Published online Aug 26, 2023. doi: 10.4252/wjsc.v15.i8.866
Peer-review started: May 7, 2023
First decision: June 9, 2023
Revised: June 21, 2023
Accepted: July 19, 2023
Article in press: July 19, 2023
Published online: August 26, 2023
Processing time: 109 Days and 20.6 Hours
Local mesenchymal stem cell (MSC) therapy for complex perianal fistulas (PFs) has shown considerable promise. But, the long-term safety and efficacy of MSC therapy in complex PFs remain unknown.
To explore the long-term effectiveness and safety of local MSC therapy for complex PFs.
Sources included the PubMed, EMBASE, and Cochrane Library databases. A standard meta-analysis was performed using RevMan 5.3.
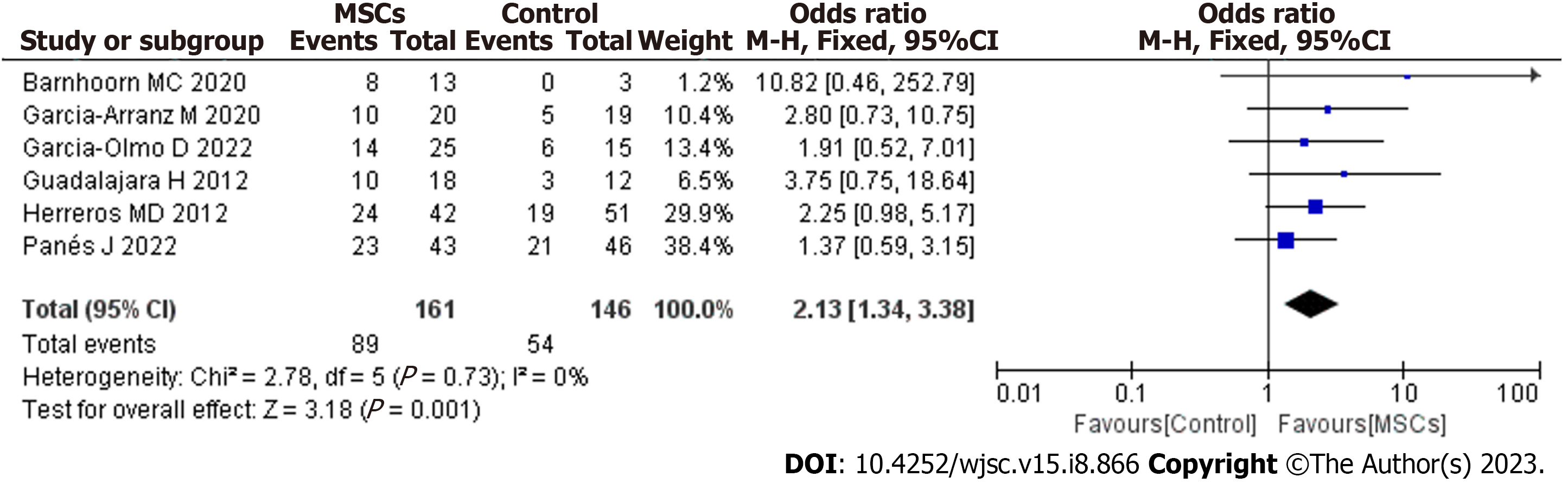
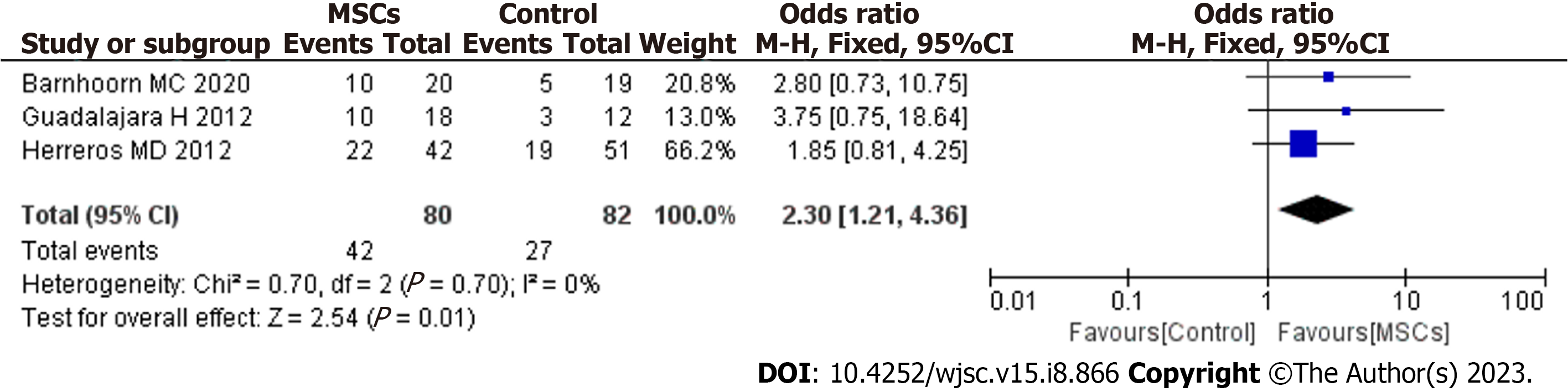
After screening, 6 studies met the inclusion criteria. MSC therapy was associated with an improved long-term healing rate (HR) compared with the control condition [odds ratio (OR) = 2.13; 95% confidence interval (95%CI): 1.34 to 3.38; P = 0.001]. Compared with fibrin glue (FG) therapy alone, MSC plus FG therapy was associated with an improved long-term HR (OR = 2.30; 95%CI: 1.21 to 4.36; P = 0.01). When magnetic resonance imaging was used to evaluate fistula healing, MSC therapy was found to achieve a higher long-term HR than the control treatment (OR = 2.79; 95%CI: 1.37 to 5.67; P = 0.005). There were no significant differences in long-term safety (OR = 0.77; 95%CI: 0.27 to 2.24; P = 0.64).
Our study indicated that local MSC therapy promotes long-term and sustained healing of complex PFs and that this method is safe.
Core Tip: The long-term safety and efficacy of mesenchymal stem cell (MSC) therapy for complex perianal fistulas (PFs) remain unknown. So, we explored the long-term effectiveness and safety of local MSC therapy for complex PFs. We found that MSC treatment is a safe and effective method that can significantly improve the long-term healing of complex PFs, and this method confers no risk of MSC-related adverse events.
- Citation: Cheng F, Zhong H, Huang Z, Li Z. Up-to-date meta-analysis of long-term evaluations of mesenchymal stem cell therapy for complex perianal fistula. World J Stem Cells 2023; 15(8): 866-875
- URL: https://www.wjgnet.com/1948-0210/full/v15/i8/866.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i8.866
A perianal fistula (PF) is an epithelialized tract that connects the luminal surface of the anal canal or rectum with the perianal or perineal skin. It affects approximately 2 in 10000 people and represents a difficult therapeutic challenge and a source of physical and psychological morbidity with a long-term risk of proctectomy[1]. The most severe form is complex PF, which is difficult to manage, as it has a high rate of recurrence and may cause sphincter damage and fecal incontinence. Complex PF is defined as cases with more muscle involvement or anterior fistulas in female patients, as well as recurrent fistulas, suprasphincteric fistulas, extrasphincteric fistulas, horseshoe fistulas, fistulas associated with irritable bowel disease, transsphincteric fistulas that involve greater than 30% of the external sphincter and fistulas associated with preexisting fecal incontinence, inflammatory bowel disease, radiation, malignancy, or chronic diarrhea. PFs are also a probable consequence of Crohn’s disease (CD) since as many as 26% of CD patients eventually develop a PF within 20 years after diagnosis[2,3]. Complex PFs in patients with CD have a high recurrence rate and cause a vast range of complications that significantly reduce quality of life (QoL)[4]. In addition, there is a risk of developing a neoplasm in the PF area related to the complexity and perianal disease duration[5]. The results of one study showed that complex PFs may lead to anal cancer in approximately 28% of patients within 20 years after diagnosis[6]. Complex PF is a chronic, recurrent immune-mediated disease with a variety of treatment options. Its exact pathogenesis is unknown. The key long-term therapeutic goals for the treatment of complex PF are to: (1) Resolve fistula discharge; (2) achieve fistula healing; (3) prevent fistula recurrence; (4) maintain fecal continence; (5) avoid long-term diversion (protectomy with stoma), and hence; and (6) improve and maintain QoL for patients. Although current treatments for PFs include a range of medical and surgical options, managing this condition is difficult. Patients with complex PFs tend to have poor treatment outcomes or experience frequent relapses, and most interventions are ineffective in providing long-term healing[7-10]. Immunomodulators can have serious side effects. Additionally, there is a risk of opportunistic infection associated with the use of biological treatments. In more severe cases of complex PF, fecal incontinence can occur, furthering morbidity. Complex PF is often not permanently cured by surgery, leading to multiple procedures and complications such as fecal incontinence. Therefore, there is a need for an effective therapy that provides long-term healing of complex PFs without the risk of fecal incontinence.
In recent years, local injection of mesenchymal stem cells (MSCs) has shown notable promising results in the treatment of PFs[11]. MSCs are a heterogeneous subset of stromal stem cells. They can be isolated from a wide variety of tissues and expanded in vitro to obtain large quantities. MSCs are characterized by multilineage differentiation and powerful immunomodulatory effects and are able to mitigate inflammatory states. Complex PF is thought to arise from an epithelial defect, which may be caused by ongoing inflammation. Current treatments cannot maintain long-term healing of the disease. Possible alternative treatments include cell therapy, especially MSC therapy. Local administration is the most performed approach to deliver MSCs. After being delivered directly to fistula tracts, MSCs induce peripheral tolerance and migrate to injured tissues, where they can inhibit the release of proinflammatory cytokines and promote the survival of damaged cells. Consequently, MSCs are capable of repairing damaged tissues and promoting tissue healing, which can lead to long-term fistula healing, significantly improving patients’ quality of life. An increasing number of studies have indicated that local MSC therapy is safe and efficacious for complex PFs. In 2020, to evaluate whether local MSC therapy for complex PFs is effective and safe, we conducted a meta-analysis. That study, with a follow-up of 8 wk to 2 years, showed that local MSC therapy for complex PFs was safe and feasible. However, the efficacy and safety evaluation period of the study was short and middle term[12]. Therefore, the long-term efficacy of MSC therapy is unclear. To date, an increasing number of studies have aimed to perform long-term evaluations of MSC therapy for PFs. Thus, based on a previous study[12], this study aimed to explore the long-term effectiveness and safety of MSC therapy for complex PFs (48 wk to 4 years of follow-up after MSC therapy).
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[13]. In August 2022, a search was performed in the PubMed, EMBASE and Cochrane Library databases for clinical studies written in English regarding local MSC treatment in patients with complex PFs. The following search terms were used: “Mesenchymal stem cell’’, “mesenchymal stromal cell”, “complex perianal fistula”, “perianal fistula”, “complex perianal Crohn’s disease”, “Crohn’s perianal fistula”, “perianal Crohn’s disease”, “Crohn’s disease”, “perianal fistulizing Crohn’s disease”, “cryptoglandular perianal fistula”, and “long-term”. We also checked the reference lists of the screened full-text studies to identify other potentially eligible trials.
Two authors independently assessed studies for inclusion by screening titles and abstracts. The inclusion criteria were: (1) Studies of human subjects; (2) randomized clinical trials (RCTs) and retrospective studies or cohort studies of patients with complex PFs treated with local injection(s) of autologous or allogeneic MSCs from any source; (3) local injection of MSCs or MSCs combined with fibrin glue (FG) for complex PFs; and (4) assessment of the efficacy and/or safety of MSC therapy at least 48 wk after treatment.
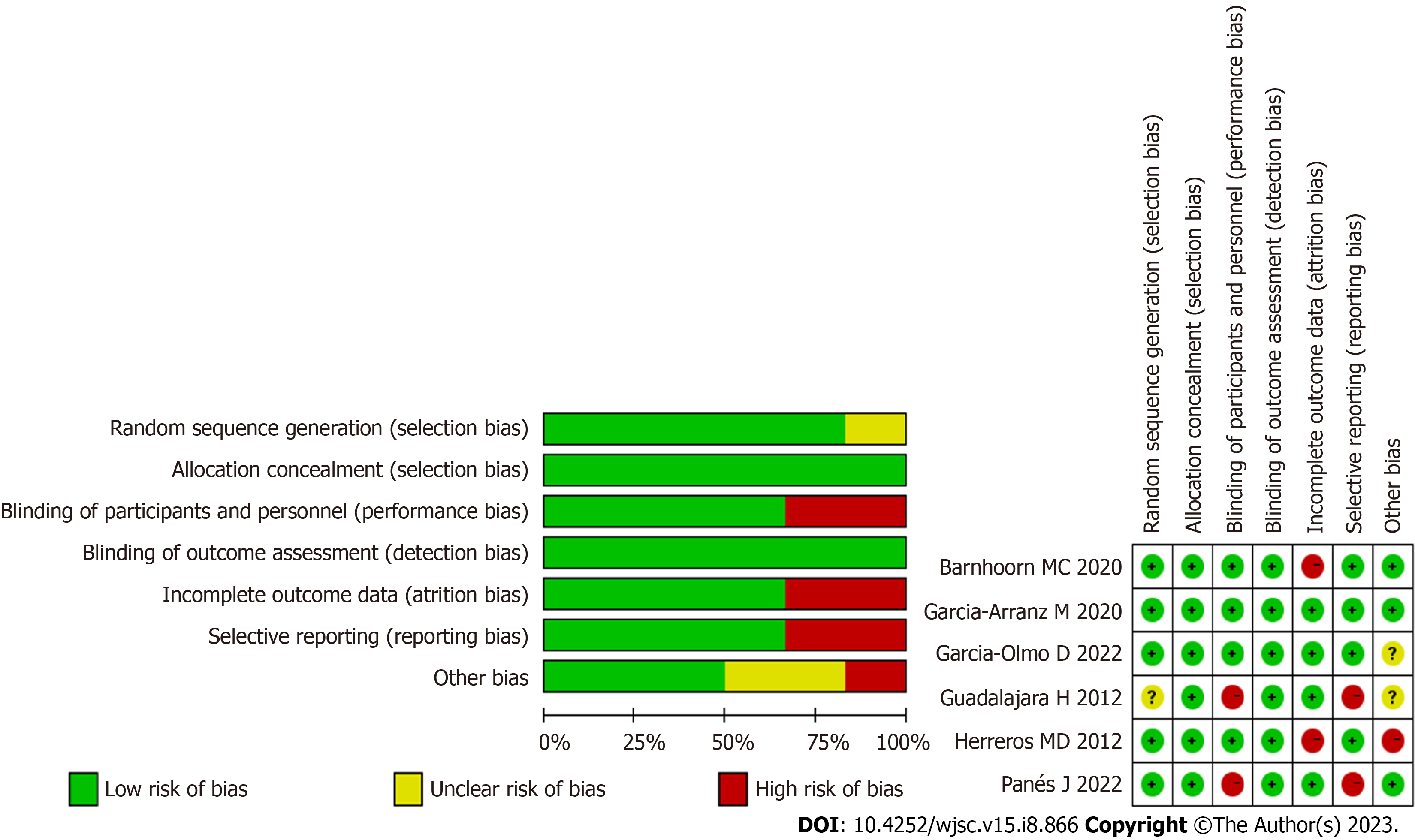
A customized data extraction form was used by two authors to extract data. These authors extracted study data features including: (1) Study characteristics (first author, publication year); (2) MSC origin; (3) definition of fistula healing [clinical and/or magnetic resonance imaging (MRI)]; (4) study outcomes; (5) dosage and modalities of intervention administration; (6) adverse events (AEs); and (7) concomitant treatment (anti-TNF). Table 1 lists the basic characteristics of the 6 identified studies. Quality assessment was performed using Review Manager (version 5.3) according to the recommendations from the Cochrane Collaboration. The bias risk assessment tool recommended by Cochrane was used to assess the quality of all enrolled studies. Each item of studies was judged as having a high, low or unclear risk of bias.
| Ref. | Cell type and source | Outcome assessment | Study outcomes | Intervention (mean) | AEs | Concurrent use of anti-TNF |
| Guadalajara et al[14], 2012 | Autologous, ASCs | Re-epithelialization + MRI | 10/18 for MSCs + FG; 3/12 for FG at 1 yr | First: 2 × 107 MSCs; second: 4 × 107 MSCs | / | Yes |
| Herreros et al[15], 2012 | Autologous, ASCs | Re-epithelialization +MRI | 24/42 for MSCs; 22/42 for MSCs + FG; 19/51 for FG at 48 wk | First: 2 × 107 MSCs; second: 4 × 107 MSCs | / | Yes |
| Garcia-Arranz et al[16], 2020 | Autologous, ASCs | Re-epithelialization | 10/20 for MSCs + FG; 5/19 for FG at 2 yr | First: 10 × 107 MSCs; second: 10 × 107 MSCs | 7/23 for MSCs + FG vs 9/21 for FG | Yes |
| Barnhoorn et al[17], 2020 | Allogeneic, BMSCs | MRI | 8/13 for MSCs; 0/3 for placebo group at 4 yr | A: 1 × 107 MSCs; B: 3 × 107 MSCs; C: 9 × 107 MSCs | / | / |
| Panés et al[18], 2022 | Allogeneic, ASCs | Re-epithelialization | 23/43 for MSC; 21/46 for saline solution at 156 wk | 12 × 107 MSCs | / | Yes |
| Garcia-Olmo et al[19], 2022 | Allogeneic, ASCs | Re-epithelialization | 14/25 for MSCs; 6/15 for saline solution at 104 wk | 12 × 107 MSCs | 3/25 for MSCs vs 1/15 for placebo | Yes |
Based on the included studies, odds ratios (ORs) and corresponding 95% confidence intervals (95%CIs) were calculated to compare the MSC groups and control groups. Heterogeneity was quantified using the I2 statistic. If the I2value was ≤ 50%, heterogeneity was considered low, and we employed a fixed-effect model. If the I2 value was > 50%, heterogeneity was considered high, and we employed a random-effect model. All statistical analyses were performed using Review Manager (version 5.3). A value of P < 0.05 was considered statistically significant.
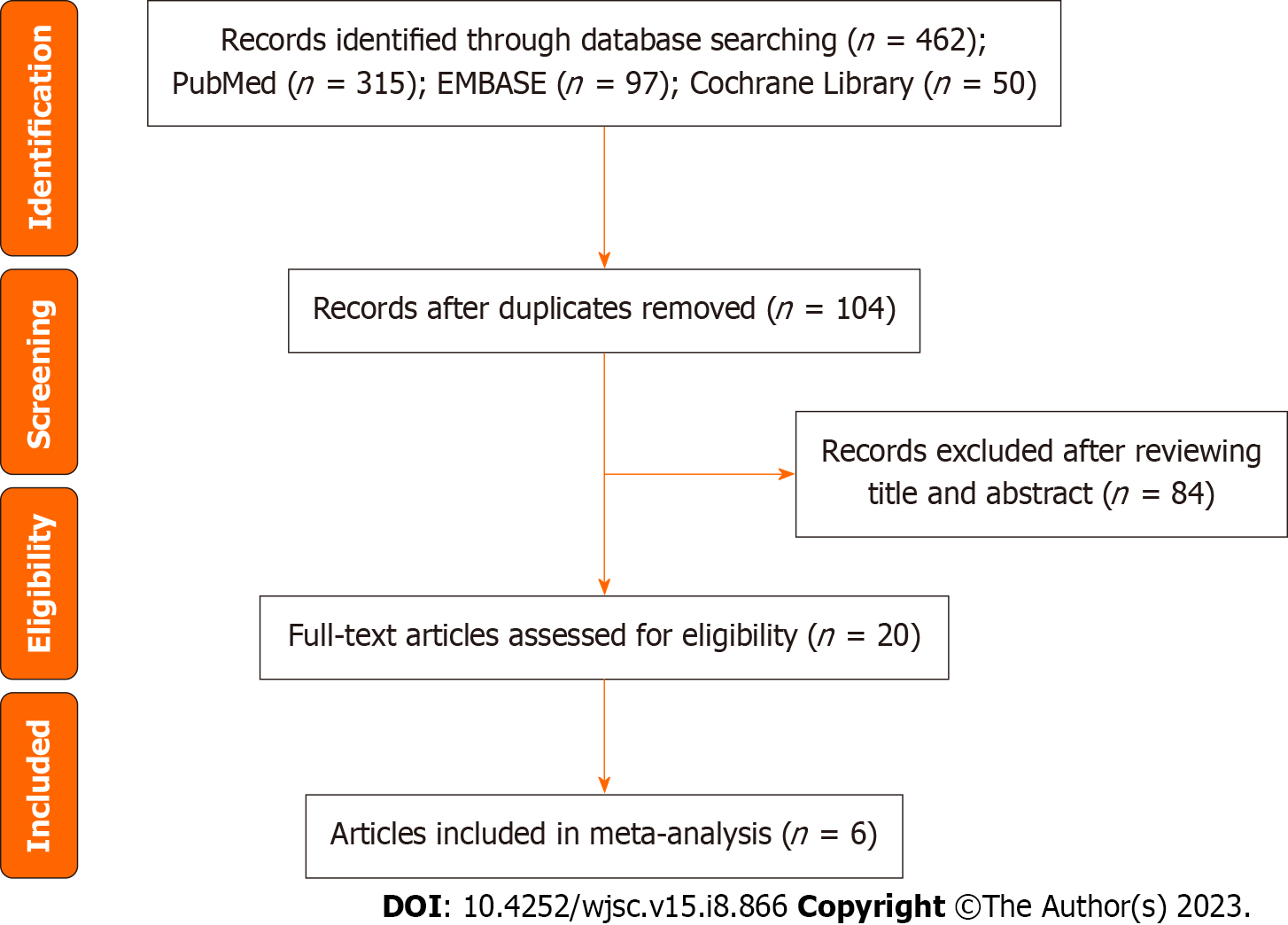
The literature search process is illustrated in Figure 1. Using this search strategy, we identified 462 references related to local MSC treatment for complex PF, of which 358 duplicate articles were removed. After reading the titles and abstracts, we identified 20 articles for full-text review. Ultimately, after all criteria were applied, 6 articles were included in the meta-analysis[14-19]. A summary of the risk of bias in the included articles is presented in Figure 2.
Our meta-analysis included 6 studies that assessed the long-term efficacy of post-MSC treatment. The pooled analysis showed that MSC therapy was associated with an improved long-term healing rate (HR) compared with the control condition (OR = 2.13; 95%CI: 1.34 to 3.38; P = 0.001) (Figure 3). The benefit was sustained for at least 48 wk of follow-up after MSC therapy.
Cell therapy strategies using MSCs carried in FG have shown promising results in regenerative medicine. FG is a natural polymer involved in the coagulation process. In regenerative medicine, FG can be used as a delivery system for drugs, biomolecules, growth factors and cells. FG also provides a temporary structure that favors angiogenesis, extracellular matrix deposition and cell-matrix interactions and it also FG maintains the local and paracrine functions of MSCs, providing tissue regeneration through less invasive clinical procedures. The biological properties of FG as a growth environment for MSCs have been reported in several studies[20]. Now, local FG combined with MSCs therapy is still a relatively new treatment and has not yet gained popularity. So, the need for the local FG combined with MSCs therapy remains unknown. In our meta-analysis, three studies were included that reported local FG combined with MSCs therapy for PF[14,15,17], with low heterogeneity between the studies (I2 = 0%). In a fixed-effects model, MSCs plus FG had more long-term efficacy for fistula healing than FG alone (OR = 2.30; 95%CI: 1.21 to 4.36; P = 0.01) (Figure 4). So, we think local FG combined with MSCs therapy have synergistic effect on PF.
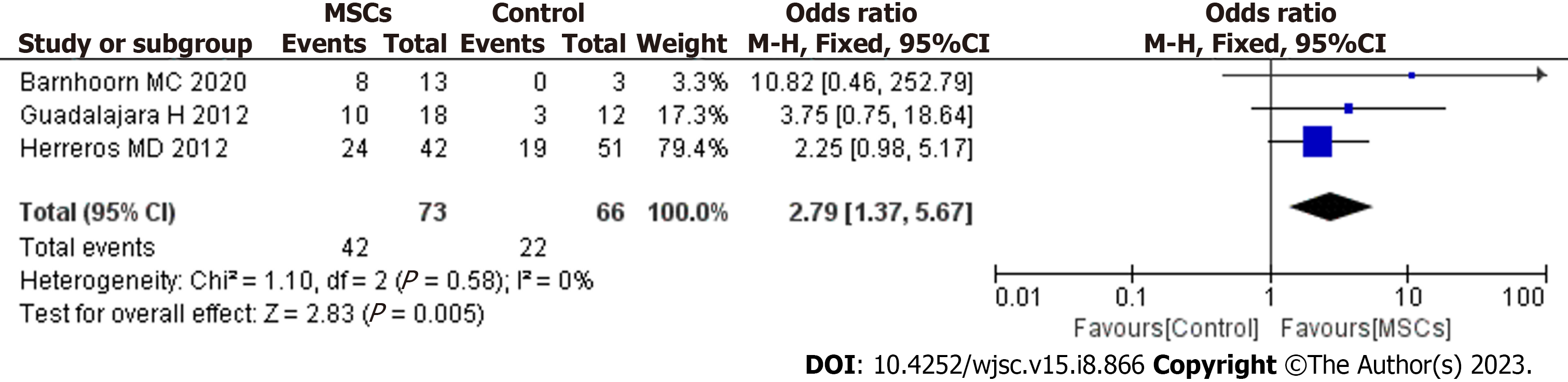
In our meta-analysis, MRI was used to evaluate fistula healing in 3 studies. The pooled analysis showed that MSC therapy was associated with improved long-term HR (OR = 2.79; 95%CI: 1.37 to 5.67; P = 0.005) (Figure 5).
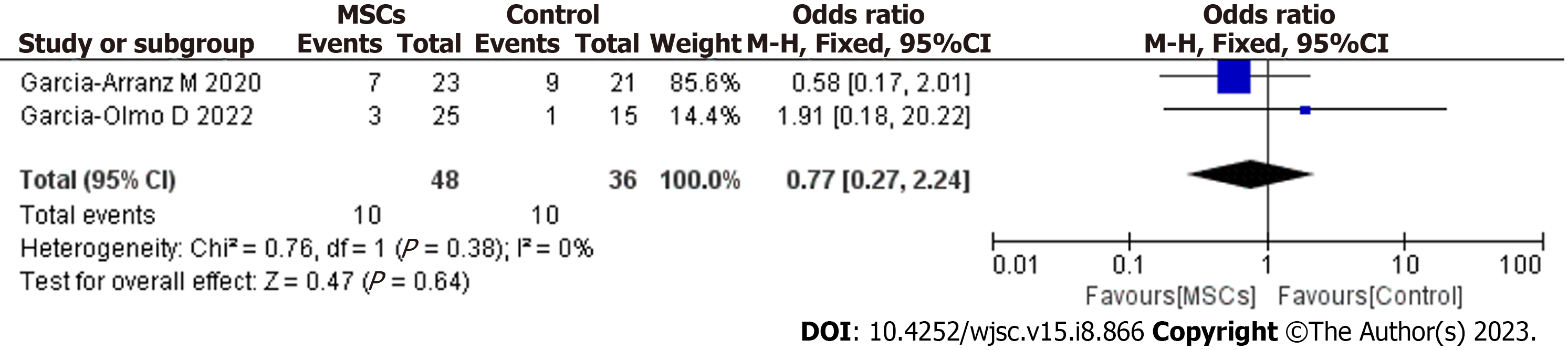
Only two studies[16,19] in this review assessed the long-term safety of MSC treatment for complex PF. The pooled results demonstrated that MSC treatment did not increase the risk of any long-term adverse or serious AEs (OR = 0.77; 95%CI: 0.27 to 2.24; P = 0.64) (Figure 6). No serious AEs related to MSC therapy were found.
During the past decade, cell therapy-based treatments have been developed to manage several digestive tract diseases, including PF[21,22]. Previous research has shown that MSCs have a variety of mechanisms that promote wound healing. These cells also lack substantial immunogenicity and are thus suitable for use across human leukocyte antigen (HLA) barriers[23,24]. An increasing number of studies have shown that MSC therapy is a safe and efficacious option for the short-term closure of PFs. However, maintaining continuous long-term fistula closure is also very important. A recurrent fistula is considered more difficult to treat surgically than the original fistula. Recently, an increasing number of studies have aimed to perform long-term follow-up of patients undergoing MSC administration to treat complex PFs. However, there has been no meta-analysis to comprehensively evaluate the long-term effectiveness and safety of MSC treatment. Therefore, this study aimed to find a treatment to maintain long-term PF healing and to provide a basis for clinical application.
To our knowledge, this is the first meta-analysis to evaluate the long-term safety and efficacy of local MSC therapy for complex PFs. Findings from our study show that MSC therapy promotes sustained healing of complex PFs and that this therapy alone or combined with FG treatment can promote the long-term healing of complex PFs (OR = 2.13; 95%CI: 1.34 to 3.38; P = 0.001). The benefit over the control was sustained for at least 48 wk after local injection of MSCs. In our study, the timepoint for the assessment of fistula healing fluctuated greatly (48 wk-4 years), and all included studies showed good long-term healing of fistulas post-MSC treatment. Therefore, we believe that fistula healing persisted after MSC treatment. Barnhoorn et al[17] also reported that in addition to high fistula closure rates, MSC-treated patients had a higher QoL after therapy than at baseline. Some studies have also shown that in patients receiving MSC transplantation, the PF closure rate is significantly higher and the time to closure significantly shorter than that with anti-TNF drugs and fistulotomy, and MSC transplantation yields a decreased frequency of recurrence of the disease[25-27]. Therefore, these data confirm that MSC therapy promotes the long-term healing of complex PFs and significantly improves the QoL of patients. In the future, in evaluating MSC therapy for PF, it might be useful to consider work productivity and lifestyle restrictions.
Cell therapy strategies using MSCs carried in FG have shown promising results in regenerative medicine. MSCs have angiogenic, anti-apoptotic and immunomodulatory properties. FG provides a temporary structure that favors angiogenesis, extracellular matrix deposition and cell-matrix interactions. Additionally, FG maintains the local and paracrine functions of MSCs, providing tissue regeneration through less invasive clinical procedures[28]. The use of FG has been found to be uniformly safe, with minimal adverse effects, an early return to normal activity, and no negative impact on continence. One study showed that FG had a short-term effectiveness in the treatment of PF. At week 8 of the study, more than one-third of patients had healed fistulas, and half showed clinical improvement. Most of the patients in clinical remission at week 8 maintained clinical remission at week 16[29]. There was also a study indicating that FG was effective over the long term for the treatment of PF, and nearly 2 years after the use of FG to treat PF, over half of the patients showed clinical signs of remission[30]. Therefore, in association with MSCs, the use of FG has shown promising results in the field of regenerative medicine[31]. However, there is a lack of long-term success data on the combination of FG and other treatments for complex PF. In our study, MSCs plus FG had more long-term efficacy for fistula healing than FG alone (OR = 2.30; 95%CI: 1.21 to 4.36; P = 0.01). Therefore, we believe that the stimulation of the cellular adhesion and growth action of FG and the differentiation ability of MSCs may have a synergistic effect on the healing of fistulas. In our study, all patients received cleaning surgery before MSC treatment. Deep curettage had a positive effect on fistula closure in both groups (MSC recipients and controls). However, Garcia-Arranz et al[16] observed an increased number of long-term recurrences among control participants. Therefore, we speculate that the inflammatory focus persists, explaining why “deep curettage” may not provide a lasting resolution. In this scenario, MSCs and their anti-inflammatory and immunomodulatory effects can promote long-term healing.
Questions persist regarding the safety of MSC treatment for PF. Although MSC therapy has not raised any major safety concerns thus far in clinical trials, it is important to evaluate the safety of cell therapy in the long term. Our study provides evidence that MSC therapy has a good long-term safety profile as a treatment for complex PF (OR = 0.77; 95%CI: 0.27 to 2.24; P = 0.64). In our study, the surgical management was fistula tract curettage and internal opening closure before MSC therapy. Notably, this is a minimally invasive surgery (involving perioperative antibiotic use, anesthesia, antisepsis, internal fistula orifice location, de-epithelization of the fistula tract, cleaning of the cavities and fistula tracts, closure of the internal opening, stem cell handling and resuspension, and cell injection) and does not produce fecal incontinence[32]. In contrast, anti-TNF therapies used for the treatment of PFs are associated with an increased risk of opportunistic infections, and surgical procedures often result in fecal incontinence. Tumorigenicity and ectopic tissue formation are the main concerns with the use of MSCs, and the risk of these SAEs is especially high during long-term MSC treatment. To date, MSCs have not been reported to cause tumors. Nevertheless, neoplasm development may become apparent only after longer follow-up periods. In another study conducted by our group, no neoplasm development was reported over the longest follow-up period of approximately 4 years[17]. To further confirm that neoplasms are not a concern with MSC treatment, biopsies taken from the fistula region were examined in that study. However, publication of these results is still pending, and we can continue the follow-up. While MSCs have not been shown to cause tumors in existing studies, long-term follow-up should be carried out to investigate the risk of cancer development. In the future, more long-term safety data are needed to fully assess the safety aspects of local MSC treatment.
Studies included in our meta-analysis used variable definitions of fistula healing. We believe that defining a healed fistula as complete re-epithelialization of external openings is not objective or accurate, as it does not account for the inside of the entire fistula. To evaluate the efficacy of MSC treatment, we should use more accurate methods for evaluating the inside of the entire fistula. MRI is the reference standard and cornerstone of fistula imaging. It demonstrates high sensitivity and specificity for the number and location of fistula tracts, detecting complexities frequently missed on clinical examination alone[33]. Therefore, MRI plays a crucial role in the evaluation, detection and follow-up of complex PFs[34]. In our meta-analysis, 3 RCTs based fistula healing on a combination of clinical examination and MRI imaging. The results showed that the MSC group had a higher HR than the control group (OR = 2.79; 95%CI: 1.37 to 5.67; P = 0.005). The absence of MRI examinations in some of the included studies at the end of the long-term follow-up is a limitation of this meta-analysis. In the future, if possible, blood should be drawn for standard measure
In this study, we aimed to evaluate the long-term efficacy and safety of MSC therapy through a meta-analysis. However, there are some unresolved questions. In the current clinical research, there are two ways to inject stem cells: (1) Systemic (mainly intravenous) injection; and (2) local injection. In our study, all patients received local MSC treatment. We speculate that for localized digestive tract diseases, local application and delivery seems more logical because side effects can be minimized and the cells are kept in direct contact with the at-risk tissue. Therefore, local MSC therapy seems to be a more promising treatment approach for further research. In our study, all eligible patients received a fixed dose of MSCs (one-time local injection or a second dose). Unfortunately, not all of the studies have compared various doses of MSCs or the benefit of repeat injections after the initial treatment. Most patients in our study received two doses of MSCs. Some studies have indicated a relationship between cell dose—or even the number of doses and efficacy[35,36]. In addition, all eligible patients with complex PFs may have branches with multiple tracks involving an extensive area that cannot always be adequately treated with a fixed dose of cells. Perhaps the cell dosage is related to the length of the fistula tracts and cavities. Due to the limitations of studies, it is difficult to provide recommendations on the optimal dose. In future research, we should pay attention to these unresolved questions (such as MSC origin, dosage and modality of intervention) to ensure that PF patients receive optimal treatment.
Our meta-analysis is the first to evaluate the long-term efficacy and safety of MSCs for PF treatment. Inevitably, this article has some limitations: (1) The studies used MSCs of different origins (adipose tissue and bone marrow from autologous as well as allogeneic sources); (2) some included studies defined the healed fistula as re-epithelialization of the external opening of the fistula. This may cause our results to be overestimated; (3) all patients underwent surgical procedures such as deep curettage. This may be beneficial to the short-term clinical remission of the fistulas. However, whether deep curettage will benefit long-term healing remains uncertain; (4) the follow-up period of the included studies varied significantly. Indeed, some of the studies lacked long-term follow-up data. In the future, we need more patients to enter the extended follow-up period so that the long-term safety and efficacy of MSCs can be assessed; and (5) the number of included studies and the sample size were limited, and extrapolation of the meta-analysis results was limited to some extent. So, our study was limited by its multiple centers and heterogeneity in the study inclusion criteria, mesenchymal stem cell origin, dose and frequency of delivery, and definition and time point of fistula healing. In the future, more patients must be evaluated in long-term follow-ups to optimize the efficacy and safety of MSCs for PF treatment.
In summary, MSC treatment is a safe and effective method that can significantly improve the long-term healing of complex PFs, and this method confers no risk of MSC-related AEs.
An increasing number of studies have indicated that local mesenchymal stem cell (MSC) therapy is safe and efficacious for complex perianal fistulas (PFs). But, the long-term efficacy of MSC therapy is unclear. To date, an increasing number of studies have aimed to perform long-term evaluations of MSC therapy for PFs.
Local MSC therapy for PFs has shown considerable promise. But, the long-term safety and efficacy of MSC therapy in complex PFs is unknown.
To explore the long-term effectiveness and safety of MSC therapy for complex PFs.
PubMed, EMBASE and Cochrane Library databases were searched that reported the long-term evaluation of local MSC therapy for complex PFs. The effectiveness and safety data analysis were conducted using RevMan5.3.
After screening, 6 studies met the inclusion criteria. MSC therapy was associated with an improved long-term healing rate (HR) compared with the control condition [odds ratio (OR) = 2.13; 95% confidence interval (95%CI): 1.34 to 3.38; P = 0.001]. Compared with fibrin glue (FG) therapy alone, MSC plus FG therapy was associated with an improved long-term HR (OR = 2.30; 95%CI: 1.21 to 4.36; P = 0.01). When magnetic resonance imaging was used to evaluate fistula healing, MSC therapy was found to achieve a higher long-term HR than the control treatment (OR = 2.79; 95%CI: 1.37 to 5.67; P = 0.005). There were no significant differences in long-term safety (OR = 0.77; 95%CI: 0.27 to 2.24; P = 0.64).
Our study indicated that local MSC therapy promotes long-term and sustained healing of complex PFs and that this method is safe.
In complex PFs treatment, local MSC therapy should be paid more attention to. Considering that this small number may not be enough to represent the whole complex PFs population, In the future, to improve on the quality of research, future studies should be carefully designed and reported.
We appreciate the contributions of all the doctors, coworkers, and friends involved in this study and thank the editors and reviewers for their help with this manuscript.
| 1. | Zanotti C, Martinez-Puente C, Pascual I, Pascual M, Herreros D, García-Olmo D. An assessment of the incidence of fistula-in-ano in four countries of the European Union. Int J Colorectal Dis. 2007;22:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Bubbers EJ, Cologne KG. Management of Complex Anal Fistulas. Clin Colon Rectal Surg. 2016;29:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 3. | Park SH, Aniwan S, Scott Harmsen W, Tremaine WJ, Lightner AL, Faubion WA, Loftus EV. Update on the Natural Course of Fistulizing Perianal Crohn's Disease in a Population-Based Cohort. Inflamm Bowel Dis. 2019;25:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Mahadev S, Young JM, Selby W, Solomon MJ. Quality of life in perianal Crohn's disease: what do patients consider important? Dis Colon Rectum. 2011;54:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Panés J, Rimola J. Perianal fistulizing Crohn's disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:652-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 6. | Eglinton TW, Barclay ML, Gearry RB, Frizelle FA. The spectrum of perianal Crohn's disease in a population-based cohort. Dis Colon Rectum. 2012;55:773-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Kotze PG, Shen B, Lightner A, Yamamoto T, Spinelli A, Ghosh S, Panaccione R. Modern management of perianal fistulas in Crohn's disease: future directions. Gut. 2018;67:1181-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, Rodriguez-de-Santiago E, Lopez-Sanroman A. Management of complex perianal Crohn's disease. Ann Gastroenterol. 2017;30:33-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Panes J, Reinisch W, Rupniewska E, Khan S, Forns J, Khalid JM, Bojic D, Patel H. Burden and outcomes for complex perianal fistulas in Crohn's disease: Systematic review. World J Gastroenterol. 2018;24:4821-4834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 10. | Pedersen KE, Lightner AL. Managing Complex Perianal Fistulizing Disease. J Laparoendosc Adv Surg Tech A. 2021;31:890-897. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Sheikholeslami A, Fazaeli H, Kalhor N, Khoshandam M, Eshagh Hoseini SJ, Sheykhhasan M. Use of Mesenchymal Stem Cells in Crohn's Disease and Perianal Fistulas: A Narrative Review. Curr Stem Cell Res Ther. 2023;18:76-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Cheng F, Huang Z, Li Z. Efficacy and Safety of Mesenchymal Stem Cells in Treatment of Complex Perianal Fistulas: A Meta-Analysis. Stem Cells Int. 2020;2020:8816737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48561] [Article Influence: 2856.5] [Reference Citation Analysis (3)] |
| 14. | Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 15. | Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D; FATT Collaborative Group. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 16. | Garcia-Arranz M, Garcia-Olmo D, Herreros MD, Gracia-Solana J, Guadalajara H, de la Portilla F, Baixauli J, Garcia-Garcia J, Ramirez JM, Sanchez-Guijo F, Prosper F; FISPAC Collaborative Group. Autologous adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistula: A randomized clinical trial with long-term follow-up. Stem Cells Transl Med. 2020;9:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Barnhoorn MC, Wasser MNJM, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, Oosten LEM, Dijkstra G, van der Woude CJ, Roelen DL, Zwaginga JJ, Verspaget HW, Fibbe WE, Hommes DW, Peeters KCMJ, van der Meulen-de Jong AE. Long-term Evaluation of Allogeneic Bone Marrow-derived Mesenchymal Stromal Cell Therapy for Crohn's Disease Perianal Fistulas. J Crohns Colitis. 2020;14:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Panés J, Bouma G, Ferrante M, Kucharzik T, Nachury M, de la Portilla de Juan F, Reinisch W, Selvaggi F, Tschmelitsch J, Brett NR, Ladouceur M, Binek M, Hantsbarger G, Campbell-Hill S, Karki C, Buskens C. INSPECT: A Retrospective Study to Evaluate Long-term Effectiveness and Safety of Darvadstrocel in Patients With Perianal Fistulizing Crohn's Disease Treated in the ADMIRE-CD Trial. Inflamm Bowel Dis. 2022;28:1737-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Garcia-Olmo D, Gilaberte I, Binek M, D Hoore AJL, Lindner D, Selvaggi F, Spinelli A, Panés J. Follow-up Study to Evaluate the Long-term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients With Perianal Fistulizing Crohn's Disease: ADMIRE-CD Phase 3 Randomized Controlled Trial. Dis Colon Rectum. 2022;65:713-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 20. | Süloğlu AK, Karacaoğlu E, Bilgic HA, Selmanoğlu G, Koçkaya EA, Karaaslan C. Osteogenic differentiation of adipose tissue-derived mesenchymal stem cells on fibrin glue- or fibronectin-coated Ceraform®. J Biomater Appl. 2019;34:375-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Trebol Lopez J, Georgiev Hristov T, García-Arranz M, García-Olmo D. Stem cell therapy for digestive tract diseases: current state and future perspectives. Stem Cells Dev. 2011;20:1113-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | García-Olmo D, García-Arranz M, García LG, Cuellar ES, Blanco IF, Prianes LA, Montes JA, Pinto FL, Marcos DH, García-Sancho L. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn's disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 233] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 23. | Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576-6583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 505] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 24. | Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 324] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 25. | Park MY, Yoon YS, Lee JL, Park SH, Ye BD, Yang SK, Yu CS. Comparative perianal fistula closure rates following autologous adipose tissue-derived stem cell transplantation or treatment with anti-tumor necrosis factor agents after seton placement in patients with Crohn's disease: a retrospective observational study. Stem Cell Res Ther. 2021;12:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Knyazev OV, Fadeeva NA, Kagramanova AV, Belyakov NI, Orlova NV, Lishchinskaya AA, Konoplyannikov AG, Parfenov AI. Stem Cell Therapy for Perianal Crohn's Disease. Ter Arkh. 2018;90:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Park MY, Yoon YS, Kim HE, Lee JL, Park IJ, Lim SB, Yu CS, Kim JC. Surgical options for perianal fistula in patients with Crohn's disease: A comparison of seton placement, fistulotomy, and stem cell therapy. Asian J Surg. 2021;44:1383-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Ortiz AC, Fideles SOM, Pomini KT, Reis CHB, Bueno CRS, Pereira ESBM, Rossi JO, Novais PC, Pilon JPG, Rosa Junior GM, Buchaim DV, Buchaim RL. Effects of Therapy with Fibrin Glue combined with Mesenchymal Stem Cells (MSCs) on Bone Regeneration: A Systematic Review. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Grimaud JC, Munoz-Bongrand N, Siproudhis L, Abramowitz L, Sénéjoux A, Vitton V, Gambiez L, Flourié B, Hébuterne X, Louis E, Coffin B, De Parades V, Savoye G, Soulé JC, Bouhnik Y, Colombel JF, Contou JF, François Y, Mary JY, Lémann M; Groupe d'Etude Thérapeutique des Affections Inflammatoires du Tube Digestif. Fibrin glue is effective healing perianal fistulas in patients with Crohn's disease. Gastroenterology. 2010;138:2275-2281, 2281.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Vitton V, Gasmi M, Barthet M, Desjeux A, Orsoni P, Grimaud JC. Long-term healing of Crohn's anal fistulas with fibrin glue injection. Aliment Pharmacol Ther. 2005;21:1453-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Ravari H, Hamidi-Almadari D, Salimifar M, Bonakdaran S, Parizadeh MR, Koliakos G. Treatment of non-healing wounds with autologous bone marrow cells, platelets, fibrin glue and collagen matrix. Cytotherapy. 2011;13:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Georgiev-Hristov T, Guadalajara H, Herreros MD, Lightner AL, Dozois EJ, García-Arranz M, García-Olmo D. A Step-By-Step Surgical Protocol for the Treatment of Perianal Fistula with Adipose-Derived Mesenchymal Stem Cells. J Gastrointest Surg. 2018;22:2003-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Williams G, Williams A, Tozer P, Phillips R, Ahmad A, Jayne D, Maxwell-Armstrong C. The treatment of anal fistula: second ACPGBI Position Statement - 2018. Colorectal Dis. 2018;20 Suppl 3:5-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Thipphavong S, Costa AF, Ali HA, Wang DC, Brar MS, Jhaveri KS. Structured reporting of MRI for perianal fistula. Abdom Radiol (NY). 2019;44:1295-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Skific M, Golemovic M, Crkvenac-Gornik K, Vrhovac R, Golubic Cepulic B. Comparative Analysis of Biological and Functional Properties of Bone Marrow Mesenchymal Stromal Cells Expanded in Media with Different Platelet Lysate Content. Cells Tissues Organs. 2018;205:226-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Lightner AL, Wang Z, Zubair AC, Dozois EJ. A Systematic Review and Meta-analysis of Mesenchymal Stem Cell Injections for the Treatment of Perianal Crohn's Disease: Progress Made and Future Directions. Dis Colon Rectum. 2018;61:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salaga M, Poland; Trebol J, Spain; Li SC, United States S-Editor: Chen YL L-Editor: A P-Editor: Xu ZH