Published online Oct 26, 2025. doi: 10.4252/wjsc.v17.i10.112397
Revised: August 22, 2025
Accepted: October 9, 2025
Published online: October 26, 2025
Processing time: 91 Days and 8.6 Hours
Tendon and ligament injuries represent a major orthopedic challenge with limited effective regenerative options. In an original research study by Yang et al de
Core Tip: Yang et al demonstrate that aligned nanofiber scaffolds combined with cyclic strain synergistically promote tenogenic differentiation of mesenchymal stem cells. This biomimetic strategy mimics native ligament cues and remains effective even under Rho-associated protein kinase inhibition, offering a promising, clinically translatable approach for engineering functional ligament grafts in regenerative medicine.
- Citation: Mahajan AA, Rajendran RL, Gangadaran P, Ahn BC. Engineering ligament tissues: Synergistic power of aligned nanofibers and cyclic stretch. World J Stem Cells 2025; 17(10): 112397
- URL: https://www.wjgnet.com/1948-0210/full/v17/i10/112397.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i10.112397
Ligament injuries, particularly those affecting the anterior cruciate ligament (ACL), pose a significant clinical challenge due to the tissue’s poor capacity for self-repair[1]. These injuries are common in athletes and individuals engaged in high-impact or high-intensity activities and often result from abrupt directional changes or trauma to the knee[2]. Once damaged, ligaments have diminished regenerative ability because they are largely avascular, composed of densely packed collagen fibers with limited cellular content. Their poor vascularization impedes nutrient and oxygen delivery to the injury site, undermines the healing cascade, and often leads to suboptimal tissue remodeling[3]. Moreover, unlike bone or skin, ligaments cannot regenerate authentic tissue architecture once disrupted, usually healing with disorganized scar tissue that lacks the strength and elasticity of the native ligament.
Current clinical interventions for ligament injuries, such as ACL reconstruction, typically involve autografts or allografts to replace the damaged tissue[4]. While somewhat effective, these approaches have notable limitations. Autografts-harvested from a patient's tendons, such as those from the hamstring or patellar tendon-can cause donor site morbidity, pain, and recovery challenges[5]. Allografts, which are donor tissues, carry risks of immune rejection, disease transmission, and inferior mechanical performance over time, particularly in younger or more active patients[6]. Despite advances in surgical techniques, reconstructive procedures often fail to fully replicate the biomechanical function of a healthy ligament, resulting in a heightened risk of re-injury, chronic joint instability, and the eventual development of osteoarthritis[7].
In light of these limitations, tissue engineering has emerged as a promising strategy to develop biological ligament replacements that can replicate both the structure and function of native tissue. This multidisciplinary approach combines stem cells, scaffold biomaterials, and bioactive cues to support tissue regeneration[8]. Mesenchymal stem cells (MSCs) encompass multipotent stromal cells that are capable of differentiating into various connective tissue lineages. In particular, bone marrow-derived MSCs (BMSCs) differentiate efficiently into tenocytes, making them ideal for ligament regeneration. Yet, seeding BMSCs onto simple scaffolds falls short of replicating native ligament structure and function. Electrospinning produces nanofibrous scaffolds with high surface area, tunable porosity, and precisely aligned fibers that guide cell elongation, enhance extracellular matrix (ECM) deposition, and promote tenogenic differentiation[9,10]. Complementarily, bioreactor-based cyclic stretching delivers controlled strain magnitude, frequency, and duration to mimic physiological loading, thereby driving mechanotransduction pathways that enhance cell viability, alignment, and collagen synthesis, and improve tensile strength compared to static culture. Together, electrospun scaffolds and mechanical conditioning synergistically recreate the structural and biomechanical cues essential for effective ligament tissue engineering[11]. Through innovations in biomaterial fabrication (such as electrospinning) and bioreactor-enabled mechanostimulation, researchers can mimic the sophisticated biomechanical and structural cues found in native ligament tissue[12].
The manuscript by Yang et al[13] advances this field by systematically studying how the interplay between nanofiber scaffold alignment and cyclic tensile strain directs BMSC differentiation toward ligamentous phenotypes. Using poly-lactic acid (PLA)-based scaffolds with well-controlled fiber orientation, they explore the effects of mechanical loading on cell viability, morphology, and gene/protein expression. Notably, the investigation extends to the impact of Rho-associated protein kinase (ROCK) pathway inhibition - a previously established repressor of tenogenic differentiation - to probe compensatory mechanisms activated under optimized biomechanical environments. This comprehensive approach sheds light on both the practical strategies and molecular underpinnings of soft tissue engineering.
Canonical RhoA/ROCK activity supports cytoskeletal tension and collaborates with transforming growth factor-beta/Smad and mechanical cues during tenogenic/tendinous differentiation, whereas ROCK inhibition modulates (and can dampen) this transformation. Y-27632 frequently enhances cell survival/viability and suppresses myofibroblast differentiation. Lineage outcomes under ROCK inhibition are context-dependent across MSC systems. Against this backdrop, we emphasize that the persistence of ligament/tenogenic markers after inhibitor withdrawal has not been systematically shown for ligament constructs, underscoring the uniqueness and breakthrough significance of Yang et al’s finding[13] in comparison with previous reports[14-19].
The regenerative microenvironment of musculoskeletal tissues, such as ligaments, is highly dependent on structural organization and mechanical loading[20]. Native ligaments feature a highly aligned, anisotropic collagen fiber architecture that provides tensile strength and directs cellular orientation and fate through biomechanical signaling[21]. Replicating this unique hierarchical structure in vitro is essential for successful ligament tissue engineering. In their study, Yang et al[13] explored the effects of combining aligned nanofiber scaffolds with cyclic mechanical loading. They demonstrated that the interplay between scaffold topography and biomechanical stimuli significantly promotes the tenogenic differentiation of BMSCs.
Yang et al[13] used electrospun PLA nanofibre scaffolds with precisely controlled alignment to emulate the aligned fiber orientation of natural ligament ECM. These scaffolds significantly influenced BMSC morphology, encouraging the cells to elongate and align parallel to the direction of the fibers - an appearance strikingly similar to tenocytes in native ligament tissue. In contrast, scaffolds with randomly oriented fibers failed to guide BMSC morphology in a uniform direction, resulting in less effective cellular organization, reduced elongation, and lowered expression of ligament-specific markers. This confirms that the physical anisotropy of the scaffold plays a critical instructive role in stem cell behavior, serving as a biomimetic template for tissue development.
Beyond scaffold structure, the study also focused on the effect of mechanical stimulation - specifically, the application of cyclic uniaxial tensile strain. The authors implemented a loading regimen of 2% strain at a frequency of 0.5 Hz for 2 hours daily, parameters that align with physiological strain regimens experienced by ligaments during regular movement and mechanical loading. This mild mechanical stimulus reinforced BMSC alignment and elongation along the direction of applied strain while enhancing cellular viability, metabolic activity, and ECM production, particularly when combined with aligned scaffold architecture. This mirrors biologically relevant conditions in vivo, where ligament fibroblasts experience repetitive tensile forces that influence their phenotypic stability and ECM remodeling capacity.
At the molecular level, the dual application of aligned topography and cyclic stretch yielded a marked elevation in the expression of tenogenic gene and protein markers, including collagen type I alpha 2 chain, collagen type III alpha 1 chain, tenomodulin, and tenascin-C. These markers are critical indicators of tenocyte identity and ligament integrity, and their upregulation signals successful lineage commitment. Importantly, these effects were far more pronounced when aligned scaffolds were used in conjunction with mechanical stimulation compared to either treatment in isolation, underscoring the synergy between mechanical and structural cues.
Additionally, cytoskeletal analysis via confocal microscopy provided insight into cellular mechanobiology. Cells subjected to mechanical stimulation exhibited organized and polarized actin filaments (F-actin) aligned along the same axis as the applied strain and the scaffold fibers. This reinforcement of cellular polarity and internal tension is a key feature of tenocyte maturation and suggests that scaffold architecture and physical forces orchestrate a cohesive cytoskeletal response that precedes ECM organization and function.
In vivo, the human ACL routinely experiences tensile strains of 5%-10% during normal gait and up to 15%-20% during higher-impact activities such as jumping or sudden deceleration. By contrast, the bioreactor regimen in Yang et al[13] applied only 2% cyclic strain at 0.5 Hz. While this low strain level effectively drove tenogenic differentiation in vitro, it represents less than half of the physiological loading amplitude and only a fraction of the dynamic frequency spectrum encountered in vivo (walking at approximately 1 Hz, running at approximately 2-3 Hz). To better replicate joint biomechanics, future studies should test higher strain amplitudes (5%-10%)[22], increased loading frequencies (1-2 Hz), and multi-phase waveforms that mimic the loading-unloading cycles of daily activities. Incorporating variable duty cycles (e.g., alternating periods of high and low strain) and multi-axial loading patterns will further improve the fidelity of the in vitro model and strengthen its predictive relevance for in vivo ligament performance[23].
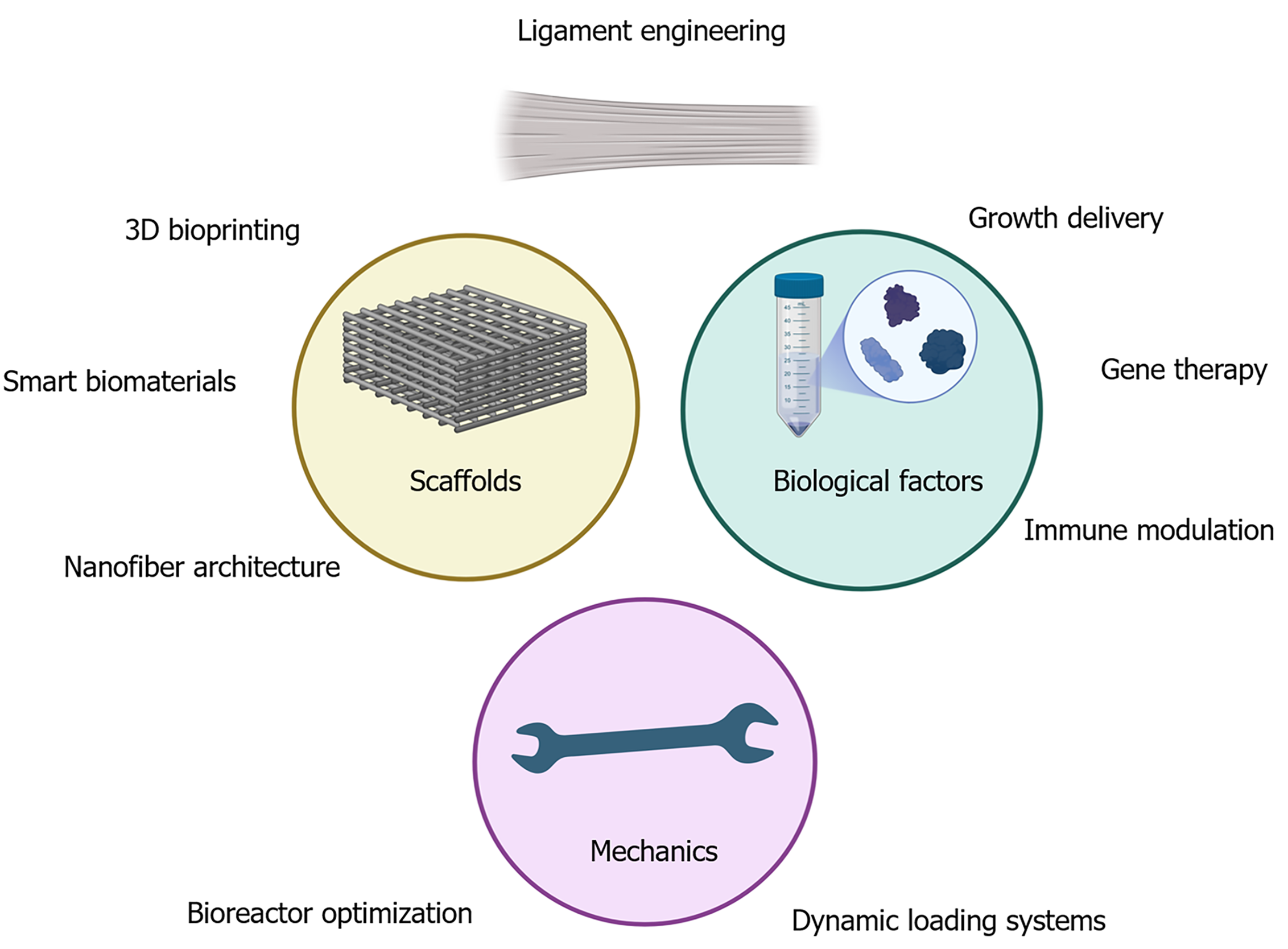
Together, the findings by Yang et al[13] reinforce the concept that mechanical stimuli and scaffold topography are not merely additive but synergistic tools in ligament tissue engineering. By mimicking the native mechanical microenvironment and applying structural templates that guide cell orientation, this dual approach effectively recapitulates critical aspects of in vivo ligament development. Such strategies offer a pathway toward creating functional tissue replacements and deepen our understanding of how stem cells sense and respond to physical and structural cues (Figure 1). This insight could benefit other applications in musculoskeletal and soft tissue regeneration.
While the study by Yang et al[13] presents significant advancements in ligament tissue engineering by combining aligned nanofiber scaffolds with cyclic mechanical stimulation, several notable limitations may affect the direct translation of these findings into clinical applications. A primary limitation is the exclusive reliance on in vitro experimentation. Although the controlled environment allowed for detailed observations of cell behavior, molecular changes, and scaffold interactions, it cannot fully replicate the complex milieu present in living organisms. Key factors such as joint biomechanics, immune system interactions, vascularization, and dynamic host tissue integration influence the success of implanted engineered grafts[24]. Without in vivo validation, it remains uncertain whether these scaffold-conditioned cells will survive, integrate, and perform effectively under physiological loading conditions over time. Thus, preclinical animal studies that incorporate mechanical loading in a joint environment, assess immune responses, and evaluate long-term tissue remodeling are critical next steps to confirm these constructs’ safety, durability, and functional capacity before moving toward human trials.
A unique aspect of the investigation was its focus on interfering with intracellular signaling using the ROCK inhibitor Y27632, which modulates cytoskeletal tension. The study reported no negative impact on cell viability or morphology following ROCK inhibition, suggesting that the inhibitor is biologically tolerated, at least in the short term. Nonetheless, the long-term biological consequences of ROCK pathway modulation remain uncertain, especially in vivo, where ROCK signaling plays key roles in angiogenesis, fibrosis, immune cell behavior, and tissue remodeling. Determining the safe and effective dose of Y27632 for therapeutic applications and its systemic effects will require further animal model testing and toxicological assessments.
Another limitation is the narrow mechanical regimen - 2% cyclic strain at 0.5 Hz for 2 hours daily - which overlooks the diverse loading conditions ligaments encounter. Studies show that higher strain magnitudes (8%-10%) enhance osteogenic and tenogenic markers via extracellular signal-regulated kinases 1 and 2 and Runt-related transcription factor 2 (Runx2) activation compared to 2%-2.5% strain. Frequency also matters: 1 Hz cyclic stretch yields stronger differentiation responses and YAP/TAZ nuclear translocation than 0.17-0.5 Hz, while high-frequency (30-40 Hz) microvibration further boosts osteogenesis via p38 mitogen-activated protein kinases signaling[25]. Varying stimulation duration - from intermittent bouts (30 minutes every 12 hours) to extended daily loading (6 hours) - has been shown to differentially affect cell viability and lineage commitment. Moreover, biaxial or triaxial loading systems better replicate physiological multi-directional forces and improve ECM synthesis over uniaxial stretch. Systematic exploration of these parameters is therefore essential to optimize tenogenic differentiation and develop clinically translatable conditioning protocols[26].
Another consideration is PLA’s slow in vivo degradation (10 months to 4 years), which may impede ECM remodeling and integration. To achieve a balanced resorption profile, blending PLA with faster-degrading polymers is effective[27]. For example, PLA/Lactic-co-glycolic acid (PLGA) (50:50) copolymers fully degrade in 6-8 weeks, with degradation rate tunable by lactic/glycolic ratio. Electrospun PLA/collagen hybrids maintain initial strength while collagen promotes cell-scaffold interactions and accelerates PLA hydrolysis. Similarly, PLGA/silk composite scaffolds leverage silk’s slower erosion to support early mechanical integrity and PLGA’s rapid bulk degradation to match tissue regeneration. Incorporating natural polymers such as gelatin or chitosan into PLA or PLGA matrices has also been shown to increase hydrophilicity and degradation rate without compromising biocompatibility[28]. Future studies should evaluate these formulations in BMSC-seeded ligament constructs to align scaffold resorption with new tissue formation.
While convenient for initial exploration, using rabbit-derived BMSCs as the cellular model introduces another limitation regarding clinical translation. Species differences in stem cell physiology, mechanosensitivity, and differentiation potential may affect how closely these results replicate human cellular responses[29]. For translational relevance, it is essential to validate findings with human MSCs or other clinically applicable stem cell sources, as these cells may react differently to scaffold cues and mechanical stimuli.
Additionally, Yang et al[13] used only BMSCs, yet native ligament repair involves the coordinated action of multiple cell types. For example, co-culturing BMSCs with primary tenocytes enhances tenogenic marker expression and ECM alignment in electrospun scaffolds. Incorporating endothelial cells (e.g., human umbilical vein endothelial cells) promotes early vascular network formation and supports nutrient delivery. Macrophages, particularly M2-polarized subtypes, facilitate inflammation resolution and secrete pro-regenerative cytokines that improve scaffold remodeling[30]. Fibroblasts or tendon-derived cells can further guide matrix organization and collagen crosslinking for mechanical integrity. Therefore, future studies should evaluate co-culture systems combining BMSCs with tenocytes, endothelial cells, macrophages, and fibroblasts to better replicate the multicellular milieu of ligament healing.
The relatively short culture duration (7 days) used in the study is another constraint. Ligament regeneration is a prolonged process involving ECM remodeling, collagen crosslinking, and maturation that can take weeks to months. A longer culture period would provide insight into the engineered tissue’s durability, mechanical strength, quality, and potential late-stage cellular behaviors crucial for clinical success.
Finally, the manuscript does not address scaffold-host integration or immune compatibility upon implantation. How well would the engineered construct vascularize, avoid fibrotic encapsulation, and integrate mechanically and biologically with surrounding native ligament tissue? Similarly, acute and chronic immune reactions to the scaffold or donor cells could impact graft survival and function. These factors are vital to ensure safety and long-term efficacy. Table 1 summarizes the preclinical-to-clinical pathway, key assessments, and regulatory milestones.
| Stage | Model & actions | Key assessments | Regulatory milestones |
| Small-animal studies | Rat ACL defect model GLP-compliant protocols | Immune: ISO 10993 cytokine panels; histology for inflammation. Degradation: In vivo micro-CT scaffold mass loss. Mechanics: Uniaxial tensile tests at weeks 4, 8, 12 | Pre-IND meeting with FDA |
| Large-animal validation | Sheep or goat ACL repair model | Immune: Serum CRP & IL-10; synovial fluid analysis. Vascular: MRI/ultrasound neovascularization. Mechanics: Cyclic fatigue of explants; gait analysis | IDE submission; ISO 10993 certification |
| Regulatory submission | Compile preclinical dossier CMC documentation (PLA formulation, fabrication controls) | Data aggregation: Safety, efficacy, reproducibility | IND filing (FDA) or CTD submission (EMA) |
| Phase I trial | Small human cohort | Immune: Serum cytokines; biopsy histopathology. Mechanics: Instrumented laxity testing; MRI | IRB approval; informed consent protocols |
| Phase II trial | Scaffold dose/size optimization; preliminary efficacy | Function: KT-1000 arthrometer; IKDC & Tegner scores. Degradation: MRI at months 3, 6, 12 | Interim safety reviews; DSMB oversight |
| Phase III trial | Large-scale efficacy and long-term safety | Biomechanics: Contralateral leg control; return-to-activity metrics. Integration: Second-look arthroscopy; histological biopsy | PMA submission (FDA) or CE marking application |
Ensuring the safety and biocompatibility of engineered scaffolds and associated treatments is critical in developing any tissue engineering strategy[31]. In the study by Yang et al[13], the preclinical safety assessment was primarily focused on in vitro analyses, providing an initial but essential lens into the compatibility of the scaffold system with MSCs. The results from cell viability assays, particularly the Cell Counting Kit-8 assay over a 7-day culture period, revealed no signs of cytotoxicity associated with the PLA nanofiber scaffolds. Cells seeded on aligned and randomly oriented PLA scaffolds retained normal morphology, maintained metabolic activity, and did not exhibit elevated cell death rates, demonstrating that the material is biocompatible and non-toxic under the tested conditions.
In terms of mechanical stimulation, the study employed a low-amplitude, physiologically relevant strain of 2% at 0.5 Hz, designed to mimic normal joint motion without inducing cellular stress or overloading. The chosen loading parameters did not result in any observable structural damage to the scaffold or deleterious outcomes on the embedded BMSCs. This confirms the mechanical safety of the loading protocols, which is critical for future translation into bioreactors or preconditioning systems used before implantation. However, it is essential to note that while low strain levels are safe, the tissue-engineered construct must also perform under more rigorous loading conditions post-implantation - a factor not yet evaluated in this study.
To minimize contamination, the team followed strict sterilization protocols during scaffold fabrication, an essential prerequisite for any biomaterial that may ultimately be used in clinical settings. Proper sterilization is crucial in ensuring both aseptic conditions during cell seeding and the absence of endotoxins or microbial agents that could interfere with cellular responses or provoke adverse immune reactions.
However, several essential safety gaps have not yet been addressed. The study did not investigate the immunogenicity of the scaffold or its potential to elicit inflammation following implantation. These are critical endpoints for clinical translation, as even biocompatible materials can trigger foreign body responses, fibrotic encapsulation, or chronic inflammation depending on patient age, immune status, and anatomical implantation site. The absence of vascularization and innervation assessments also raises questions about the construct’s ability to integrate functionally with host tissue. Long-term in vivo studies are necessary to evaluate scaffold degradation products, systemic responses, vascular integration, tissue remodeling, and functional performance metrics such as tensile strength and joint mobility after implantation.
The findings presented in this study by Yang et al[13] hold significant translational potential, offering actionable strategies to bridge the gap between laboratory research and clinical implementation in ligament repair. A considerable strength of the approach lies in the use of electrospinning technology to produce aligned PLA nanofiber scaffolds that closely mimic the anisotropic architecture of native ligaments. This fabrication method is not only scalable and reproducible, but also allows for customization of fiber parameters such as diameter, alignment, and porosity[32]. By fine-tuning these characteristics, researchers can tailor scaffolds to target specific anatomical sites or patient needs, enhancing their clinical versatility. The widespread use and regulatory familiarity with PLA as a medical-grade material also increase the clinical feasibility and regulatory appeal of this approach, simplifying pathways for future implantation trials.
Another valuable advantage lies in the demonstrated benefit of mechanical loading during in vitro culture. The study’s low-frequency cyclic uniaxial stretch resulted in significant gains in cell alignment, viability, and tenogenic differentiation. These results support the development of bioreactor systems aimed at conditioning engineered constructs before implantation. Preconditioning tissues under controlled mechanical stimuli could improve ECM organization, collagen fiber alignment, and overall mechanical strength, making the grafts more robust and functionally compatible when introduced into the dynamic environment of a joint. This approach also reduces the time needed for the implant to “mature” post-implantation, potentially accelerating recovery and improving patient outcomes.
Perhaps the most novel and clinically relevant finding is that BMSCs can differentiate tenogenically even with ROCK inhibition. The upregulation of alternative signaling molecules such as focal adhesion kinase (FAK) and RUNX2 suggests that tenogenic pathways are redundant and adaptable, allowing for modulation under various biochemical contexts[14]. This finding holds therapeutic promise, particularly for patients who may be on medications or have conditions that alter traditional mechanotransduction signaling. It also opens the door to custom-tailored interventions that exploit different mechanosensitive or cytoskeletal signaling routes to guide cell fate.
This study also lays the groundwork for expanding the engineered scaffold system to include multifactorial integrative strategies. While mechanical and structural guidance are critical, future designs could incorporate localized delivery of growth factors (e.g., transforming growth factor-beta and connective tissue growth factor), ECM proteins, or gene therapies to further promote ligamentous regeneration. Similarly, vascularization strategies such as co-culturing with endothelial cells or using angiogenic biomolecules will be essential for larger grafts or those intended for load-bearing ligaments that require sustained tissue perfusion. Additionally, hybrid scaffolds or decellularized matrices may be explored to mimic native tissue composition and minimize immune rejection while enhancing bioactivity.
Lastly, this study lays important groundwork for regulatory approval of tissue-engineered ligament constructs by using Food and Drug Administration-approved materials (PLA) and demonstrating cytocompatibility, controlled degradation, and tenogenic potential in vitro. Aligning scaffold fabrication with good laboratory practice standards and adhering to ISO biocompatibility guidelines further strengthens its regulatory readiness. Before progressing to human trials, however, a comprehensive preclinical evaluation is essential. Long-term animal studies should assess scaffold degradation kinetics, host immune response and fibrosis, neovascularization, and mechanical durability under physiological loading in anatomically relevant models (e.g., rabbit or sheep ACL repair). Functional endpoints - such as tensile strength recovery, joint stability, and gait analysis - will be critical to establish efficacy. The reproducible manufacturing of aligned nanofiber scaffolds combined with robust tenogenic outcomes supports a clear, evidence-based path toward clinical translation once these safety and efficacy parameters are validated.
The study by Yang et al[13] highlights a crucial turning point in the field of ligament tissue engineering, where biomimetic scaffold design and mechanical conditioning converge to create an environment that closely replicates native ligament architecture and function. Their work demonstrates that BMSCs, when seeded onto aligned electrospun PLA nanofiber scaffolds and subjected to low-amplitude cyclic tensile loading, undergo profound tenogenic differentiation marked by organized cell elongation, cytoskeletal alignment, and upregulation of tendon/Ligament-specific genes and proteins. The successfully engineered constructs display enhanced ECM deposition, including major ligamentous proteins such as collagen types I and III, tenascin-C, and tenomodulin, essential hallmarks of functional ligament tissue.
A particularly compelling aspect of this study is its new insight into the flexibility of tenogenic signaling pathways. The authors observed that even under pharmacological inhibition of the ROCK pathway - widely considered a key regulator of cytoskeletal dynamics and differentiation - BMSCs continued to commit to a ligament-like fate when scaffold alignment and mechanical stimuli were present. This implies the existence of redundant or compensatory signaling networks, such as those involving FAK and RUNX2, that can sustain tenogenic differentiation in supportive physical microenvironments. This mechanistic adaptability greatly expands the therapeutic relevance of this platform, suggesting it may be viable even in complex pathological or pharmacological scenarios where specific signaling pathways are compromised.
As promising as these findings are, translating them into clinical success requires addressing key limitations through carefully designed preclinical studies. Longer-term cultures are needed to better understand how the mechanical properties of the scaffold evolve and how stable the tenogenic phenotype remains as cells mature. Moreover, in vivo studies must evaluate immune responses, vascularization potential, scaffold degradation dynamics, and mechanical durability following implantation. These studies should aim to recreate joint-specific loading conditions, establish co-culturing with vascular and immune cells, and assess the scaffold’s ability to integrate and remodel in response to local signaling cues.
Furthermore, as we gain a deeper understanding of how stem cells interpret multiple, often competing, biophysical and biochemical cues, it will become possible to design increasingly sophisticated constructs that are preconditioned for in vivo success. Future iterations of engineered ligament grafts may include bioreactor-mediated preconditioning, controlled release of bioactive agents, or patient-specific modifications based on genetic, lifestyle, or injury-related factors.
Ultimately, the convergence of precision-engineered scaffolds, dynamic mechanical environments, and systems-level biological understanding paves the way for developing off-the-shelf, biofunctional ligament grafts. Such constructs hold the potential to more effectively restore structural integrity and joint function, reduce reliance on donor tissues, and significantly improve outcomes for patients suffering from debilitating ligament injuries. The work by Yang et al[13] represents a pivotal step forward and a beacon of progress toward next-generation therapies in orthopedic and regenerative medicine.
The present review synthesizes of Yang et al’s findings[13] within the broader context of ligament tissue engineering by emphasizing three key themes. First, it underscores the importance of biomimetic scaffold design, detailing how aligned electrospun PLA nanofibers replicate native ECM topography to guide BMSC morphology and enhance tenogenic gene expression. Second, it highlights the role of mechanical conditioning, explaining how low-amplitude cyclic tensile loading synergizes with scaffold alignment to activate mechanotransduction pathways (e.g., FAK, RUNX2) and promote robust ECM deposition of collagen I/III, tenascin-C, and tenomodulin. Third, it explores the molecular adaptability revealed by ROCK inhibition experiments, which demonstrate that tenogenic commitment can be maintained through compensatory signaling networks when physical cues are optimized. By integrating detailed discussion of fabrication technologies, mechanobiology, and signaling mechanisms, this review provides a cohesive interpretation of how Yang et al’s study[13] advances our understanding of stem cell-scaffold interactions and lays a foundation for future preclinical validation and clinical translation.
| 1. | Robinson JD Jr, Williamson T, Carson T, Whelan RJ, Abelow SP, Gilmer BB. Primary anterior cruciate ligament repair: Current concepts. J ISAKOS. 2023;8:456-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Forelli F, Riera J, Mazeas J, Coulondre C, Putnis S, Neri T, Rambaud A. Ligament Healing After Anterior Cruciate Ligament Rupture: An Important New Patient Pathway? Int J Sports Phys Ther. 2023;18:1032-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Bosco F, Rovere G, Giustra F, Masoni V, Cassaro S, Capella M, Risitano S, Sabatini L, Lucenti L, Camarda L. Advancements in Anterior Cruciate Ligament Repair—Current State of the Art. Surgeries. 2024;5:234-247. [DOI] [Full Text] |
| 4. | Neufeld EV, Sgaglione J, Sgaglione NA. Anterior Cruciate Ligament Reconstruction Graft Options. Arthroscopy. 2025;41:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Ostojic M, Indelli PF, Lovrekovic B, Volcarenghi J, Juric D, Hakam HT, Salzmann M, Ramadanov N, Królikowska A, Becker R, Prill R. Graft Selection in Anterior Cruciate Ligament Reconstruction: A Comprehensive Review of Current Trends. Medicina (Kaunas). 2024;60:2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 6. | Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 341] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Lu Y, Till SE, Labott JR, Reinholz AK, Hevesi M, Krych AJ, Camp CL, Okoroha KR. Graft Failure and Contralateral ACL Injuries After Primary ACL Reconstruction: An Analysis of Risk Factors Using Interpretable Machine Learning. Orthop J Sports Med. 2024;12:23259671241282316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Tang Y, Wang Z, Xiang L, Zhao Z, Cui W. Functional biomaterials for tendon/ligament repair and regeneration. Regen Biomater. 2022;9:rbac062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 9. | Wang J, Huang D, Ren H, Zhao Y. Bioinspired Spatially Ordered Multicellular Lobules for Liver Regeneration. Research (Wash D C). 2025;8:0634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Song T, Shen Q, Cheng Y, Zhi Y, Liu G, Ren H, Wang J. Mechanically Tunable Hydrogel Microfibers for Biomimetic Tumor Drug Testing. Adv Funct Mater. 2025. [DOI] [Full Text] |
| 11. | Owida HA, Al-Nabulsi JI, Alnaimat F, Al-Ayyad M, Turab NM, Al Sharah A, Shakur M. Recent Applications of Electrospun Nanofibrous Scaffold in Tissue Engineering. Appl Bionics Biomech. 2022;2022:1953861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Sheng R, Jiang Y, Backman LJ, Zhang W, Chen J. The Application of Mechanical Stimulations in Tendon Tissue Engineering. Stem Cells Int. 2020;2020:8824783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Yang CW, Zhang YQ, Chang H, Gao R, Chen D, Yao H. Aligned nanofiber scaffolds combined with cyclic stretch facilitate mesenchymal stem cell differentiation for ligament engineering. World J Stem Cells. 2025;17:107124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Melzer M, Schubert S, Müller SF, Geyer J, Hagen A, Niebert S, Burk J. Rho/ROCK Inhibition Promotes TGF-β3-Induced Tenogenic Differentiation in Mesenchymal Stromal Cells. Stem Cells Int. 2021;2021:8284690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Subramanian A, Kanzaki LF, Galloway JL, Schilling TF. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. Elife. 2018;7:e38069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (18)] |
| 16. | Wang T, Kang W, Du L, Ge S. Rho-kinase inhibitor Y-27632 facilitates the proliferation, migration and pluripotency of human periodontal ligament stem cells. J Cell Mol Med. 2017;21:3100-3112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Gegg C, Yang F. The Effects of ROCK Inhibition on Mesenchymal Stem Cell Chondrogenesis Are Culture Model Dependent. Tissue Eng Part A. 2020;26:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Xie Z, Xu Q, Sun L, Li R, Shi J, Yang Q, Zong M, Qin J. Effects of Y-27632 on the osteogenic and adipogenic potential of human dental pulp stem cells in vitro. Hum Exp Toxicol. 2022;41:9603271221089003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Wei YH, Liao SL, Wang SH, Wang CC, Yang CH. Simvastatin and ROCK Inhibitor Y-27632 Inhibit Myofibroblast Differentiation of Graves' Ophthalmopathy-Derived Orbital Fibroblasts via RhoA-Mediated ERK and p38 Signaling Pathways. Front Endocrinol (Lausanne). 2020;11:607968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Lei T, Zhang T, Ju W, Chen X, Heng BC, Shen W, Yin Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact Mater. 2021;6:2491-2510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | Kew SJ, Gwynne JH, Enea D, Abu-Rub M, Pandit A, Zeugolis D, Brooks RA, Rushton N, Best SM, Cameron RE. Regeneration and repair of tendon and ligament tissue using collagen fibre biomaterials. Acta Biomater. 2011;7:3237-3247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Mohtadi NG, Webster-Bogaert S, Fowler PJ. Limitation of motion following anterior cruciate ligament reconstruction. A case-control study. Am J Sports Med. 1991;19:620-4; discussion 624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 124] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | MacQueen L, Sun Y, Simmons CA. Mesenchymal stem cell mechanobiology and emerging experimental platforms. J R Soc Interface. 2013;10:20130179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Banovetz MT, Kennedy NI, LaPrade RF, Engebretsen L, Moatshe G. Biomechanical considerations for graft choice in anterior cruciate ligament reconstruction. Ann Jt. 2023;8:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 25. | Aiello BR, Iriarte-Diaz J, Blob RW, Butcher MT, Carrano MT, Espinoza NR, Main RP, Ross CF. Bone strain magnitude is correlated with bone strain rate in tetrapods: implications for models of mechanotransduction. Proc Biol Sci. 2015;282:20150321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Raman N, Imran SAM, Ahmad Amin Noordin KB, Zaman WSWK, Nordin F. Mechanotransduction in Mesenchymal Stem Cells (MSCs) Differentiation: A Review. Int J Mol Sci. 2022;23:4580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | da Silva D, Kaduri M, Poley M, Adir O, Krinsky N, Shainsky-Roitman J, Schroeder A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem Eng J. 2018;340:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 423] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 28. | Park SH, Gil ES, Kim HJ, Lee K, Kaplan DL. Relationships between degradability of silk scaffolds and osteogenesis. Biomaterials. 2010;31:6162-6172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Lee TC, Lee TH, Huang YH, Chang NK, Lin YJ, Chien PW, Yang WH, Lin MH. Comparison of surface markers between human and rabbit mesenchymal stem cells. PLoS One. 2014;9:e111390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Silva M, Ferreira FN, Alves NM, Paiva MC. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J Nanobiotechnology. 2020;18:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Antmen E, Vrana NE, Hasirci V. The role of biomaterials and scaffolds in immune responses in regenerative medicine: macrophage phenotype modulation by biomaterial properties and scaffold architectures. Biomater Sci. 2021;9:8090-8110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 32. | Dong X, Zhang J, Pang L, Chen J, Qi M, You S, Ren N. An anisotropic three-dimensional electrospun micro/nanofibrous hybrid PLA/PCL scaffold. RSC Adv. 2019;9:9838-9844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/