Published online Oct 26, 2025. doi: 10.4252/wjsc.v17.i10.111978
Revised: August 28, 2025
Accepted: October 13, 2025
Published online: October 26, 2025
Processing time: 102 Days and 9.3 Hours
Mesenchymal stem cells (MSCs), as a living bio-drug, are being considered as a potential treatment for coronavirus disease 2019 (COVID-19)-induced acute res
To synthesize the existing evidence on MSCs and their derivative exosomes for treating COVID-19-induced ARDS, with a focus on the key outcomes of safety and efficacy.
Four databases were systematically searched for randomized controlled trials assessing MSCs and their derived exosomes for COVID-19-induced ARDS trea
Sixteen randomized controlled trials involving 1027 ARDS patients were in
MSC and exosome-based therapies were found to be safe and associated with a reduced duration of mechanical ventilation in patients with ARDS. NMA showed that exosome-based therapy matched the benefits of its parent cells, but with practical and logistical advantages.
Core Tip: Mesenchymal stem cells, as a living bio-drug, are being considered as a potential treatment for coronavirus disease 2019-induced acute respiratory distress syndrome due to their immunomodulatory and reparative properties. Our systematic review and meta-analysis, involving 16 randomized controlled trials (n = 1027 acute respiratory distress syndrome patients), is the first study to directly compare the therapeutic benefits of mesenchymal stem cell-based and their derivative exosome-based therapeutic approaches. Although we did not find significant differences in their safety profiles and efficacy, the exosome-based treatment ranked higher in terms of therapeutic benefits in a network meta-analysis, offering practical and logistical advantages.
- Citation: Safwan M, Bourgleh MS, Al-Ruqi A, Shrebaty O, Almujaydil F, AlOthaim B, AlRashidi N, Haider KH. Living bio-drug therapies using mesenchymal stem cells and exosomes for mechanically ventilated patients with acute respiratory distress syndrome: A systematic review and meta-analysis. World J Stem Cells 2025; 17(10): 111978
- URL: https://www.wjgnet.com/1948-0210/full/v17/i10/111978.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i10.111978
Coronavirus disease 2019 (COVID-19) was responsible for the global pandemic health crisis in late 2019, which led to millions of deaths worldwide[1]. While most individuals experienced mild symptoms, a subset developed severe respiratory complications, such as acute respiratory distress syndrome (ARDS), which was characterized by rapid respiratory failure and high mortality. ARDS was ubiquitous among patients with certain risk factors such as advanced age, obesity, and pre-existing cardiopulmonary conditions[2].
Classical ARDS and COVID-19-associated ARDS share core features, such as alveolar fluid accumulation, respiratory failure, cytokine storm, and dysregulated inflammation; however, the two do show critical pathophysiological distinctions. While classical ARDS has diverse etiologies (e.g., sepsis, trauma, aspiration) and is marked by reduced lung compliance, COVID-19-associated ARDS is exclusively driven by severe acute respiratory syndrome coronavirus-2 and often exhibits preserved compliance, increased thrombotic activity (e.g., D-dimer), suppressed interferon responses, and a reduced neutrophil-to-lymphocyte ratio linked to disease severity[3]. These differences underscore the need for targeted strategies that address the unique immune-thrombotic dysregulation associated with COVID-19-induced lung injury.
The current management of COVID-19-induced ARDS is primarily supportive, including prone positioning, fluid replacement, mechanical ventilation, and pharmacological interventions using corticosteroids; however, effective targeted therapies remain limited[4]. Mesenchymal stem cells (MSCs), due to their anti-inflammatory and immunomodulatory properties, are emerging rapidly as living bio-drugs with potential therapeutic applications. Sourced from tissues such as bone marrow, adipose tissue, and umbilical cord, MSCs secrete soluble paracrine factors that mitigate the ARDS-associated hyperinflammatory response[5]. More recently, MSC-derived exosomes, which constitute the insoluble fraction of their secretome and contain bioactive cargo, i.e., microRNAs, proteins, and lipids, have garnered interest as a cell-free therapy approach. These vesicles mediate intercellular communication, suppressing hyperinflammation and restoring immune homeostasis, while avoiding the risks associated with whole-cell therapies, such as immunogenicity and tumorigenesis[6].
Preclinical studies in murine models of ARDS have demonstrated that MSC administration can reduce pulmonary inflammation, restore immune metabolic balance, enhance macrophage phagocytosis, and decrease alveolar apoptosis, ultimately contributing to improved survival outcomes[7,8]. Moreover, recent mechanistic insights reveal that MSCs promote an anti-inflammatory, pro-phagocytic macrophage phenotype via extracellular vesicles (EV)-mediated mitochondrial transfer, which enhances oxidative phosphorylation in macrophages. This EV-driven metabolic reprogramming suppresses cytokine storms and ameliorates lung injury in vivo, underscoring mitochondrial transfer as a critical therapeutic mechanism[9]. Similarly, clinical evidence has also shown promise for MSC-based therapy in the management of ARDS. A recent meta-analysis by Fang et al[10] in 2024, which included 17 randomized controlled trials (RCTs) involving 796 patients with ARDS, reported that MSC-based treatment did not increase the risk of adverse events. MSC-based treatment was associated with reduced mortality, improved oxygenation parameters (PaO2/FiO2 ratio), and decreased levels of inflammatory biomarkers, including C-reactive protein and interleukin-6[10].
Despite these encouraging results, data specific to MSCs and MSC-derived exosomes in the context of COVID-19-related ARDS is limited. In particular, a comprehensive data synthesis directly comparing the efficacy and safety of MSCs and their exosomes in this population has not been reported. This systematic review and network meta-analysis (NMA) provide a head-to-head comparison of MSCs and MSC-derived exosome-based interventions on key clinical outcomes, including mortality, duration of mechanical ventilation, hospital and intensive care unit (ICU) stay, and rate of adverse events in patients with COVID-19-induced ARDS.
Following the guidelines of the PRISMA[11], the study protocol was prospectively registered on PROSPERO under registration number (CRD420251064267). No protocol changes were made after obtaining study approval.
The following eligibility criteria were followed for reports inclusion: (1) At least phase I/II RCTs; (2) Study population had COVID-19-induced ARDS; (3) The intervention groups were MSCs or MSC-derived exosomes; (4) A control group was included for comparison; and (5) Reported at least one of the following outcomes: Number of mortality cases, number of treatment-related adverse events, length of hospital and ICU stay, duration of mechanical ventilation, mechanical ventilation-free days, and 6-minute walk distance (6-MWD) test. Studies that did not meet our stipulated inclusion criteria (such as single-arm studies) or lacked full-text availability were excluded from the analysis. Two independent researchers from our team assessed the eligibility of the reports (Al-Ruqi A and Shrebaty O). In the event of disagreement between the authors, a third independent author (Haider KH) was consulted to reach a consensus through review and discussion.
Four databases, including PubMed, Cochrane Library, EMBASE, and ClinicalTrials.gov, were systematically searched from their inception to May 2025 by two independent reviewers (Safwan M and Bourgleh MS) to identify relevant RCTs. The whole search strategy is available in Supplementary Table 1. Briefly, we used common text words and medical subject headings, including: “COVID-19”, “ARDS”, “MSCs”, and “Exosomes”. These terms were combined using specific algorithms, for instance COVID-19 ARDS “AND” Mesenchymal stem cells. We also performed a manual search of the reference lists in the included reports to identify relevant trials. Our search was not restricted to the English language.
The following data were extracted from the included studies: (1) First author and year of publication; (2) Study location; (3) Type of intervention (MSCs or exosomes); (4) Source of MSCs (such as umbilical cord, bone marrow, or adipose tissue derived); (5) Type of control; (6) Follow-up duration; (7) Sample size; (8) Number of patients included in each arm; (9) Mean age of the patients included in the two arms; and (10) The reported study outcomes. These data were collected by two independent authors (Shrebaty O and Mujaydil F) and reported descriptively in the results, along with a table for easier representation of the collected data.
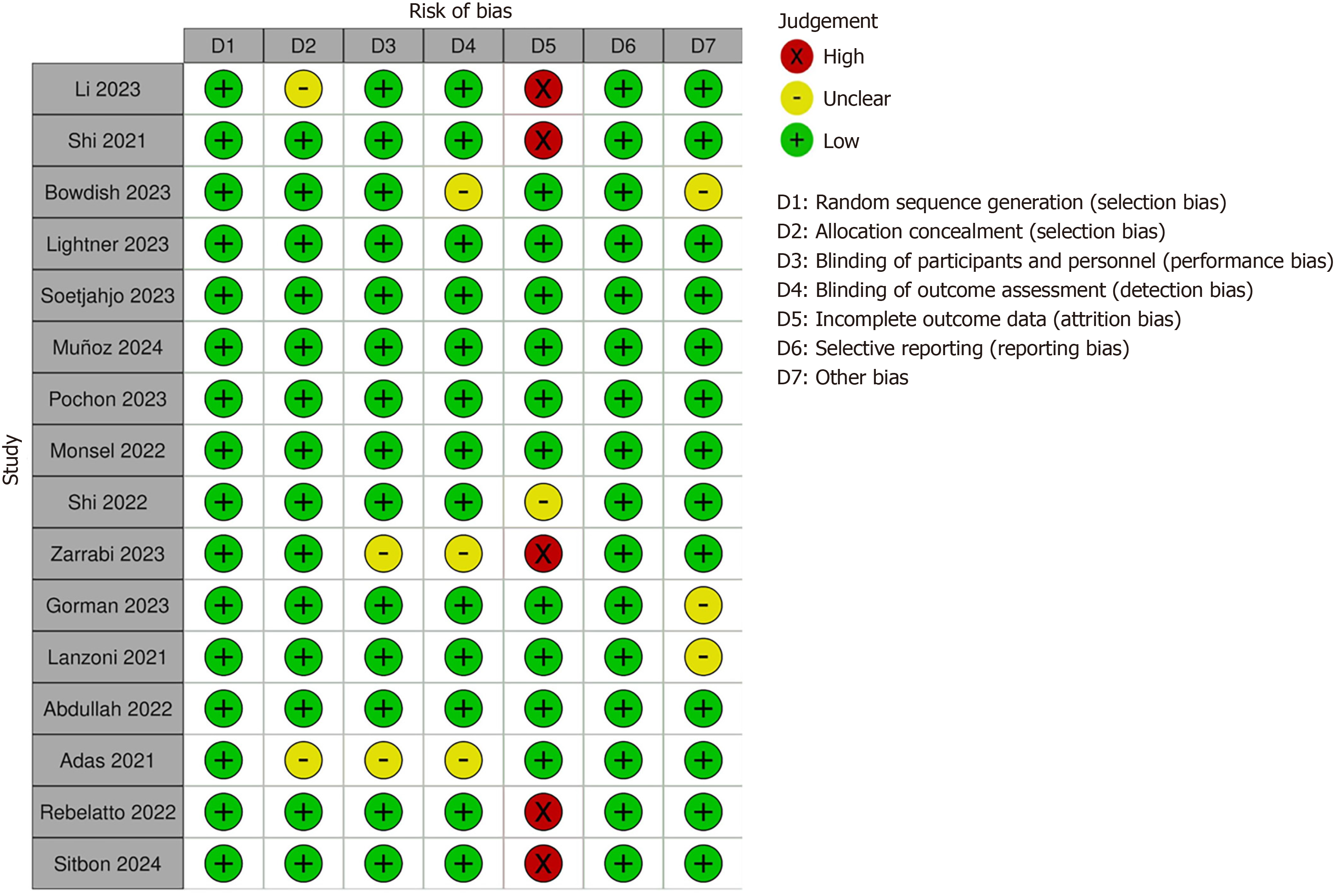
The quality of the eligible RCTs was comprehensively evaluated using the Cochrane Collaboration’s tool for assessing risk of bias. This validated tool assessed RCTs based on six domains of bias: Selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias, categorizing studies as high or low risk of bias[12]. After complete evaluation of the studies, we generated a risk of bias graph to display the overall risk of bias across each study.
Data extraction and preparation: This systematic review and meta-analysis assessed the effects of MSCs on various outcomes in patients with COVID-19 and ARDS. Key outcomes included respiratory metrics, the duration of mechanical ventilation, mechanical ventilation-free days, and overall patient care duration, which encompassed the length of hospital and ICU stay. Safety outcomes were also assessed, specifically mortality rates, adverse events, and the 6-MWD test, which serves as a clinical measure of functional capacity. The most continuous outcomes were reported as medians with ranges or medians with interquartile ranges. To perform the weighted mean difference (WMD) analysis, all reported values were converted into means and SDs using the method outlined by Wan et al[13]. Dichotomous outcomes were extracted as events and totals for each study.
Conventional pairwise meta-analysis: Pooled odds ratios (ORs) were calculated to evaluate the treatment effects of MSCs, while WMDs were calculated for continuous outcomes. The analysis results were considered statistically significant if the 95% confidence interval (CI) did not include the null hypothesis values, zero for the WMD and one for the OR. Conventional meta-analyses (CMAs) were performed using RevMan 5.4.1 software[14].
Assessment of heterogeneity and sensitivity analysis: Heterogeneity among studies was assessed using the I2 statistic, with values greater than 50% indicating significant heterogeneity. This necessitated the application of a random-effects model and a sensitivity analysis to evaluate the influence of individual studies on the overall results.
Publication bias assessment: Publication bias was assessed using Egger’s regression test and visual inspection of the funnel plot for asymmetry. P < 0.05 in Egger’s test indicated significant evidence of publication bias. As the funnel plot is not suitable for outcomes with a limited number of studies, a Deeks and Higgins (DH) plot was generated, and the DH index was calculated to quantify the asymmetry of the DH plot for outcomes with fewer than ten studies. An LFK value between -1 and +1 suggests an absence of publication bias[15]. Publication bias testing was performed using Jamovi software[16].
NMA: In addition to CMA, we performed an NMA to enable indirect comparisons between interventions (MSCs and exosomes) where direct head-to-head trials were lacking. The NMA was conducted for outcomes with more than two treatment groups, including mechanical ventilation-free days, mortality, and adverse events. Given the absence of direct comparisons between some interventions, this approach allowed us to integrate both direct and indirect evidence, providing a comparative assessment and ranking of treatments. As the number of included studies was limited, the NMA should be considered exploratory in nature.
A network plot was generated to visualize the treatment arms, where treatments are represented as nodes, with the size of each node corresponding to the number of participants. Edges connecting the nodes represent direct comparisons between treatments, with the thickness of each edge proportional to the number of studies comparing the two treatments. The network treatment effect was estimated by combining both direct and indirect treatment effects for each intervention and was presented in a forest plot. To rank the treatment arms from most to least effective, surface under the cumulative ranking curve (SUCRA) values were calculated and displayed in rankograms. A higher SUCRA value indicates a greater likelihood that a treatment will be the most effective. NMA and sensitivity analyses were conducted using the netmeta and metasens packages in R Studio[17-19].
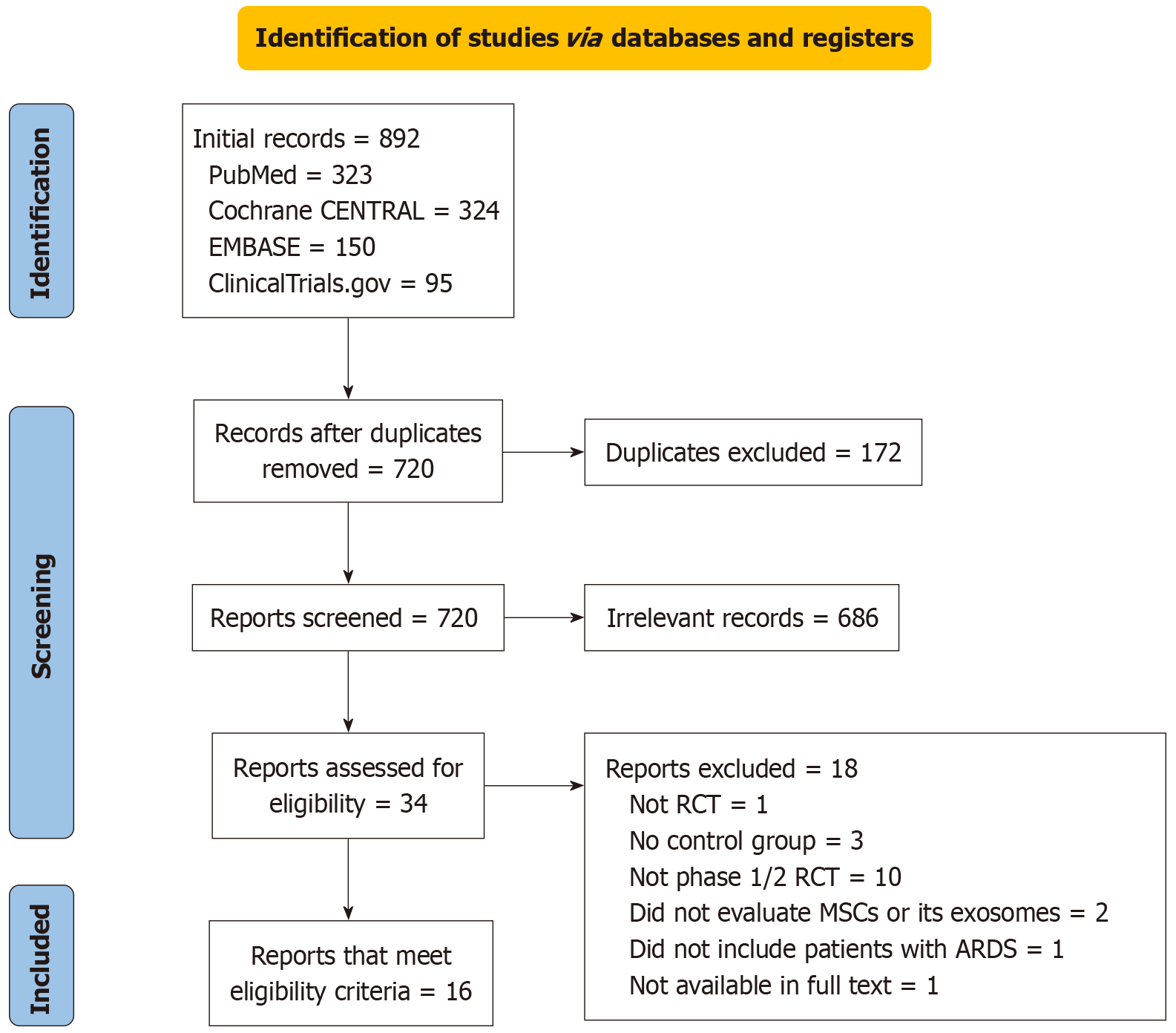
The PRISMA flow diagram illustrates the study selection process from the four databases stipulated in our study (Figure 1). From the 892 identified records, a total of 16 RCTs met the inclusion criteria for the final analysis[20-35].
The 16 included studies (n = 1027 ARDS patients) were conducted between 2021 and 2024. Of these, ten studies investigated MSCs, two examined exosomes, and one compared both treatments with a control in the intervention group. These studies provided a global perspective, with research conducted in several countries: Three studies each from China[20,24,32], France[26,28,34], and the United States[22,25,31]; two from Indonesia[23,33]; and one each from Iran[21], Turkey[35], Spain[27], Brazil[29], and the United Kingdom[30]. The follow-up duration ranged from 14 days to 24 months. The mean age of the patients in the intervention group was 63.06 years, compared to 58.23 years in the control group.
Regarding tissue MSC sources, umbilical cord-derived MSCs were the most commonly used in 11 RCTs, followed by bone marrow-derived MSCs and perinatal MSCs, with two studies utilizing both umbilical cord-derived MSCs and bone marrow-derived MSCs (Table 1). The Cochrane Collaboration tool for risk of bias assessment identified 11 RCTs as having a low risk of bias. The remaining five RCTs were considered to have a high risk, primarily due to the lack of intention-to-treat analysis. Figure 2 summarizes the authors’ final assessment.
| Ref. | Intervention | Control | F/U period | Sample size | Mean age (years) | Outcome measures | |||
| Total | Intervention (males) | Control (males) | Intervention | Control | |||||
| Shi et al[20], 2022, China | UC-MSCs | Placebo | 12 months | 100 | 65 (56.92) | 35 (54.29) | 60.72 | 59.94 | Safety; mortality; 6-MWD; length of hospitalization |
| Zarrabi et al[21], 2023, Iran | Perinatal MSCs and MSCs-Exo | Standard therapy | 28 days | 43 | MSCs group: 11 (90.9), MSCs + Exo: 8 (62.5) | 24 (66.7) | MSCs group = 50, MSCs + Exo group = 47.75 | 49.4 | Safety mortality |
| Lightner et al[22], 2023, United States | BM-MSCs-Exo (Exoflo) | Placebo | 60 days | 102 | Exoflo-10: 34 (61.8), MSCs Exoflo-15: 34 (64.7) | 34 (70.6) | MSCs group Exoflo-10 = 62.1, MSCs group Exoflo-15 = 56.8 | 58.5 | Safety; mortality; ventilation-free days; length of hospitalization |
| Soetjahjo et al[23], 2023, Indonesia | UC-MSCs | Placebo | 22 days | 42 | 21 (47.6) | 21 (57.1) | 56.10 | 55.86 | Safety mortality-duration of hospitalization; 6-MWD |
| Li et al[24], 2023, China | UC-MSCs | Placebo | 24 months | 100 | 65 (56.92) | 35 (54.29) | 60.72 | 59.94 | 6-MWD test, adverse events |
| Bowdish et al[25], 2023, United States | UC-MSCs | Placebo | 12 months | 222 | 112 (70.54) | 110 (68.18) | 61.8 | 59.6 | Mortality, hospitalizations; adverse events: Mechanical ventilatory support |
| Pochon et al[26], 2023, France | UC-MSCs | Placebo | 90 days | 30 | 15 (87) | 15 (47) | 49.13 | 61 | Survival at day 90, ventilation-free days for 28 days |
| Martínez-Muñoz et al[27], 2024, Spain | BM-MSCs | Placebo | 12 months | 20 | 10 (50) | 10 (80) | 59.5 | 65.5 | Hospital length of stay |
| Sitbon et al[28], 2024, France | UC-MSCs | Placebo | 12 months | 45 | 21 (81) | 24 (83.3) | 64 | 63.2 | Adverse events |
| Rebelatto et al[29], 2022, Brazil | UC-MSCs | Placebo | 4 months | 17 | 11 (72.7) | 6 (66.6) | 53 | 61.7 | Mortality, safety |
| Gorman et al[30], 2023, United Kingdom | UC-MSCs | Placebo | 24 months | 59 | 30 (80) | 29 (69) | 58.4 | 58.4 | Adverse events, mortality, duration of ventilation, and duration of hospital and ICU stay |
| Lanzoni et al[31], 2021, United States | UC-MSCs | Placebo | 59 days | 24 | 12 (41.7) | 12 (66.7) | 58.58 | 58.83 | Safety, mortality |
| Shi et al[32], 2021, China | UC-MSCs | Placebo | 3 months | 100 | 65 (56.92) | 35 (54.29) | 60.72 | 59.94 | Safety, mortality; 6-MWD; length of hospitalization |
| Abdullah et al[33], 2022, Indonesia | UC-MSC-secretome | Placebo | 14 days | 40 | 20 (27.5) | 20 (27.5) | 51.25 | 49.9 | Mortality, safety |
| Monsel et al[34], 2022, France | UC-MSCs | Placebo | 28 days | 45 | 21 (81) | 24 (83.3) | 64 | 63.2 | Mortality, safety |
| Adas et al[35], 2021, Turkey | UC-MSCs | Standard therapy | 108 days | 38 | 19 (63) | 19 (63) | 56 | 56 | Mortality |
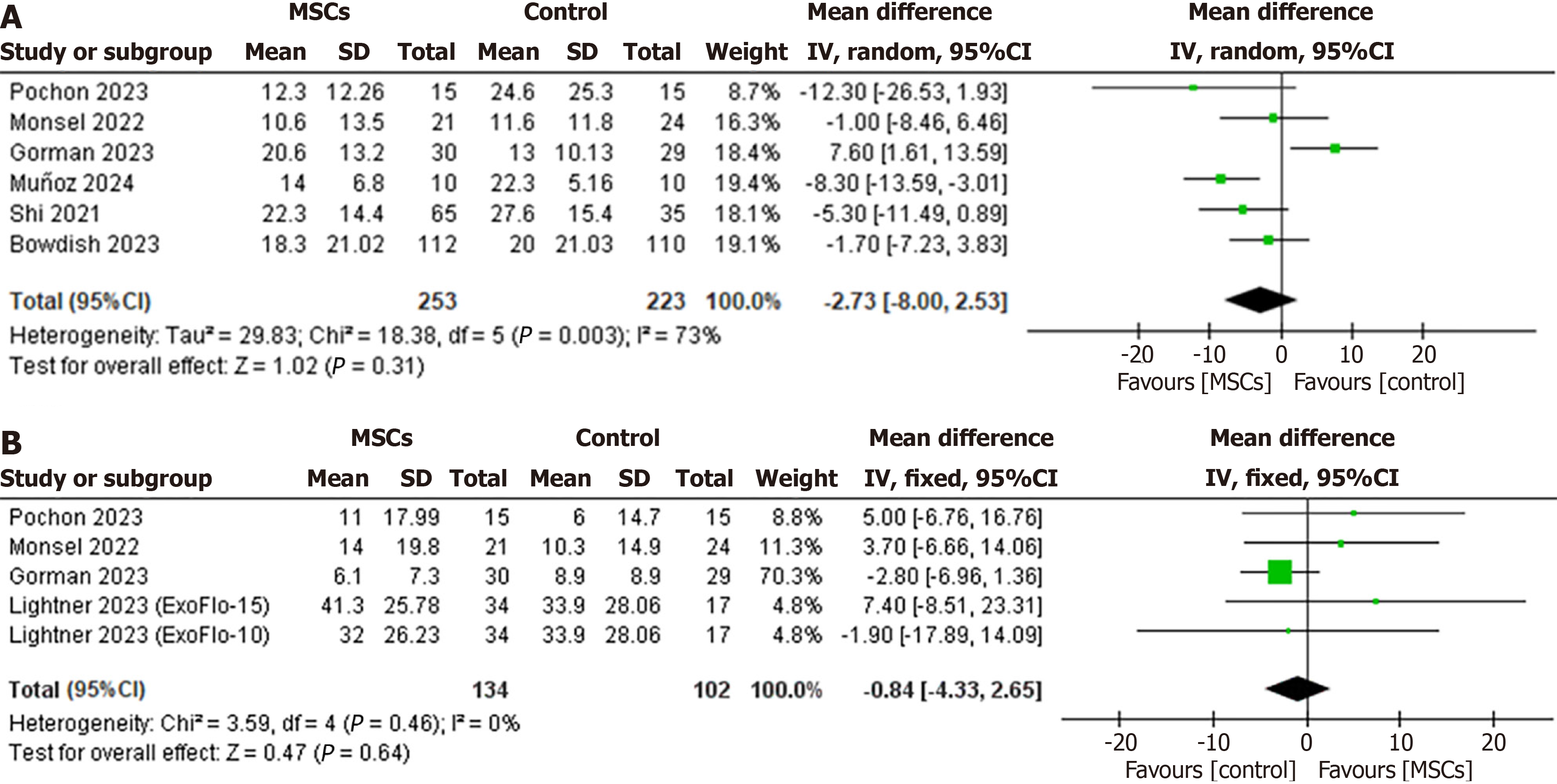
Duration of mechanical ventilation: Six RCTs reported the duration of mechanical ventilation for patients in the intervention group (n = 253) and the control group (n = 223). The pooled analysis revealed no significant difference between the MSCs group and the control group (WMD: -2.73, 95%CI: -8.00 to 2.53, P = 0.31, I2 = 73%) (Figure 3A). Due to the high heterogeneity, a sensitivity analysis was performed. The study by Gorman et al[30] in 2023 had the most significant influence on the between-study heterogeneity. Omitting this study reduced the heterogeneity to 20% and revealed a significant reduction of 4.84 days in mechanical ventilation for the MSCs group compared to the control group (WMD: -4.84, 95%CI: -8.21 to -1.47, I2 = 20%) (Supplementary Figure 1).
Mechanical ventilation-free days: Four RCTs (MSCs group, n = 134; control group, n = 102) reported the treatment effect on mechanical ventilation-free days. The CMA revealed a statistically nonsignificant difference between the two groups (WMD: -0.84, 95%CI: -4.33 to 2.65, P = 0.64, I2 = 0%) (Figure 3B).
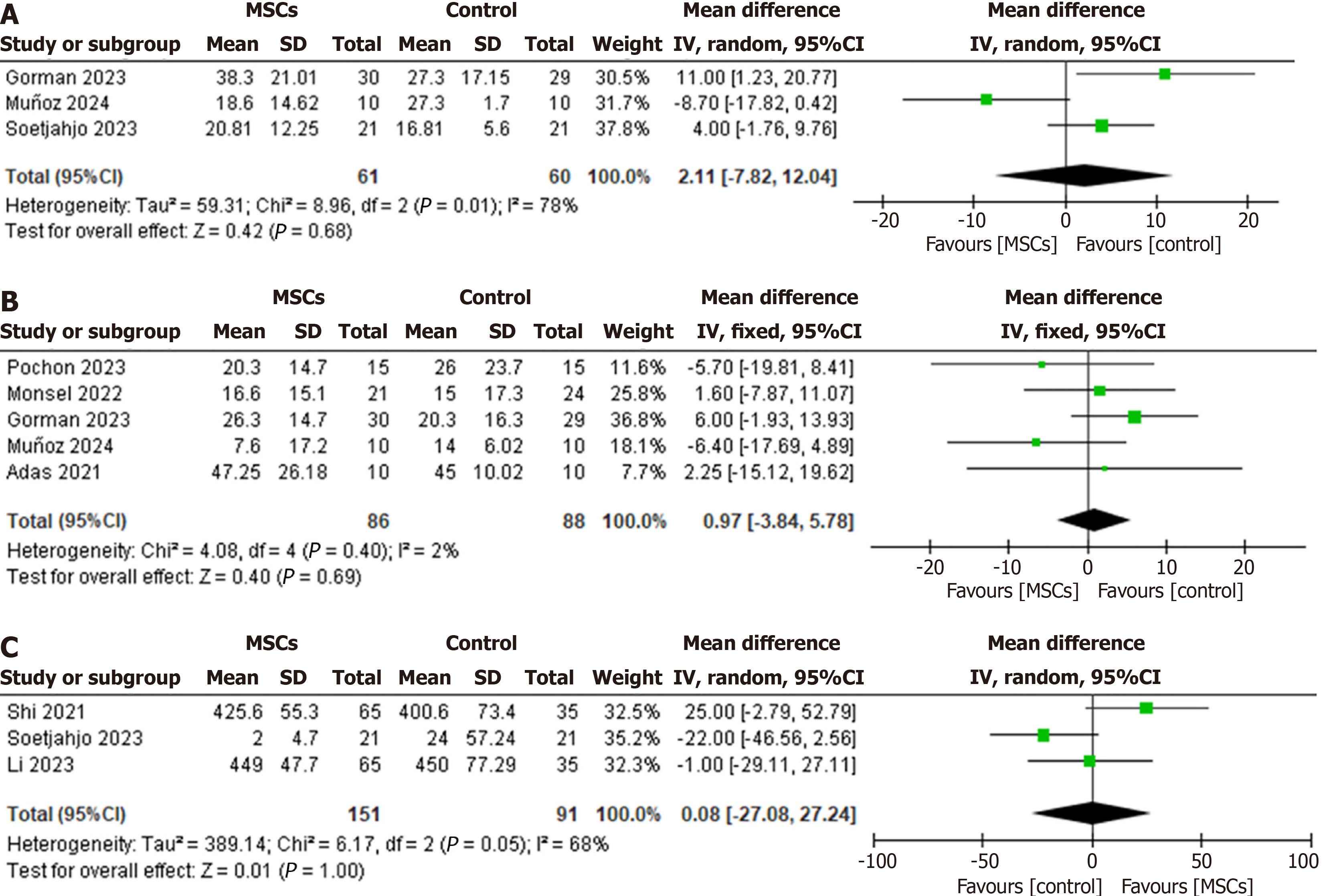
Length of hospital stay: The pooled WMD indicated no significant difference in the length of hospital stay between the MSC-treated patients and the control group patients (WMD: 2.11, 95%CI: -7.82 to 12.04, P = 0.68, I2 = 78%) (Figure 4A). Omitting Martínez-Muñoz et al’s study[27] in 2024 from the analysis as an outlier had the most significant impact on the heterogeneity of the results. It reduced the I2 statistic to 32% without significantly affecting the duration of hospital stay (WMD: 6.34, 95%CI: -0.13 to 12.81, I2 = 32%) (Supplementary Figure 2).
Length of ICU stay: The mean length of ICU stay for patients at the end of the follow-up period was reported by five of the included studies, involving patients from the MSCs group (n = 86), and the control group (n = 88). The WMD analysis showed no statistically significant difference between the MSC-treated group and the control group (WMD: 0.97, 95%CI: -3.84 to 5.78, P = 0.69, I2 = 2%) (Figure 4B).
Only three of the included studies reported the 6-MWD test results (MSCs group, n = 151; control group, n = 91). The pooled analysis revealed no statistically significant difference between the two groups (WMD: 0.08, 95%CI: -27.08 to 27.08, P = 1.00, I2 = 68%) (Figure 4C). Given the significant heterogeneity, sensitivity analysis showed that the study by Shi et al[32] in 2021 was an outlier, exclusion of which reduced the I2 statistic to 18% without significantly affecting the treatment effect on 6MWD (WMD: -12.66, 95%CI: -33.11 to 7.80, P = 0.23, I2 = 18%) (Supplementary Figure 3).
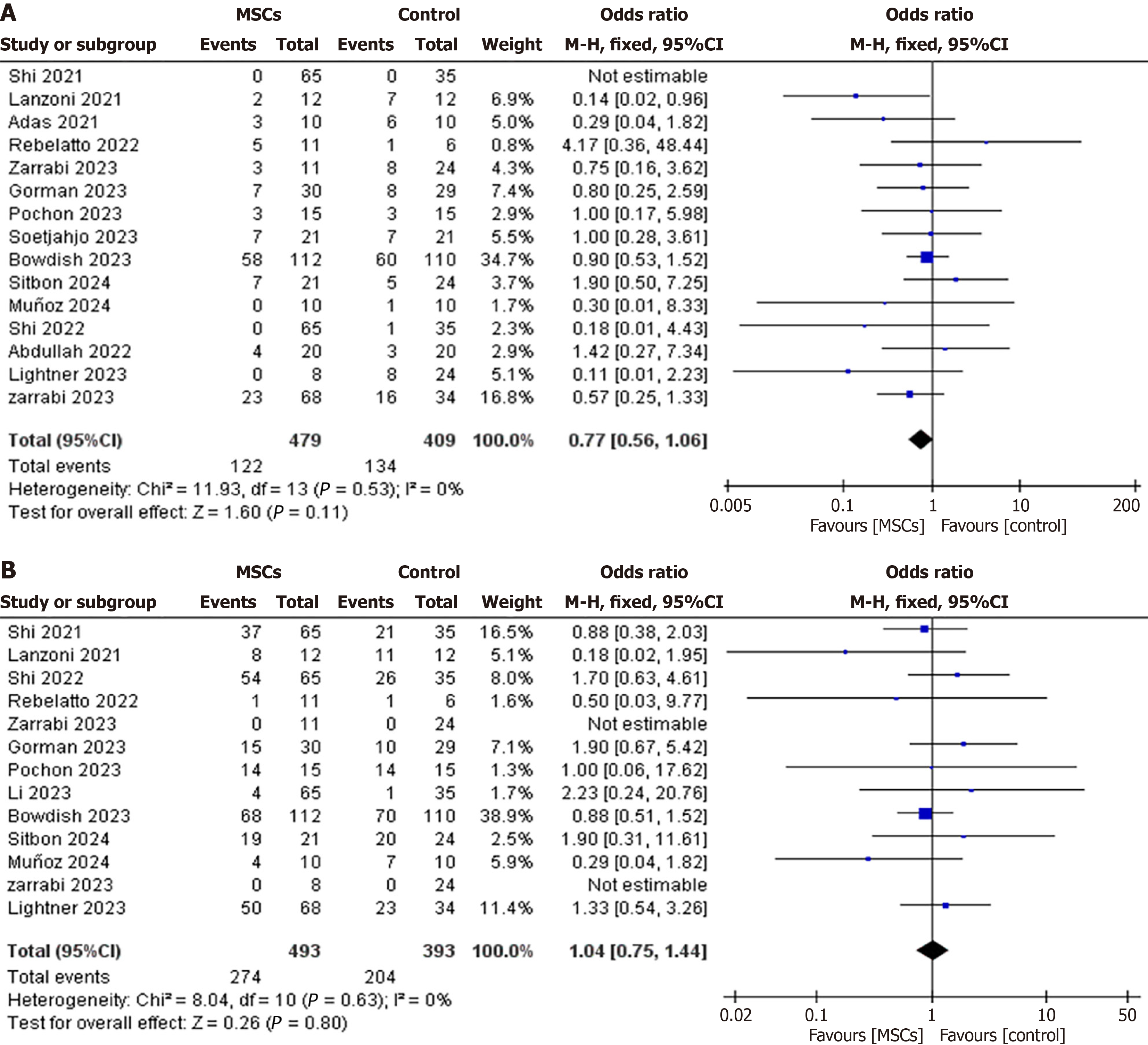
Mortality: Fifteen RCTs (intervention group, n = 479; control group, n = 409) reported mortality rates. Among these, twelve RCTs compared MSCs to the control group, two RCTs compared exosomes to the control group, and one RCT evaluated MSCs vs exosomes vs control. The CMA revealed a statistically nonsignificant reduction in overall mortality in the MSCs and MSC-derived exosome groups compared to the control (OR: 0.77, 95%CI: 0.56-1.06, P = 0.11, I2 = 0%) (Figure 5A).
Adverse events: Thirteen RCTs (intervention group, n = 493, control group, n = 393) reported adverse events during the follow-up period. Among these, ten RCTs compared MSCs to the control group, two compared exosomes to the control group, and one compared MSCs to exosomes (Figure 6A). The pooled OR analysis revealed a statistically nonsignificant difference in adverse events between the intervention group and the control group (OR: 1.04, 95%CI: 0.75-1.44, P = 0.80, I2 = 0%) (Figure 5B).
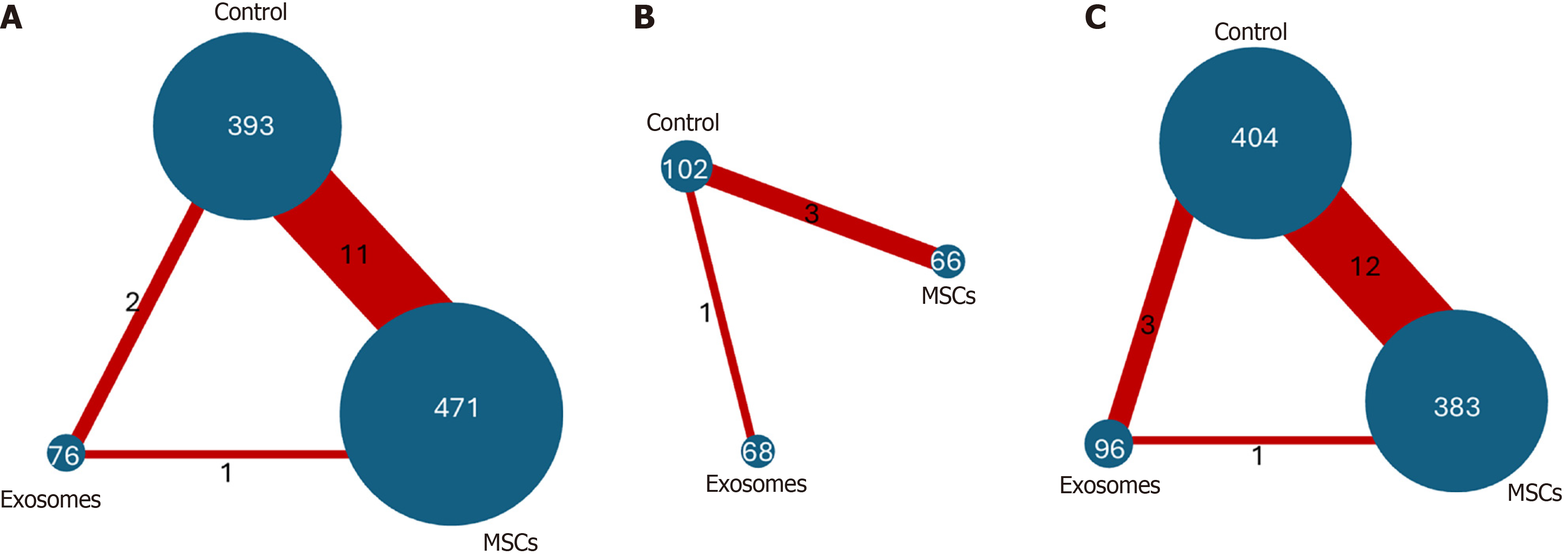
As the included studies evaluating MSC-derived exosome treatment reported only mechanical ventilation-free days, mortality, and adverse events, an NMA was performed to indirectly compare MSCs, exosomes, and control groups across these shared outcomes (Table 2).
| Outcome | Analysis | Comparison(s) | Effect estimate (95%CI), P value | Notes |
| Duration of MV | CMA | MSCs vs control | WMD: -2.73 (-8.00 to 2.53), P = 0.31 | Not significant; sensitivity analysis showed -4.84 days |
| MV-free days | CMA | MSCs vs control | WMD: -0.84 (-4.33 to 2.65), P = 0.64 | No difference |
| NMA | MSCs vs control | WMD: -0.59 (-5.23 to 4.04) | ||
| NMA | Exosomes vs control | WMD: 2.78 (-6.69 to 12.26) | ||
| NMA | MSCs vs exosomes | WMD: 3.38 (-7.17 to 13.93) | ||
| Length of hospital stay | CMA | MSCs vs control | WMD: 2.11 (-7.82 to 12.04), P = 0.68 | No difference; heterogeneity reduced after sensitivity analysis |
| Length of ICU stay | CMA | MSCs vs control | WMD: 0.97 (-3.84 to 5.78), P = 0.69 | No difference |
| 6-MWD | CMA | MSCs vs control | WMD: 0.08 (-27.08 to 27.08), P = 1.00 | No difference; heterogeneity reduced after sensitivity analysis |
| Mortality | CMA | MSCs/exosomes vs control | OR: 0.77 (0.56-1.06), P = 0.11 | Trend toward reduction, not significant |
| NMA | MSCs vs control | OR: 0.84 (0.58-1.22) | ||
| NMA | Exosomes vs control | OR: 0.62 (0.30-1.28) | ||
| NMA | Exosomes vs MSCs | OR: 0.74 (0.33-1.66) | ||
| Adverse events | CMA | MSCs/exosomes vs control | OR: 1.04 (0.75-1.44), P = 0.80 | No difference |
| NMA | MSCs vs control | OR: 1.02 (0.72-1.45) | ||
| NMA | Exosomes vs control | OR: 1.34 (0.56-3.20) | ||
| NMA | Exosomes vs MSCs | OR: 1.31 (0.52-3.34) |
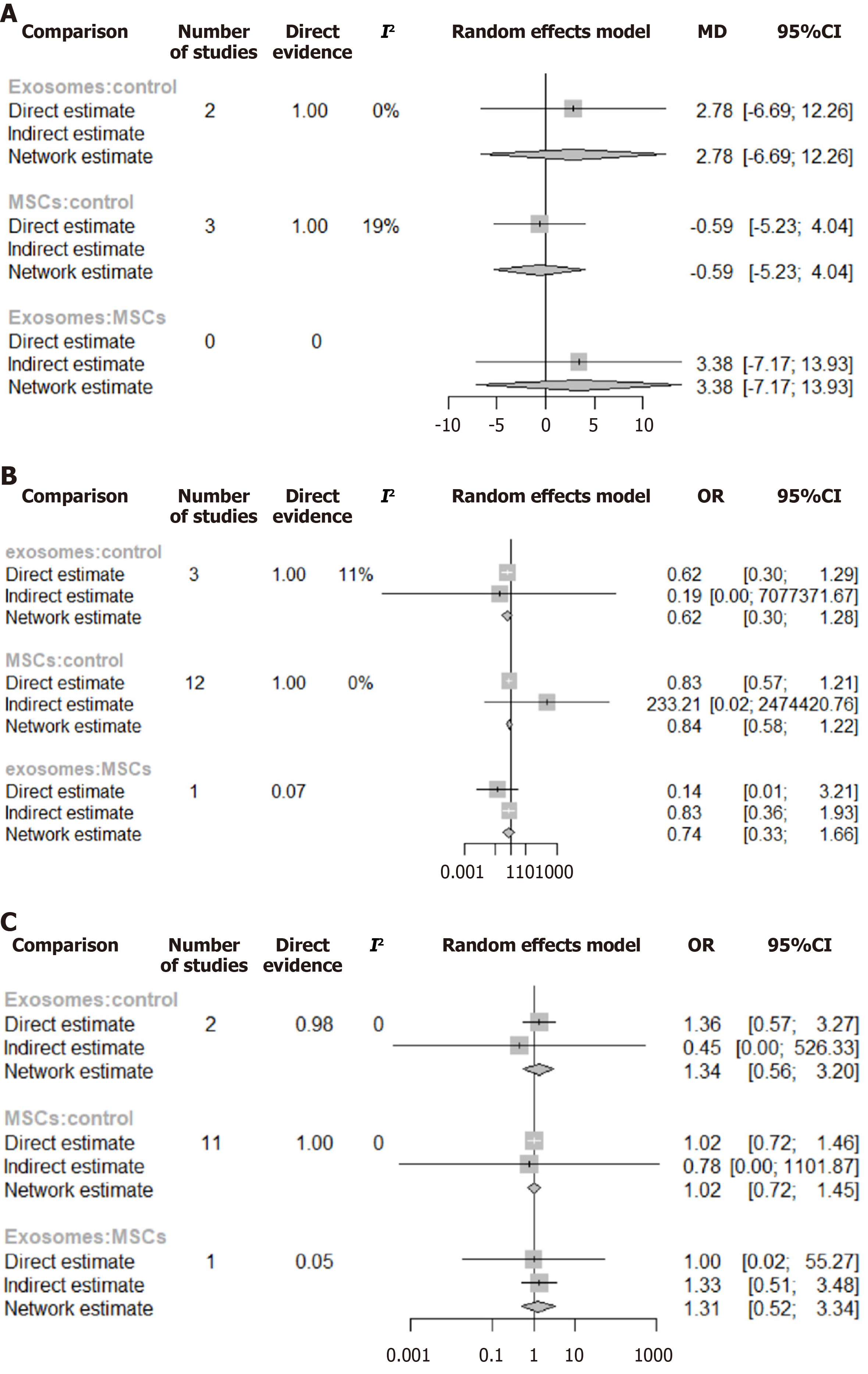
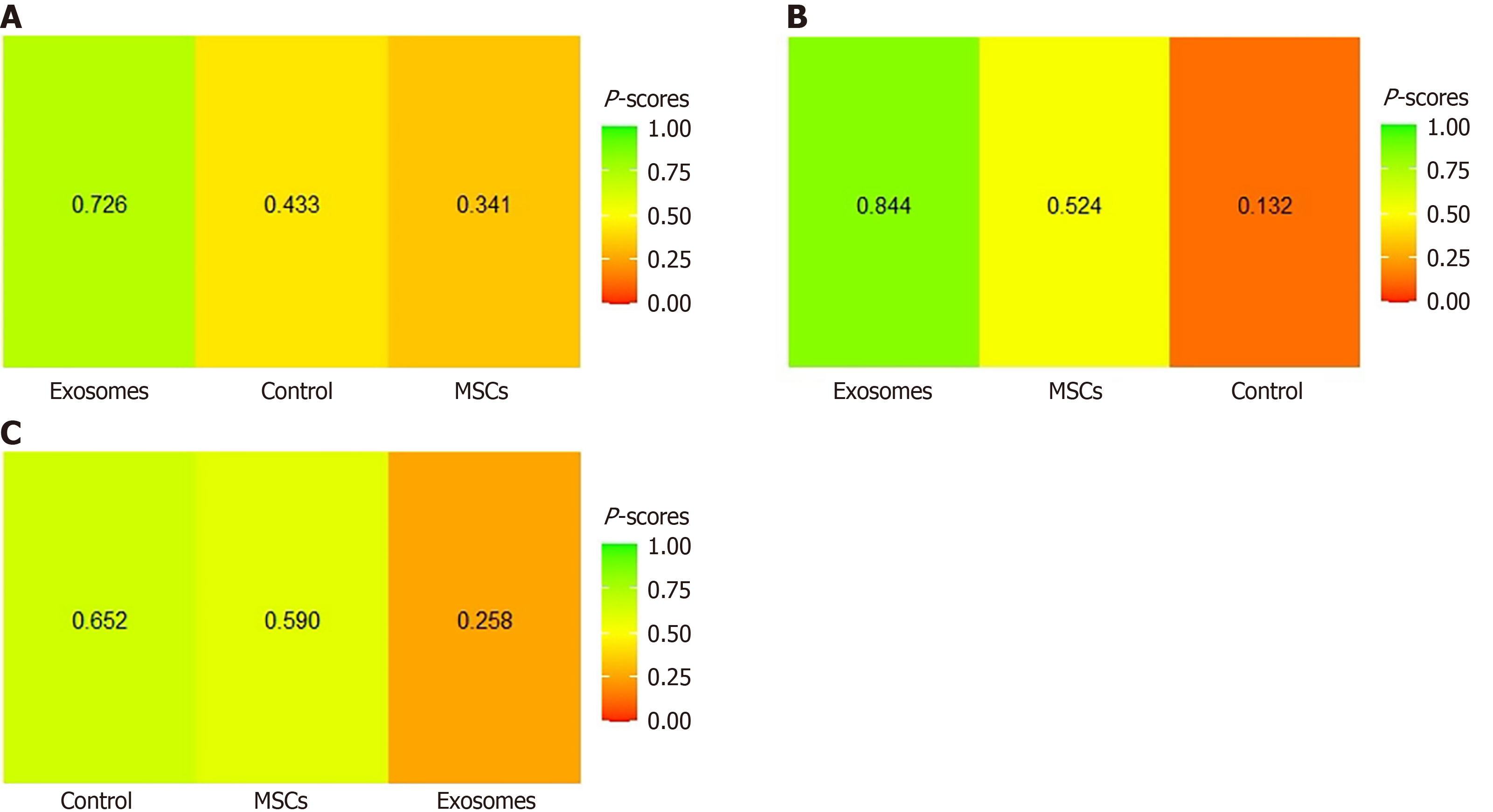
Mechanical ventilation-free days: Three studies[26,30,34] directly compared MSC-based treatment vs control, while one study[22] directly compared treatment with different exosome doses vs control, as shown in Figure 6B. The network comparison estimates showed no statistically significant differences between MSC-based intervention vs control (WMD: -0.59, 95%CI: -5.23 to 4.04), exosome-based treatment vs control (WMD: 2.78, 95%CI: -6.69 to 12.26), and MSC-based treatment vs exosome-based treatment (WMD: 3.38, 95%CI: -7.17 to 13.93) (Figure 7A). Based on the SUCRA ranking, the exosome-based treatment had the highest probability of being the most effective treatment (73%), followed by the control (43%). In comparison, the MSC-based treatment had the lowest probability (34%) (Figure 8A). Given the overlapping CIs and the lack of statistically significant results in the forest plots, these rankings should be interpreted with caution.
Mortality: Twelve studies directly compared MSC-based treatment vs the control group[20,21,23,25-32,35], two compared exosomes with the control group[22,23], and one compared MSCs with exosomes[21] as shown in Figure 6C. The NMA estimate showed a non-statistically significant trend toward reduced mortality in both the exosomes and MSC groups (OR: 0.62, 95%CI: 0.30-1.28) and (OR: 0.84, 95%CI: 0.58-1.22), respectively. When exosome-based treatment was compared to MSC-based treatment, the former showed a 26% greater reduction in mortality; however, this difference was statistically nonsignificant (OR: 0.74, 95%CI: 0.33-1.66) (Figure 7B). Based on the SUCRA ranking, exosome-based treatment was ranked as the most effective treatment (84%), followed by MSC-based treatment (52%) and the control (13%) (Figure 8B).
Adverse events: Eleven studies directly compared MSC-based treatment vs the control group[20,21,24-32], one compared exosomes with the control group[22], and one compared MSCs with exosomes[21], as shown in Figure 6A. The NMA estimates for the three treatment arms revealed no significant difference in adverse event rates between the exosome-based treatment and the control group (OR: 1.34, 95%CI: 0.56-3.20) or between the MSC-based treatment and the control group (OR: 1.02, 95%CI: 0.72-1.46). When exosomes were compared to MSC-based treatment, they showed a 30% higher rate of adverse events; however, this difference was statistically nonsignificant (OR: 1.31, 95%CI: 0.52-3.34) (Figure 7C). According to the SUCRA ranking, the control group was the least likely to experience adverse events (65%), followed by MSC-based treatment (59%), and exosome-based treatment (26%) (Figure 8C).
Doi plots for the length of hospital stay and 6-MWD revealed a symmetrical pyramid, indicating no evidence of publication bias (LFK index = -0.51 and 0.38, respectively). However, Doi plots for the duration of mechanical ventilation, mechanical ventilation-free days, and length of ICU stay were asymmetrical pyramids, suggesting a high probability of publication bias for these outcomes (LFK index = -1.32, 5.12, and -1.92, respectively) (Supplementary Figure 4). Additionally, the funnel plots for mortality and adverse events showed a symmetrical distribution of studies, and Egger’s regression test indicated no significant evidence of publication bias (P values = 0.36 and 0.89, respectively) (Sup
We have evaluated the safety and efficacy of MSC-based and exosome-based treatments in COVID-19-induced ARDS patients across various respiratory and functional outcomes, as well as mortality risk. Our essential findings include the following: (1) MSC-based treatment reduced the duration of mechanical ventilation by 4.84 days compared to control; (2) No significant effects were observed in mortality risk, duration of care, or the 6-MWD; (3) Both MSC-based and exosome-based treatment were not associated with an increased risk of adverse events; and (4) Although statistically nonsignificant, NMA suggested that exosome-based treatment achieved better outcomes than MSC-based treatment in mortality and ventilation-free days.
ARDS is associated with high mortality rates, both in COVID-19 and non-COVID-19 patients. Pre-pandemic studies reported mortality rates ranging from 34.9% to 46.1%. At the same time, meta-analyses estimate that approximately one-third of hospitalized COVID-19 patients developed ARDS, with fatality rates ranging from 23% to 56%, depending on the healthcare settings and quality of treatment availability[36,37]. Despite advancements such as lung-protective ventilation, there remains an urgent need for novel therapies that address the underlying inflammatory injury associated with ARDS. MSCs and their derivative exosomes have garnered significant interest due to their anti-inflammatory and immunomodulatory properties, rendering them potential candidates for ARDS treatment.
Our analysis did not reveal a significant reduction in mortality with MSC-based or exosome-based treatments compared to the control group. These findings diverge from the published data from Wang et al[38] and Yao et al[39], which reported 44% and 48% reductions in mortality, respectively, in COVID-19 ARDS patients treated with MSCs. For example, Wang et al[38] found an OR of 0.66 (95%CI: 0.46-0.96, I2 = 10%), while Yao et al[39] reported an OR of 0.52 (95%CI: 0.32-0.84, I2 = 0%). Their differences, compared with our findings, may stem from differences in study cohorts and treatment contexts. Both meta-analyses relied on trials conducted during the early pandemic when standard therapies, such as dexamethasone, tocilizumab, and optimized ventilatory protocols, were not yet implemented[40]. However, our analysis included RCTs conducted in 2023 to 2024, when these therapies were already established as standard practice, potentially attenuating the additional survival benefit of MSC-based or exosome-based therapies. Furthermore, our analysis included a larger cohort of 888 patients (compared to 593 patients in Wang et al’s study[38]), thus offering greater statistical power but potentially yielding a more conservative estimate of efficacy. More importantly, heterogeneity in our study was low (I2 = 0%), reflecting homogeneous results across trials, while Wang et al’s analysis[38] showed slightly higher heterogeneity (I2 = 20%).
Yao et al’s meta-analysis[39], which reported a 48% reduction in in-hospital mortality, included prospective cohort studies that are inherently vulnerable to confounding factors and selection bias. In addition, their study population comprised patients with severe COVID-19, who may or may not have developed ARDS. In contrast, our analysis was limited strictly to RCTs enrolling patients with a confirmed diagnosis of ARDS, thereby ensuring a more homogeneous population and providing more reliable and internally valid estimates of efficacy.
Despite these differences, all three meta-analyses, including ours, consistently demonstrate that MSC-based treatment is not associated with any increased risk of adverse events. This favorable safety profile is particularly notable given the adverse effects associated with many standard ARDS therapies. For instance, dexamethasone, while life-saving, carries risks of hyperglycemia, secondary infections, and muscle wasting[41]; tocilizumab is associated with neutropenia, hepatotoxicity, and reactivation of latent infections[42]; and antiviral agents, such as remdesivir, are linked to hepatotoxicity, metabolic disturbances, and anemia[43]. Against this backdrop, MSCs and their derived exosomes are novel yet promising therapeutic alternatives with a favorable safety profile, particularly for patients with contraindications to standard pharmacological modalities or those who experience treatment-limiting complications.
COVID-19-related ARDS is characterized by prolonged respiratory failure, with a median intubation duration of 22 days, which is significantly longer than in non-COVID ARDS[44]. Our analysis examined ventilation duration as a key efficacy endpoint and found that MSCs tended to reduce ventilation time, although the effect was initially nonsignificant. However, sensitivity analysis excluding the study by Gorman et al[30] revealed a clear shift in results: Heterogeneity (I2) decreased from 73% to 20%, and the treatment effect became statistically significant, indicating that MSC-based treatment reduced mechanical ventilation duration by 4.84 days. This may be attributed to the 24-month follow-up period, which was significantly longer than the 3-12 months observed in other studies, potentially introducing variability by capturing long-term factors. These findings also underscore the importance of follow-up duration in assessing the efficacy of MSCs, suggesting that shorter follow-ups may yield more consistent data for acute-phase interventions.
A 4.84-day reduction in mechanical ventilation duration may represent a clinically meaningful improvement with numerous benefits. Prolonged mechanical ventilation generally increases the risk of complications such as ventilator-associated pneumonia, airway trauma, and respiratory muscle deconditioning, all of which delay recovery and worsen long-term outcomes[45]. Reducing ventilation time by nearly five days significantly lowers these risks, promoting patient safety and faster recovery. Additionally, shorter ventilation durations enable earlier mobilization and reduce sedation exposure, enhancing both physical and cognitive recovery after critical illness. When evaluating ventilation-free days, neither MSCs nor exosome-based treatment produced a statistically significant effect. However, NMA suggested that exosomes performed slightly better than MSCs in improving ventilation-free days. Although this difference was statistically nonsignificant, it indicates that exosome-based therapy may offer a marginal advantage, possibly due to its uniform, nano-sized nature and ability to deliver a payload of bioactive molecules more efficiently. Further research with larger sample sizes and robust study designs is needed to confirm whether exosomes may truly outperform MSCs in this context.
The key question remains whether MSC-derived exosomes can fully substitute for MSC-based cell therapy. Exosomes are naturally occurring nanoparticles rich in the payload of bioactive molecules, as contained in the secretome of MSCs, while avoiding the limitations of MSC-based treatments, such as uncontrolled proliferation, higher risks of immunogenicity, and tumorigenicity[46]. Previous meta-analyses have also highlighted the potential of exosomes in other clinical settings, for example, revealing a notable correlation with wound closure rates in diabetic chronic wounds and improvements in locomotion, as well as reduced cavity size in spinal cord injury models[47,48]. RCTs in our analysis suggest that exosome-based therapy, as a cell-free therapy approach, performs at least as well as MSC-based therapy, with a potential added advantage in tolerability. In addition to this, exosomes may offer logistical advantages over MSC-based treatment due to their smaller size, stability, and potential for standardized dosing; however, these benefits remain hypothetical and require confirmation in future trials. Additionally, questions regarding their payload uniformity, optimal dose, and route of administration need to be addressed before their routine clinical use can be considered.
Despite these interesting data, our study has several limitations. The analysis relied on NMA, which assumes comparability among different trials and populations, but there is potential heterogeneity in ARDS severity, concomitant treatments, and MSC/exosome preparation. Although we performed an NMA, the limited number of trials assessing exosome interventions restricts the strength of indirect comparisons and rankings. Thus, the NMA findings and the SUCRA-based rankings regarding exosome efficacy should be interpreted cautiously. These findings are exploratory rather than conclusive.
Additionally, there was variability in the reported outcomes across the included studies. For instance, six RCTs reported on the duration of mechanical ventilation, four reported ventilation-free days, three reported the duration of hospital stay, five reported the duration of ICU stay, and three reported results from the 6-MWD test. This variability introduced challenges to the study’s analysis, hindered direct comparisons, complicated the interpretation of results, and limited the generalizability of the findings.
The included trials differed in several key methodological aspects, including the source of MSCs or exosomes (e.g., bone marrow, adipose tissue, umbilical cord), the administered dose and number of doses, and the timing of administration. The contribution of these parameters has been shown to influence the outcome of the intervention[49-52]. Using a single-cell transcriptome analysis approach, a recent study reported heterogeneity in tumorigenic potential among subclones of MSCs[53]. These variations have likely contributed to heterogeneity in treatment effects across studies, complicating direct comparisons and limiting the generalizability of our findings. We did not apply the GRADE approach to assess the certainty of evidence. Given the variability in outcomes reporting, the overall certainty of evidence would likely be rated low to very low. Nevertheless, we acknowledge that the inclusion of a formal GRADE assessment would have further strengthened the interpretability of our findings.
Future research should focus on larger, high-quality RCTs directly comparing MSCs and their derived exosomes, and a placebo control, using standard predefined endpoints during short-term and long-term follow-ups, functional outcomes, and stratification by patient risk or biomarkers. Mechanistic studies could identify which subgroups benefit most, and cost-effectiveness analyses are needed to inform clinical implementation. As COVID-19 evolves and ARDS cases arise due to new variants or other causes, these therapies may become integrated into broader ARDS treatment guidelines if efficacy is confirmed.
In summary, this comprehensive analysis suggests that MSC-based therapies are associated with a reduced duration of mechanical ventilation in ARDS, without any added safety concerns. Effects on mortality risk, ventilation duration, ICU and hospital stay, and functional recovery were minor and less confident, requiring further study. Notably, NMA showed that MSC-exosomes appear to offer nearly the same benefits as parent cells and may provide practical advantages. Given the limitations, these findings should be considered hypothesis-generating yet encouraging, as they support continued research on cell therapies in severe COVID-19 and raise the possibility that MSC or exosome treatment could become a valuable adjunct in ARDS care, if confirmed by larger trials.
| 1. | Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). 2023 Aug 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 2. | Krynytska I, Marushchak M, Birchenko I, Dovgalyuk A, Tokarskyy O. COVID-19-associated acute respiratory distress syndrome versus classical acute respiratory distress syndrome (a narrative review). Iran J Microbiol. 2021;13:737-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 3. | Weiss DJ, Rolandsson Enes S. MSC-Based Cell Therapy for COVID-19-Associated ARDS and Classical ARDS: Comparative Perspectives. Curr Stem Cell Rep. 2024;10:9-19. [DOI] [Full Text] |
| 4. | Navas-Blanco JR, Dudaryk R. Management of Respiratory Distress Syndrome due to COVID-19 infection. BMC Anesthesiol. 2020;20:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Safwan M, Bourgleh MS, Alshakaki H, Molhem A, Haider KH. Morbid Cell Status and Donor Age Significantly Alter Mesenchymal Stem Cell Functionality and Reparability. In: Haider KH. Handbook of Stem Cell Applications. Singapore: Springer, 2023. [DOI] [Full Text] |
| 6. | Liang D, Liu C, Yang M. Mesenchymal stem cells and their derived exosomes for ALI/ARDS: A promising therapy. Heliyon. 2023;9:e20387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 7. | Xu Z, Huang Y, Zhou J, Deng X, He W, Liu X, Li Y, Zhong N, Sang L. Current Status of Cell-Based Therapies for COVID-19: Evidence From Mesenchymal Stromal Cells in Sepsis and ARDS. Front Immunol. 2021;12:738697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Blot M, Jacquier M, Pauchard LA, Rebaud C, Marlin C, Hamelle C, Bataille A, Croisier D, Thomas C, Jalil A, Mirfendereski H, Piroth L, Chavanet P, Bensoussan D, Laroye C, Reppel L, Charles PE. Adverse Mechanical Ventilation and Pneumococcal Pneumonia Induce Immune and Mitochondrial Dysfunctions Mitigated by Mesenchymal Stem Cells in Rabbits. Anesthesiology. 2022;136:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, Krasnodembskaya AD. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017;196:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 604] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 10. | Fang L, Hu F, Li H, Chang W, Liu L. Efficacy and safety of mesenchymal stem cell therapy for acute respiratory distress syndrome-a systematic review and meta-analysis. J Thorac Dis. 2024;16:5802-5814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 5222] [Article Influence: 1044.4] [Reference Citation Analysis (1)] |
| 12. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26303] [Article Influence: 1753.5] [Reference Citation Analysis (4)] |
| 13. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 8051] [Article Influence: 670.9] [Reference Citation Analysis (0)] |
| 14. | The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.4. [cited 30 September 2025]. Available from: https://revman.cochrane.org. |
| 15. | Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 718] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 16. | jamovi. The jamovi project (Version 2.3). Available from: https://www.jamovi.org. |
| 17. | rdrr.io. metasens: Statistical Methods for Sensitivity Analysis in Meta-Analysis. [cited 15 May 2025]. Available from: https://rdrr.io/cran/metasens/. |
| 18. | Allaire JJ. RStudio: Integrated Development Environment for R. [cited 15 May 2025]. Available from: https://www.r-project.org/conferences/useR-2011/abstracts/180111-allairejj.pdf. |
| 19. | Metelli S. A fully interactive web-application for producing and visualising network meta-analyses. [cited 15 May 2025]. Available from: https://training.cochrane.org/sites/training.cochrane.org/files/public/uploads/NMAstudio.pdf. |
| 20. | Shi L, Yuan X, Yao W, Wang S, Zhang C, Zhang B, Song J, Huang L, Xu Z, Fu JL, Li Y, Xu R, Li TT, Dong J, Cai J, Li G, Xie Y, Shi M, Li Y, Zhang Y, Xie WF, Wang FS. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;75:103789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Zarrabi M, Shahrbaf MA, Nouri M, Shekari F, Hosseini SE, Hashemian SR, Aliannejad R, Jamaati H, Khavandgar N, Alemi H, Madani H, Nazari A, Amini A, Hassani SN, Abbasi F, Jarooghi N, Fallah N, Taghiyar L, Ganjibakhsh M, Hajizadeh-Saffar E, Vosough M, Baharvand H. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: a randomized controlled trial. Stem Cell Res Ther. 2023;14:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 22. | Lightner AL, Sengupta V, Qian S, Ransom JT, Suzuki S, Park DJ, Melson TI, Williams BP, Walsh JJ, Awili M. Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicle Infusion for the Treatment of Respiratory Failure From COVID-19: A Randomized, Placebo-Controlled Dosing Clinical Trial. Chest. 2023;164:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 23. | Soetjahjo B, Malueka RG, Nurudhin A, Purwoko, Sumardi, Wisaksana R, Adhiputri A, Sudadi, Soeroto AY, Sidharta BRA, Thobari JA, Murni TW, Soewondo W, Herningtyas EH, Sudjud RW, Trisnawati I, Ananda NR, Faried A. Effectiveness and safety of normoxic allogenic umbilical cord mesenchymal stem cells administered as adjunctive treatment in patients with severe COVID-19. Sci Rep. 2023;13:12520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Li TT, Zhang B, Fang H, Shi M, Yao WQ, Li Y, Zhang C, Song J, Huang L, Xu Z, Yuan X, Fu JL, Zhen C, Zhang Y, Wang ZR, Zhang ZY, Yuan MQ, Dong T, Bai R, Zhao L, Cai J, Dong J, Zhang J, Xie WF, Li Y, Shi L, Wang FS. Human mesenchymal stem cell therapy in severe COVID-19 patients: 2-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2023;92:104600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 25. | Bowdish ME, Barkauskas CE, Overbey JR, Gottlieb RL, Osman K, Duggal A, Marks ME, Hupf J, Fernandes E, Leshnower BG, Golob JL, Iribarne A, Rassias AJ, Moquete EG, O'Sullivan K, Chang HL, Williams JB, Parnia S, Patel NC, Desai ND, Vekstein AM, Hollister BA, Possemato T, Romero C, Hou PC, Burke E, Hayes J, Grossman F, Itescu S, Gillinov M, Pagani FD, O'Gara PT, Mack MJ, Smith PK, Bagiella E, Moskowitz AJ, Gelijns AC. A Randomized Trial of Mesenchymal Stromal Cells for Moderate to Severe Acute Respiratory Distress Syndrome from COVID-19. Am J Respir Crit Care Med. 2023;207:261-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 26. | Pochon C, Laroye C, Kimmoun A, Reppel L, Dhuyser A, Rousseau H, Gauthier M, Petitpain N, Chabot JF, Valentin S, de Carvalho Bittencourt M, Peres M, Aarnink A, Decot V, Bensoussan D, Gibot S. Efficacy of Wharton Jelly Mesenchymal Stromal Cells infusions in moderate to severe SARS-Cov-2 related acute respiratory distress syndrome: a phase 2a double-blind randomized controlled trial. Front Med (Lausanne). 2023;10:1224865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Martínez-Muñoz ME, Payares-Herrera C, Lipperheide I, Malo de Molina R, Salcedo I, Alonso R, Martín-Donaire T, Sánchez R, Zafra R, García-Berciano M, Trisán-Alonso A, Pérez-Torres M, Ramos-Martínez A, Ussetti P, Rubio JJ, Avendaño-Solà C, Duarte RF. Mesenchymal stromal cell therapy for COVID-19 acute respiratory distress syndrome: a double-blind randomised controlled trial. Bone Marrow Transplant. 2024;59:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Sitbon A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Diehl JL, Demoule A, Annane D, Marois C, Demeret S, Weiss E, Voiriot G, Fartoukh M, Constantin JM, Mégarbane B, Plantefève G, Boucher-Pillet H, Churlaud G, Cras A, Maheux C, Pezzana C, Diallo MH, Lebbah S, Ropers J, Salem JE, Straus C, Menasché P, Larghero J, Monsel A; APHP STROMA–CoV‐2 Collaborative Research Group. Treatment of COVID-19-associated ARDS with umbilical cord-derived mesenchymal stromal cells in the STROMA-CoV-2 multicenter randomized double-blind trial: long-term safety, respiratory function, and quality of life. Stem Cell Res Ther. 2024;15:109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Rebelatto CLK, Senegaglia AC, Franck CL, Daga DR, Shigunov P, Stimamiglio MA, Marsaro DB, Schaidt B, Micosky A, de Azambuja AP, Leitão CA, Petterle RR, Jamur VR, Vaz IM, Mallmann AP, Carraro Junior H, Ditzel E, Brofman PRS, Correa A. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther. 2022;13:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Gorman EA, Rynne J, Gardiner HJ, Rostron AJ, Bannard-Smith J, Bentley AM, Brealey D, Campbell C, Curley G, Clarke M, Dushianthan A, Hopkins P, Jackson C, Kefela K, Krasnodembskaya A, Laffey JG, McDowell C, McFarland M, McFerran J, McGuigan P, Perkins GD, Silversides J, Smythe J, Thompson J, Tunnicliffe WS, Welters IDM, Amado-Rodríguez L, Albaiceta G, Williams B, Shankar-Hari M, McAuley DF, O'Kane CM. Repair of Acute Respiratory Distress Syndrome in COVID-19 by Stromal Cells (REALIST-COVID Trial): A Multicenter, Randomized, Controlled Clinical Trial. Am J Respir Crit Care Med. 2023;208:256-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, Alvarez Gil A, Poggioli R, Ruiz P, Marttos AC, Hirani K, Bell CA, Kusack H, Rafkin L, Baidal D, Pastewski A, Gawri K, Leñero C, Mantero AMA, Metalonis SW, Wang X, Roque L, Masters B, Kenyon NS, Ginzburg E, Xu X, Tan J, Caplan AI, Glassberg MK, Alejandro R, Ricordi C. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10:660-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 298] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 32. | Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, Wang S, Zhang C, Yuan X, Xu Z, Huang L, Fu JL, Li Y, Zhang Y, Yao WQ, Liu T, Song J, Sun L, Yang F, Zhang X, Zhang B, Shi M, Meng F, Song Y, Yu Y, Wen J, Li Q, Mao Q, Maeurer M, Zumla A, Yao C, Xie WF, Wang FS. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 33. | Abdullah M, Pawitan JA, Irawan C, - R, Aditianingsih D, Liem IK, Sinto R, Susilo A, Yulianti M, Handayani RRD, Pratomo IP, Burhan E, Damayanti T, Wibowo H, Dilogo IH, Muliawan HS, Elhidsi M. Effectiveness and safety profile of mesenchymal stem cell secretome as a treatment for severe cases of COVID-19: a randomized controlled trial. F1000Res. 2022;11:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Nguekap Tchoumba O, Diehl JL, Demoule A, Annane D, Marois C, Demeret S, Weiss E, Voiriot G, Fartoukh M, Constantin JM, Mégarbane B, Plantefève G, Malard-Castagnet S, Burrel S, Rosenzwajg M, Tchitchek N, Boucher-Pillet H, Churlaud G, Cras A, Maheux C, Pezzana C, Diallo MH, Ropers J, Menasché P, Larghero J; APHP STROMA–CoV-2 Collaborative Research Group. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care. 2022;26:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 35. | Adas G, Cukurova Z, Yasar KK, Yilmaz R, Isiksacan N, Kasapoglu P, Yesilbag Z, Koyuncu ID, Karaoz E. The Systematic Effect of Mesenchymal Stem Cell Therapy in Critical COVID-19 Patients: A Prospective Double Controlled Trial. Cell Transplant. 2021;30:9636897211024942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 36. | Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2627] [Cited by in RCA: 3884] [Article Influence: 388.4] [Reference Citation Analysis (0)] |
| 37. | Azagew AW, Beko ZW, Ferede YM, Mekonnen HS, Abate HK, Mekonnen CK. Global prevalence of COVID-19-induced acute respiratory distress syndrome: systematic review and meta-analysis. Syst Rev. 2023;12:212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 38. | Wang F, Li Y, Wang B, Li J, Peng Z. The safety and efficacy of mesenchymal stromal cells in ARDS: a meta-analysis of randomized controlled trials. Crit Care. 2023;27:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 39. | Yao W, Dong H, Qi J, Zhang Y, Shi L. Safety and efficacy of mesenchymal stem cells in severe/critical patients with COVID-19: A systematic review and meta-analysis. EClinicalMedicine. 2022;51:101545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat Rev Drug Discov. 2023;22:449-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 443] [Article Influence: 147.7] [Reference Citation Analysis (0)] |
| 41. | Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA, Martín-Rodríguez C, Díaz-Domínguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Añón JM, Fernández RL, González-Martín JM; dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 809] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 42. | Charan J, Dutta S, Kaur R, Bhardwaj P, Sharma P, Ambwani S, Jahan I, Abubakar AR, Islam S, Hardcastle TC, Rahman NAA, Lugova H, Haque M. Tocilizumab in COVID-19: a study of adverse drug events reported in the WHO database. Expert Opin Drug Saf. 2021;20:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 43. | Kang H, Kang CK, Im JH, Cho Y, Kang DY, Lee JY. Adverse Drug Events Associated With Remdesivir in Real-World Hospitalized Patients With COVID-19, Including Vulnerable Populations: A Retrospective Multicenter Study. J Korean Med Sci. 2023;38:e346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 44. | Weinberger J, Rhee C, Klompas M. Incidence, Characteristics, and Outcomes of Ventilator-associated Events during the COVID-19 Pandemic. Ann Am Thorac Soc. 2022;19:82-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Haribhai S, Mahboobi SK. Ventilator Complications. 2022 Sep 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 46. | Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142-4157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 617] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 47. | Gunjan, Himanshu, Pandey RP, Mukherjee R, Chang CM. Advanced meta-analysis on therapeutic strategies of mesenchymal derived exosome for diabetic chronic wound healing and tissue remodeling. Mol Cell Probes. 2024;77:101974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 48. | Jabermoradi S, Paridari P, Ramawad HA, Gharin P, Roshdi S, Toloui A, Yousefifard M. Stem Cell-Derived Exosomes as a Therapeutic Option for Spinal Cord Injuries; a Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 2025;13:e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Safwan M, Bourgleh MS, Aldoush M, Haider KH. Tissue-source effect on mesenchymal stem cells as living biodrugs for heart failure: Systematic review and meta-analysis. World J Cardiol. 2024;16:469-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 50. | Jihwaprani MC, Sula I, Charbat MA, Haider KH. Establishing delivery route-dependent safety and efficacy of living biodrug mesenchymal stem cells in heart failure patients. World J Cardiol. 2024;16:339-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Ahmed ZT, Zain Al-Abeden MS, Al Abdin MG, Muqresh MA, Al Jowf GI, Eijssen LMT, Haider KH. Dose-response relationship of MSCs as living Bio-drugs in HFrEF patients: a systematic review and meta-analysis of RCTs. Stem Cell Res Ther. 2024;15:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Ahmed OTF, Ahmed ZT, Dairi AW, Zain Al-Abeden MS, Alkahlot MH, Alkahlot RH, Al Jowf GI, Eijssen LMT, Haider KH. The inconclusive superiority debate of allogeneic versus autologous MSCs in treating patients with HFrEF: a systematic review and meta-analysis of RCTs. Stem Cell Res Ther. 2025;16:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Stucky A, Gao L, Li SC, Tu L, Luo J, Huang X, Chen X, Li X, Park TH, Cai J, Kabeer MH, Plant AS, Sun L, Zhang X, Zhong JF. Molecular Characterization of Differentiated-Resistance MSC Subclones by Single-Cell Transcriptomes. Front Cell Dev Biol. 2022;10:699144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/