Published online Oct 26, 2020. doi: 10.4252/wjsc.v12.i10.1152
Peer-review started: May 15, 2020
First decision: June 7, 2020
Revised: June 18, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: October 26, 2020
Processing time: 163 Days and 18.5 Hours
Adipose-derived mesenchymal stem cells (ASCs) are characterized by long-term self-renewal and a high proliferation rate. Under adequate conditions, they may differentiate into cells belonging to mesodermal, endodermal or ectodermal lineages. Pericytes support endothelial cells and play an important role in stabilizing the vessel wall at the microcirculation level. The loss of pericytes, as occurs in diabetic retinopathy, results in a breakdown of the blood-retina barrier (BRB) and infiltration of inflammatory cells. In this context, the use of pericyte-like differentiated ASCs may represent a valuable therapeutic strategy for restoring BRB damage.
To test in vitro strategies to obtain pericyte-like differentiation of human ASCs (hASCs).
Different culture conditions were tested: hASCs cultured in a basal medium supplemented with transforming growth factor β1; and hASCs cultured in a specific pericyte medium (PM-hASCs). In a further sample, pericyte growth supplement was omitted from the PM. In addition, cultures of human retinal pericytes (hRPCs) were used for comparison. Pericyte-like differentiation of hASCs was tested by immunocytochemical staining and western blotting to evaluate the expression of α-smooth muscle actin (α-SMA) and neural/glial antigen 2 (NG2). Interactions between human retinal endothelial cells (hRECs) and different groups of hASCs were investigated in co-culture experiments. In these cases, the expression of typical junctional proteins such as vascular endothelial-Cadherin, zonula occludens-1 and Occludin were assessed in hRECs. In an in vitro model of the BRB, values of trans-endothelial electrical resistance were measured when hRECs were co-cultured with various groups of pretreated hASCs. The values observed were compared with co-cultures of hRECs and hRPCs as well as with cultures of hRECs alone. Three-dimensional co-cultures of hRECs and hRPCs or pericyte-like hASCs in Matrigel were designed to assess their reciprocal localization.
After 3-6 d of culture, α-SMA and NG2 immunocytochemistry showed that the closest pericyte-like phenotype was observed when hASCs were cultured in Pericyte Medium (PM-hASCs). In particular, α-SMA immunoreactivity, already visible at the basal level in pericytes and ASCs, was strongly increased only when transforming growth factor was added to the culture medium. NG2 expression, almost undetectable in most conditions, was substantially increased only in PM-hASCs. Immunocytochemical results were confirmed by western blot analysis. The presence of pericyte growth supplement seems to increase NG2 expression rather than α-SMA, in agreement with its role in maintaining pericytes in the proliferative state. In co-culture experiments, immunoreactivity of vascular endothelial-Cadherin, zonula occludens-1 and Occludin was considerably increased in hRECs when hRPCs or PM-hASCs were also present. Supporting results were found by trans-endothelial electrical resistance measurements, gathered at 3 and 6 d of co-culture. The highest resistance values were obtained when hRECs were co-cultured with hRPCs or PM-hASCs. The pericyte-like phenotype of PM-hASCs was also confirmed in three-dimensional co-cultures in Matrigel, where PM-hASCs and hRPCs similarly localized around the tubular formations made by hRECs.
PM-hASCs seem able to strengthen the intercellular junctions between hRECs, likely reinforcing the BRB; thus, hASC-based therapeutic approaches may be developed to restore the integrity of retinal microcirculation.
Core Tip: Pericyte-like differentiation was achieved in human adipose-derived mesenchymal stem cells (hASCs) by a culture medium specific for optimal pericyte growth. When co-cultured with retinal endothelial cells, pre-differentiated hASCs increased endothelial junction protein expression and trans-endothelial electrical resistance. Similar to pericytes, differentiated hASCs localized in the typical perivascular position.
- Citation: Mannino G, Gennuso F, Giurdanella G, Conti F, Drago F, Salomone S, Lo Furno D, Bucolo C, Giuffrida R. Pericyte-like differentiation of human adipose-derived mesenchymal stem cells: An in vitro study. World J Stem Cells 2020; 12(10): 1152-1170
- URL: https://www.wjgnet.com/1948-0210/full/v12/i10/1152.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i10.1152
Human adipose-derived mesenchymal stem cells (hASCs) have been widely studied in the last few decades due to their multipotent differentiation ability. In fact, they can differentiate not only into typical mesodermal cells such as adipocytes[1], chondrocytes[2] and osteocytes[3], but also into cells of other lineages[4]. For this reason, they have been extensively investigated as a tool for potential therapeutic applications in a variety of diseases[5].
In the present investigation, the differentiation of ASCs toward a pericyte phenotype was tested in vitro. Pericytes share several mesenchymal stem cell (MSC) features[6-8]. Both types of cells originate from the embryonic mesodermal layer, show some similar surface markers, and share some differentiation capabilities[9].
In blood vessels, pericytes surround endothelial cells and help to stabilize the vessel wall, preventing vascular leakage. Pericytes have also been associated with vessel plasticity, regression and remodeling of vascular networks[10]. Pericytes participate in the formation of the blood-retina barrier (BRB), as they do in brain tissue, together with neurons and astrocytes, in the formation of the blood-brain barrier.
The loss of pericytes has been associated with microcirculation damage and inflammation processes[11,12], such as those occurring in diabetic retinopathy (DR). Loss of pericytes is one of the earliest hallmarks of DR[13,14]. As a result, the BRB is disrupted, leading to macular edema as well as new vessel formation[15]. Since pericyte loss is virtually irreversible, only limited beneficial effects have been obtained so far with available therapeutic strategies. Therefore, every effort should be made to recover pericyte physiology and BRB integrity.
Data in the literature report that cultured hASCs may spontaneously acquire a pericyte-like phenotype[16,17]. However, depending on culture conditions, spontaneous differentiation towards endothelial cells has also been described.
Indeed, the use of stem cells has been explored to counteract DR-induced damage. Encouraging effects were reported by in vitro and in vivo experiments carried out in rodent models of DR[18]. In these animals, a functional vascular protection was reported after intravitreal administration of naïve or TGF-β pretreated hASCs. In particular, an acceleration of vascular growth and a recovery of the central retinal vasculature was described. In diabetic rodent models, beneficial effects in the stabilization of BRB breakdown were also described by Kim et al[13], by intravitreal injections of multipotent perivascular progenitor cells derived from human embryonic stem cells.
In the present work, various culture strategies were tested to induce a pericyte-like differentiation of hASCs. The expression of α-smooth muscle actin (α-SMA) and neural/glial antigen 2 (NG2) in hASCs was evaluated by immunocytochemistry and western blot analysis and compared to that of native pericytes. In addition, co-cultures of human retinal endothelial cells (hRECs) with variously pretreated hASCs or human retinal pericytes (hRPCs) were also prepared to evaluate their interactions. Previous studies showed that a pericyte coverage of microvessels enhances the expression of junction proteins in endothelial cells and decreases vascular leakage[19]. Therefore, the expression of vascular endothelial (VE)–Cadherin, zonula occludens-1 (ZO-1) and Occludin were assessed in an in vitro equivalent of the BRB, comparing co-cultures of hRECs and hASCs to co-cultures of hRECs and hRPCs. Furthermore, the tightness of the barrier formed by these co-cultures was assessed by measuring the trans-endothelial electrical resistance (TEER). Finally, three-dimensional co-cultures of hRECs with hRPCs or pericyte-like differentiated hASCs in Matrigel were evaluated. Successful pericyte-like differentiation in vitro may be further developed in in vivo experiments for potential therapeutic approaches aimed at restoring the integrity of retinal microcirculation.
Adipose tissue was harvested from 4 healthy female donors (32-38 years old) undergoing liposuction procedures at the Cannizzaro Hospital, Catania (Italy). The donors were non-smokers and did not take estrogen replacement therapy. Lipoaspirate was obtained from the abdominal region after donors signed an informed consent to use the lipoaspirate for experimental procedures, in accordance with the Declaration of Helsinki (2000). The protocol was approved by the local ethics committee (Comitato etico Catania1; Authorization n. 155/2018/PO).
The raw lipoaspirate (50–100 mL) was washed with sterile phosphate-buffered saline (PBS; Invitrogen, Monza, Italy) to remove red blood cells and debris, and incubated for 3 h at 37°C with an equal volume of serum-free low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM-lg; Sigma-Aldrich, Milan, Italy) containing 0.075% type I collagenase (Invitrogen). After inactivation of collagenase activity by adding an equal volume of DMEM-lg containing 10% of heat-inactivated fetal bovine serum (FBS, Gibco, Monza, Italy), the digested lipoaspirate was centrifuged at 1200 rpm for 10 min. The pellets were then resuspended in PBS and filtered through a 100 µm nylon cell strainer (Falcon BD Biosciences, Milan, Italy). After a further centrifugation (1200 rpm for 10 min), cells were plated in T75 culture flasks (Falcon BD Biosciences) with DMEM-1g containing 10% FBS, 1% penicillin/streptomycin, and 1% MSC growth supplement (ScienCell Research Laboratories, Milan, Italy). After 24 h incubation at 37°C with 5% CO2, non-adherent cells were removed by replacing the growth medium. After reaching confluence (about 80% of total flask surface), all cultures were trypsinized and, after resuspension, cells were expanded for 2-3 passages and plated for the following procedures.
Some cell samples were used to verify their MSC nature, according to procedures previously described[20]. In particular, their immunopositivity for typical MSC markers (CD44, CD73, CD90 and CD105) was confirmed by immunocytochemistry and flow cytometry. At the same time, their immunonegativity for typical hematopoietic stem cell markers (CD14, CD34 and CD45) was verified.
For the purpose of the present investigation, several groups of cultures were prepared: The first group served as the control, consisting of hASCs in basal growth medium containing 5% FBS; in the second group, TGF-β1 (R and D Systems, Minneapolis, MN, United States) was added to simulate physiological conditions, where TGF-β is released by endothelial cells (TGF-hASCs); in the third group, hASCs were cultured in pericyte medium (PM) (Innoprot, Elexalde, Derio, Spain) instead of the hASCs pre-cultured in complete pericyte medium (PM-hASCs); and, in the fourth group, hASCs were cultured in PM, in which pericyte growth supplement (PGS) was omitted (noPGS-hASCs). PGS (Innoprot) is a medium supplement containing growth factors, hormones, and proteins, specifically designed for the optimal growth of human pericytes in vitro. When added to PM, each mL of the final solution contained bovine serum albumin (10 μg), apo-transferrin (10 μg), insulin (5 mg), EGF-2 (2 ng), fibroblast growth factor-2 (FGF-2; 2 ng), IGF-I (2 ng) and hydrocortisone (1 mg).
Some samples from each group were stopped at 1 d, 3 d and 6 d of culture. These samples were assessed for α-SMA and NG2 expression by immunocytochemical staining. Other samples stopped at 3 d were processed for western blotting. Further samples of each group were trypsinized after 3 d of growth and used to prepare co-cultures with hRECs.
hRPCs were purchased from Innoprot, cryopreserved at secondary culture after purification and delivered frozen. hRPCs were seeded in poly-L-lysine (0.01 mg/mL solution, Innoprot) coated culture flasks and incubated at 37°C with 5% CO2 in PM, containing 2% FBS, 1% PGS, and 1% penicillin/streptomycin (Innoprot). At about 70% confluence, the cells were trypsinized and plated for comparison with hASCs cultured in the different conditions. For this purpose, some samples were immunostained for α-SMA and NG2 at 1, 3 and 6 d of culture. Other samples of each group, stopped at 3 d, were used for western blotting or to prepare co-cultures with hRECs.
hRECs, already characterized by their immunopositivity for von Willebrand factor and CD31, were purchased from Innoprot. They were seeded in culture flasks and incubated at 37°C with 5% CO2 in endothelial cell medium (ECM) (Innoprot), containing 5% FBS, 1% endothelial cell supplement factor, and 1% penicillin/streptomycin (Innoprot). When cells reached about 70% confluence, they were trypsinized and plated for co-culture experiments.
hRECs were plated in multi-well plates (10000 cells/well) and cultured for 3 d before adding different cell populations (5000 of each type in each well). Thus, the following groups of co-cultures were prepared: (1) hRECs with untreated hASCs; (2) hRECs with TGF-hASCs; (3) hRECs with PM-hASCs; (4) hRECs with noPGS-hASCs; and (5) hRECs with hRPCs. The first group was cultured in a mixed medium containing 50% ECM and 50% basal growth medium for hASCs. A combination of 50% ECM and 50% PM was used for the other groups. In addition, a monoculture of only hRECs was used as a control. From each group, some samples were processed after 1 d and others after 4 d of growth. A double immune-labeling procedure was carried out to reveal simultaneously α-SMA and some typical junctional proteins, as follows: (1) α-SMA and VE–Cadherin; (2) α-SMA and ZO-1; and (3) α-SMA and Occludin.
To further evaluate reciprocal interactions between hRECs and hPRCs or pretreated hASCs, a BRB-like model was set up in vitro using transwell inserts (Falcon permeable transparent PET membrane inserts for 12-well plates, with a pore size of 0.4 µm) in which hRECs were plated on the underside and hPRCs or pretreated hASCs were seeded on the topside[21].
hRECs were first plated on the underside of the transwell inserts with a density of 15000 cells/well. After 3 h, the supports were turned upside down in the culture plate. After 5 d, hPRCs (10000 cells/well) or pretreated hASCs (10000 cells/well) were added to the topside of the transwell inserts, opposite the hRECs. These cultures were grown in mixed media as previously described (50% ECM and 50% PM). An additional sample, containing only hRECs, seeded on the underside, was used as a reference. These indirect co-cultures were used to evaluate the TEER and hREC mRNA levels of junctional proteins by quantitative RT-PCR (qRT-PCR).
Immunocytochemical staining was carried out following the same procedures described previously[20]. Cells were washed with PBS and fixed. Fixation with 4% paraformaldehyde for 20 min was carried out for hASC and hRPC monocultures. Fixation at -20°C with acetone (15 min) and methanol (20 min) was preferred in co-culture experiments, when hRECs were also present. In fact, junctional proteins were easier to detect after this fixation method. In the following step, cells were incubated for 30 min with a 5% solution of normal goat serum (Sigma-Aldrich) in PBS containing 0.1% Triton (Sigma-Aldrich). They were then exposed overnight at 4°C to primary antibodies: Mouse anti-α-SMA (1:200; Dako M0851, Milan, Italy); rabbit anti-NG2 (1:200; Abcam ab129051, Milan, Italy); rabbit anti-VE-Cadherin (1:400; Cell Signaling Technology, D87F2, Danvers, MA, United States); rabbit anti-ZO-1 (1:100; Invitrogen 61-7300); rabbit anti-Occludin (1:100; Abcam ab31721).
The following day, the cells were washed with PBS and incubated for 60 min at room temperature with secondary antibodies conjugated to different fluorochromes: FITC-conjugated goat anti-rabbit (1:500; Abcam) and/or Cy3-conjugated goat anti-mouse (1:500; Abcam). The specificity of immunostaining was verified in control experiments by omitting the primary antibody. Finally, DAPI staining was used to visualize cell nuclei (10 min).
Immunoblots were carried out on samples of each monoculture group (hASCs, TGF-hASCs, PM-hASCs, noPGS-hASCs and hRPCs), after 3 d of growth. Cells were trypsinized, homogenized and sonicated in RIPA buffer (Life Technologies, Monza, Italy), in the presence of a protease inhibitor cocktail (Sigma P2714), serine/threonine phosphatase inhibitors (Sigma P0044) and tyrosine protein phosphatase inhibitors (Sigma P5726). Protein concentrations were determined by the BCA protein assay using bovine serum albumin as the standard. Cell lysates (40 μg protein) were loaded into SDS-PAGE, blotted and probed for different target proteins.
Membranes were incubated overnight at 4°C with primary antibodies: mouse anti-αSMA (1:500, Dako M0851); and rabbit anti-NG2 (1:500, Abcam ab 129051). The following day, the membranes were incubated with secondary fluorescent antibodies (1:15000) for 1 h at room temperature, and the immunocomplexes were detected by the Odyssey imaging system (LI-COR, Lincoln, NE, United States). All blots were checked for equal loading by probing with β-actin antibody (rabbit, 1:700; Sigma A2066). Densitometric analysis was performed using free software Image J (NIH, Bethesda, MD United States).
After 4 d of co-culture in transwell inserts, qRT-PCR was performed to determine hREC mRNA levels of VE-Cadherin, ZO-1 and Occludin genes. Briefly, total cellular RNA was extracted from hRECs of each co-culture group using TRIzol reagent (Invitrogen) according to the manufacturer's instructions and re-dissolved in 30 μL of RNase-free water. RNA concentrations and purity were estimated by optical density at 260 and 280 nm. Reverse transcription of RNA (2 µg) into first-strand cDNA was accomplished by using 200 U of SuperScript III in a 20 µL reaction volume with 50 ng random hexamers, 1.25 mM dNTP, 10 mM dithiothreitol, 50 mM Tris-HCl, pH 8.3, 75 mM KCl, and 3 mM MgCl2 (Invitrogen). cDNA synthesis was carried out at 50°C for 50 min and subsequently stopped and the temperature raised to 85°C for 5 min. Aliquots of cDNA (50 ng) were amplified by employing iTaq Universal SYBR Green Supermix (Biorad, Milan, Italy) in a final volume of 20 µL (0.5 µM primers, 1.6 mM Mg2+, 1 × SYBR Green). Specific primers were used to amplify VE-Cadherin (forward: 5’-GGCAAGATCAAGTCAAGCGTG-3’, reverse: 5’-ACGTCTCCTGTCTCTGCATCG-3’), ZO1 (forward: 5’-CAGCCGGTCACGATCTCCT-3’, reverse: 5’-TCCGGAGACTGCCATTGC-3’), Occludin (forward: 5’-CACACAGGACGTGCCTTCAC-3’, reverse: 5’-GAGTATGCCATGGGACTGTCAA-3’) and 18S ribosomal rRNA (forward: 5′-AGTCCCTGCCCTTTGTACACA-3′, reverse: 5′-GATCCGAGGGCCTCACTAAAC-3′) purchased from Eurofins Genomics Germany GmbH. Negative controls (no template control) were included in each assay. Amplifications were carried out in a Light Cycler 1.5 instrument (Roche Diagnostics, Indianapolis, IN, United States). The relative mRNA variations of target genes in each experimental group were calculated using the ΔCycle Threshold (CT) method by comparing the CT value of the gene of interest to the CT value of the selected 18S rRNA gene, considered as the internal reference control gene.
In transwell insert co-cultures, TEER was measured daily (Ohm/cm2) for the following 8 d with the Millicell Electrical Resistance System-2 (Merck Millipore). An increase in TEER is considered indicative of barrier formation and tightness[22]. The value of cell-free transwell insert resistance was assumed to be the background resistance. Values are expressed as Ω/cm2 and were calculated by the following formula: (Average resistance of experimental wells − average resistance of blank wells) × 0.33 (the area of the transwell membrane).
A tube formation assay was performed in three-dimensional co-cultures in vitro with the Matrigel Basement Membrane Matrix system (BD Discovery Labware, Bedford, MA, United States). The experimental protocol was run according to the manufacturer’s instructions. Briefly, the gel solution was thawed at 4°C overnight, then 96-well plates were coated with 50 µL of Matrigel per well and allowed to solidify at 37°C for 2 h. A total of 15000 cells were seeded in each well. Of these, 10000 were hRECs and 5000 were hRPCs or different groups of hASCs, as previously specified. Each condition was run in triplicate. After 8 h of incubation, tube-like structures were photographed using an inverted microscope[23]. In two additional samples, hRPCs or PM-hASCs were pre-labeled with a fluorescent dye (Di-alkyl Indocarbocyanine, DiI; Invitrogen, Monza, Italy) in order to visualize their location in the co-culture.
Statistical analysis was carried out with GraphPad Prism (GraphPad Software, La Jolla, CA, United States); Descriptive statistics (mean ± SEM, median and 25%-75% percentiles) are reported in the figures. Comparisons between groups were evaluated by one-way analysis of variance, followed by Tukey’s post hoc test for multiple comparisons; P values < 0.05 were considered statistically significant. The statistical methods of this study were reviewed by Dr V. Guardabasso from the University Teaching Hospital (Catania, Italy).
At 24 h, hASCs cultured in the basal MSC medium exhibited a typical fibroblast-like shape. After 7 d, the cell population appeared much denser, but showed a shape similar to that observed at day 1. The stem cell profile of hASCs was verified by immunocytochemistry and flow cytometry and was analogous to that reported previously[20]; that is the cells were immunopositive for typical MSC markers (CD44, CD73, CD90, and CD105) and immunonegative for typical hematopoietic stem cell markers (CD14, CD34, and CD45). Furthermore, as widely demonstrated in the literature, they were able to differentiate into chondrocytes, adipocytes and osteocytes[3].
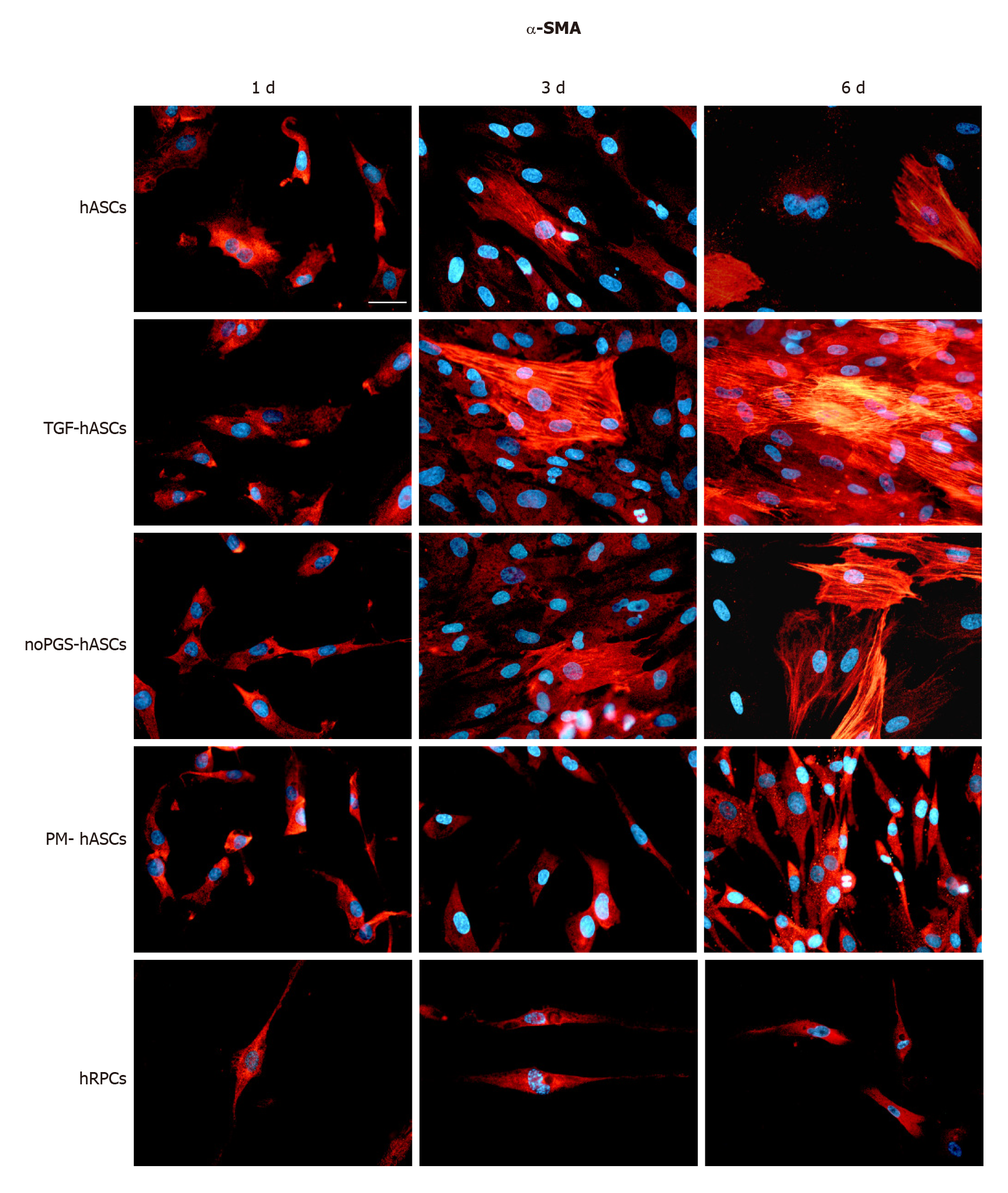
Immunofluorescence: The potential for differentiation of hASCs into pericyte-like cells was examined by their immunopositivity for α-SMA (Figure 1) and NG2 (Figure 2), generally acknowledged as pericyte markers. Immunostaining for each marker was carried out at 1 d, 3 d and 6 d of culture. Four groups of differently treated hASCs (hASCs, TGF-hASCs, noPGS-hASCs, and PM-hASCs) were examined and compared to native pericytes (hRPCs). The results showed that only basal expression of α-SMA was detectable at day 1 in hRPCs and all hASC groups (Figure 1, left column). Observations at day 3 (Figure 1, middle column) and day 6 (Figure 1, right column) showed increased α-SMA expression in control hASCs, in TGF-hASCs, and in noPGS-hASCs. In these cases, a typical filamentous pattern of α-SMA organization was clearly detected. In hRPCs and PM-hASCs, the basal expression of α-SMA remained virtually unmodified (last two rows).
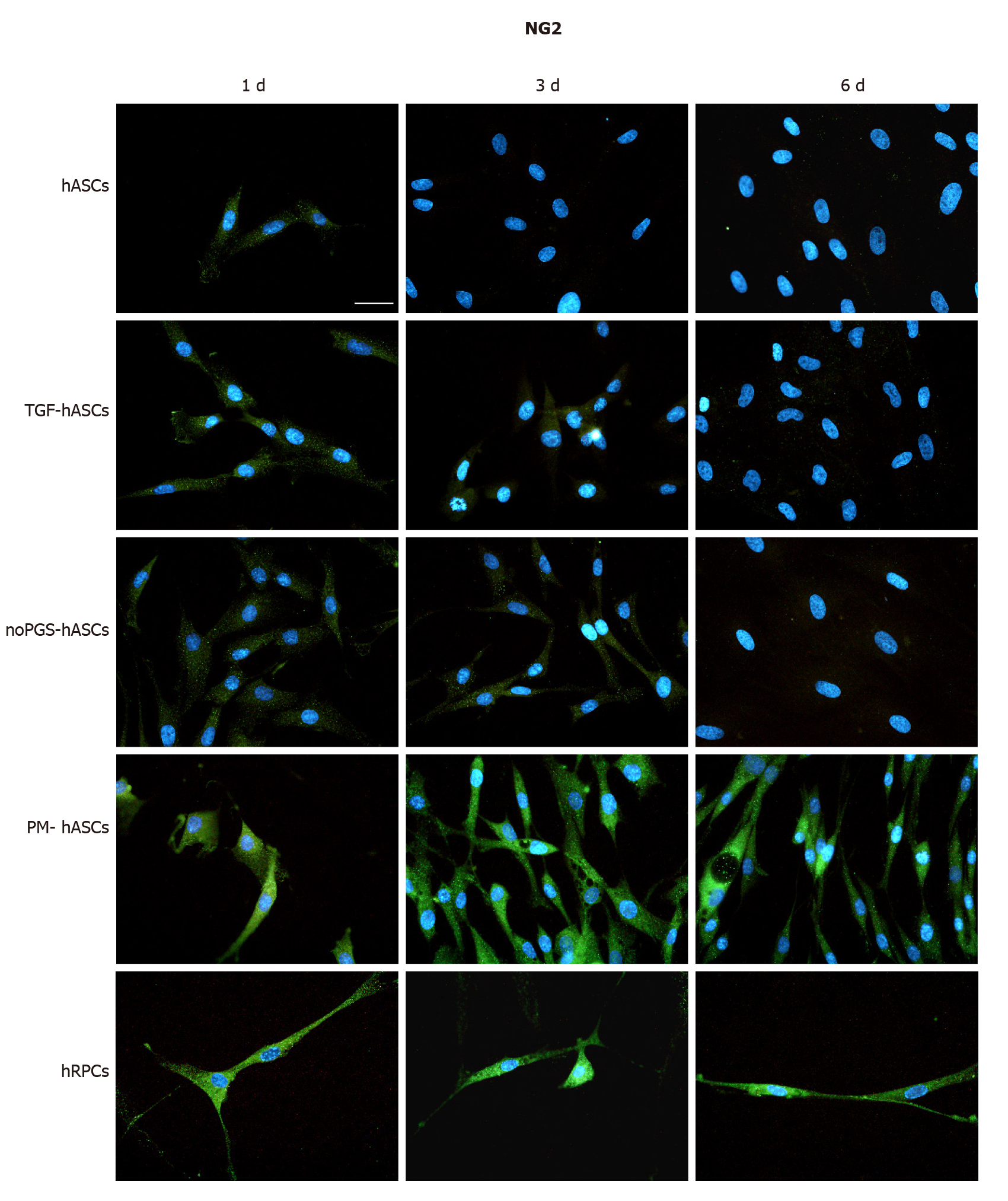
NG2 expression was clearly exhibited by hRPCs at day 1 and was similarly detectable at day 3 and day 6 (Figure 2, last row). Among the hASC groups, a similar immunoreactivity was observed only in PM-hASCs (Figure 2, second-last row), whereas NG2 immunostaining was much weaker in the other conditions.
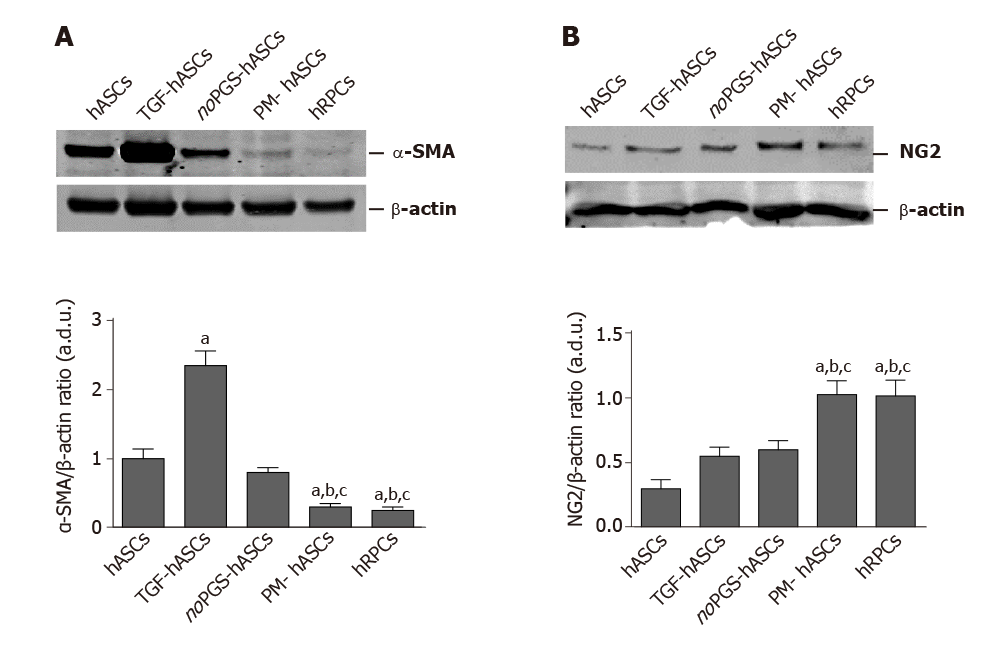
Western blot analysis: As immunostaining modifications were already evident at day 3, this time point was chosen to further evaluate α-SMA and NG2 expression by western blot analysis (Figure 3). These data corroborated the immunofluorescence observations. In fact, the histograms in Figure 3A show that α-SMA levels present in control hASCs were similar to noPGS-hASCs, while much higher levels were found in TGF-hASCs; the lowest levels were observed in PM-hASCs, close to those for hRPCs.
NG2 levels (Figure 3B) measured in control hASCs were slightly higher in TGF-hASCs and noPGS-hASCs; a significant increase in NG2 was observed in PM-hASCs, where it reached levels similar to hRPCs.
Co-culture strategies were designed to evaluate interactions between hRECs and hASCs pre-cultured in different conditions, compared to interactions between hRECs and hRPCs. In these co-cultures, double immunostaining was carried out to visualize VE-Cadherin, ZO-1 and Occludin in hRECs, and α-SMA in the various groups of hASCs or hRPCs.
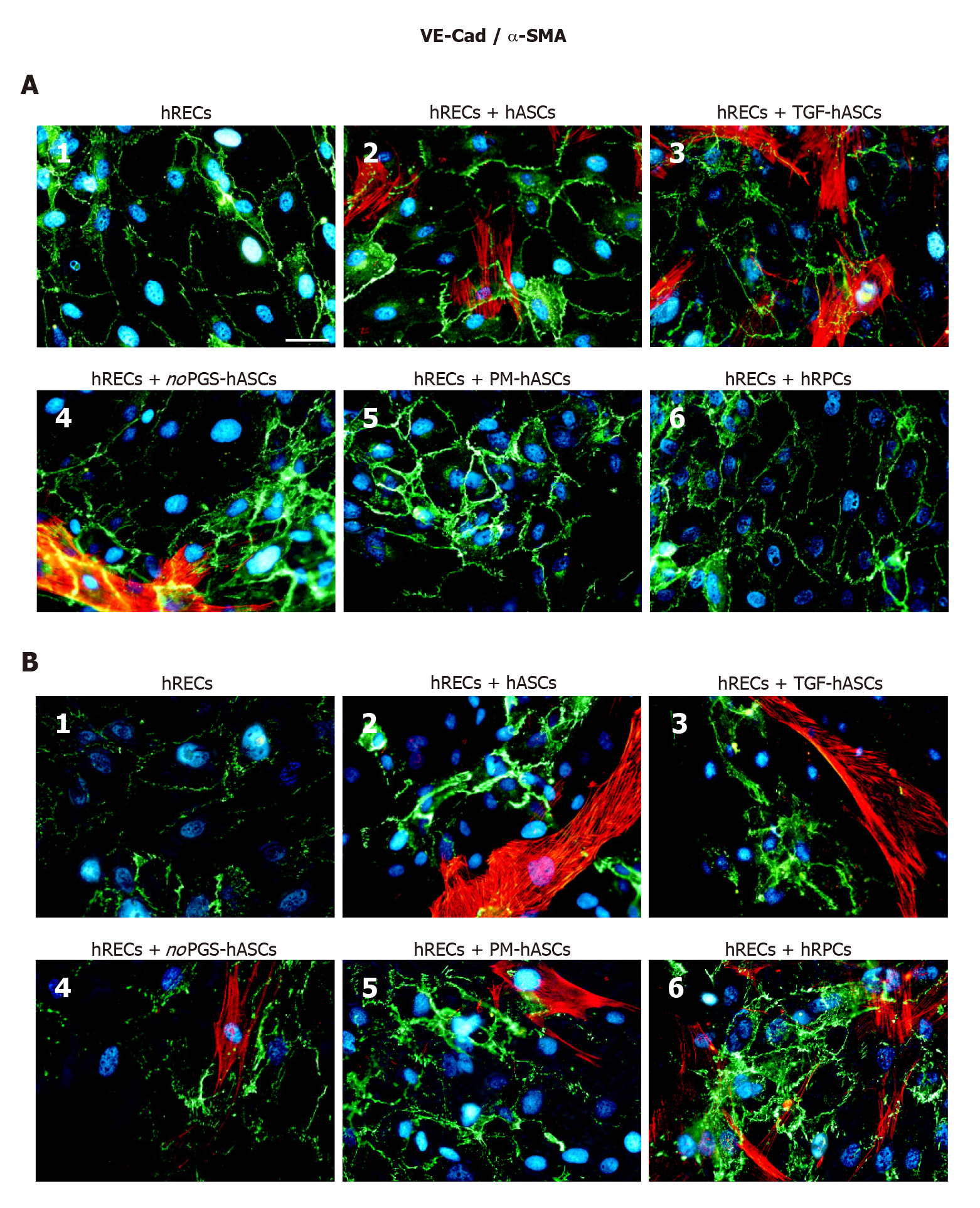
Junction protein immunostaining in hRECs: At day 1 (Figure 4A) and day 4 (Figure 4B) VE-Cadherin immunostaining was carried out in hRECs, which exhibited the typical distribution at the level of the plasma membranes of adjacent cells (Figure 4A1 and B1). VE-Cadherin expression was markedly increased when these cells were co-cultured with hRPCs (Figure 4A6 and B6) or PM-hASCs (Figure 4A5 and B5). Lower increases were, however, also detectable in co-cultures of hRECs with the other groups of hASCs. Overall, a comparable immunostaining pattern was observed after 1 d and 4 d of co-culture.
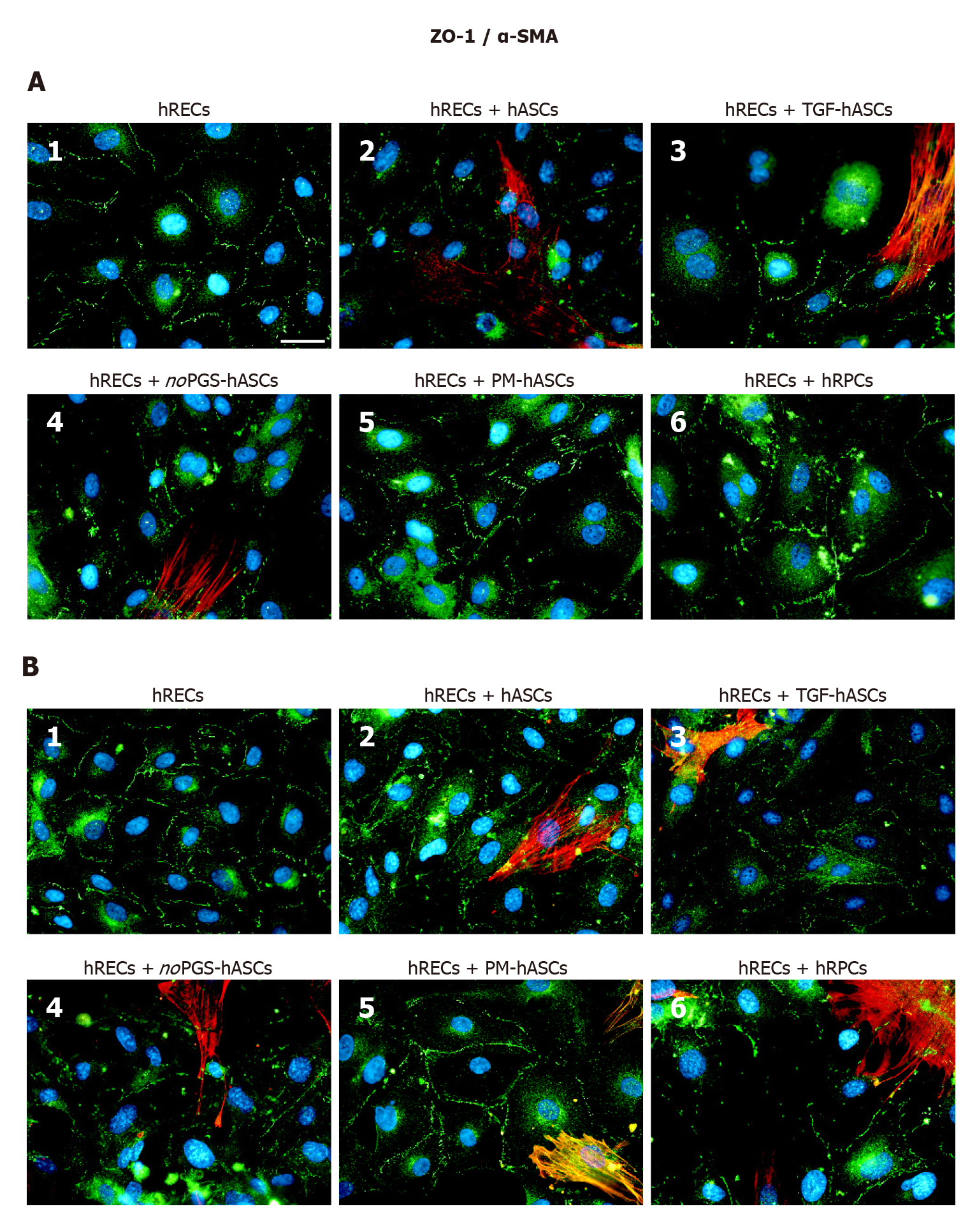
ZO-1 expression was already visible on the plasma membranes of hRECs at day 1 (Figure 5A1) and day 4 (Figure 5B1). ZO-1 expression increased only when hRECs were co-cultured with hRPCs (Figure 5A6 and B6) or PM-hASCs (Figure 5A5 and B5). A similar pattern of immunostaining was observed after 1 d and 4 d of co-culture.
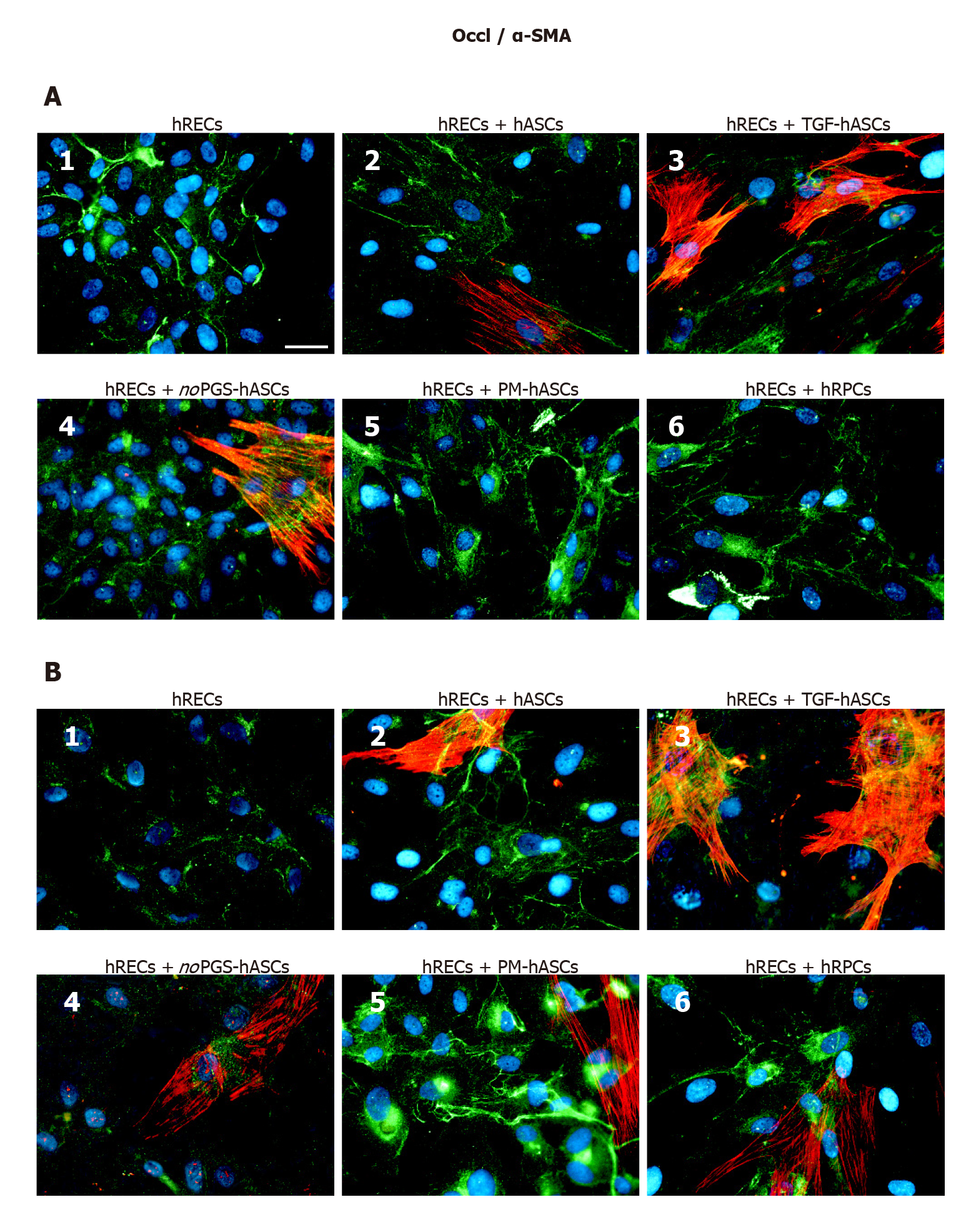
Occludin immunostaining at day 1 (Figure 6A) and day 4 (Figure 6B) largely matched those described for VE-Cadherin and ZO-1. In fact, Occludin expression was already visible on the plasma membranes of hRECs alone (Figure 5A1 and B1), and increased when hRECs were co-cultured with hRPCs (Figure 6A6 and B6) or PM-hASCs (Figure 5A5 and B5). Similar results were observed after 1 d and 4 d of co-culture.
α-SMA immunostaining in hASCs or hRPCs: Overall, the presence of hRECs stimulated α-SMA expression in both hRPCs and hASCs, especially after 4 d of co-culture. In detail, a strong filamentous α-SMA expression was visible at day 1 in co-cultures of hRECs and hASCs, in co-cultures of hRECs and TGF-hASCs and in co-cultures of hRECs and noPGS-hASCs [Figures 4-6 (A2, A3, A4)]. In contrast, no evident α-SMA immunoreactivity was detectable in co-cultures of hRECs and PM-hASCs (Figures 4-6, A5) or in co-cultures of hRECs and hRPCs (Figures 4-6, A6). After 4 d of co-culture with hRECs, α-SMA immunoreactivity was also visible in hRPCs and PM-hASCs [Figures 4-6 (B5, B6)]. In the other hASC groups (hASCs, TGF-hASCs and noPGS-hASCs), strong α-SMA expression was still observed [Figures 4-6 (A2, A3, A4)]. As expected, no α-SMA immunoreactivity was detectable in cultures of hRECs alone [Figures 4-6 (A1, B1)].
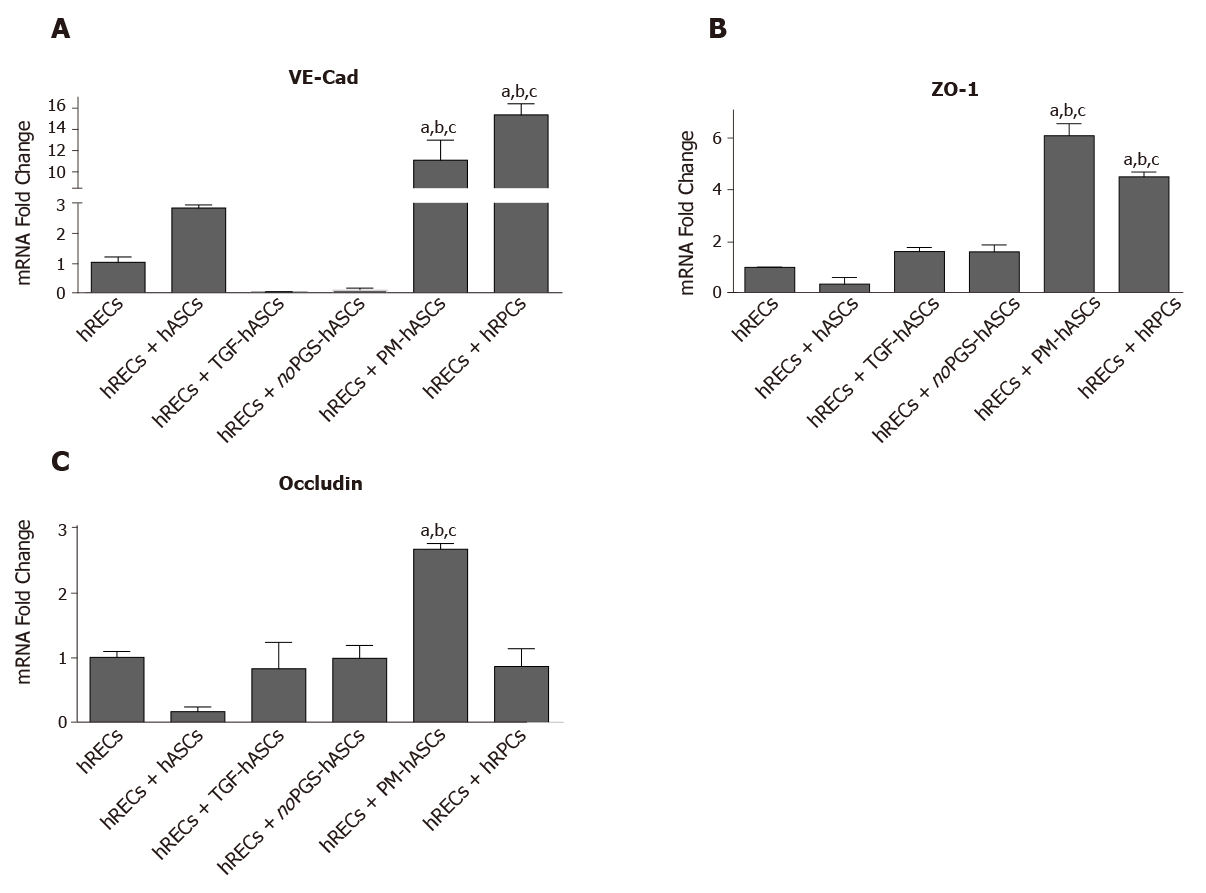
Measurements of mRNA levels (Figure 7) of junctional proteins in hRECs largely matched the results obtained by double-labeling experiments. When co-cultured with hRPCs, very high increases were observed in endothelial cells for VE-Cadherin mRNA; moderate increases were found for ZO-1, whereas no significant modifications were detected for Occludin. Increased levels of gap junction protein mRNA were also observed when hRECs were co-cultured with PM-hASCs. Compared to co-cultures with hRPCs, VE-Cadherin levels were nearly as high, whereas even higher mRNA levels were obtained for ZO-1 and Occludin. Less evident modifications were observed when hRECs were co-cultured with other hASC groups.
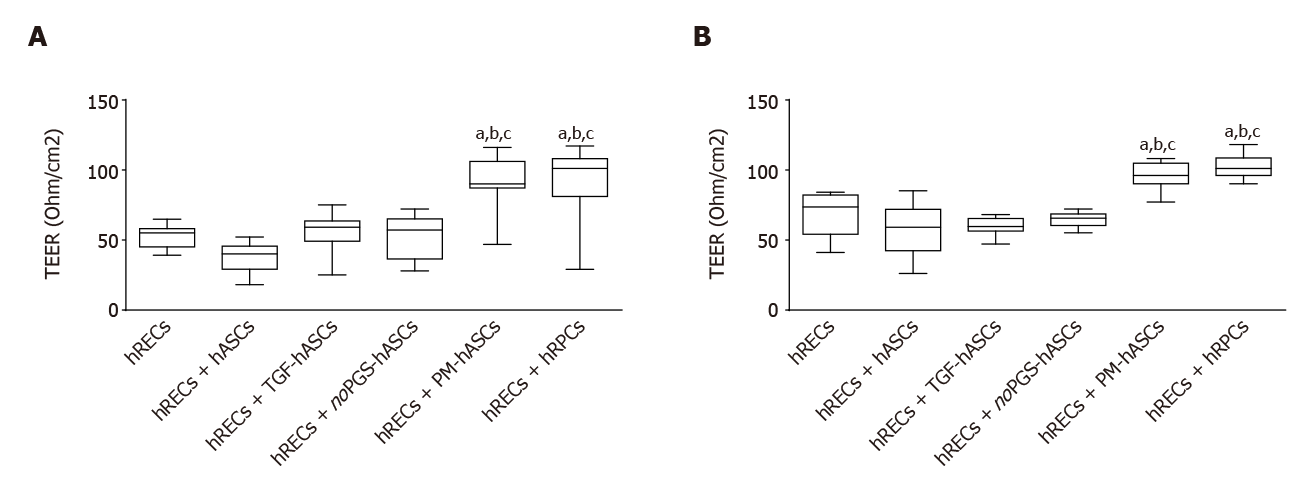
Measurements of TEER values were carried out in transwell co-cultures, where a model of the BRB was established. TEER values were considered indicative of the junctional membrane interactions between hRECs. Measurements at day 3 and 6 are reported in Figure 8. When compared with cultures of hRECs alone, values of TEER significantly increased when hRECs had been co-cultured with hRPCs or PM-hASCs. The increase in TEER was evident at day 3 and persisted to day 6. No significant differences in TEER values were detected in the other groups of co-cultures (hRECs and hASCs, hRECs and TGF-hASCs, hRECs and noPGS-hASCs) when compared with hRECs alone.
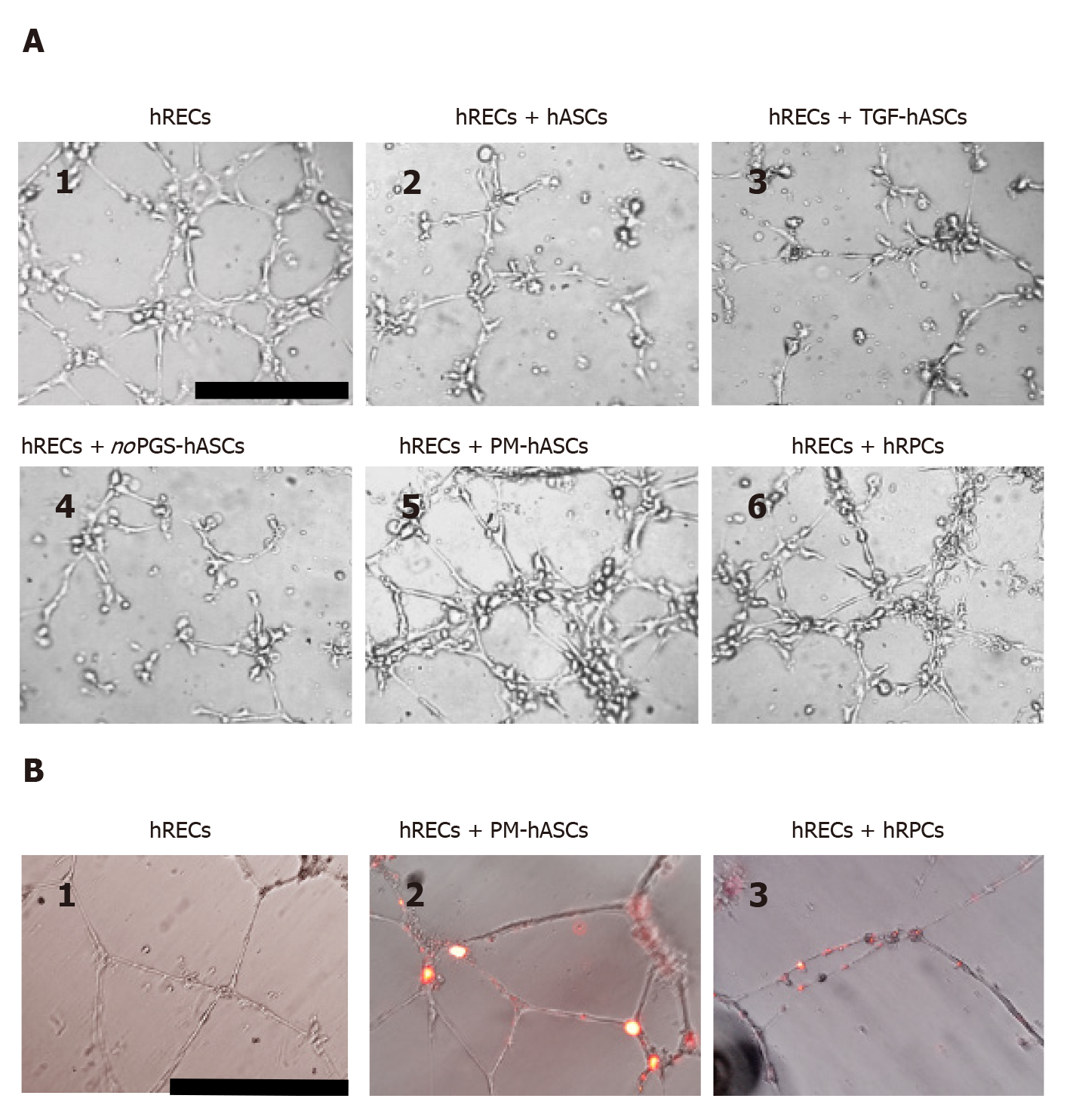
Interactions between hRECs and hRPCs or different groups of hASCs were further investigated in three-dimensional co-cultures in Matrigel, where vessel-like tubular structures are spontaneously formed by endothelial cells[24]. As shown in Figure 9, tubular structures were already visible in monoculture of hRECs after 6 h from seeding (Figure 9A1); the capability of forming tubular structures was maintained or improved in the presence of PM-hASCs (Figure 9A5) or hRPCs (Figure 9A6), but not in the other co-culture conditions (Figure 9A2-A4).
A pericyte-like phenotype of PM-hASCs was also confirmed in three-dimensional co-cultures in Matrigel after 20 h. Figure 9B shows that in these conditions well-defined tubular structures were spontaneously assembled by hRECs alone (Figure 9B1). When DiI prelabeled PM-hASCs (Figure 9B2) or hRPCs (Figure 9B3) were also present, a similar localization around the tubular formations was clearly recognizable in both cases.
Data available in the literature indicate that an unequivocal immunocytochemical characterization of pericytes is hard to assess, especially as their phenotype depends on resident tissue and may change according to their activity state[9,25]. Because α-SMA and NG2 are recognized as pericyte markers[26,27], their modifications were considered indicative of a pericyte-like differentiation of hASCs. In addition, hASC immunocytochemical expression of these markers was compared to that of native pericytes (hRPCs), used as a positive control. In this way, the best culture strategy for driving hASCs to a pericyte-like phenotype was recognized. Overall, the present results indicate that a closer pericyte-like differentiation can be achieved when hASCs are grown in a culture medium specifically designed for pericytes.
Only basal levels of α-SMA were detected at the early stages (1 d) of growth, both in hRPCs and hASCs. These basal levels remained unchanged in hRPCs and PM-hASCs, whereas in the other conditions (hASCs, TGF-hASCs and noPGS-hASCs), α-SMA expression increased considerably, especially after 3-6 d. Consistent with published data[18], the most evident increase in α-SMA expression was found when TGF-β1 was added to the culture medium. Indeed, the increased α-SMA expression by itself is not definitely indicative of pericyte differentiation, since TGF-β1 is a strong inductor of α-SMA expression in various cell types, including pulmonary fibroblasts[28]. Therefore, a pericyte-like differentiation of hASCs was more confidently assumed by assessing the expression of NG2, which was better induced when hASCs had been cultured in PM. In this case, NG2 immunoreactivity of hASCs closely overlapped that of bona fide pericytes (hRPCs).
The pattern of marker expression observed in PM-hASC was strictly related to the presence of PGS in the culture medium. In fact, when PGS was omitted, NG2 expression was barely detectable, as in control hASCs. On the other hand, the absence of PGS provoked a marked increase in α-SMA expression. Among PGS components, FGF is probably responsible for these effects. In fact, it has been reported that FGF maintains cultured pericytes in a proliferative state, characterized by low levels of α-SMA[29]; consequently, the absence of FGF induces a pericyte switch from a proliferative to a contractile phenotype, where α-SMA is upregulated. Moreover, FGF is able to antagonize TGF-β-induced α-SMA expression in pericytes and smooth muscle cells[30]. FGF-mediated proliferative effects normally decline as pericytes interact with endothelial cells, whose TGF-β production induces a switch toward the contractile phenotype[29]. It has also been reported that mesenchymal cells express smooth muscle cell markers when treated with TGF-β1 or co-cultured with endothelial cells, thus inducing precursor cells to differentiate into pericytes or smooth muscle cells[31].
We further validated the pericyte-like phenotype induced in hASCs by assessing their interaction with endothelial cells, in co-culture experiments. Compared with hRECs cultured alone, the expression of junction proteins (VE-Cadherin, ZO-1 and Occludin) was more consistently increased when hRECs were co-cultured with hRPCs or PM-hASCs, the condition that more closely matches native pericytes, rather than with hASCs cultured in other conditions. Similar conclusions may be drawn from the qRT-PCR experiments. Thus, it can be assumed that these pericyte-like differentiated ASCs may mimic the functional role normally played by pericytes in strengthening the BRB. In fact, these junction proteins are mainly responsible for maintaining the tight and adherens junctions between adjacent endothelial cells[23,32,33].
In these co-cultures, it was also observed that the presence of hRECs was able to influence α-SMA expression in hRPCs or hASCs. In particular, the typical filamentous pattern of α-SMA expression was clearly detectable in hRPCs and PM-hASCs after only 4 d of co-culture with hRECs. Instead, α-SMA immunoreactivity was poorly detectable in these two conditions after 1 d of co-culture. This was likely due to the presence of FGF in the culture medium, which induces a pericyte proliferative state. After 4 d, probably because of the endothelial secretion of growth factors such as TGF-β or platelet derived growth factor, a pericyte switching toward the contractile phenotype, characterized by a strong α-SMA expression, occurs[9,29,34]. Similar data were also reported by Rajashekhar et al[35], showing that a significant increase in α-SMA fibrous expression occurs in ASCs located in close proximity to hRECs.
We believe that the differentiation protocol adopted in the present work has the advantage of obtaining pericyte-like hASCs still in the proliferative stage, the most suitable to interact with endothelial cells to build up an efficient BRB. In fact, especially during the early days of treatment, PM-hASCs already overexpress NG2 but not the filamentous α-SMA pattern, typical of pericytes in the contractile phenotype. Moreover, a pericyte induction protocol would avoid ASC differentiation towards other cell lines.
A positive interaction between PM-hASCs and hRECs was confirmed by results obtained from TEER experiments. In fact, significantly increased resistance values were observed when hRECs were co-cultured with hRPCs or PM-hASCs, likely a result of reinforced cell-to-cell adhesion.
Supporting observations were also obtained from experiments of three-dimensional co-cultures, where a comparable phenotype was observed for hRPCs and PM-hASCs. Both cell types tended to localize at the typical perivascular physiological position, close to the tubular structures formed in vitro by hRECs. Notably, this perivascular localization, together with the immunocytochemical phenotype, is considered very indicative of pericyte-like differentiation[36]. It remains to be demonstrated whether this in vitro differentiation strategy actually provides beneficial effects when tested in vivo, for example by intraocular administration of PM-hASCs in animal models of DR.
MSCs from other sources have been tested for pericyte-like differentiation. Encouraging results have also been obtained using MSCs isolated from bone marrow and umbilical cord blood[37]. In fact, positive outcomes have been reported for bone marrow MSCs in an in vitro three-dimensional model of the blood brain barrier[38].
However, hASCs feature some undeniable advantages for therapeutic applications in the field of regenerative medicine. First of all, they can be harvested in large amounts from subcutaneous fat tissue, with minimal discomfort for the patient; the yield is considerably higher than that from other tissues such as bone marrow; they feature a higher rate of proliferation and, most importantly, they are readily available for autologous treatments. Hopefully, when safe therapeutic protocols are developed, hASC-based therapeutic approaches may be successfully used to restore the integrity of disrupted retinal microcirculation.
The loss of pericytes, which occurs in diabetic retinopathy, results in a breakdown of the blood-retina barrier (BRB) and infiltration of inflammatory cells. Lost retinal pericytes might be replaced by adipose-derived mesenchymal stem cells (ASCs) after differentiating into a pericyte-like phenotype.
The use of pericyte-like differentiated ASCs may represent a valuable therapeutic strategy for restoring BRB damage.
The purpose of this study was to develop in vitro strategies to obtain pericyte-like differentiation of human ASCs.
Different ASC culture conditions were tested and compared to human retinal pericytes (hRPCs). The expression of α-smooth muscle actin (α-SMA) and neural/glial antigen 2 (NG2) was assessed by immunocytochemical staining and western blotting. In co-cultures of human retinal endothelial cells (hRECs) with hRPCs or different groups of hASCs, the endothelial expression of typical junctional proteins such as vascular endothelial-Cadherin, zonula occludens-1 and Occludin were evaluated. In an in vitro model of BRB, the trans-endothelial electrical resistance was measured. Three-dimensional co-cultures in Matrigel of hRECs and hRPCs or pericyte-like hASCs were designed to assess their reciprocal localization.
Immunocytochemical results and western blot analysis for α-SMA and NG2 indicated that the closest pericyte-like phenotype was observed when hASCs were cultured in pericyte medium (PM-hASCs). α-SMA immunoreactivity was strongly increased only when TGF was added to the culture medium. NG2 expression, almost undetectable in most conditions, was substantially increased in PM-hASCs.
In co-culture experiments, immunoreactivity of vascular endothelia-Cadherin, zonula occludens-1 and Occludin was considerably increased in hRECs when hRPCs or PM-hASCs were also present. Supporting results were found by trans-endothelial electrical resistance measurements, showing the highest values in analogous conditions. The pericyte-like phenotype of PM-hASCs was also confirmed in three-dimensional co-cultures in Matrigel, where PM-hASCs and hRPCs similarly localized around the tubular formations made by hRECs.
PM-hASCs seem able to strengthen the intercellular junctions between hRECs, likely reinforcing the BRB; thus, hASC-based therapeutic approaches may be usefully developed to restore the integrity of retinal microcirculation.
Future in vivo experiments will be designed to test pericyte-like ASC engraftment following intraocular administration. Possible beneficial effects will be evaluated in animal models of diabetic retinopathy. Eventually, when safe therapeutic protocols are developed, ASC-based therapeutic approaches may be successfully used in diabetic patients to restore disrupted retinal microcirculation.
| 1. | Dicker A, Le Blanc K, Aström G, van Harmelen V, Götherström C, Blomqvist L, Arner P, Rydén M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Musumeci G, Lo Furno D, Loreto C, Giuffrida R, Caggia S, Leonardi R, Cardile V. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp Biol Med (Maywood). 2011;236:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Calabrese G, Giuffrida R, Lo Furno D, Parrinello NL, Forte S, Gulino R, Colarossi C, Schinocca LR, Giuffrida R, Cardile V, Memeo L. Potential Effect of CD271 on Human Mesenchymal Stromal Cell Proliferation and Differentiation. Int J Mol Sci. 2015;16:15609-15624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Lo Furno D, Mannino G, Giuffrida R, Gili E, Vancheri C, Tarico MS, Perrotta RE, Pellitteri R. Neural differentiation of human adipose-derived mesenchymal stem cells induced by glial cell conditioned media. J Cell Physiol. 2018;233:7091-7100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Lo Furno D, Mannino G, Cardile V, Parenti R, Giuffrida R. Potential Therapeutic Applications of Adipose-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2016;25:1615-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Gonçalves R, Mintz A, Delbono O. How Plastic Are Pericytes? Stem Cells Dev. 2017;26:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Caporarello N, D'Angeli F, Cambria MT, Candido S, Giallongo C, Salmeri M, Lombardo C, Longo A, Giurdanella G, Anfuso CD, Lupo G. Pericytes in Microvessels: From "Mural" Function to Brain and Retina Regeneration. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Esteves CL, Sheldrake TA, Dawson L, Menghini T, Rink BE, Amilon K, Khan N, Péault B, Donadeu FX. Equine Mesenchymal Stromal Cells Retain a Pericyte-Like Phenotype. Stem Cells Dev. 2017;26:964-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Geevarghese A, Herman IM. Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res. 2014;163:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Berrone E, Beltramo E, Buttiglieri S, Tarallo S, Rosso A, Hammes HP, Porta M. Establishment and characterization of a human retinal pericyte line: a novel tool for the study of diabetic retinopathy. Int J Mol Med. 2009;23:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Platania CBM, Giurdanella G, Di Paola L, Leggio GM, Drago F, Salomone S, Bucolo C. P2X7 receptor antagonism: Implications in diabetic retinopathy. Biochem Pharmacol. 2017;138:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Platania CBM, Lazzara F, Fidilio A, Fresta CG, Conti F, Giurdanella G, Leggio GM, Salomone S, Drago F, Bucolo C. Blood-retinal barrier protection against high glucose damage: The role of P2X7 receptor. Biochem Pharmacol. 2019;168:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Kim JM, Hong KS, Song WK, Bae D, Hwang IK, Kim JS, Chung HM. Perivascular Progenitor Cells Derived From Human Embryonic Stem Cells Exhibit Functional Characteristics of Pericytes and Improve the Retinal Vasculature in a Rodent Model of Diabetic Retinopathy. Stem Cells Transl Med. 2016;5:1268-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Santos GSP, Prazeres PHDM, Mintz A, Birbrair A. Role of pericytes in the retina. Eye (Lond). 2018;32:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Ogura S, Kurata K, Hattori Y, Takase H, Ishiguro-Oonuma T, Hwang Y, Ahn S, Park I, Ikeda W, Kusuhara S, Fukushima Y, Nara H, Sakai H, Fujiwara T, Matsushita J, Ema M, Hirashima M, Minami T, Shibuya M, Takakura N, Kim P, Miyata T, Ogura Y, Uemura A. Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight. 2017;2:e90905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 16. | Amos PJ, Shang H, Bailey AM, Taylor A, Katz AJ, Peirce SM. IFATS collection: The role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008;26:2682-2690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Natesan S, Zhang G, Baer DG, Walters TJ, Christy RJ, Suggs LJ. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng Part A. 2011;17:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Mendel TA, Clabough EB, Kao DS, Demidova-Rice TN, Durham JT, Zotter BC, Seaman SA, Cronk SM, Rakoczy EP, Katz AJ, Herman IM, Peirce SM, Yates PA. Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS One. 2013;8:e65691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Ting KK, Zhao Y, Shen W, Coleman P, Yam M, Chan-Ling T, Li J, Moller T, Gillies M, Vadas MA, Gamble JR. Therapeutic regulation of VE-cadherin with a novel oligonucleotide drug for diabetic eye complications using retinopathy mouse models. Diabetologia. 2019;62:322-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Lo Furno D, Tamburino S, Mannino G, Gili E, Lombardo G, Tarico MS, Vancheri C, Giuffrida R, Perrotta RE. Nanofat 2. Physiol Res. 2017;66:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Lupo G, Motta C, Giurdanella G, Anfuso CD, Alberghina M, Drago F, Salomone S, Bucolo C. Role of phospholipases A2 in diabetic retinopathy: in vitro and in vivo studies. Biochem Pharmacol. 2013;86:1603-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20:107-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1564] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 23. | Giurdanella G, Lazzara F, Caporarello N, Lupo G, Anfuso CD, Eandi CM, Leggio GM, Drago F, Bucolo C, Salomone S. Sulodexide prevents activation of the PLA2/COX-2/VEGF inflammatory pathway in human retinal endothelial cells by blocking the effect of AGE/RAGE. Biochem Pharmacol. 2017;142:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Arutyunyan IV, Fatkhudinov TH, El'chaninov AV, Makarov AV, Kananykhina EY, Usman NY, Raimova ESh, Goldshtein DV, Bol'shakova GB. Effect of Endothelial Cells on Angiogenic Properties of Multipotent Stromal Cells from the Umbilical Cord during Angiogenesis Modeling in the Basement Membrane Matrix. Bull Exp Biol Med. 2016;160:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Schmitt BM, Laschke MW, Rössler OG, Huang W, Scheller A, Menger MD, Ampofo E. Nerve/glial antigen (NG) 2 is a crucial regulator of intercellular adhesion molecule (ICAM)-1 expression. Biochim Biophys Acta Mol Cell Res. 2018;1865:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL. Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res. 2014;51:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Xu J, Gong T, Heng BC, Zhang CF. A systematic review: differentiation of stem cells into functional pericytes. FASEB J. 2017;31:1775-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Vancheri C, Gili E, Failla M, Mastruzzo C, Salinaro ET, Lofurno D, Pistorio MP, La Rosa C, Caruso M, Crimi N. Bradykinin differentiates human lung fibroblasts to a myofibroblast phenotype via the B2 receptor. J Allergy Clin Immunol. 2005;116:1242-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Chen PY, Qin L, Li G, Tellides G, Simons M. Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFβ)-dependent smooth muscle cell phenotype modulation. Sci Rep. 2016;6:33407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 32. | McGuire PG, Rangasamy S, Maestas J, Das A. Pericyte-derived sphingosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler Thromb Vasc Biol. 2011;31:e107-e115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Wisniewska-Kruk J, Hoeben KA, Vogels IM, Gaillard PJ, Van Noorden CJ, Schlingemann RO, Klaassen I. A novel co-culture model of the blood-retinal barrier based on primary retinal endothelial cells, pericytes and astrocytes. Exp Eye Res. 2012;96:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Rustenhoven J, Aalderink M, Scotter EL, Oldfield RL, Bergin PS, Mee EW, Graham ES, Faull RL, Curtis MA, Park TI, Dragunow M. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation. 2016;13:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 35. | Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, Evans-Molina C, Maturi R, Harris A, Kern TS, March KL. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One. 2014;9:e84671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Trost A, Lange S, Schroedl F, Bruckner D, Motloch KA, Bogner B, Kaser-Eichberger A, Strohmaier C, Runge C, Aigner L, Rivera FJ, Reitsamer HA. Brain and Retinal Pericytes: Origin, Function and Role. Front Cell Neurosci. 2016;10:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 37. | Salehi H, Amirpour N, Razavi S, Esfandiari E, Zavar R. Overview of retinal differentiation potential of mesenchymal stem cells: A promising approach for retinal cell therapy. Ann Anat. 2017;210:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Tian X, Brookes O, Battaglia G. Pericytes from Mesenchymal Stem Cells as a model for the blood-brain barrier. Sci Rep. 2017;7:39676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Long X S-Editor: Zhang L L-Editor: Webster JR P-Editor: Wu YXJ