced obese mice. Sci Rep. 2021;11:6291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 76. | Mikłosz A, Chabowski A. Adipose-derived Mesenchymal Stem Cells Therapy as a new Treatment Option for Diabetes Mellitus. J Clin Endocrinol Metab. 2023;108:1889-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 77. | Shafiei G, Saheli M, Ganjalikhan-Hakemi S, Haghpanah T, Nematollahi-Mahani SN. Administration of adipose-derived mesenchymal stem cell conditioned medium improves ovarian function in polycystic ovary syndrome rats: involvement of epigenetic modifiers system. J Ovarian Res. 2023;16:238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 78. | Horino M, Ikeda K, Yamada T. The Role of Thermogenic Fat Tissue in Energy Consumption. Curr Issues Mol Biol. 2022;44:3166-3179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 79. | Silva FJ, Holt DJ, Vargas V, Yockman J, Boudina S, Atkinson D, Grainger DW, Revelo MP, Sherman W, Bull DA, Patel AN. Metabolically active human brown adipose tissue derived stem cells. Stem Cells. 2014;32:572-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Singh AM, Zhang L, Avery J, Yin A, Du Y, Wang H, Li Z, Fu H, Yin H, Dalton S. Human beige adipocytes for drug discovery and cell therapy in metabolic diseases. Nat Commun. 2020;11:2758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 81. | Cruciani S, Garroni G, Pala R, Coradduzza D, Cossu ML, Ginesu GC, Capobianco G, Dessole S, Ventura C, Maioli M. Metformin and vitamin D modulate adipose-derived stem cell differentiation towards the beige phenotype. Adipocyte. 2022;11:356-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Zhou Z, Tao Y, Zhao H, Wang Q. Adipose Extracellular Vesicles: Messengers From and to Macrophages in Regulating Immunometabolic Homeostasis or Disorders. Front Immunol. 2021;12:666344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Heo JS, Choi Y, Kim HO. Adipose-Derived Mesenchymal Stem Cells Promote M2 Macrophage Phenotype through Exosomes. Stem Cells Int. 2019;2019:7921760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 84. | Michurina S, Stafeev I, Podkuychenko N, Sklyanik I, Shestakova E, Yah'yaev K, Yurasov A, Ratner E, Menshikov M, Parfyonova Y, Shestakova M. Decreased UCP-1 expression in beige adipocytes from adipose-derived stem cells of type 2 diabetes patients associates with mitochondrial ROS accumulation during obesity. Diabetes Res Clin Pract. 2020;169:108410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Ong WK, Chakraborty S, Sugii S. Adipose Tissue: Understanding the Heterogeneity of Stem Cells for Regenerative Medicine. Biomolecules. 2021;11:918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 86. | Oestreich AK, Collins KH, Harasymowicz NS, Wu CL, Guilak F. Is Obesity a Disease of Stem Cells? Cell Stem Cell. 2020;27:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 87. | Pham DV, Nguyen TK, Park PH. Adipokines at the crossroads of obesity and mesenchymal stem cell therapy. Exp Mol Med. 2023;55:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 88. | Yin D, Shen G. Exosomes from adipose-derived stem cells regulate macrophage polarization and accelerate diabetic wound healing via the circ-Rps5/miR-124-3p axis. Immun Inflamm Dis. 2024;12:e1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 89. | Dong Z, Fu Y, Cai Z, Dai H, He Y. Recent advances in adipose-derived mesenchymal stem cell-derived exosomes for regulating macrophage polarization. Front Immunol. 2025;16:1525466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 90. | Yao JM, Ying HZ, Zhang HH, Qiu FS, Wu JQ, Yu CH. Exosomal RBP4 potentiated hepatic lipid accumulation and inflammation in high-fat-diet-fed mice by promoting M1 polarization of Kupffer cells. Free Radic Biol Med. 2023;195:58-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 91. | Zhang Y, Mei H, Chang X, Chen F, Zhu Y, Han X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J Mol Cell Biol. 2016;8:505-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 92. | Patra D, Ramprasad P, Sharma S, Dey U, Kumar V, Singh S, Dasgupta S, Kumar A, Tikoo K, Pal D. Adipose tissue macrophage-derived microRNA-210-3p disrupts systemic insulin sensitivity by silencing GLUT4 in obesity. J Biol Chem. 2024;300:107328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 93. | Tanti JF, Jager J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol. 2009;9:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 94. | Barra NG, Henriksbo BD, Anhê FF, Schertzer JD. The NLRP3 inflammasome regulates adipose tissue metabolism. Biochem J. 2020;477:1089-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 95. | Lee SH, Park SY, Choi CS. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab J. 2022;46:15-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 643] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 96. | Malaguarnera M, Cauli O, Cabrera-Pastor A. Obesity and Adipose-Derived Extracellular Vesicles: Implications for Metabolic Regulation and Disease. Biomolecules. 2025;15:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 97. | Zhou B, Chen Q, Zhang Q, Tian W, Chen T, Liu Z. Therapeutic potential of adipose-derived stem cell extracellular vesicles: from inflammation regulation to tissue repair. Stem Cell Res Ther. 2024;15:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 98. | Xia W, Veeragandham P, Cao Y, Xu Y, Rhyne TE, Qian J, Hung CW, Zhao P, Jones Y, Gao H, Liddle C, Yu RT, Downes M, Evans RM, Rydén M, Wabitsch M, Wang Z, Hakozaki H, Schöneberg J, Reilly SM, Huang J, Saltiel AR. Obesity causes mitochondrial fragmentation and dysfunction in white adipocytes due to RalA activation. Nat Metab. 2024;6:273-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 99. | Woo CY, Jang JE, Lee SE, Koh EH, Lee KU. Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation. Diabetes Metab J. 2019;43:247-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 100. | Zhang Q, Piao C, Ma H, Xu J, Wang Y, Liu T, Liu G, Wang H. Exosomes from adipose-derived mesenchymal stem cells alleviate liver ischaemia reperfusion injury subsequent to hepatectomy in rats by regulating mitochondrial dynamics and biogenesis. J Cell Mol Med. 2021;25:10152-10163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 101. | Yan W, Diao S, Fan Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res Ther. 2021;12:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 102. | Qian L, Zhu Y, Deng C, Liang Z, Chen J, Chen Y, Wang X, Liu Y, Tian Y, Yang Y. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases. Signal Transduct Target Ther. 2024;9:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 178] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 103. | Shi Z, Wang S, Deng J, Gong Z. PGC-1α attenuates the oxidative stress-induced impaired osteogenesis and angiogenesis regulation effects of mesenchymal stem cells in the presence of diabetic serum. Biochem Biophys Rep. 2021;27:101070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Petersen MC, Shulman GI. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol Sci. 2017;38:649-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 295] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 105. | Xu W, Zhang D, Ma Y, Gaspar RC, Kahn M, Nasiri A, Murray S, Samuel VT, Shulman GI. Ceramide synthesis inhibitors prevent lipid-induced insulin resistance through the DAG-PKCε-insulin receptor(T1150) phosphorylation pathway. Cell Rep. 2024;43:114746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 106. | Ikeda K, Yamada T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front Endocrinol (Lausanne). 2020;11:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 107. | Xia T, Zhang M, Lei W, Yang R, Fu S, Fan Z, Yang Y, Zhang T. Advances in the role of STAT3 in macrophage polarization. Front Immunol. 2023;14:1160719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 196] [Reference Citation Analysis (0)] |
| 108. | Mitchell R, Mellows B, Sheard J, Antonioli M, Kretz O, Chambers D, Zeuner MT, Tomkins JE, Denecke B, Musante L, Joch B, Debacq-Chainiaux F, Holthofer H, Ray S, Huber TB, Dengjel J, De Coppi P, Widera D, Patel K. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res Ther. 2019;10:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 109. | Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 504] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 110. | Habib HA, Heeba GH, Khalifa MMA. Effect of combined therapy of mesenchymal stem cells with GLP-1 receptor agonist, exenatide, on early-onset nephropathy induced in diabetic rats. Eur J Pharmacol. 2021;892:173721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 111. | Yang CC, Chen YL, Sung PH, Chiang JY, Chen CH, Li YC, Yip HK. Repeated administration of adipose-derived mesenchymal stem cells added on beneficial effects of empagliflozin on protecting renal function in diabetic kidney disease rat. Biomed J. 2024;47:100613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 112. | Malekzadeh H, Surucu Y, Chinnapaka S, Yang KS, Arellano JA, Samadi Y, Epperly MW, Greenberger JS, Rubin JP, Ejaz A. Metformin and adipose-derived stem cell combination therapy alleviates radiation-induced skin fibrosis in mice. Stem Cell Res Ther. 2024;15:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 113. | Bjerre FA, Nielsen JV, Burton M, Dhumale P, Jørgensen MG, Hansen ST, Lund L, Thomassen M, Sørensen JA, Andersen DC, Jensen CH. Single-cell transcriptomics of clinical grade adipose-derived regenerative cells reveals consistency between donors independent of gender and BMI. Stem Cell Res Ther. 2025;16:109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 114. | Riis S, Stensballe A, Emmersen J, Pennisi CP, Birkelund S, Zachar V, Fink T. Mass spectrometry analysis of adipose-derived stem cells reveals a significant effect of hypoxia on pathways regulating extracellular matrix. Stem Cell Res Ther. 2016;7:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 115. | Bian X, Conley SM, Eirin A, Zimmerman Zuckerman EA, Smith AL, Gowan CC, Snow ZK, Jarmi T, Farres H, Erben YM, Hakaim AG, Dietz MA, Zubair AC, Wyles SP, Wolfram JV, Lerman LO, Hickson LJ. Diabetic kidney disease induces transcriptome alterations associated with angiogenesis activity in human mesenchymal stromal cells. Stem Cell Res Ther. 2023;14:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 116. | Hu W, Jiang C, Guan D, Dierickx P, Zhang R, Moscati A, Nadkarni GN, Steger DJ, Loos RJF, Hu C, Jia W, Soccio RE, Lazar MA. Patient Adipose Stem Cell-Derived Adipocytes Reveal Genetic Variation that Predicts Antidiabetic Drug Response. Cell Stem Cell. 2019;24:299-308.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 117. | de Dios C, Vij R, Kim H, Park H, Chang D. Safety of multiple intravenous infusions of adipose-derived mesenchymal stem cells for hospitalized cases of COVID-19: a randomized controlled trial. Front Med (Lausanne). 2023;10:1321303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 118. | Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 119. | Cremona M, Rusconi G, Ferrario A, Mariotta L, Gola M, Soldati G. Processing Adipose Tissue Samples in a GMP Environment Standardizes the Use of SVF in Cell Therapy Treatments: Data on 302 Patients. Biomedicines. 2023;11:2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 120. | Adare AF, Tiyare FT, Marine BT. Time to development of macrovascular complications and its predictors among type 2 diabetes mellitus patients at Jimma University Medical Center. BMC Endocr Disord. 2024;24:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 121. | Chait A, den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 2020;7:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 924] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 122. | Li M, Qian M, Kyler K, Xu J. Adipose Tissue-Endothelial Cell Interactions in Obesity-Induced Endothelial Dysfunction. Front Cardiovasc Med. 2021;8:681581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 123. | American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S207-S238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 124. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1921] [Cited by in RCA: 2032] [Article Influence: 290.3] [Reference Citation Analysis (1)] |
| 125. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 5291] [Article Influence: 529.1] [Reference Citation Analysis (0)] |
| 126. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8729] [Article Influence: 793.5] [Reference Citation Analysis (2)] |
| 127. | Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020;5:632-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 637] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 128. | Deng S, Zhou X, Ge Z, Song Y, Wang H, Liu X, Zhang D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114:105564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 129. | Rautiainen S, Laaksonen T, Koivuniemi R. Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells. Int J Mol Sci. 2021;22:10890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 130. | Yang S, Sun Y, Yan C. Recent advances in the use of extracellular vesicles from adipose-derived stem cells for regenerative medical therapeutics. J Nanobiotechnology. 2024;22:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 131. | Levin A, Ahmed SB, Carrero JJ, Foster B, Francis A, Hall RK, Herrington WG, Hill G, Inker LA, Kazancıoğlu R, Lamb E, Lin P, Madero M, McIntyre N, Morrow K, Roberts G, Sabanayagam D, Schaeffner E, Shlipak M, Shroff R, Tangri N, Thanachayanont T, Ulasi I, Wong G, Yang CW, Zhang L, Robinson KA, Wilson L, Wilson RF, Kasiske BL, Cheung M, Earley A, Stevens PE. Executive summary of the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: known knowns and known unknowns. Kidney Int. 2024;105:684-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 186] [Reference Citation Analysis (0)] |
| 132. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 4310] [Article Influence: 615.7] [Reference Citation Analysis (0)] |
| 133. | American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S239-S251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 134. | Rossing P, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, Liew A, Michos ED, Navaneethan SD, Olowu WA, Sadusky T, Tandon N, Tuttle KR, Wanner C, Wilkens KG, Zoungas S, Craig JC, Tunnicliffe DJ, Tonelli MA, Cheung M, Earley A, de Boer IH. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney Int. 2022;102:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (1)] |
| 135. | Habiba UE, Khan N, Greene DL, Shamim S, Umer A. The therapeutic effect of mesenchymal stem cells in diabetic kidney disease. J Mol Med (Berl). 2024;102:537-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 136. | Ren P, Qian F, Fu L, He W, He Q, Jin J, Zheng D. Adipose-derived stem cell exosomes regulate Nrf2/Keap1 in diabetic nephropathy by targeting FAM129B. Diabetol Metab Syndr. 2023;15:149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 137. | Perico N, Remuzzi G, Griffin MD, Cockwell P, Maxwell AP, Casiraghi F, Rubis N, Peracchi T, Villa A, Todeschini M, Carrara F, Magee BA, Ruggenenti PL, Rota S, Cappelletti L, McInerney V, Griffin TP, Islam MN, Introna M, Pedrini O, Golay J, Finnerty AA, Smythe J, Fibbe WE, Elliman SJ, O'Brien T; NEPHSTROM Trial Consortium. Safety and Preliminary Efficacy of Mesenchymal Stromal Cell (ORBCEL-M) Therapy in Diabetic Kidney Disease: A Randomized Clinical Trial (NEPHSTROM). J Am Soc Nephrol. 2023;34:1733-1751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 138. | American Diabetes Association Professional Practice Committee. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S252-S265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 139. | Dhanapalaratnam R, Issar T, Lee ATK, Poynten AM, Milner KL, Kwai NCG, Krishnan AV. Glucagon-like peptide-1 receptor agonists reverse nerve morphological abnormalities in diabetic peripheral neuropathy. Diabetologia. 2024;67:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 140. | Liu C, Wu T, Ren N. Glucagon-like peptide-1 receptor agonists for the management of diabetic peripheral neuropathy. Front Endocrinol (Lausanne). 2023;14:1268619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 141. | American Diabetes Association Professional Practice Committee. Summary of Revisions: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S6-S13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 142. | Alvarez M, Prieto AE, Portilla N, Moya D, Rincon O, Guzman I. Metformin-induced vitamin B12 deficiency: An underdiagnosed cause of diabetic neuropathy. World J Diabetes. 2025;16:107514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 143. | Chen JJ, Lin C, Lo MT, Lin LY, Chang HC, Liu GC. Autonomic modulation by SGLT2i or DPP4i in patients with diabetes favors cardiovascular outcomes as revealed by skin sympathetic nerve activity. Front Pharmacol. 2024;15:1424544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 144. | Ahmed LA, Al-Massri KF. Exploring the Role of Mesenchymal Stem Cell-Derived Exosomes in Diabetic and Chemotherapy-Induced Peripheral Neuropathy. Mol Neurobiol. 2024;61:5916-5927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 145. | Alizadeh SD, Jahani S, Rukerd MRZ, Tabrizi R, Masoomi R, Banihashemian SZ, Tabatabaei MSHZ, Ghodsi Z, Pour-Rashidi A, Harrop J, Rahimi-Movaghar V. Human studies of the efficacy and safety of stem cells in the treatment of diabetic peripheral neuropathy: a systematic review and meta-analysis. Stem Cell Res Ther. 2024;15:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 146. | Petropoulos IN, Ponirakis G, Ferdousi M, Azmi S, Kalteniece A, Khan A, Gad H, Bashir B, Marshall A, Boulton AJM, Soran H, Malik RA. Corneal Confocal Microscopy: A Biomarker for Diabetic Peripheral Neuropathy. Clin Ther. 2021;43:1457-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 147. | Kim H, Choi CU, Rhew K, Park J, Lim Y, Kim MG, Kim K. Comparative effects of glucose-lowering agents on endothelial function and arterial stiffness in patients with type 2 diabetes: A network meta-analysis. Atherosclerosis. 2024;391:117490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 148. | Sposito AC, Breder I, Soares AAS, Kimura-Medorima ST, Munhoz DB, Cintra RMR, Bonilha I, Oliveira DC, Breder JC, Cavalcante P, Moreira C, Moura FA, de Lima-Junior JC, do Carmo HRP, Barreto J, Nadruz W, Carvalho LSF, Quinaglia T; ADDENDA-BHS2 trial investigators. Dapagliflozin effect on endothelial dysfunction in diabetic patients with atherosclerotic disease: a randomized active-controlled trial. Cardiovasc Diabetol. 2021;20:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 149. | Mosenzon O, Capehorn MS, De Remigis A, Rasmussen S, Weimers P, Rosenstock J. Impact of semaglutide on high-sensitivity C-reactive protein: exploratory patient-level analyses of SUSTAIN and PIONEER randomized clinical trials. Cardiovasc Diabetol. 2022;21:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 150. | Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 397] [Article Influence: 198.5] [Reference Citation Analysis (0)] |
| 151. | Che D, Xiang X, Xie J, Chen Z, Bao Q, Cao D. Exosomes Derived from Adipose Stem Cells Enhance Angiogenesis in Diabetic Wound Via miR-146a-5p/JAZF1 Axis. Stem Cell Rev Rep. 2024;20:1026-1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 152. | American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S181-S206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 271] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 153. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1618] [Cited by in RCA: 1636] [Article Influence: 409.0] [Reference Citation Analysis (0)] |
| 154. | American Diabetes Association Professional Practice Committee. 16. Diabetes Care in the Hospital: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S321-S334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 155. | Ayoub M, Chela H, Amin N, Hunter R, Anwar J, Tahan V, Daglilar E. Pancreatitis Risk Associated with GLP-1 Receptor Agonists, Considered as a Single Class, in a Comorbidity-Free Subgroup of Type 2 Diabetes Patients in the United States: A Propensity Score-Matched Analysis. J Clin Med. 2025;14:944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 156. | Mehta AE, Lomeli LD, Pantalone KM. Glucagon-like peptide-1 receptor agonists and pancreatitis: A reconcilable divorce. Cleve Clin J Med. 2025;92:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 157. | Viscoli CM, Inzucchi SE, Young LH, Insogna KL, Conwit R, Furie KL, Gorman M, Kelly MA, Lovejoy AM, Kernan WN; IRIS Trial Investigators. Pioglitazone and Risk for Bone Fracture: Safety Data From a Randomized Clinical Trial. J Clin Endocrinol Metab. 2017;102:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 158. | Lovell-Badge R, Anthony E, Barker RA, Bubela T, Brivanlou AH, Carpenter M, Charo RA, Clark A, Clayton E, Cong Y, Daley GQ, Fu J, Fujita M, Greenfield A, Goldman SA, Hill L, Hyun I, Isasi R, Kahn J, Kato K, Kim JS, Kimmelman J, Knoblich JA, Mathews D, Montserrat N, Mosher J, Munsie M, Nakauchi H, Naldini L, Naughton G, Niakan K, Ogbogu U, Pedersen R, Rivron N, Rooke H, Rossant J, Round J, Saitou M, Sipp D, Steffann J, Sugarman J, Surani A, Takahashi J, Tang F, Turner L, Zettler PJ, Zhai X. ISSCR Guidelines for Stem Cell Research and Clinical Translation: The 2021 update. Stem Cell Reports. 2021;16:1398-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 159. | Upadhya D, Shetty AK. MISEV2023 provides an updated and key reference for researchers studying the basic biology and applications of extracellular vesicles. Stem Cells Transl Med. 2024;13:848-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 160. | Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, Falcon-Perez JM, Fu QL, Hill AF, Lenassi M, Lim SK, Mahoney MG, Mohanty S, Möller A, Nieuwland R, Ochiya T, Sahoo S, Torrecilhas AC, Zheng L, Zijlstra A, Abuelreich S, Bagabas R, Bergese P, Bridges EM, Brucale M, Burger D, Carney RP, Cocucci E, Crescitelli R, Hanser E, Harris AL, Haughey NJ, Hendrix A, Ivanov AR, Jovanovic-Talisman T, Kruh-Garcia NA, Ku'ulei-Lyn Faustino V, Kyburz D, Lässer C, Lennon KM, Lötvall J, Maddox AL, Martens-Uzunova ES, Mizenko RR, Newman LA, Ridolfi A, Rohde E, Rojalin T, Rowland A, Saftics A, Sandau US, Saugstad JA, Shekari F, Swift S, Ter-Ovanesyan D, Tosar JP, Useckaite Z, Valle F, Varga Z, van der Pol E, van Herwijnen MJC, Wauben MHM, Wehman AM, Williams S, Zendrini A, Zimmerman AJ; MISEV Consortium, Théry C, Witwer KW. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1603] [Cited by in RCA: 2256] [Article Influence: 1128.0] [Reference Citation Analysis (0)] |
| 161. | Mizuta Y, Akahoshi T, Guo J, Zhang S, Narahara S, Kawano T, Murata M, Tokuda K, Eto M, Hashizume M, Yamaura K. Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the PI3K/Akt pathway in endothelial cells. Stem Cell Res Ther. 2020;11:508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 162. | Yu JW, Deng YP, Han X, Ren GF, Cai J, Jiang GJ. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc Diabetol. 2016;15:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 163. | Pei CZ, Liu B, Li YT, Fang L, Zhang Y, Li YG, Meng S. MicroRNA-126 protects against vascular injury by promoting homing and maintaining stemness of late outgrowth endothelial progenitor cells. Stem Cell Res Ther. 2020;11:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 164. | Ding Y, Zhou Y, Ling P, Feng X, Luo S, Zheng X, Little PJ, Xu S, Weng J. Metformin in cardiovascular diabetology: a focused review of its impact on endothelial function. Theranostics. 2021;11:9376-9396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 165. | Wang Y, Li Q, Zhou S, Tan P. Contents of exosomes derived from adipose tissue and their regulation on inflammation, tumors, and diabetes. Front Endocrinol (Lausanne). 2024;15:1374715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 166. | Zheng Z, Zong Y, Ma Y, Tian Y, Pang Y, Zhang C, Gao J. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 438] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 167. | Chen Y, Yang X, Feng M, Yu Y, Hu Y, Jiang W. Exosomal miR-223-3p from bone marrow mesenchymal stem cells targets HDAC2 to downregulate STAT3 phosphorylation to alleviate HBx-induced ferroptosis in podocytes. Front Pharmacol. 2024;15:1327149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 168. | Houshmandfar S, Saeedi-Boroujeni A, Rashno M, Khodadadi A, Mahmoudian-Sani MR. miRNA-223 as a regulator of inflammation and NLRP3 inflammasome, the main fragments in the puzzle of immunopathogenesis of different inflammatory diseases and COVID-19. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:2187-2195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 169. | Shao Z, Zhang X, Cai J, Lu F. Glucagon-like peptide-1: a new potential regulator for mesenchymal stem cells in the treatment of type 2 diabetes mellitus and its complication. Stem Cell Res Ther. 2025;16:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 170. | Kim W, Lee SK, Kwon YW, Chung SG, Kim S. Pioglitazone-Primed Mesenchymal Stem Cells Stimulate Cell Proliferation, Collagen Synthesis and Matrix Gene Expression in Tenocytes. Int J Mol Sci. 2019;20:472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 171. | Mori D, Miyagawa S, Matsuura R, Sougawa N, Fukushima S, Ueno T, Toda K, Kuratani T, Tomita K, Maeda N, Shimomura I, Sawa Y. Pioglitazone strengthen therapeutic effect of adipose-derived regenerative cells against ischemic cardiomyopathy through enhanced expression of adiponectin and modulation of macrophage phenotype. Cardiovasc Diabetol. 2019;18:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/