Published online Aug 26, 2023. doi: 10.4252/wjsc.v15.i8.842
Peer-review started: April 18, 2023
First decision: May 17, 2023
Revised: May 25, 2023
Accepted: June 27, 2023
Article in press: June 27, 2023
Published online: August 26, 2023
Processing time: 129 Days and 0.1 Hours
Intervertebral disc degeneration (IDD) is a main contributor to low back pain. Oxidative stress, which is highly associated with the progression of IDD, increases senescence of nucleus pulposus-derived mesenchymal stem cells (NPMSCs) and weakens the differentiation ability of NPMSCs in degenerated intervertebral discs (IVDs). Quercetin (Que) has been demonstrated to reduce oxidative stress in diverse degenerative diseases.
To investigate the role of Que in oxidative stress-induced NPMSC damage and to elucidate the underlying mechanism.
In vitro, NPMSCs were isolated from rat tails. Senescence-associated β-galactosidase (SA-β-Gal) staining, cell cycle, reactive oxygen species (ROS), real-time quantitative polymerase chain reaction (RT-qPCR), immunofluorescence, and western blot analyses were used to evaluated the protective effects of Que. Meanwhile the relationship between miR-34a-5p and Sirtuins 1 (SIRT1) was evaluated by dual-luciferase reporter assay. To explore whether Que modulates tert-butyl hydroperoxide (TBHP)-induced senescence of NPMSCs via the miR-34a-5p/SIRT1 pathway, we used adenovirus vectors to overexpress and downregulate the expression of miR-34a-5p and used SIRT1 siRNA to knockdown SIRT1 expression. In vivo, a puncture-induced rat IDD model was constructed, and X rays and histological analysis were used to assess whether Que could alleviate IDD in vivo.
We found that TBHP can cause NPMSCs senescence changes, such as reduced cell proliferation ability, increased SA-β-Gal activity, cell cycle arrest, the accumulation of ROS, and increased expression of senescence-related proteins. While abovementioned senescence indicators were significantly alleviated by Que treatment. Que decreased the expression levels of senescence-related proteins (p16, p21, and p53) and senescence-associated secreted phenotype (SASP), including IL-1β, IL-6, and MMP-13, and it increased the expression of SIRT1. In addition, the protective effects of Que on cell senescence were partially reversed by miR-34a-5p overexpression and SIRT1 knockdown. In vivo, X-ray, and histological analyses indicated that Que alleviated IDD in a puncture-induced rat model.
In summary, the present study provides evidence that Que reduces oxidative stress-induced senescence of NPMSCs via the miR-34a/SIRT1 signaling pathway, suggesting that Que may be a potential agent for the treatment of IDD.
Core Tip: In our article, we provide the evidence that quercetin (Que) can prevent oxidative stress induced senescence of nucleus pulposus-derived mesenchymal stem cells via miR-34a/SIRT1 signaling pathway. Moreover, Que could ameliorate the progression of intervertebral disc degeneration (IDD) in rat model. Thus, Que can be considered as a potential agent for the treatment of IDD.
- Citation: Zhao WJ, Liu X, Hu M, Zhang Y, Shi PZ, Wang JW, Lu XH, Cheng XF, Tao YP, Feng XM, Wang YX, Zhang L. Quercetin ameliorates oxidative stress-induced senescence in rat nucleus pulposus-derived mesenchymal stem cells via the miR-34a-5p/SIRT1 axis. World J Stem Cells 2023; 15(8): 842-865
- URL: https://www.wjgnet.com/1948-0210/full/v15/i8/842.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i8.842
As a widely known musculoskeletal disorder, low back pain (LBP) is the leading cause of disability and results in a huge economic burden on the family and society[1,2]. Intervertebral disc degeneration (IDD) is a main contributor to LBP, but the etiology of IDD is multifactorial[3]. Therefore, elucidating the underlying molecular mechanisms of IDD will help develop solutions for the prevention and treatment of IDD.
Several previous studies have indicated that oxidative stress and reactive oxygen species (ROS) are highly associated with the progression of IDD[4-7]. While ROS production is inevitable during the metabolic processes of disc cells, the progression of IDD is considered to be the result of disc cell senescence caused by ROS accumulation[7,8]. Recently, stem cell-based endogenous repair has been shown to play a significant role in the repair and regeneration of degenerative intervertebral discs (IVDs)[9,10]. Nucleus pulposus-derived mesenchymal stem cells (NPMSCs) were first reported by Risbud et al[11] in 2007, which rendered a basis for the endogenous repair of IDD[11]. We also previously found NPMSCs in normal and degenerated IVDs[12-15]. Wu et al[16] indicated that the regenerative potential of NPMSCs decreases with aging and degeneration of IVDs[16]. In addition, Ma et al[17] proposed that the decrease in endogenous NPMSCs caused by adverse microenvironments, such as oxidative stress, low nutrition, and inflammation, is one of the main reasons for the failure of endogenous repair of IDD[17]. Therefore, inhibition of oxidative stress-induced senescence of NPMSCs may be of great significance in alleviating IDD.
Quercetin (Que), a natural flavonoid widely present in various plants, has antioxidative stress, anti-inflammatory, and antiaging properties. Que has been used as a senolytic to prevent diverse degenerative diseases[18-20]. Zhu et al[21] found that Que reduces senescent cells by selectively killing senescent human epithelial cells and mouse bone marrow mesenchymal stem cells[21]. Feng et al[22] reported that Que attenuates oxidative stress-induced apoptosis in rat chondrocytes and prevents the progression of osteoarthritis in a rat model[22]. Previous clinical studies have also demonstrated that Que reduces the expression levels of senescence-related markers[23].
MicroRNAs (miRNAs) are small double-stranded noncoding RNAs that are approximately 20 nucleotides in length and interfere with RNA translation by binding to the 3’UTR sequence of the target gene RNA. Abnormal expression of miRNAs has been observed in degenerative IVD, indicating that miRNAs play an important role in the patho
In the present study, tert-butyl hydroperoxide (TBHP) was used to trigger oxidative stress in NPMSCs, which is widely accepted as an apoptosis and senescence in vitro model. The effects of Que on senescence in NPMSCs under oxidative stress and the role of the miR-34a-5p/SIRT1 pathway were investigated. Finally, the therapeutic effects were also evaluated in a puncture-induced rat IDD model.
All the procedures performed in this study were approved by the Ethical Committee of the Clinical Medical College of Yangzhou University. Sprague-Dawley (SD) rats (2-4 mo old and weighing 200-300 g) were obtained from Yangzhou University, No. SYXK (Su) 2017-0044. The separation of nucleus pulposus (NP) tissues and the isolation of NPMSCs were performed as previously described[14]. Briefly, the NP tissues obtained from SD rats were carefully separated by a microscope under sterile conditions, and the NP tissue was mechanically fragmented into 1 mm3 pieces and digested with 0.2% collagenase type II (Gibco, United States) at 37 °C in 5% CO2 for 12 h. Cells were filtered through a 75-µm cellular filter, centrifuged at 1000 rpm for 3 min, washed with phosphate-buffered saline (PBS), resuspended in Mesenchymal Stem Cell Complete Medium (Cyagen, United States) with 10% fetal bovine serum (FBS; HyClone, United States) and 1% antibiotics (Gibco, United States), and cultured in an incubator at 37 °C with 5% CO2. NPMSCs were subcultured at a ratio of 1:3 when they reached 80% confluence. The third passages of NPMSCs were used for the subsequent experiments.
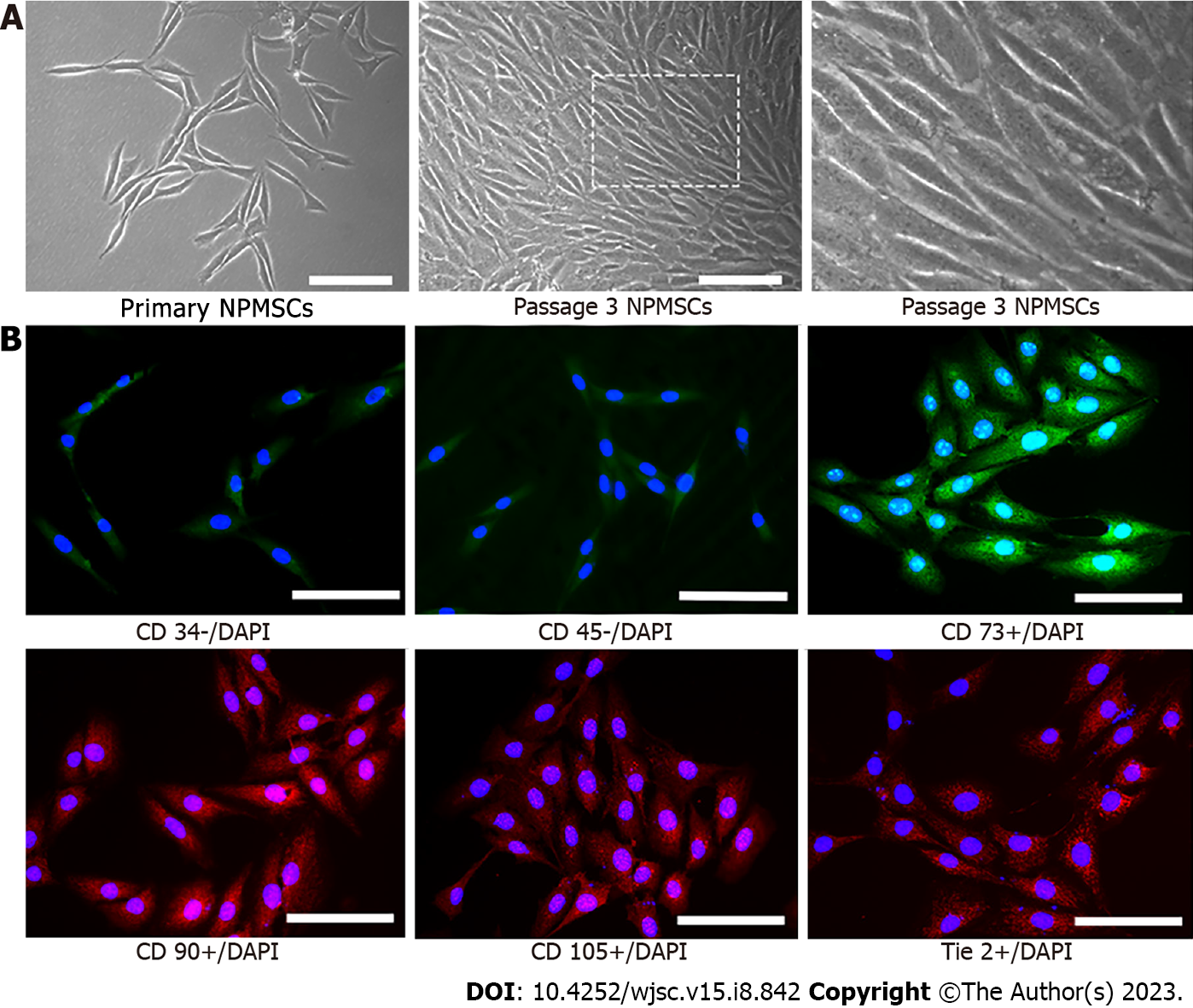
NPMSCs were cultured in a 12-well plate with a 25 mm diameter cell slide in complete medium. Cells were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 15 min. Subsequently, cells were blocked with 10% bovine serum albumin for 1 h at room temperature and incubated with the following primary antibodies (1:100) overnight at 4 °C according to the International Society for Cellular Therapy (ISCT): CD34 (ABclonal, China), CD45 (ABclonal, China), CD73 (ABclonal, China), CD90 (ABclonal, China), CD105 (Proteintech, China), and tyrosine kinase with Ig and EGF homology domains 2 (Tie2, a disc NP progenitor marker) (Biodragon, China). Cells were then incubated with FITC- or Cy3-conjugated secondary antibodies (1:200) at room temperature for 1 h in the dark. After treatment with 4’,6-diamidino-2-phenylindole (DAPI) for 5 min, the cell slides were observed under a fluorescence microscope (Leica, Wetzlar, Germany).
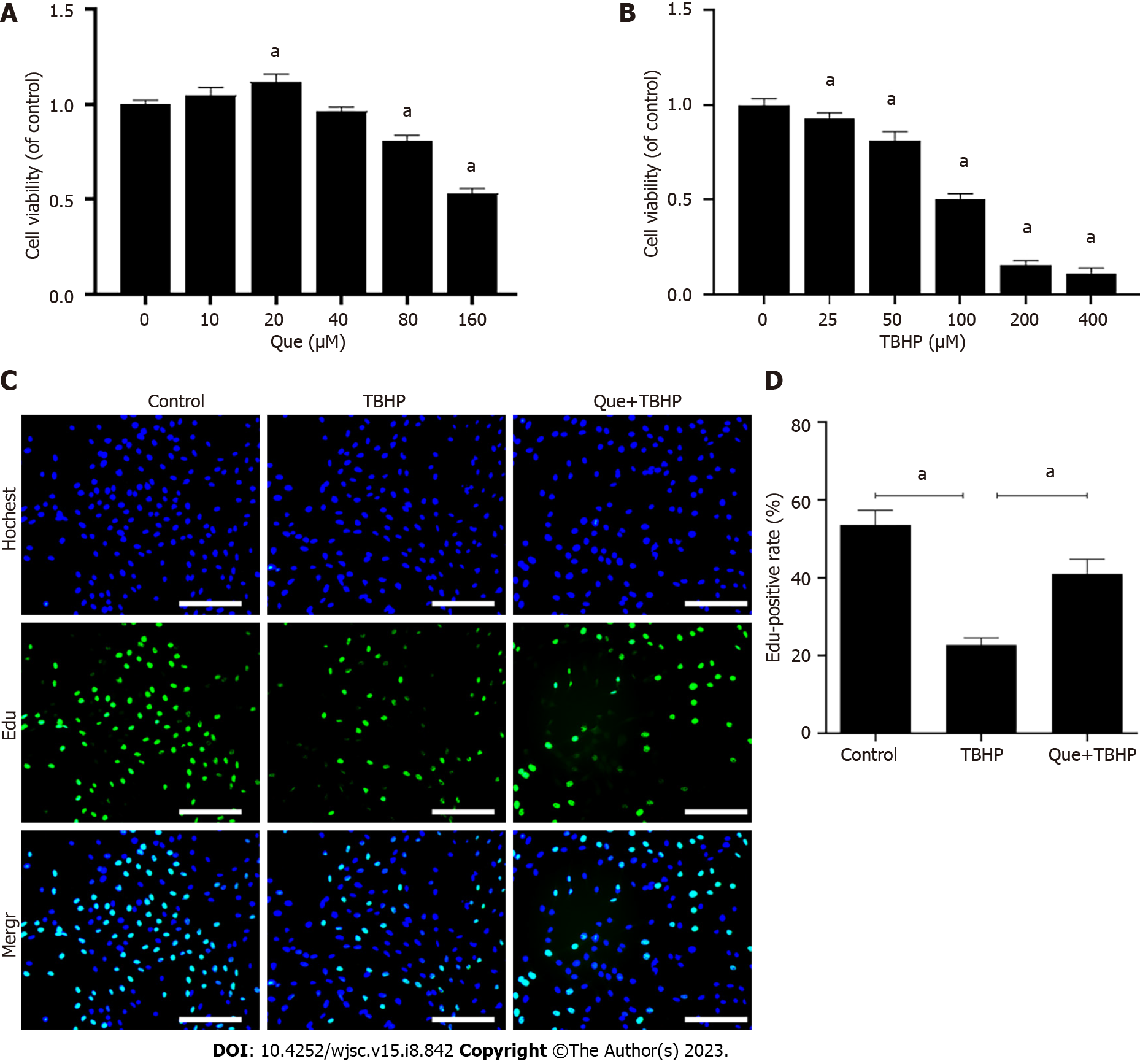
To establish an oxidative stress-induced model of NPMSCs, NPMSCs were cultured in complete medium with different concentrations (0, 25, 50, 100, 200, and 400 µM) of TBHP (Sigma-Aldrich, United States) for 12 h. Cells were pretreated with different concentrations (0, 10, 20, 40, 80, and 160 µM) of Que (MedChem Express, China) for 24 h before the addition of a suitable concentration of TBHP to investigate the suitable dose of Que. A cell counting kit-8 (CCK-8, Beyotime, China) was used to evaluate cell viability according to the manufacturer’s instructions. Briefly, 2 × 103 cells/well were seeded in 96-well plates and cultured overnight at 37 °C in 5% CO2. After the cells were treated as described above, a mixture of 10 µL of CCK-8 reagent and 100 µL of fresh medium was added to each well for 1 h at 37 °C. The optical density (OD) value of each well was measured at 450 nm by a microplate reader (Bio-Rad, United States). Cells were divided into the following groups: Control group (untreated), TBHP group (treated with 100 µM TBHP) and Que + TBHP group (treated with 20 µM Que and 100 µM TBHP).
Cell proliferation was evaluated by an 5-ethynyl-2’-deoxyuridine (EdU) assay (Beyotime, China). Cells (5 × 105 cells/well) were seeded in 12-well plates and cultured at 37 °C in 5% CO2. According to the manufacturer’s instructions, NPMSCs were incubated with EdU for 2 h, fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.5% Triton X-100 for 15 min, and incubated with Click Reaction Mixture for 30 min. After washing with PBS, cells were counterstained with Hoechst 33342 in the dark for 10 min. Finally, cells were observed under a fluorescence microscope and analyzed by ImageJ software (NIH, United States).
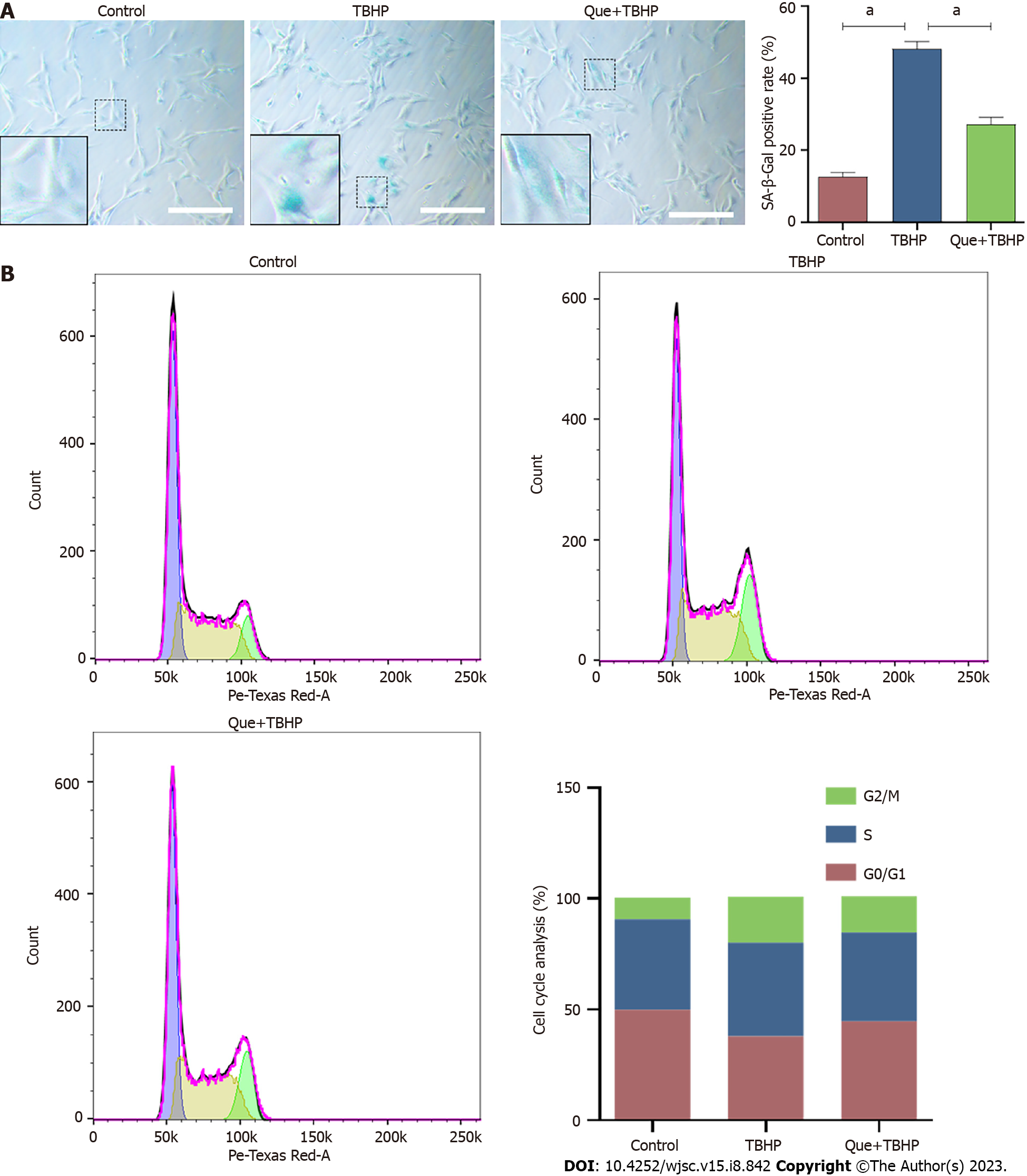
NPMSCs were seeded in a 6-well plate at a density of 4 × 105 cells/well at 37 °C in 5% CO2. Cells were stained with SA-β-Gal using a SA-β-Gal staining kit (Beyotime, China) according to the manufacturer’s instructions. Cells were fixed with SA-β-gal fixing solution for 15 min at room temperature and then incubated overnight with an SA-β-Gal working solution at 37 °C without CO2. Cells were observed under a microscope and analyzed by ImageJ software.
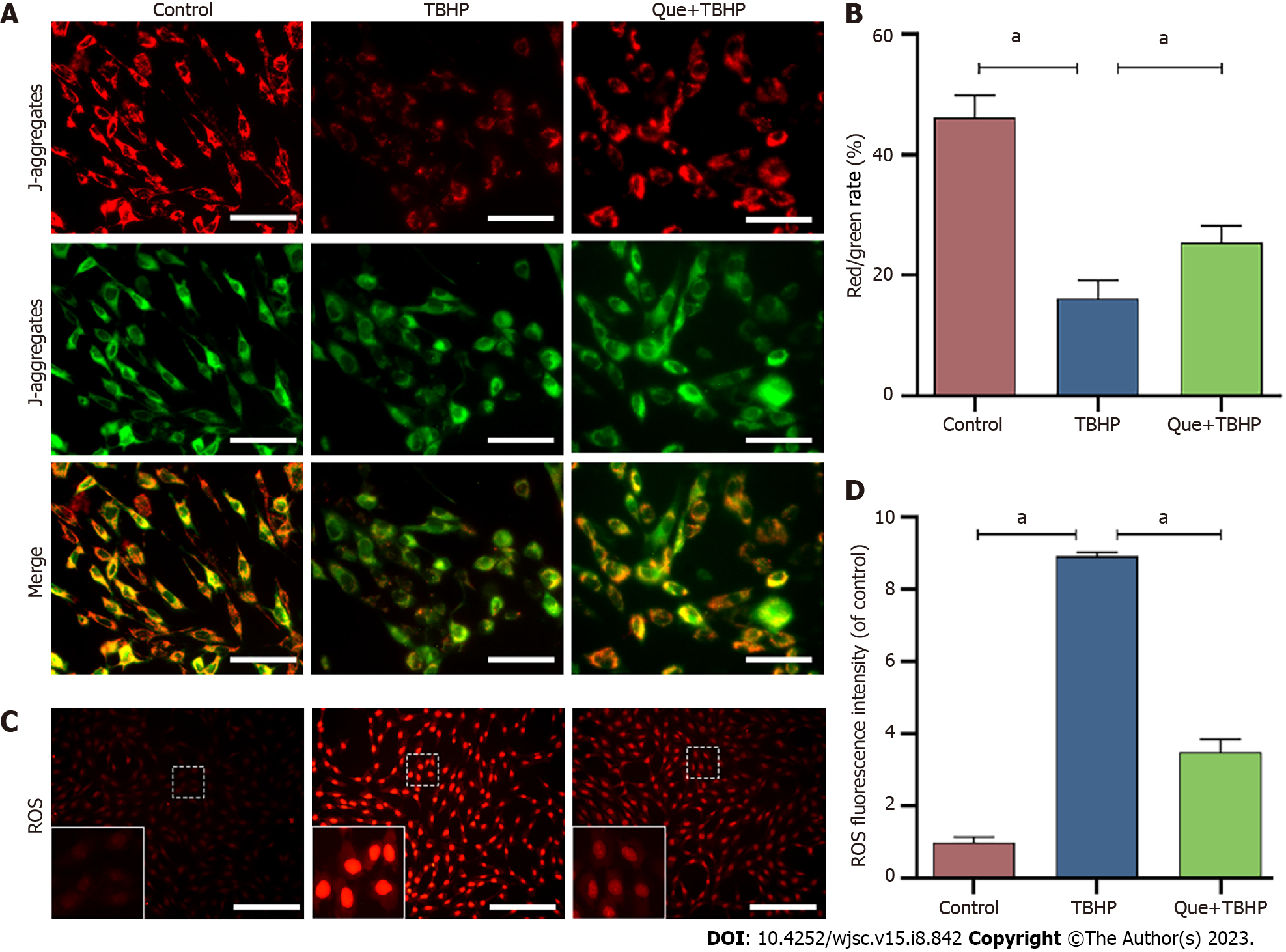
According to the manufacturer’s instructions provided by a ROS detection fluorescent probe-DHE kit (Keygen, China), NPMSCs were incubated with 20 µM DHE for 30 min at 37 °C. Cells were then observed under a fluorescence microscope and analyzed by ImageJ software.
A JC-1 Detection Kit (Keygen, China) was used to measure mitochondrial membrane potential (MMP). Briefly, NPMSCs were cultured in medium containing 2 µM JC-1 dye, a cationic dye, for 20 min. After being washed twice with incubation buffer, cells were cultured in complete medium, observed by fluorescence microscopy, and analyzed by ImageJ software.
The cell cycle phases of TBHP-induced senescent NPMSCs were determined by flow cytometric analyses using a Cell Cycle Detection Kit (Keygen, China). Briefly, NPMSCs were harvested from 6-well plates, washed twice with ice-cold PBS, fixed with 75% ethanol at 4 °C for 2 h, and incubated with a mixed solution of propidium iodide (PI) and RNase A for 30 min in the dark. Flow cytometry (BD Company, USA) was used to analyze the cell cycle phases.
Adenovirus vectors containing miR-34a-5p mimic, miR-34a-5p inhibitor, and their corresponding negative controls were obtained from GeneChem (Shanghai, China). SIRT1 siRNA and scrambled siRNA were obtained from Gene Pharma (Shanghai, China). NPMSCs (5×105/well) were seeded in 6-well plates and then transfected with miR-34a-5p mimic or miR-34a-5p inhibitor using Lipofectamine 2000 (Thermo Fisher, UT, USA) for 12 h at 37 °C according to the manufacturer’s protocol. After replacing the medium with fresh complete medium, NPMSCs were cultured for an additional 24 h. Cells were then harvested for subsequent experiments.
The TargetScan Human database (https://www.targetscan.org/vert_80/) was used to predict the complementary binding site of miR-34a-5p in the 3'-UTR of SIRT1 mRNA. According to the Lipofectamine 2000 transfection protocol, SIRT1 3'-UTR wild-type plasmids or mutant plasmids were cotransfected with miR-34a-5p mimic and mimic control into cells. After 48 h, a dual-luciferase reporter assay kit was used to detect luciferase activity.
TRIzol reagent (Invitrogen, United States) was used to extract total RNA from NPMSCs. To measure the expression levels of mRNA, RNA was reverse-transcribed into complementary DNA (cDNA) by a Prime Script-RT reagent kit (Vazyme Biotech, China) according to the manufacturer’s protocol. cDNA was amplified by SYBR Premix Ex Taq (Vazyme Biotech, China). To measure the expression levels of miR-34a, reverse transcription of RNA and amplification of cDNA were performed using the miRNA 1st Strand cDNA Synthesis Kit (by stem-loop) (Vazyme Biotech, China) and miRNA Universal SYBR qPCR Master Mix (Vazyme Biotech, China). The 2-ΔΔCT method was used to calculate the expression level of target genes. The expression levels of mRNA or miRNA were normalized to Gapdh or U6. The primers for the target genes used in this study are listed in Table 1.
| Gapdh | F: CTGGAGAAACCTGCCAAGTATG |
| R: GGTGGAAGAATGGGAGTTGCT | |
| SIRT1 | F: TGACCTCCTCATTGTTATTGGG |
| R: GGCATACTCGCCACCTAACCT | |
| U6 | F: CTCGCTTCGGCAGCACA |
| R: AACGCTTCACGAATTTGCGT | |
| miR-34a-5p | F: ACACTCCAGCTGGGTGGCAGTGTCTTAGCT |
| R: TGGTGTCGTGGAGTCG |
The total protein of NPMSCs was extracted by a Whole Cell Lysis assay (Keygen Biotech, China), and the protein concentration was determined by a BCA protein assay kit (Beyotime, China). Each sample containing 30 µg of protein was separated by 10%-12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (Millipore, United States). After being blocked in 5% skim milk for 2 h at room temperature, the membranes were incubated overnight with the following primary antibodies at 4 °C: P16 (1:1000; Proteintech, China), p21 (1:1000; Proteintech, China), p53 (1:5000; Proteintech, China), SIRT1 (1:1000; ABclonal, China), IL-1β (1:1000; ABclonal, China), IL-6 (1:1000; ABclonal, China), matrix metalloproteinase-13 (MMP-13, 1:1000; ABclonal, China) and Gapdh (1:1000; Servicebio, China). The membranes were then incubated with horseradish peroxidase (HRP)-labeled secondary antibodies (1:5000; Abcam). The protein bands were observed with an enhanced chemiluminescence system, and the expression of protein was analyzed by ImageJ software.
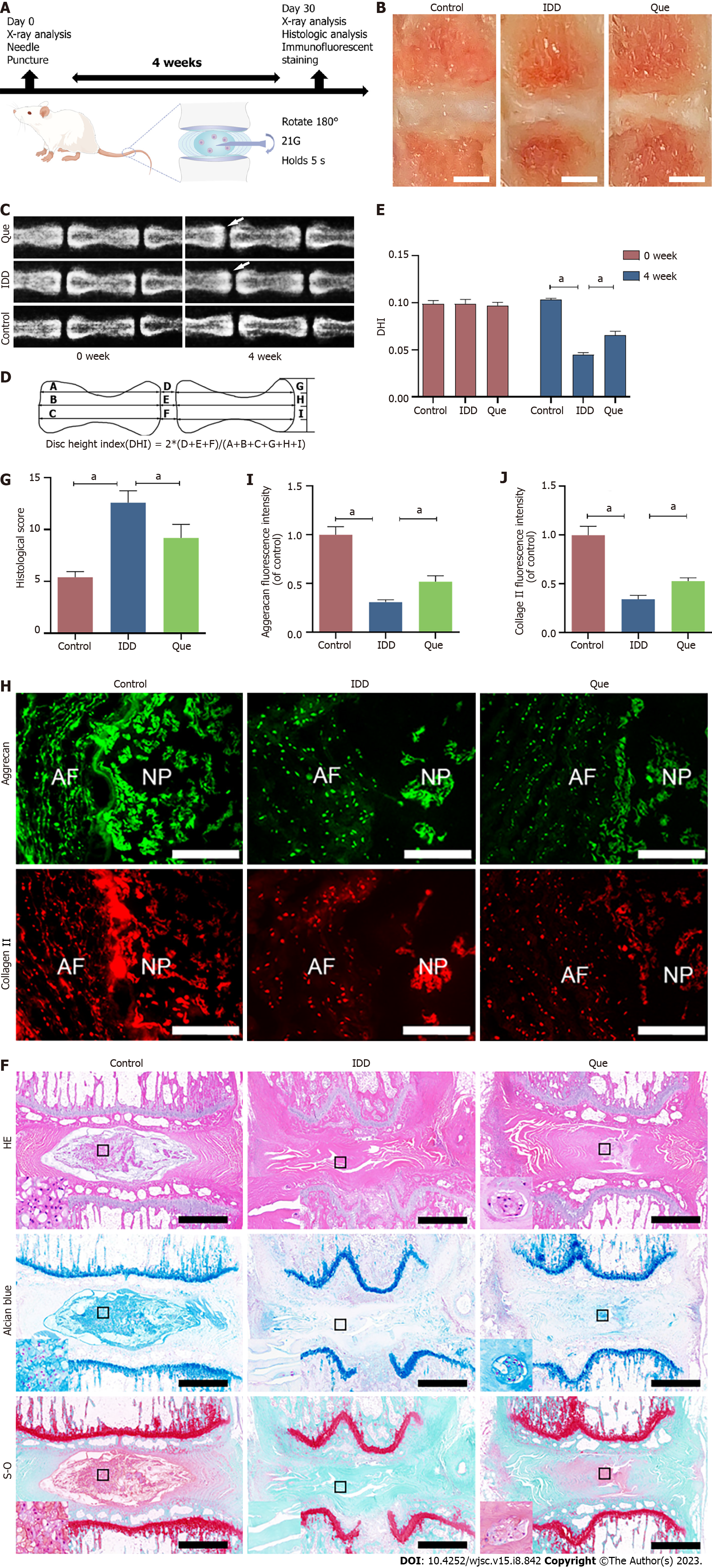
In total, 15 male SD rats (2-4 mo old and weighing 200-300 g) were randomly divided into the following three groups: Control group (n = 5), IDD group (n = 5), and Que group (n = 5). The IDD model of SD rats was established as previously described[13]. Briefly, the rats were anesthetized with pentobarbital, and the tail skin was sterilized by povidone iodine. A 21 G needle was used to puncture the coccygeal IVD (Co 6-7), and the needle was rotated 180° and kept in the disc for 5 s at a depth of 5 mm. After surgery, the Que group was treated with Que (100 mg/kg, 5 mg/mL of Que suspended in 0.5% sodium carboxymethyl cellulose solution) every other day by intragastric administration for 4 wk. The control group and IDD group were treated with 0.5% sodium carboxymethyl cellulose solution for 4 wk.
At 4 wk postinjury, all rats were placed in a prone position after anesthetization with pentobarbital, and X-ray images were acquired. ImageJ software was used to calculate the disc height index (DHI) as previously described[31]. The tails were harvested after the rats were euthanized with an overdose of sodium pentobarbital. The specimens were fixed in 4% paraformaldehyde for 48 h, decalcified in EDTA for 1 mo, dehydrated in gradient alcohol, and embedded in paraffin. The specimens were cut into 5 µm sections, and the sections were stained with hematoxylin-eosin (HE), Alcian blue, and Safranin O-fast Green. Histological scores were evaluated as previously described[31,32] based on the following scale: 5, normal discs; 6-11, moderately degenerated discs; and 12-14, severely degenerated discs.
After the IVD specimens were prepared, a freezing microtome (Leica, Wetzlar, Germany) was used to cut the specimens into 5-µm sections. The sections were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 15 min. Subsequently, the sections were blocked with 10% bovine serum albumin for 1 h at room temperature and incubated with the following primary antibodies (1:100) at 4 °C overnight: Collagen type II (ABclonal, China) and aggrecan (ABclonal, China). The sections were then incubated with FITC- or Cy3-conjugated secondary antibodies (1:200) at room temperature for 1 h in the dark. Fluorescence microscopy and ImageJ software were used to observe and analyze the sections.
All data were analyzed by GraphPad Prism 8 (GraphPad, La Jolla). Data are expressed as the mean ± SD. Student’s t test and one-way analysis of variance (ANOVA) was used to analyze the data of two groups and multiple independent groups, respectively. The Kruskal-Wallis H test was used to analyze the histological score. A P value < 0.05 was considered significant.
Elongated spindle-shaped NPMSCs were successfully isolated and cultured from rat IVDs (Figure 1A). Based on the immunofluorescence staining analysis, the MSC-associated markers, namely, CD73, CD90, and CD105 were highly expressed, whereas the expression of CD34 and CD45 was low (Figure 1B). Moreover, the expression of Tie2, a disc NP progenitor marker, was also high in NPMSCs (Figure 1B). These results indicated that NPMSCs isolated from IVD correspond to the standards of stem cells described by ISCT.
A CCK-8 assay was used to evaluate the effect of Que and TBHP on NPMSC viability. As shown in Figure 2A, Que showed no significant cytotoxic effect at concentrations up to 20 µM for 24 h. Therefore, a concentration of 20 µM Que was selected for the subsequent experiments. NPMSC viability was decreased after treatment with TBHP in a dose-dependent manner (Figure 2B), and 100 µM TBHP was selected for use in subsequent experiments. The effect of Que on NPMSC proliferation was detected by EdU staining. Compared to the control group, the positive rate of EdU in the TBHP group was significantly lower (P < 0.05), and the positive rate of EdU was partially increased in the Que + TBHP group (P < 0.05) (Figure 2C and D).
SA-β-Gal accumulation is an indicator of cellular senescence. Compared to the control group, the number of SA-β-Gal-positive senescent NPMSCs in the TBHP group was significantly higher (P < 0.05), while the number of SA-β-Gal-positive cells was decreased in Que + TBHP group (Figure 3A). Compared to the control group, the percentage of NPMSC in the G2/M phase in TBHP group was higher, which indicated cell cycle arrest, but treatment with Que decreased the percentage of NPMSCs arrested in G2/M phase (Figure 3B).
When the MMP is high, JC-1 exists in the mitochondria as J-aggregates of polymers, which produce red fluorescence. When the MMP is low, JC-1 is released from the mitochondria matrix and exists in the cytoplasm in the form of monomers, which produce green fluorescence. As shown in Figure 4A and B, the MMP was decreased after TBHP treatment, and this effect was partially reversed by pretreatment with Que. Figure 4C and D show that NPMSCs in the TBHP group had higher ROS levels compared to the control group, and pretreatment with Que partially reversed the effect of TBHP on ROS generation.
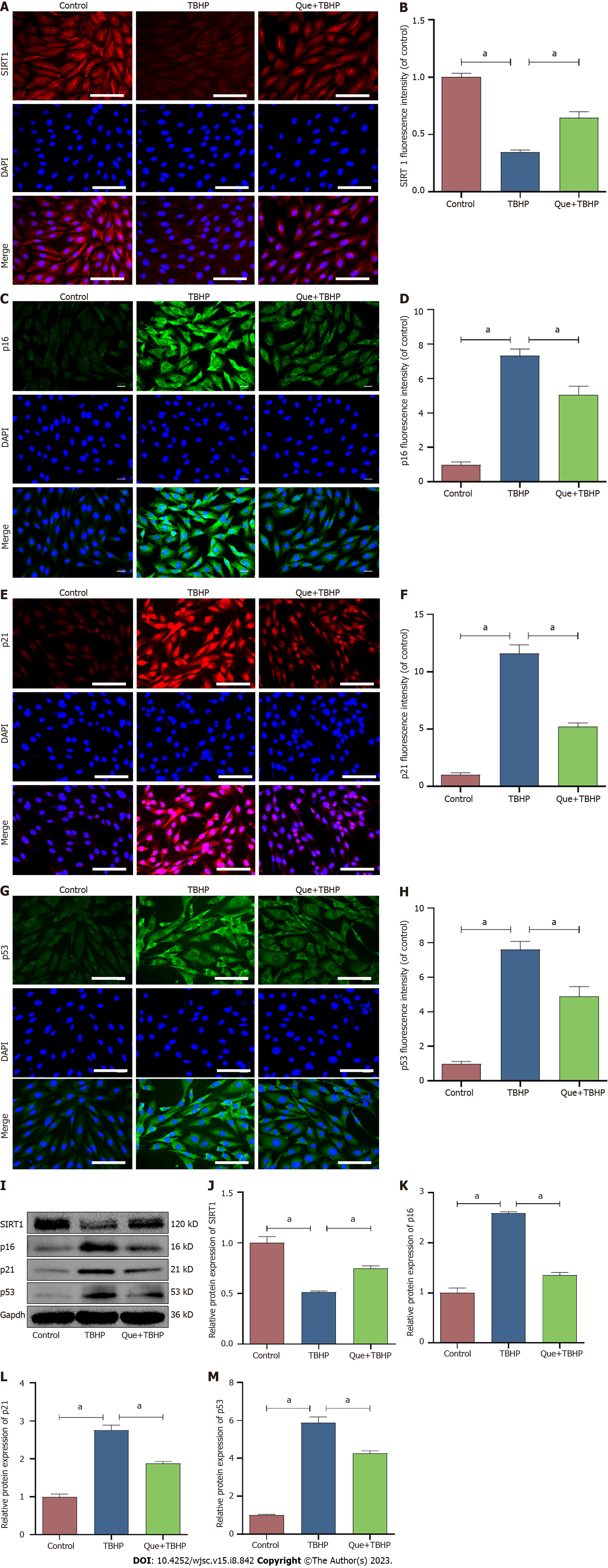
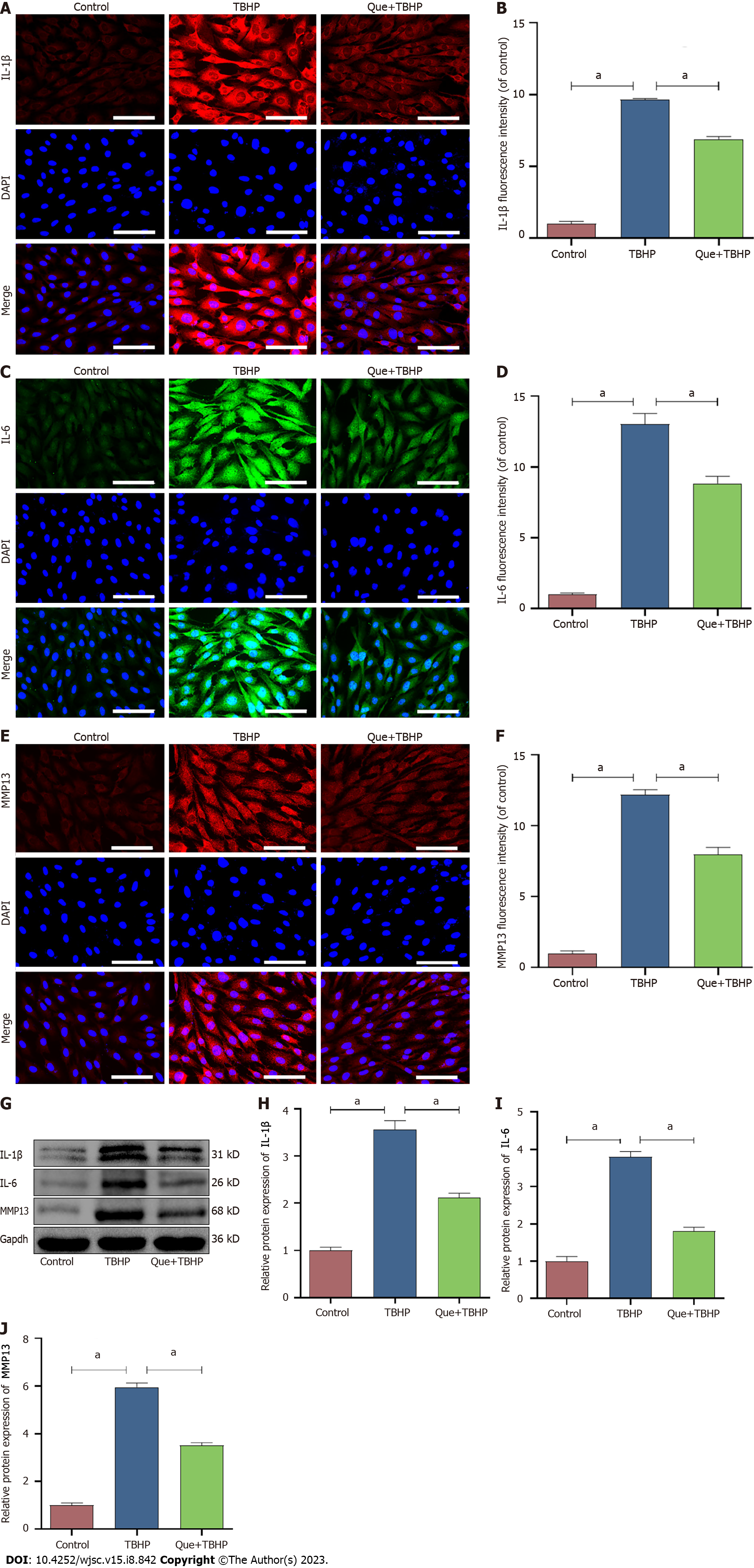
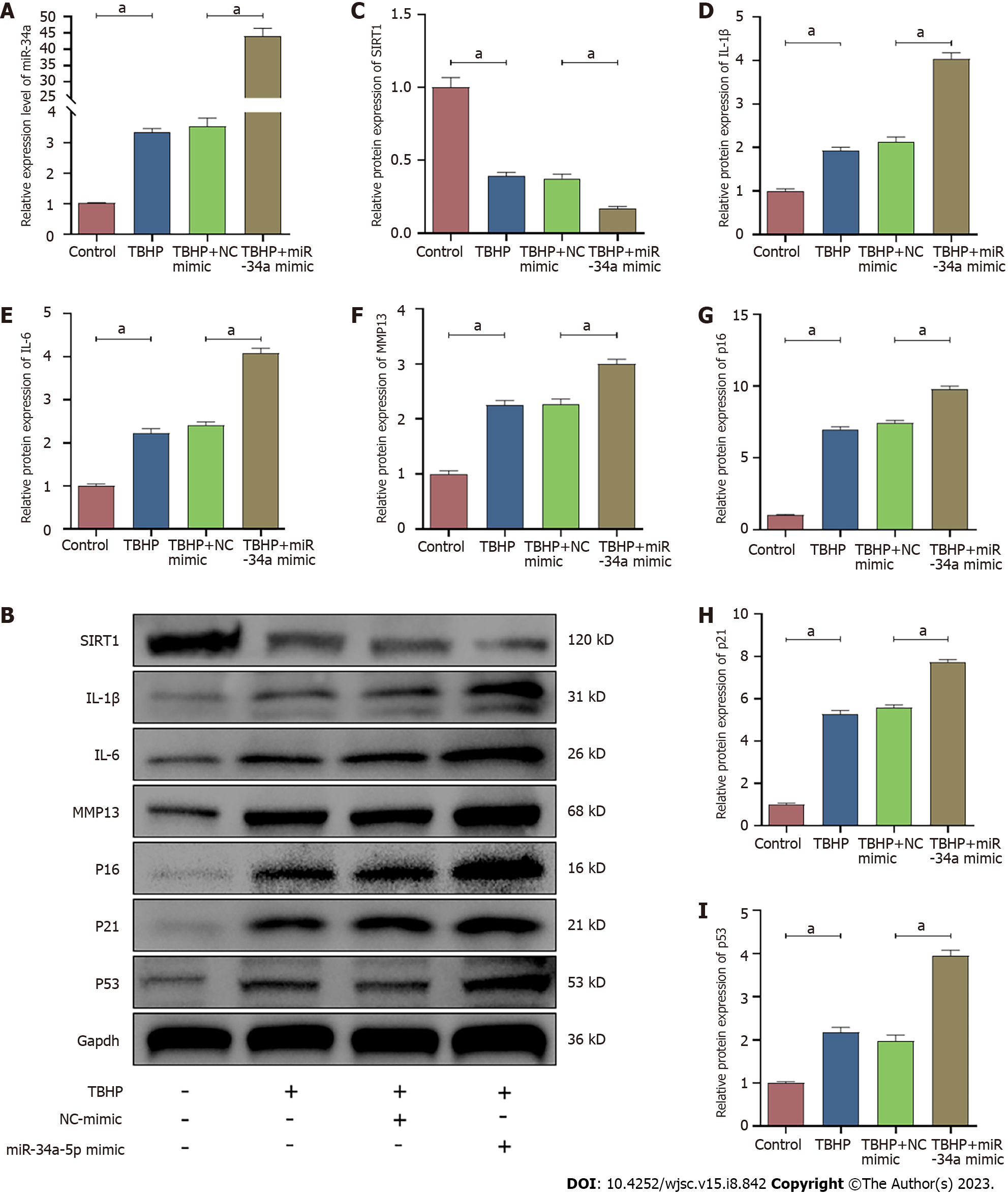
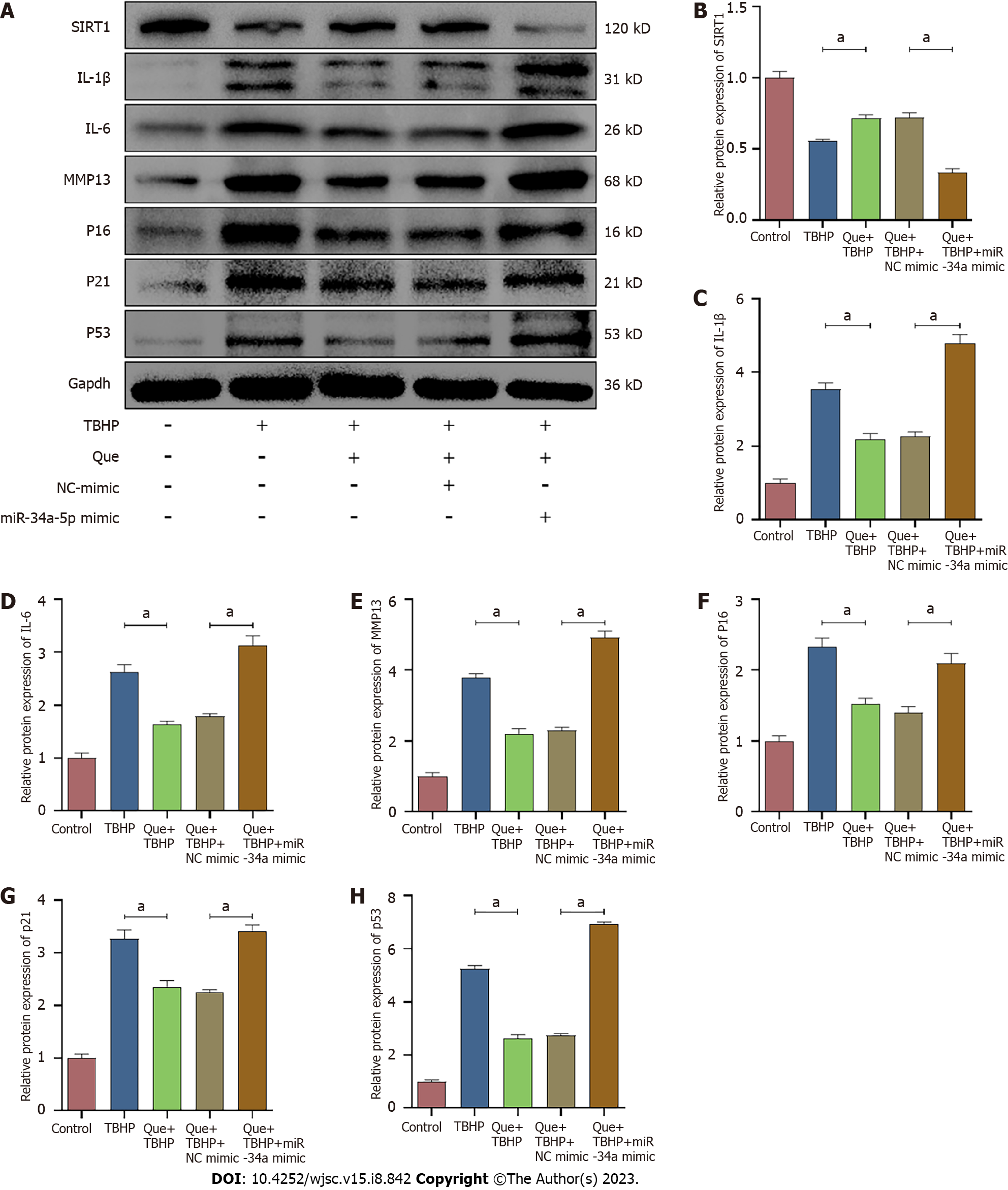
Cell senescence is broadly characterized by cell cycle arrest and the production of SASP. To explore the effect of Que in TBHP-induced NPMSCs, western blot analysis and immunofluorescence staining were used to evaluate the expression of SIRT1, cell senescence-related proteins (p16, p21, and p53), and SASP (IL-1β, IL-6, and MMP-13). The expression of SIRT1 was downregulated after TBHP treatment, and this effect was partially reversed by Que pretreatment (P < 0.05; Figure 5). Moreover, Que treatment significantly decreased the upregulation of p16, p21, p53, IL-1β, IL-6, and MMP-13 expression induced by TBHP (P < 0.05; Figures 5 and 6). Taken together, these results indicated that Que may have a protective role in TBHP-induced oxidative stress injury of NPMSCs.
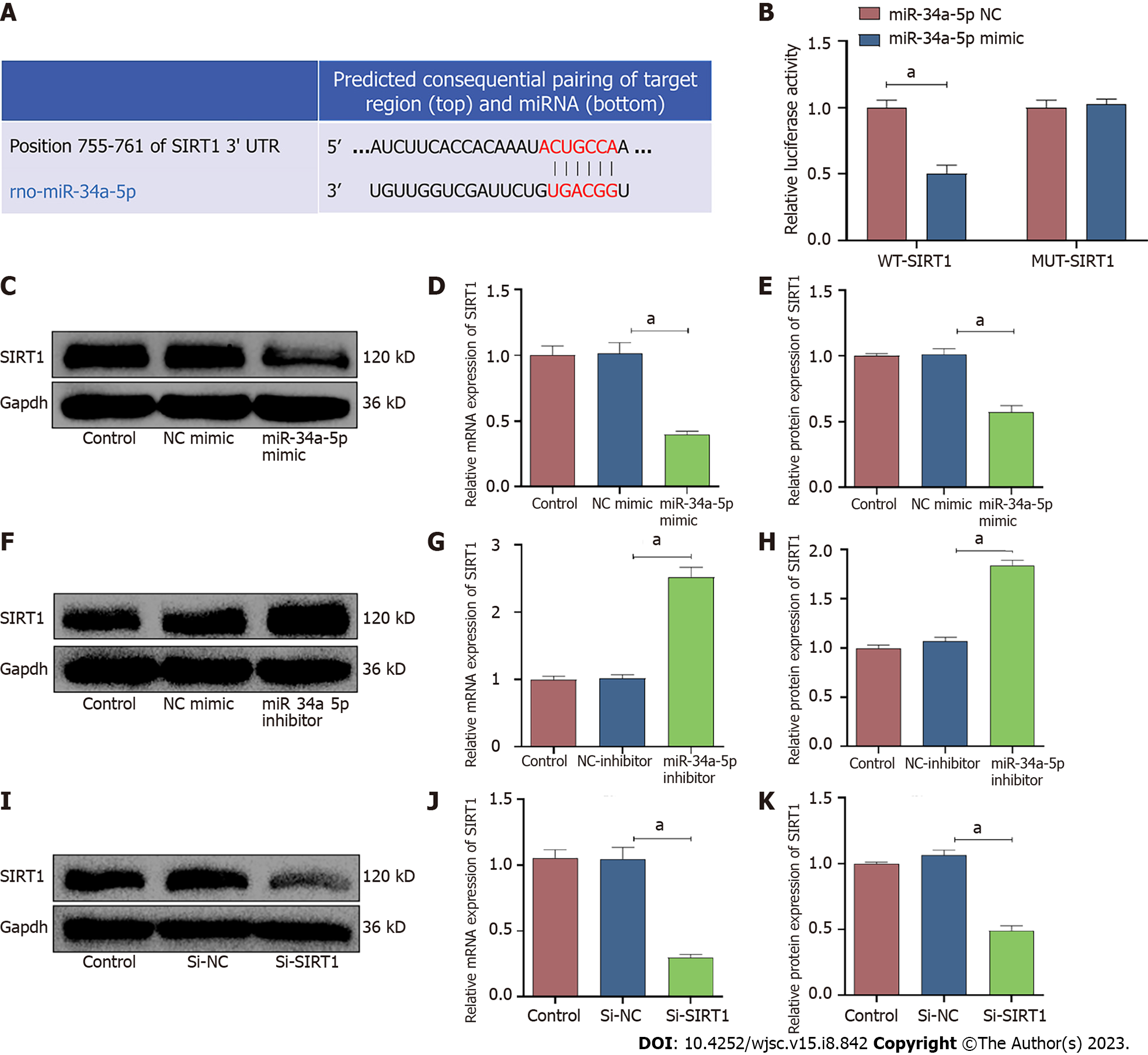
According to the TargetScan online prediction database, SIRT1 is a potential target of miR-34a-5p (Figure 7A). A dual-luciferase reporter assay revealed that the miR-34a-5p mimic significantly inhibited the relative luciferase activity in the wild-type SIRT1 reporter vector (WT-SIRT1), whereas there was no significant change in the luciferase activity in the mutant SIRT1 reporter vector (MUT-SIRT1) (Figure 7B). These data indicated that miR-34a-5p inhibits SIRT1 expression by directly binding to the 3UTR of SIRT1. In addition, the mRNA and protein expression levels of SIRT1 were significantly inhibited by overexpression of miR-34a-5p (Figure 7C-E). Conversely, downregulation of miR-34a-5p increased the expression level of SIRT1 (Figure 7F-H). Moreover, the expression of SIRT1 was successfully downregulated by siRNA (Figure 7I-K).
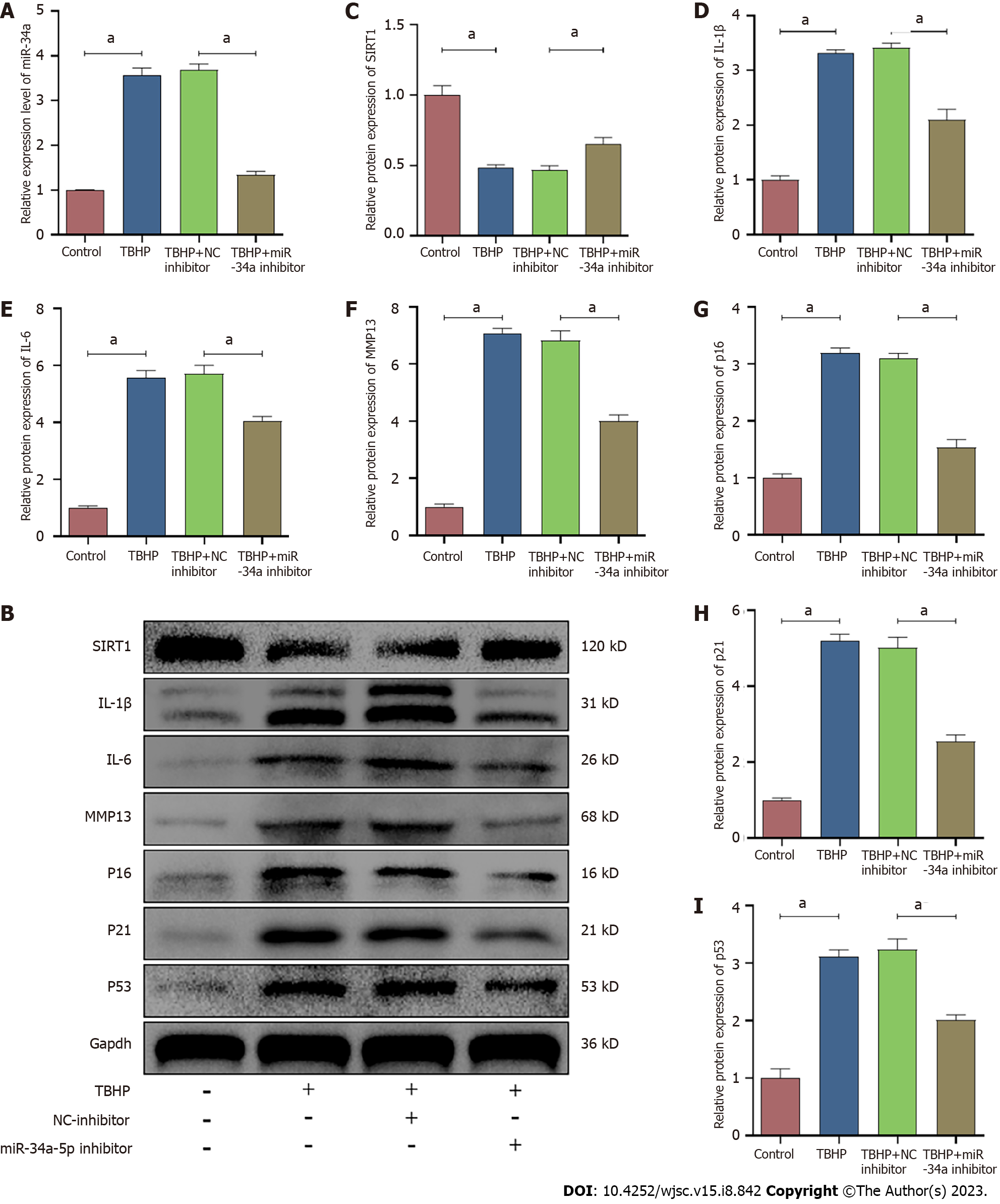
According to the qRT-PCR analysis, the expression of miR-34a-5p was increased by TBHP treatment, and it was suppressed and promoted by the miR-34a-5p inhibitor and miR-34a-5p mimic, respectively (Figures 8A and 9A). In contrast, the expression of SIRT1 was suppressed after TBHP treatment, and it was promoted and suppressed and by miR-34a-5p inhibitor and miR-34a-5p mimic, respectively (Figures 8 and 9). The TBHP-enhanced senescence-related proteins (p16, p21, and p53) and SASP (IL-1β, IL-6, and MMP-13) were significantly suppressed after miR-34a-5p knockdown (Figure 8). In contrast, the expression levels of senescence-related proteins and SASP were increased when cells were treated with the miR-34a-5p mimic (Figure 9).
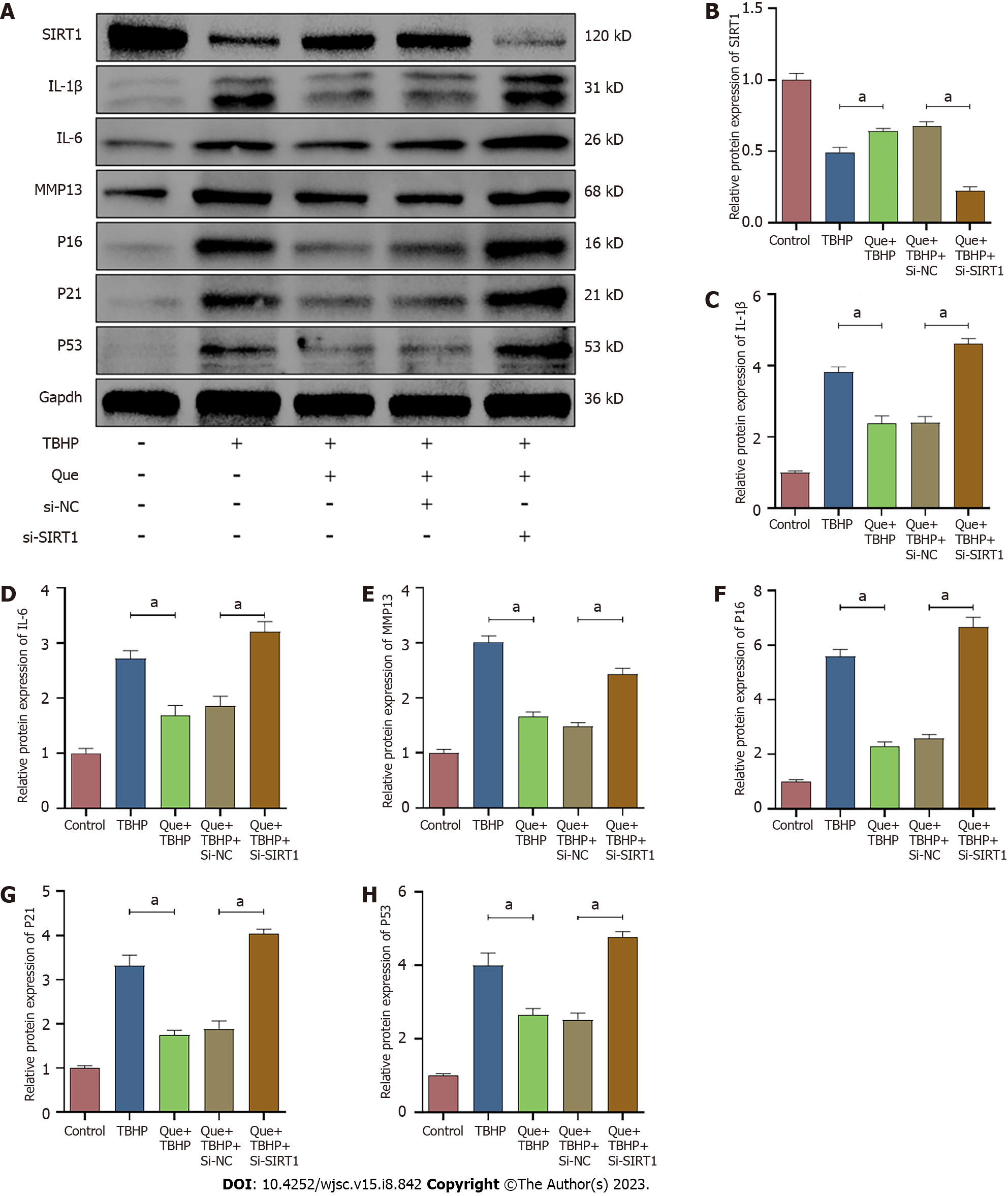
To explore the relationship between miR-34a-5p/SIRT1 and oxidative stress-induced senescence in Que-treated NPMSCs, the miR-34a-5p mimic and siSIRT1 were used to investigate whether overexpression of miR-34a-5p and knockdown of SIRT1, respectively, counteracts the effects of Que on the expression of senescence-related proteins (p16, p21, and p53), SASP (IL-1β, IL-6, and MMP-13), and SIRT1. As shown in Figures 10 and 11, Que suppressed the expression of senescence-related proteins and SASP but promoted the expression of SIRT1, whereas overexpression of miR-34a-5p and knockdown of SIRT1 impaired this protective effect in TBHP-treated NPMSCs. These results suggested that Que may protect NPMSCs from TBHP-induced senescence via the miR-34a-5p/SIRT1 pathway.
A puncture-induced rat model was established to investigate the effect of Que in IDD in vivo, and disc height was measured by X-ray images at Week 0 and Week 4 after puncture (Figure 12A). Compared to the control group, the DHI of the IDD group was significantly decreased (Figure 12B-E). Interestingly, the DHI of the Que group was significantly higher than that of the IDD group (P < 0.05) (Figure 12B-E). According to the Hematoxylin & eosin staining, NP tissues occupied most of the area in the discs, and NP cells were well dispersed in the matrix. The annulus fibrosus (AF) was well organized in the remaining area. In the IDD group, the NP tissues almost disappeared, and the border between NP and AF was severely disrupted (Figure 12F). However, Que treatment alleviated the degeneration and morphological changes in the NP and AF (Figure 12F). The histological score was higher in the IDD group compared to the control group (P < 0.05) (Figure 12G). Both Safranin-O Fast Green and Alcian blue staining showed that the proteoglycan matrix area was lower in the IDD group compared to the control group (Figure 12F). However, Que treatment alleviated the proteoglycan decrease compared to the IDD group (Figure 12H). Immunofluorescence was also used to assess the expression of collagen II and aggrecan in the disc tissue. Compared to the control group, the expression of collagen type II and aggrecan was significantly lower in the IDD group, and Que treatment significantly attenuated the decrease in collagen type II and aggrecan compared to the IDD group (Figure 12I and J). Taken together, these results suggested that Que alleviates the IDD process in a puncture-induced rat model.
In the present study, the results demonstrated that Que ameliorates oxidative stress-induced senescence in NPMSCs via the miR-34a-5p/SIRT1 axis. The in vivo results also demonstrated that Que inhibits the expression of senescence-related proteins (p16, p21, and p53) and SASP (IL-1β, IL-6, and MMP-13) as well as promotes the expression of SIRT1, suggesting that it has a therapeutic effect in the IDD rat model.
Several previous studies have found that oxidative stress is highly associated with the progression of IDD[4,7]. Oxidative stress is triggered in the microenvironment of degenerative IVD when the balance between generation and elimination of ROS is broken[33]. ROS not only trigger oxidative damage of the extracellular matrix of IVD but also induce oxidative damage to DNA, proteins, and mitochondria[33]. Moreover, ROS promote the production of ROS in IVD cells, forming a positive feedback loop[5-7,34]. The discovery of NPMSCs provides a theoretical basis for the endogenous repair of IDD[9,11]. The endogenous repair ability of NPMSCs in degenerative IVD is achieved by differentiation into NP cells and inhibiting cell apoptosis and/or senescence[10,35]. Oxidative stress in the microenvironment of IVD induces NPMSC senescence, and senescent NPMSCs decrease multipotency and self-renew ability, thus decreasing the regenerative potential and endogenous repair ability of NPMSCs[17,36-38]. In the present study, we found increased expression of senescence-related proteins (p16, p21, and p53), increased SASP (IL-1β, IL-6, and MMP-13), increased SA-β-Gal-positive senescent cells, decreased expression of SIRT1, decreased proliferation, and decreased viability after TBHP treatment in NPMSCs. Therefore, it is important to understand how to maintain the quantity and quality of NPMSCs under adverse microenvironments in degenerative IVD, such as oxidative stress, hypoxia, nutrient deficiency, and compression.
As a potent antioxidant, Que scavenges ROS, scavenges free radicals, inhibits lipid peroxidation, and inhibits xanthine oxidase activity[18-20,39]. Previous studies have reported that Que is an effective activator of SIRT1[40-42]. Feng et al[22] reported that Que attenuates oxidative stress-induced apoptosis of rat chondrocytes via the SIRT1/mitogen-activated protein kinase (AMPK) pathway and prevents the progression of osteoarthritis in a rat model[22]. Lou et al[43] confirmed that Que inhibits HepG2 cell apoptosis through the p53/miRNA-34a/SIRT1 pathway[43]. To evaluate the effect of Que in TBHP-induced NPMSCs, we pretreated cells with Que (20 µM) for 24 h before the addition of TBHP (100 µM) in the present study. Pretreatment with Que decreased ROS generation, decreased SA-β-Gal activity, and partially restored cell proliferation in oxidative stress induced-NPMSCs. Further, Que significantly reduced the expression of senescence-related proteins (p16, p21, and p53), reduced SASP (IL-1β, IL-6, and MMP-13), and increased the expression of SIRT1. Therefore, these results indicated that Que may have a protective effect against TBHP-induced oxidative stress in NPMSCs.
A previous study has also demonstrated that the expression of miR-34a-5p is significantly upregulated in degenerative IVD and that miR-34a-5p increases extracellular matrix degradation and cell apoptosis. Chen et al[44] reported that miR-34a increases apoptosis in degenerated cartilage endplate (CEP) chondrocytes. In addition, inhibition of miR-34a reduces cell apoptosis, while upregulation of miR-34a has an opposite effect in CEP cells[44]. In the present study, TBHP significantly upregulated miR-34a-5p expression. Overexpression miR-34a aggravated the oxidative stress damage induced by TBHP in NPMSCs, including increased the expression of senescence-related proteins and SASP as well as and decreased the expression of SIRT1, whereas knockdown of miR-34a had an opposite effect in NPMSCs. Accumulating studies have demonstrated that SIRT1 exerts important function in the progression of IDD. The expression level of SIRT1 in IVD cells decreases with the progression of IDD[45], and SIRT1 expression is negatively correlated with Pfirrmann grade in IDD[46]. It has been reported that SIRT1 decreases the oxidative stress-induced senescence in CEP cells through the p53/p21 pathway[47]. H2O2-induced oxidative stress reduces the expression of SIRT1 as the expression of SIRT1 is downregulated with the increasing concentration of H2O2, and SIRT1 activation alleviates oxidative stress-induced senescence in NP cells[29,48]. Xia et al[49] found that the expression of p16 is downregulated by activating SIRT1 in rat IDD model[49]. The present study found that SIRT1 expression was downregulated in TBHP induced-NPMSCs, which was rescued by Que and inhibiting miR-34a-5p. These results suggested that SIRT1 plays a protective role in TBHP induced-NPMSCs. miR-34a-5p overexpression and SIRT1 downregulation in NPMSCs were utilized to investigate whether Que reduces oxidative stress damage via the miR-34a/SIRT1 pathway to delay NPMSC senescence. The results demonstrated that the protective effect of Que is reversed by miR-34a-5p overexpression and SIRT1 knockdown.
In the present study, Que was also administered to IDD animal models to evaluate the protective effect of Que in vivo. X-ray analysis indicated the loss of DHI in the IDD model, but Que treatment ameliorated the decreased DHI. Moreover, histological analysis using HE, Alcian blue, and S-O staining as well as immunofluorescence analysis of collagen type II and aggrecan confirmed that Que had a positive effect on delaying the degree of IDD.
The present study had several limitations. Previous studies have shown Que has pleiotropic properties. In addition to the activation of SIRT1, Que has anti-inflammatory and antioxidative effects via activating nuclear factor erythroid 2-related factor 2 (NRF2)[39], mitogen-activated protein kinase (MAPK)[39], and phosphatidylinositol 3-kinases/protein kinase B (PI3K/AKT)[50]. Hence, it remains unknown whether Que plays a role in NPMSC senescence induced by oxidative stress via other signaling pathways, suggesting that further study is needed to explore the other mechanisms underlying the protective effect of Que on NPMSCs. Moreover, future clinical studies are needed to evaluate the effect of Que on IDD progress.
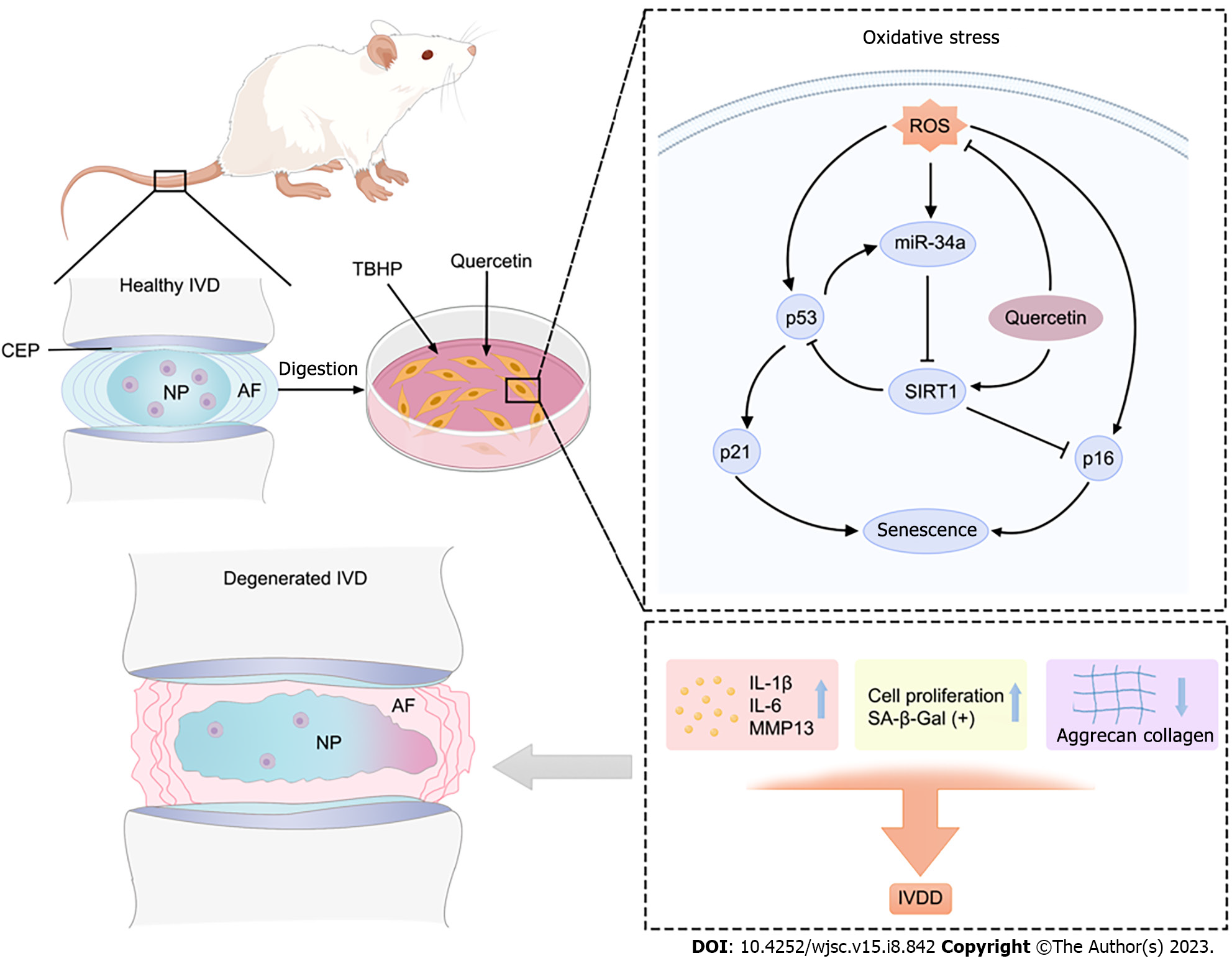
In summary, the present study demonstrated that Que prevents oxidative stress-induced senescence of NPMSCs via the miR-34a/SIRT1 signaling pathway (Figure 13). Moreover, Que ameliorates the progression of IDD in a rat model. These findings suggested that Que may be a potential agent for the treatment of IDD.
Intervertebral disc degeneration (IDD) is a main contributor to low back pain. Oxidative stress, which is highly associated with the progression of IDD, increases senescence of nucleus pulposus-derived mesenchymal stem cells (NPMSCs) and weakens the differentiation ability of NPMSCs in degenerated intervertebral discs (IVDs). Quercetin (Que) has been demonstrated to reduce oxidative stress in diverse degenerative diseases.
An adverse microenvironments of degenerative intervertebral disc such as oxidative stress, low nutrition, and inflammation leads to increased senescence NPMSCs, which severely affects endogenous repair. Therefore, inhibition senescence of NPMSCs may be of great significance in alleviating IDD.
The present study aimed to investigate the role of Que in oxidative stress-induced NPMSC damage and to elucidate the underlying mechanism.
In vitro, NPMSCs were isolated from rat tails. Senescence-associated β-galactosidase (SA-β-Gal) staining, cell cycle, reactive oxygen species (ROS), real-time quantitative polymerase chain reaction (RT-qPCR), immunofluorescence, and western blot analyses were used to evaluate the protective effects of Que. Meanwhile the relationship between miR-34a-5p and Sirtuins 1(SIRT1) was evaluated by dual-luciferase reporter assay. To explore whether Que modulates tert-butyl hydroperoxide (TBHP)-induced senescence of NPMSCs via the miR-34a-5p/SIRT1 pathway, we used adenovirus vectors to overexpress and downregulate the expression of miR-34a-5p, and used SIRT1 siRNA to knockdown SIRT1 expression. In vivo, a puncture-induced rat IDD model was constructed, and X rays and histological analysis were used to assess whether Que could alleviate IDD in vivo.
We found that TBHP can cause NPMSCs senescence changes, such as reduced cell proliferation ability, increased SA-β-Gal activity, cell cycle arrest, the accumulation of ROS, and increased expression of senescence-related proteins. While abovementioned senescence indicators were significantly alleviated by Que treatment. Que decreased the expression levels of senescence-related proteins (p16, p21, and p53) and senescence-associated secreted phenotype (SASP), including IL-1β, IL-6, and MMP-13, and it increased the expression of SIRT1. In addition, the protective effects of Que on cell senescence were partially reversed by miR-34a-5p overexpression and SIRT1 knockdown. In vivo, X-ray, and histological analyses indicated that Que alleviated IDD in a puncture-induced rat model.
In summary, the present study provides evidence that Que reduces oxidative stress-induced senescence of NPMSCs via the miR-34a/SIRT1 signaling pathway, suggesting that Que may be a potential agent for the treatment of IDD.
We demonstrated Que ameliorates oxidative stress-induced senescence of NPMSCs and delays the progression of IDD. Que may be a potential agent for the treatment of IDD.
We also thank Figdraw for the assistance in drawing graphical abstract.
| 1. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9354] [Cited by in RCA: 8390] [Article Influence: 1048.8] [Reference Citation Analysis (4)] |
| 2. | Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, Aremu O, Artaman A, Asgedom SW, Assadi R, Atey TM, Avila-Burgos L, Awasthi A, Ba Saleem HO, Barac A, Bennett JR, Bensenor IM, Bhakta N, Brenner H, Cahuana-Hurtado L, Castañeda-Orjuela CA, Catalá-López F, Choi JJ, Christopher DJ, Chung SC, Curado MP, Dandona L, Dandona R, das Neves J, Dey S, Dharmaratne SD, Doku DT, Driscoll TR, Dubey M, Ebrahimi H, Edessa D, El-Khatib Z, Endries AY, Fischer F, Force LM, Foreman KJ, Gebrehiwot SW, Gopalani SV, Grosso G, Gupta R, Gyawali B, Hamadeh RR, Hamidi S, Harvey J, Hassen HY, Hay RJ, Hay SI, Heibati B, Hiluf MK, Horita N, Hosgood HD, Ilesanmi OS, Innos K, Islami F, Jakovljevic MB, Johnson SC, Jonas JB, Kasaeian A, Kassa TD, Khader YS, Khan EA, Khan G, Khang YH, Khosravi MH, Khubchandani J, Kopec JA, Kumar GA, Kutz M, Lad DP, Lafranconi A, Lan Q, Legesse Y, Leigh J, Linn S, Lunevicius R, Majeed A, Malekzadeh R, Malta DC, Mantovani LG, McMahon BJ, Meier T, Melaku YA, Melku M, Memiah P, Mendoza W, Meretoja TJ, Mezgebe HB, Miller TR, Mohammed S, Mokdad AH, Moosazadeh M, Moraga P, Mousavi SM, Nangia V, Nguyen CT, Nong VM, Ogbo FA, Olagunju AT, Pa M, Park EK, Patel T, Pereira DM, Pishgar F, Postma MJ, Pourmalek F, Qorbani M, Rafay A, Rawaf S, Rawaf DL, Roshandel G, Safiri S, Salimzadeh H, Sanabria JR, Santric Milicevic MM, Sartorius B, Satpathy M, Sepanlou SG, Shackelford KA, Shaikh MA, Sharif-Alhoseini M, She J, Shin MJ, Shiue I, Shrime MG, Sinke AH, Sisay M, Sligar A, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tessema GA, Topor-Madry R, Tran TT, Tran BX, Ukwaja KN, Vlassov VV, Vollset SE, Weiderpass E, Williams HC, Yimer NB, Yonemoto N, Younis MZ, Murray CJL, Naghavi M, Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1105] [Cited by in RCA: 1196] [Article Influence: 149.5] [Reference Citation Analysis (0)] |
| 3. | Chou D, Samartzis D, Bellabarba C, Patel A, Luk KD, Kisser JM, Skelly AC. Degenerative magnetic resonance imaging changes in patients with chronic low back pain: a systematic review. Spine (Phila Pa 1976). 2011;36:S43-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Suzuki S, Fujita N, Hosogane N, Watanabe K, Ishii K, Toyama Y, Takubo K, Horiuchi K, Miyamoto T, Nakamura M, Matsumoto M. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res Ther. 2015;17:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater. 2015;30:89-102; discussion 103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 6. | Hou G, Lu H, Chen M, Yao H, Zhao H. Oxidative stress participates in age-related changes in rat lumbar intervertebral discs. Arch Gerontol Geriatr. 2014;59:665-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Feng C, Yang M, Lan M, Liu C, Zhang Y, Huang B, Liu H, Zhou Y. ROS: Crucial Intermediators in the Pathogenesis of Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2017;2017:5601593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 288] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 8. | Lee DC, Adams CS, Albert TJ, Shapiro IM, Evans SM, Koch CJ. In situ oxygen utilization in the rat intervertebral disc. J Anat. 2007;210:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 10. | Lyu FJ, Cheung KM, Zheng Z, Wang H, Sakai D, Leung VY. IVD progenitor cells: a new horizon for understanding disc homeostasis and repair. Nat Rev Rheumatol. 2019;15:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 11. | Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D, Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976). 2007;32:2537-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Liu Y, Li Y, Huang ZN, Wang ZY, Nan LP, Wang F, Zhou SF, Wang JC, Feng XM, Zhang L. The effect of intervertebral disc degenerative change on biological characteristics of nucleus pulposus mesenchymal stem cell: an in vitro study in rats. Connect Tissue Res. 2019;60:376-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Wang F, Nan LP, Zhou SF, Liu Y, Wang ZY, Wang JC, Feng XM, Zhang L. Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats. Stem Cells Int. 2019;2019:8496025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Wang JW, Zhu L, Shi PZ, Wang PC, Dai Y, Wang YX, Lu XH, Cheng XF, Feng XM, Zhang L. 1,25(OH)(2)D(3) Mitigates Oxidative Stress-Induced Damage to Nucleus Pulposus-Derived Mesenchymal Stem Cells through PI3K/Akt Pathway. Oxid Med Cell Longev. 2022;2022:1427110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Shi PZ, Wang JW, Wang PC, Han B, Lu XH, Ren YX, Feng XM, Cheng XF, Zhang L. Urolithin a alleviates oxidative stress-induced senescence in nucleus pulposus-derived mesenchymal stem cells through SIRT1/PGC-1α pathway. World J Stem Cells. 2021;13:1928-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 16. | Wu H, Shang Y, Yu J, Zeng X, Lin J, Tu M, Cheang LH, Zhang J. Regenerative potential of human nucleus pulposus resident stem/progenitor cells declines with ageing and intervertebral disc degeneration. Int J Mol Med. 2018;42:2193-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Ma K, Chen S, Li Z, Deng X, Huang D, Xiong L, Shao Z. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis Cartilage. 2019;27:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 18. | de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF. Quercetin and the mitochondria: A mechanistic view. Biotechnol Adv. 2016;34:532-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 19. | Liao YR, Lin JY. Quercetin intraperitoneal administration ameliorates lipopolysaccharide-induced systemic inflammation in mice. Life Sci. 2015;137:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Sun Y, Coppé JP, Lam EW. Cellular Senescence: The Sought or the Unwanted? Trends Mol Med. 2018;24:871-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 21. | Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1102] [Cited by in RCA: 1894] [Article Influence: 172.2] [Reference Citation Analysis (0)] |
| 22. | Feng K, Chen Z, Pengcheng L, Zhang S, Wang X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J Cell Physiol. 2019;234:18192-18205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 23. | Kim SR, Jiang K, Ogrodnik M, Chen X, Zhu XY, Lohmeier H, Ahmed L, Tang H, Tchkonia T, Hickson LJ, Kirkland JL, Lerman LO. Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl Res. 2019;213:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Li Z, Yu X, Shen J, Chan MT, Wu WK. MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015;48:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Ohrt-Nissen S, Døssing KB, Rossing M, Lajer C, Vikeså J, Nielsen FC, Friis-Hansen L, Dahl B. Characterization of miRNA expression in human degenerative lumbar disks. Connect Tissue Res. 2013;54:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Sosnowska B, Mazidi M, Penson P, Gluba-Brzózka A, Rysz J, Banach M. The sirtuin family members SIRT1, SIRT3 and SIRT6: Their role in vascular biology and atherogenesis. Atherosclerosis. 2017;265:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 27. | Mendelsohn AR, Larrick JW. The NAD+/PARP1/SIRT1 Axis in Aging. Rejuvenation Res. 2017;20:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Maiese K. Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr Neurovasc Res. 2017;14:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | He J, Zhang A, Song Z, Guo S, Chen Y, Liu Z, Zhang J, Xu X, Liu J, Chu L. The resistant effect of SIRT1 in oxidative stress-induced senescence of rat nucleus pulposus cell is regulated by Akt-FoxO1 pathway. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Ji ML, Jiang H, Zhang XJ, Shi PL, Li C, Wu H, Wu XT, Wang YT, Wang C, Lu J. Preclinical development of a microRNA-based therapy for intervertebral disc degeneration. Nat Commun. 2018;9:5051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 31. | Han B, Zhu K, Li FC, Xiao YX, Feng J, Shi ZL, Lin M, Wang J, Chen QX. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine (Phila Pa 1976). 2008;33:1925-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 353] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 32. | Mao HJ, Chen QX, Han B, Li FC, Feng J, Shi ZL, Lin M, Wang J. The effect of injection volume on disc degeneration in a rat tail model. Spine (Phila Pa 1976). 2011;36:E1062-E1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Cao G, Yang S, Cao J, Tan Z, Wu L, Dong F, Ding W, Zhang F. The Role of Oxidative Stress in Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2022;2022:2166817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 34. | Chen JW, Ni BB, Zheng XF, Li B, Jiang SD, Jiang LS. Hypoxia facilitates the survival of nucleus pulposus cells in serum deprivation by down-regulating excessive autophagy through restricting ROS generation. Int J Biochem Cell Biol. 2015;59:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Clouet J, Fusellier M, Camus A, Le Visage C, Guicheux J. Intervertebral disc regeneration: From cell therapy to the development of novel bioinspired endogenous repair strategies. Adv Drug Deliv Rev. 2019;146:306-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 36. | Vono R, Jover Garcia E, Spinetti G, Madeddu P. Oxidative Stress in Mesenchymal Stem Cell Senescence: Regulation by Coding and Noncoding RNAs. Antioxid Redox Signal. 2018;29:864-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Chen Y, Tang L. Stem Cell Senescence: the Obstacle of the Treatment of Degenerative Disk Disease. Curr Stem Cell Res Ther. 2019;14:654-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 38. | Wang F, Shi R, Cai F, Wang YT, Wu XT. Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev. 2015;24:2479-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 39. | Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 761] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 40. | Sarubbo F, Ramis MR, Kienzer C, Aparicio S, Esteban S, Miralles A, Moranta D. Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels Improving Cognition in Aged Rats. J Neuroimmune Pharmacol. 2018;13:24-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Peredo-Escárcega AE, Guarner-Lans V, Pérez-Torres I, Ortega-Ocampo S, Carreón-Torres E, Castrejón-Tellez V, Díaz-Díaz E, Rubio-Ruiz ME. The Combination of Resveratrol and Quercetin Attenuates Metabolic Syndrome in Rats by Modifying the Serum Fatty Acid Composition and by Upregulating SIRT 1 and SIRT 2 Expression in White Adipose Tissue. Evid Based Complement Alternat Med. 2015;2015:474032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Iskender H, Dokumacioglu E, Sen TM, Ince I, Kanbay Y, Saral S. The effect of hesperidin and quercetin on oxidative stress, NF-κB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed Pharmacother. 2017;90:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 43. | Lou G, Liu Y, Wu S, Xue J, Yang F, Fu H, Zheng M, Chen Z. The p53/miR-34a/SIRT1 Positive Feedback Loop in Quercetin-Induced Apoptosis. Cell Physiol Biochem. 2015;35:2192-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Chen H, Wang J, Hu B, Wu X, Chen Y, Li R, Yuan W. MiR-34a promotes Fas-mediated cartilage endplate chondrocyte apoptosis by targeting Bcl-2. Mol Cell Biochem. 2015;406:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Wang D, Hu Z, Hao J, He B, Gan Q, Zhong X, Zhang X, Shen J, Fang J, Jiang W. SIRT1 inhibits apoptosis of degenerative human disc nucleus pulposus cells through activation of Akt pathway. Age (Dordr). 2013;35:1741-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Guo J, Shao M, Lu F, Jiang J, Xia X. Role of Sirt1 Plays in Nucleus Pulposus Cells and Intervertebral Disc Degeneration. Spine (Phila Pa 1976). 2017;42:E757-E766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Zhou N, Lin X, Dong W, Huang W, Jiang W, Lin L, Qiu Q, Zhang X, Shen J, Song Z, Liang X, Hao J, Wang D, Hu Z. SIRT1 alleviates senescence of degenerative human intervertebral disc cartilage endo-plate cells via the p53/p21 pathway. Sci Rep. 2016;6:22628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Zhang GZ, Deng YJ, Xie QQ, Ren EH, Ma ZJ, He XG, Gao YC, Kang XW. Sirtuins and intervertebral disc degeneration: Roles in inflammation, oxidative stress, and mitochondrial function. Clin Chim Acta. 2020;508:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 49. | Xia X, Guo J, Lu F, Jiang J. SIRT1 Plays a Protective Role in Intervertebral Disc Degeneration in a Puncture-induced Rodent Model. Spine (Phila Pa 1976). 2015;40:E515-E524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Lu XL, Zhao CH, Yao XL, Zhang H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed Pharmacother. 2017;85:658-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SC, United States; Rezus E, Romania; Shamseldeen AM, Egypt S-Editor: Li L L-Editor: A P-Editor: Yu HG