Published online Nov 26, 2019. doi: 10.4252/wjsc.v11.i11.1005
Peer-review started: April 4, 2019
First decision: June 6, 2019
Revised: July 17, 2019
Accepted: September 4, 2019
Article in press: September 4, 2019
Published online: November 26, 2019
Processing time: 215 Days and 23.7 Hours
Mesenchymal stem cells are pluripotent cells that have the ability to generate cells from a cell line or in other cell types from different tissues but from the same origin. Although those cells have more limited differentiation capacity than embryonic stem cells, they are easily obtained from somatic tissue and can be grown in large quantities. This characteristic of undifferentiated stem cells differentiating into different cell lines arouses strategies in regenerative medicine for the treatment of different diseases such as neurodegenerative diseases.
To evaluate the cell differentiation capacity of human breastmilk stem cells for the three germ layers by a systematic review.
The searched databases were PubMed, EMBASE, OVID, and COCHRANE LIBRARY, published between 2007 and 2018 in the English language. All were in vitro studies for analysis of the "cell differentiation potential" in the literature using the keywords “human breastmilk,” “stem cells,” and keywords combined with the Boolean operator “NOT” were used to exclude those articles that had the word “CANCER” and their respective synonyms, which were previously consulted according to medical subject heading terms. PRISMA 2009 guidelines were followed in this study.
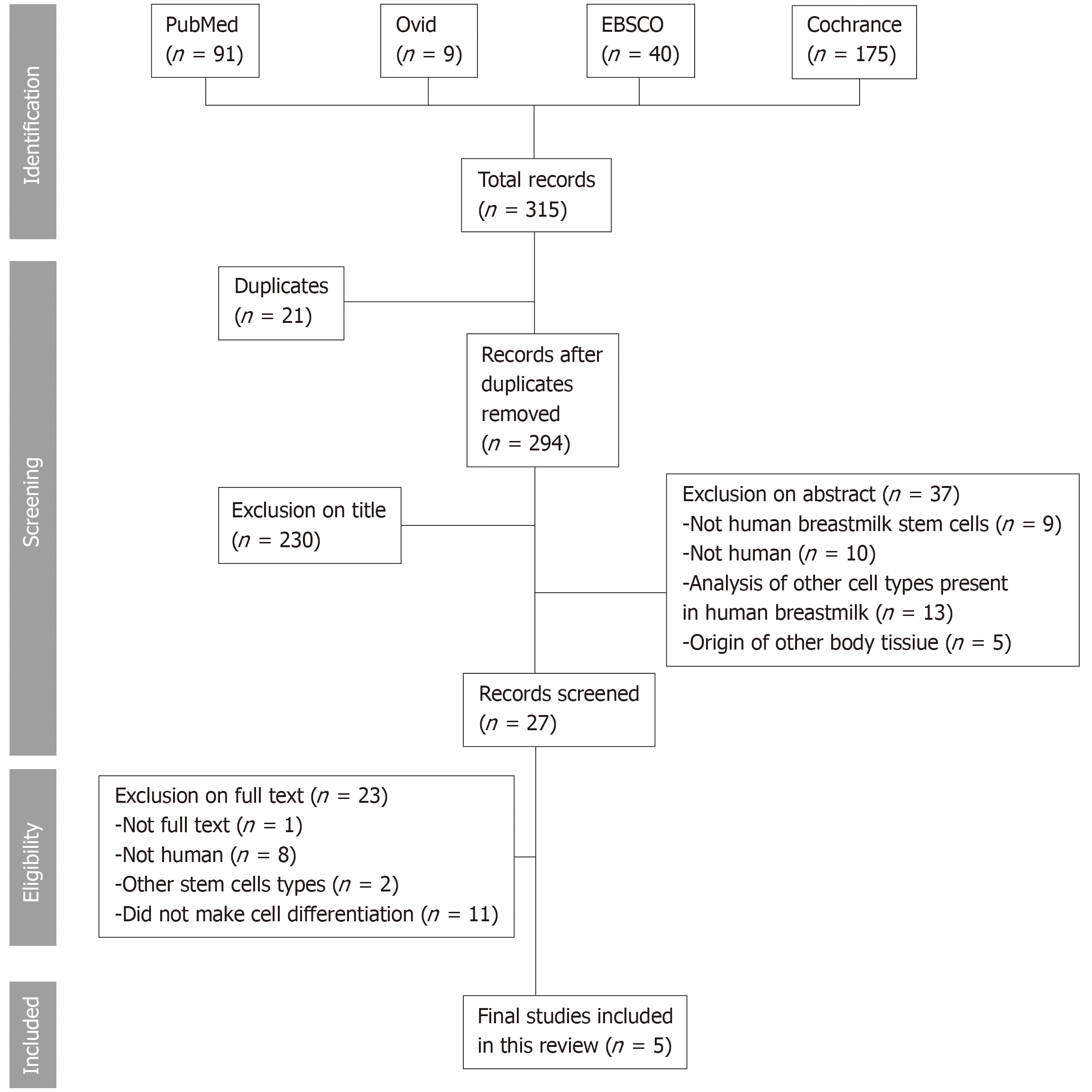
A total of 315 titles and abstracts of articles were examined. From these, 21 were in common with more than one database, leaving 294 articles for analysis. Of that total, five publications met the inclusion criteria. When analyzing the publications, it was demonstrated that human breastmilk stem cells have a high cellular plasticity, exhibiting the ability to generate cells of all three germ layers, endoderm, mesoderm, and ectoderm, demonstrating their stemness. Those cells expressed the genes, TRA-1-60/81, octamer-binding transcription factor 4, and NANOG, of which NANOG, a critical regulator for self-renewal and maintenance, was the most highly expressed. Those cells have the ability to differentiate in vitro into adipocytes, chondrocytes, osteocytes, oligodendrocytes, astrocytes, and neurons as well hepatocytes, β-pancreatic cells, and cardiomyocytes.
Although the literature has been scarce, the pluripotentiality of these cells represents great potential for tissue engineering and cellular therapy. Further studies for safe clinical translation are needed.
Core tip: Human breastmilk stem cells present interesting features that make them an alternative source of stem cells, mainly because they do not require any invasive procedure to be obtained. The objective was to investigate the literature data on their ability to differentiate into other cell lines. It was possible to verify that these cells have a high capacity of differentiation, as they are able to generate cells of the endoderm, mesoderm, and ectoderm lineages. However, the number of publications on the subject is still scarce, demonstrating that this source needs more studies and has the potential to be explored in regenerative medicine.
- Citation: Pacheco CMR, Ferreira PE, Saçaki CS, Tannous LA, Zotarelli-Filho IJ, Guarita-Souza LC, de Carvalho KAT. In vitro differentiation capacity of human breastmilk stem cells: A systematic review. World J Stem Cells 2019; 11(11): 1005-1019
- URL: https://www.wjgnet.com/1948-0210/full/v11/i11/1005.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i11.1005
Adult stem cells (ASCs) are present in small quantities in several mature tissues where they are quiescent, that is, not in the process of cell division. These cells are activated during the process of cellular replacement of tissues, being responsible for maintaining the biological homeostasis of the organism, thereby preserving the integrity of the tissues in which they are found. Similar to all other stem cells (SCs), ASCs have the property of self-renewal in that they make identical copies of themselves for long periods. Also, they may give rise to mature cell types with characteristic morphology and specialized functions[1].
Typically, SCs generate an intermediate cell type, called progenitor or precursor cells, before they become fully differentiated. These intermediate-stage cells are partially differentiated in fetal or adult tissues and give rise to different lineages after cell division. ASCs generally reach differentiation through a specific cellular development pathway; however, research has shown that their characteristics are not as definitive as previously thought[2,3].
A well-known type of ASC is mesenchymal stem cells (MSCs), which are present in several adult tissues. Among the most studied are the bone marrow (BM), adipose tissue, dental pulp, and vessel wall of the umbilical cord. MSCs are considered pluripotent cells by many researchers, because they are able to differentiate into osteoblasts, chondrocytes, adipocytes, as well as neural precursors, neurons, and glial cells. This cell population can be easily isolated and expanded in vitro because its potential of differentiation reflects the stimulatory and inhibitory factors to which they are subjected. Moreover, they are able to differentiate into specialized cells, with distinct phenotypes from their precursor, through inhibition and/or activation of certain molecular pathways[4].
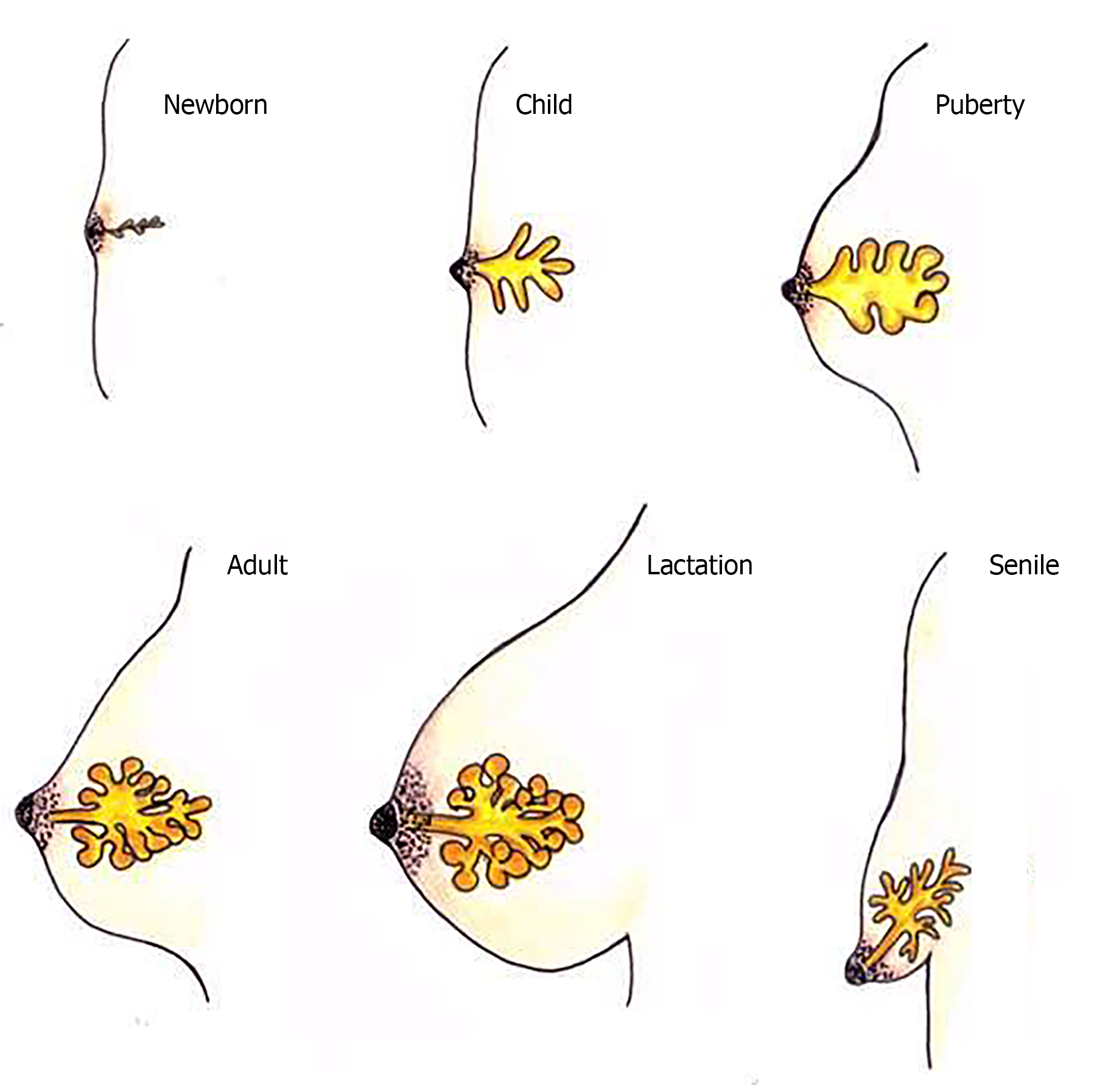
Recently, the mammary epithelium has been the focus of a large number of studies due to its remarkable population of SCs as human breastmilk stem cells (hBSCs). It is believed that the existence of these cells in this organ is related to the ability of the mammary gland to expand significantly and regress over the adult life (Figure 1)[5]. The hBSCs are found in the mammary gland in a state of latency and in low numbers; however, during gestation and lactation, they are activated and then transform the ductal structure into a secretory organ. Afterwards, when weaning occurs, milk production decays, inducing apoptosis of the mammary parenchyma cells. As a result, the ductal structure returns until a next pregnancy stimulates modifications of the tissue architecture once again[6,7].
Breastmilk has several cellular components together with hBSCs. In 2007, this population of stem cells in breastmilk was identified for the first time and when analyzed, showed Nestin (marker of neural stem cells) and different cytokeratins (CKs) such as CK5, CK14, CK18, and CK19 on their surfaces[8]. CKs are intermediate filaments expressed in the mammary epithelium depending on the differentiation that the cell undergoes. For example, CK5 expression is indicative of hBSCs of mammary origin. Cells in culture were found to be positive only for Nestin or double positive for CK5/Nestin, but no co-expression of CK14, CK18, or CK19 and Nestin was observed. It is also noteworthy that the analysis of fresh milk by real-time polymerase chain reaction (RT-PCR) only detected Nestin and CK18, indicating that breastmilk contains SCs and differentiated cells[9].
Research has shown that hBSCs have the ability to generate the following three lineages: (1) Alveolar lobe, also named myoepithelial cells, a structure of the adult gland, which constitutes the basal layer of the ducts and alveoli; (2) Ductal epithelial cells, which coat the lumen of the ducts; and (3) Alveolar epithelial cells, which are responsible for protein synthesis in breastmilk[10]. This evident ability of differentiation into cell types different from their tissue of origin raises the question of the real potential of differentiation of these cells. In this context, the purpose of this systematic review was to address the potential of differentiation of hBSCs into the three germ layers.
In order to select papers that addressed the topic “potential of cell differentiation of hBSCs” in the literature, PubMed, EMBASE, OVID, AND COCHRANE LIBRARY databases were searched for analysis of the “potential of cell differentiation of hBSCs.” The keywords used were “HUMAN BREAST MILK” and “STEM CELLS.” Also, a combination of the keywords with AND, and the Boolean operator “NOT” were used to exclude those papers that had the word “CANCER” and its respective synonyms, which were previously consulted in medical subject heading (MeSH) terms. The title and abstracts were examined in all conditions. Papers on cell differentiation were selected manually from this broader primary search by adding the term “cell differentiation” and synonyms, and a very small number of papers were generated. The research structure used in the databases is represented in Table 1.
| The same search strategy was used in the other databases | |
| PubMed | Human Breastmilk OR Human Milk OR |
| (Title/Abstract) | Breast Feeding OR Lactation |
| AND | |
| PubMed | Stem Cells Or Progenitor Cells OR |
| (Title/Abstract) | Mother Cells |
| NOT | |
| PubMed | Cancer |
| (Title/Abstract) | |
All duplicate papers were excluded, and the remaining ones were sorted t by title, abstract, and full text. Furthermore, papers in which the abstract was not available were analyzed in their entirety.
The inclusion criteria were all papers that: (1) Addressed the plasticity of stem cells derived from human breastmilk, that is, the ability of the cell to differentiate into more than one cell line; (2) Were published between 2007 and 2018; (3) Were written in the English language; and (4) Were in vitro studies. Three authors independently assessed all papers, and the reviewers reached consensus on the eligibility of the studies after discussion. The exclusion criteria were as follows: (1) Studies in which the origin of the stem cells obtained were from other animal species; (2) Studies that used stem cells from other body tissues; (3) In vivo studies; and (4) Written in languages other than English. Subsequently, using the same criteria, the abstracts were selected. Lastly, the complete texts of the papers considered relevant in the previous steps were evaluated according to the following exclusion criteria: (1) Unpublished literature; (2) Only summary of the congress available; (3) Full text not available; and (4) Does not contemplate the process of cell differentiation.
Two independent reviewers (1 and 2) performed research and study selection. Data extraction was performed by reviewer 1 and fully reviewed by reviewer 2. A third investigator decided some conflicting points and made the final decision to choose the articles. Only studies reported in English were evaluated. The Cochrane instrument was adopted to assess the quality of the included studies[11].
Considering the Cochrane tool for risk of bias, the overall evaluation resulted in five studies with a high risk of bias and three studies with uncertain risk. The domains that presented the highest risk of bias were related to a number of samples, volume of milk collected e income cells per milliliters (n = 3). Also, there was an absence of financial source in two studies and two studies did not disclose information on the conflict of interest statement.
In the review, 315 papers were initially retrieved (Figure 2), 21 of which were present in more than one database; therefore, they were excluded and considered only once, generating a total of 294 papers. Of these, only five papers met the eligibility criteria; their characteristics are summarized in Table 2.
| Ref. | Purpose | Number of samples | Vol. of milk collected | Income cellcells/ mL | Markers - FC | Markers - IF | PCR | WB | Differentiation cell | Conclusion |
| Patki et al[12] | To study the multipotent differentiation capacity of the long-term MSC culture. | 35 | 15 mL | 2.5 x 106-3.0 x 106/mL | CD29, CD33, CD34, CD44, CD45, CD73, SCA-1. | Nestin, Vimentin, Smooth Muscle Actin and E-Cadherin. | Not done | Not done | Adipogenic, chondroge-nic, osteogenic lineages. | Mesenchy-mal stem cells isolated from human breast milk have cellular potency to differentiate into different cell types. |
| Hassiotou et al[13] | To examine regulators of self-renewal of hBSCs and their plasticity and potential to differentiate into cell types outside the mammary lineage. | > 70 | 5-200 mL | Not done | SSEA-4 e TRA-1-60 / TRA-1-81. | OCT4, SOX2 and Nanog. | TRA-1-60 / TRA-1-81, Oct4, Sox2, Klf4 and Nanog genes. | Oct4, Sox2 and Nanog. | Osteogenic, adipogenic, chondroge-nic, neuronal, pancreatic, hepatocyte, cardiomyo-cytes, mammary differentiation and teratoma. | Stem cells in breast milk contain pluripotency properties. |
| Hosseini et al[14] | To differentiate the breast milk-derived stem cells toward neural stem cells and then into the neurons and neuroglia. | Not present | 5-200 mL | 4.0 x 104/mL | Not done | Nestin, Nanog, β-tubulin III, O4, GFAP, Oct4, Sox2, CD44, CD105, CD106, CD133. | Not done | Not done | Neurosphe-res, neurons, oligodendro-cytes, and astrocytes. | The breast milk-derived stem cells showed the capability to be differentia-ted into neural cell lineages and their similarity to both embryonic and mesenchy-mal stem cells makes them a good candidate for cell therapy in neurodegenerative diseases. |
| Sani et al[15] | To examine the pluripotency of the human breast milk-derived cells. | 5 | 5-50 mL | Not done | CD90 CD44, CD144, CD 271, CD123, CD133, CD73, CD 106, CD146, CD45, CD 105,CD34, SSEA-4, TRA-60–1, CD 15/SSEA-1. | Oct4, Sox2, Nanog and CK18. | SRY gene | Not done | Osteogenic and adipogenic lineages. | Most of the cells found are mesenchy-mal stem cells, but, cells similar to embryonic stem cells are also present. |
| Sani et al[16] | To examine the differentiation potential of the hBSCs into functional hepatocytes in vitro. | 15 | 5-50 mL | Not done | Not done | CK18, CK19, albumin, alpha-fetoprotein. | AFP, CK19, CYP2B6, HNF4, G6P. | Not done | Hepatocyte lineage. | The hBSCs demonstrate the ability to express many key factors that are important in liver functions. |
Although there are few studies in the literature on hBSCs, it is evident that this cell population has mesenchymal characteristics. Positivity for smooth muscle actin (SMA), Vimentin, and Nestin markers, along with the expression of surface markers [cluster of differentiations (CDs)]: CD44, CD29, CD73, CD90, CD106, CD133, CD146, CD271, and spinocerebellar ataxia type 1 (SCA1) support this claim. Low expression of adhesion molecules markers CD146 and CD144 was also detected. In addition, negativity was found for CD33, CD34, CD45, CD105, CD123, CD133, stage-specific embryonic antigen-4 (SSEA-4), and SSEA-1/CD15, confirming that such cells have no hematopoietic origin (Table 3).
| Marker | Function | Ref. |
| CD15/SSEA-1 | Stage-specific embryonic antigen, also marker for primitive mesenchymal cells in human bone marrow. | Anjos-Afonso et al[17] |
| CD29 | Expressed in myoepithelial cells including mammary gland cells. | Indumathi et al[5] |
| CD33 | Present in immune cells of myeloid lineage. | |
| CD34 | Marker of hematopoietic stem cells, vascular endothelium and fibroblasts of some tissues. | Indumathi et al[5] |
| CD44 | Marker of myoepithelial cells. | Indumathi et al[5] |
| CD45 | Membrane protein present in lymphocytes and absent in erythrocytes and platelets. This protein is lost during the maturation of red cells in the bone marrow. Because it is present in lymphocytes, it is called Common Leukocyte Antigen. | Notta et al[18] |
| CD73 | Marker expressed in mesenchymal stem cells, possessing enzymatic activity and catalytic dephosphorylation of adenosine monophosphate converting it into adenosine. | Indumathi et al[5] |
| CD90 | Marker of mesenchymal stem cells. | Indumathi et al[5] |
| CD105 | Marker of mesenchymal stem cells. | Indumathi et al[5] |
| CD106 | Acts on the cell cycle of T and B lymphocytes, in addition to being involved in tissue repair. | Indumathi et al[5] |
| CD123 | The IL-3 receptor subunit plays an important role in the growth and differentiation of hematopoietic progenitor cells. | Testa et al[19] |
| CD133 | Marker of hematopoietic stem cells. | Indumathi et al[5] |
| CD144 | Also known as VE-cadherin and cadherin-5, it is a calcium-dependent transmembrane cell adhesion molecule, located in the intercellular borders of endothelial cells, hematopoietic stem cells and perineural cells. | Dejana et al[20] |
| CD146 | Mediated cell-cell interactions, cytoskeletal remodeling, angiogenesis, and migration of endothelial cells. | Sorrentino et al[21] |
| CD271 | Transmembrane protein found in neuronal axons, Schwann cells, and perineural cells of peripheral nerves. In addition, it can be found in some epithelial, mesenchymal, and lymphoid tissues. | Lv et al[22] |
| SSEA-4 | Marker of mesenchymal stem cells derived from the BM, acting mainly on cell proliferation. | Lv et al[22] |
| TRA-60-1 | Related to pluripotency of human embryonic stem cells, and lost in cell differentiation. | Schopperle et al[23] |
| NESTIN | Multipotent, neural, medullary, pancreatic, and epithelial stem cell marker. | Toma et al[24] |
| VIMENTIN | Cytoskeletal intermediate filament protein, associated with the nuclear and plasma membrane, maintains the position of the nucleus and the mitotic spindle during the life of the cell, and is found mainly in mesenchymal stem cells. | Mendez et al[25] |
| SMA | Marker of myoepithelial cells. | Twigger et al[26] |
| E-CADHERIN | Calcium-dependent cell adhesion molecule, with important function in the formation and maintenance of normal tissue architecture, present in epithelial cells of the human mammary gland and epithelial marker of mesenchymal transition in its first passages. | Klopp et al[27] |
| NANOG | Pluripotency marker. | Riekstina et al[28] |
| β-TUBULIN III | Considered an early neuronal marker because the tubulin protein is the main constituent of the microtubules, which are tubular structures that make up the cytoskeleton and they are involved in the transport of organelles and the elongation of axons and dendrites. | Kapitein et al[29] |
| O4 | Specific marker of gangliosides expressed by pre-oligodendrocytes and premyelinated oligodendrocytes. | Girolamo et al[30] |
| GFAP | Glial fibrillary acidic protein forms subunits of the intermediate filaments of the cellular cytoskeleton, being present in the cytoplasm of the astrocytes. | Hol et al[31] |
| OCT4 | Pluripotency marker. | Riekstina et al[28] |
| SOX2 | Controls the undifferentiated and pluripotential state of the CTEs. | Young et al[32] |
| CK18 | Expressed in the luminal cells of the breast, which synthesize proteins from breast milk. | Thomas et al[33] |
| KLF 4 | Kruppel like factor 4 is a transcription factor related to pluripotency and cell proliferation potential. | Li et al[34] |
| SCA-1 | The stem cell antigen 1 is found in stem cells. | Holmes et al[35] |
The expression of transcription factors considered embryonic stem cell (ESC) markers such as SEEA4, TRA 60-1, Oct4, sex-determining region Y (SRY)-box 2 (Sox2), and NANOG was also evaluated by immunofluorescence staining. A subpopulation of cells expressing CK18 was found, and the frequency of positive cells for that population was 65.33% ± 6.1%.
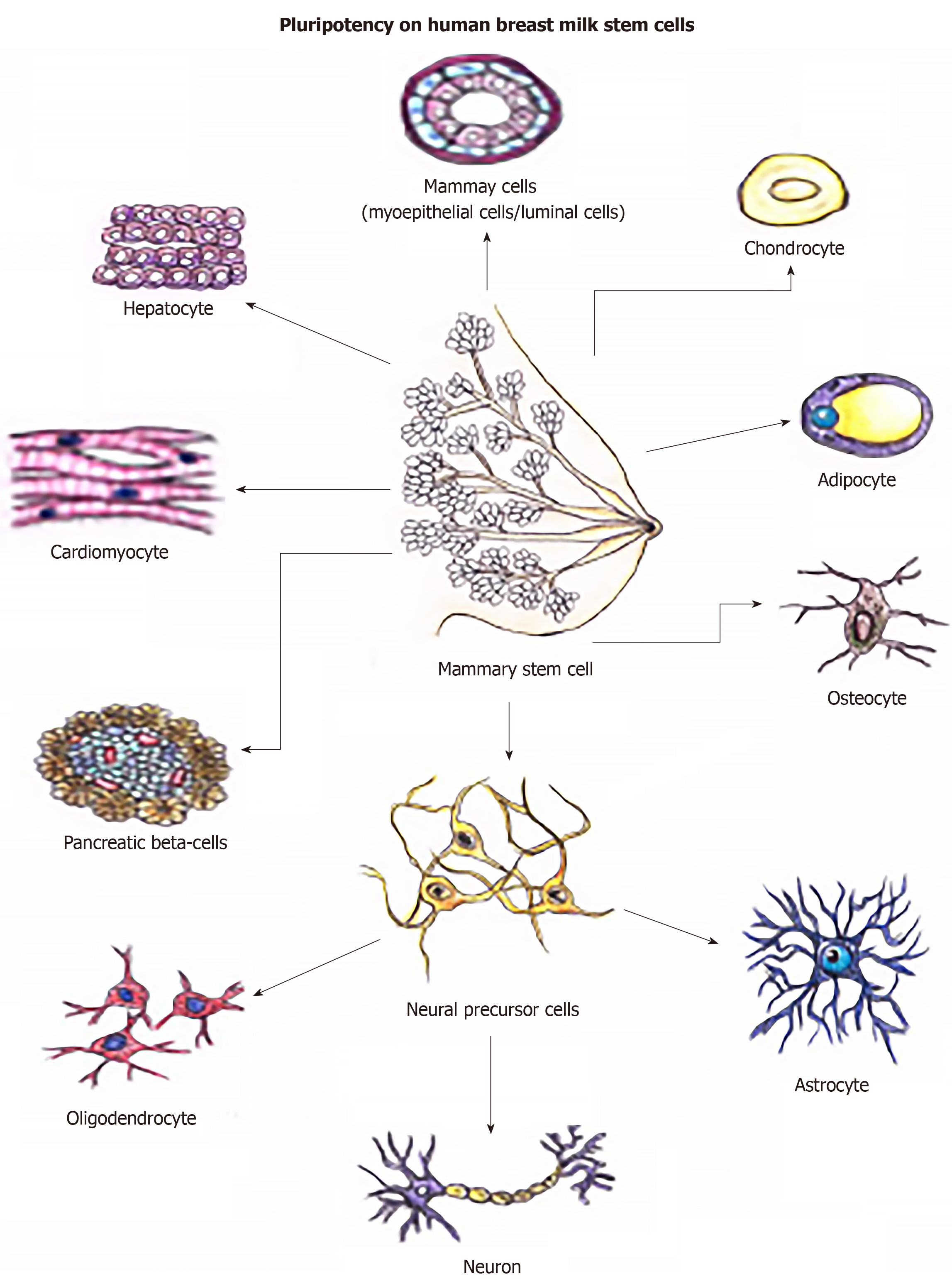
The hBSCs differentiated into different cell types of mesodermal origin in vitro including adipocytes, chondrocytes, and osteocytes (Figure 3). Moreover, they differentiated into three neural lines: oligodendrocytes, astrocytes, and neurons. They also differentiated into tissues of endodermal origin, as hepatocytes, β-pancreatic cells, and cardiomyocytes. Therefore, their property of differentiation in multiple lines is explicit (Figure 4).
These findings provide evidence of the existence of a subpopulation of pluripotent hBSCs, suggesting that these cells, when isolated from breastmilk, can be stimulated to create several types of tissues and be used in regenerative medicine. If hBSCs have great potential to differentiate into neural cell lines or even into neural precursor cells spontaneously, they would have great applicability in the treatment of neurodegenerative diseases.
Considering the results obtained in this review, it was possible to observe the scarcity of studies seeking to verify the potential of cellular differentiation of hBSCs. BM-derived MSCs were the first to be described in 1966[36], and even though in recent years there has been an increase in reports on the isolation of MSCs from different sources, they are the most frequently studied cell type[5]. MSCs can be induced in vitro to differentiate into various cell types found at various stages of development, as well as at specific anatomical sites[22].
Although the International Society of Cell Therapy advocates that human MSCs contain the positive surface markers CD105, CD73 and CD90; and have negativity to CD45, CD34, CD14 or CD11b, CD79a or CD19 and human leukocyte antigen-DR isotype, other surface antigens such as CD13, CD29, CD44, and CD10, which are often expressed in MSCs[37], should also be analyzed. Flow cytometry and immunocytochemistry analyses revealed that the hBSCs exhibited the positive surface markers CD29, CD44, CD90, CD146, CD271, and stem cell antigen-1. In addition, the markers CD33, CD34, CD45, CD73, CD123, and CD144 were considered negative by both techniques. Nevertheless, a discrepancy was observed concerning the CD105 marker, because it was negative (2.64% ± 0.55%) when analyzed by flow cytometry, and positive (68.3% ± 3.91%) when observed by immunocytochemistry[12,14,15]. Likewise, lack of expression of CD90 (7.7% ± 0.8%) and CD73 (2.1% ± 0.41%) surface markers was reported, but a positive marker was found for CD105 (47.7% ± 2.95%)[5].
CD146-positive cells have a greater potential for cell proliferation and differentiation[21]. The CD271 marker acts to maintain the clonogenicity of MSCs; however, most of these cells do not co-express CD90 and CD73, two general MSC markers. CD271 is not expressed in all MSCs, but is found at high levels in the BM, adipose tissue, and periodontal ligament. Moreover, it is expressed at low levels in MSCs derived from placenta and is not expressed in synovial membranes[22].
Regarding the transcription factors considered ESC markers that were evaluated by immunofluorescence, flow cytometry, and reverse transcription PCR (RT-PCR), the hBSCs did not express SSEA4 and SSEA1/CD15. Additionally, the markers Oct4, Sox2, NANOG, and TRA-1-60/81 were reactive. The genes, TRA-1-60/81 and NANOG were the most highly expressed, followed by Oct4. In fact, NANOG is considered a crucial regulator of self-renewal and maintenance of pluripotentiality[38].
SSEA-4 is a marker of ESCs initially used to isolate MSCs from the BM. It has been observed that when positively expressed, the cells in culture expand extensively and exhibit trilineage differentiation potency. Nevertheless, the use of SSEA-4 as a marker is questionable since its positivity was suggested to be an artificial induction of in vitro culture using fetal bovine serum (FBS), which contains glycosphingolipids and globoserms that can be recognized by an SSEA-4 antibody. These findings raise the question of the physiological relevance and reliability of SSEA-4 as a marker for MSCs[22,39].
The transcription factors Oct4 and NANOG are indispensable for maintaining the self-renewal of ESCs. In addition, Oct4 directly inhibits the signaling pathway of bone morphogenetic protein-4, which activates the mesoderm and the extra-embryonic differentiation of the ectoderm and endoderm, while NANOG acts as a repressor of the neural crest and neuroectoderma lineage and is essential for maintaining the properties of MSCs[4,28].
Pluripotency genes can be strongly correlated with and are the main transcription factors controlling the differentiation and pluripotency of multiple lines of ESCs. The presence of these genes in hBSCs allows a strong association since they play an important role in lactation and the ability to differentiate multiple breastmilk cell lines[26].
Nestin is a well-known marker of SCs of various lineages, and its expression in hBSCs indicates an increase in the characteristics of mammary progenitor cells during lactation. It is also highly expressed in lactating mammary tissues[26].
Through the observation of positivity for SMA, Vimentin, E-cadherin, and Nestin markers by flow cytochemistry, it is possible to hypothesize that the origin of the hBSCs is myoepithelial. HBSCs are positive for CK5 and SMA, and such dual positivity indicates the precursor nature of the cells. In addition, when labeling for CK18 was analyzed, it was present at a frequency of 65.33% ± 6.1% of the cell pool, revealing that there is a subpopulation of cells in breastmilk that is derived from luminal epithelial cells[8].
Currently in the MSC population, only a subpopulation contemplates the criteria recommended by the International Society of Cell Therapy since more compromised or more immature cells can be observed. There is still no consensus on the use of only one marker for the identification of MSCs from different sources, because there are phenotypic and/or functional differences that need to be better understood. It is reinforced that MSC manipulation can generate phenotypic rearrangements, losing the expression of some markers and acquiring others[2]. This phenotype can be modulated in culture and does not reflect the phenotype in vivo.
By PCR in cells derived from fresh breastmilk, absence of the SRY gene in genomic DNA was shown, indicating that there are no cells originating from fetal tissues between the isolated cells; thus, there is no fetal microchemistry. Therefore, there is no exchange of stem cells between the mother and the embryo[40].
Among the criteria established for the characterization of MSCs, their ability to differentiate into osteogenic, chondrogenic and adipogenic lineage is included[13]. Several studies have shown that these cells can also differentiate into unrelated germ lines in a process called transdifferentiation. Thus, MSCs can differentiate into cells of mesodermal lineage, such as bone, fat and cartilage, as well as have the potential for endodermal and neuroectodermal differentiation[41].
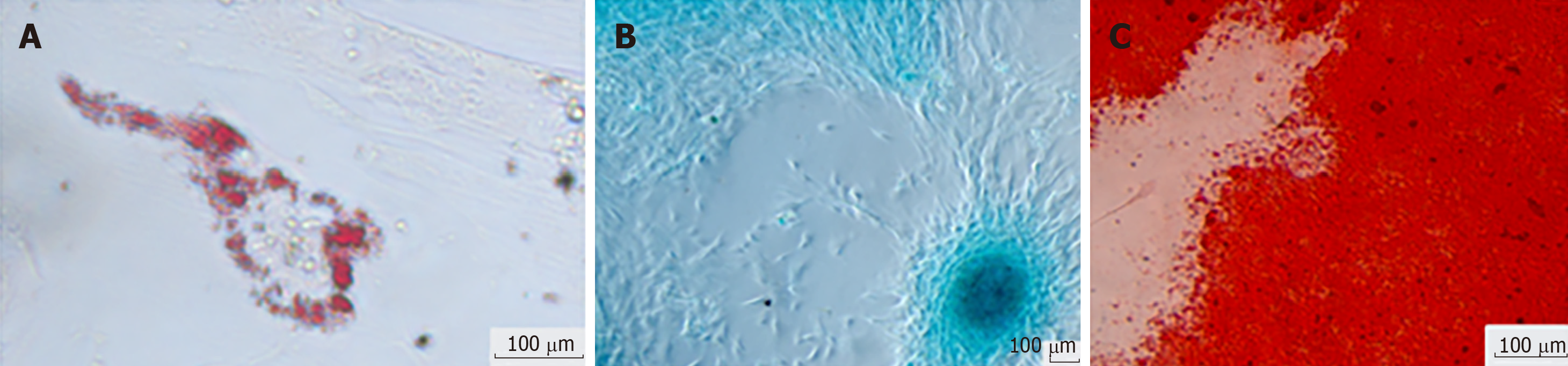
The use of culture medium containing FBS supplemented with ascorbic acid, β-glycerophosphate, and dexamethasone is needed for osteogenic differentiation from MSCs. It induces an increase in alkaline phosphatase activity and calcium deposition. It was found that by subjecting hBSCs to the osteogenic differentiation environment, for 3 to 4 wk, the presence of osteocytes, the cell acquired cuboidal morphology, and produced mineralized material were observed. Calcium phosphate mineralization was verified by positive staining by Alizarin Red S, indicating that the calcium deposits as amorphous accumulation between the cells[12,15]. Nuclear expression of Runt-related transcription factor 2 (RUNX2), an essential transcription factor for osteoblastic differentiation, was also detected. Some RUNX2-positive cells co-expressed osterix, an osteoblast-specific transcription factor required for bone formation[13].
Chondrogenic differentiation can be obtained by micromass culture utilizing an FBS-free culture medium containing transforming growth factor beta, which stimulates the production of highly sulfated proteoglycans, specific for cartilage and type II collagen. In contrast, cells synthesize extracellular matrix glycosaminoglycan, collagen type II, and aggrecan, which can be detected by immunohistological staining or by the expression of typical genes of the chondrogenic lineage via PCR. After chondrogenic differentiation environment exposure for 21 d, the hBSCs began to show changes in their cellular morphology including acquisition of the spindle shape, the presence of larger round cell aggregates, and the accumulation of cartilage characteristic sulfate proteoglycans, being ratified by staining of Safranin-O[12,15]. The transcription factors for chondrocytes, RUNX2/Sox6, were also identified in differentiated cells[13].
When exposed to culture medium containing FBS, dexamethasone, and isobutylmethylxanthine, MSCs were induced to differentiate into adipocytes. Adipogenic differentiation is characterized by the acquisition of an oval cellular morphology and the appearance of intracellular lipid droplets. Such changes can be verified in hBSCs stimulated to adipogenic differentiation for 21 d, which were confirmed by staining with Oil Red O and by positivity of the peroxisome proliferator-activated receptor gamma transcription factor, which is responsible for the regulation of the lipid metabolism[12,13,15].
Regarding the potential of hBSCs to generate different lineages, the presence of myoepithelial cells was observed in the adhered colonies after 2 wk of receiving induction medium for breast differentiation; by the 3rd wk, luminal cells were detected, some of which spontaneously synthesized milk proteins such as ß-casein, lactoferrin, and α-lactalbumin, which were detected in the culture supernatant[13].
It is interesting that many cells that constitute the liver are also the main progenitors for the formation of new hepatocytes. Moreover, when the tissue is compromised, SCs present in the bile ducts are stimulated, which leads to the differentiation of the hepatocytes. The differentiation of hepatocytes from non-hepatic ASCs has already been demonstrated by SCs originating from BM[42] in the umbilical cord blood[43] and adipose tissue[44]. The hBSCs were able to differentiate in hepatocytes using α-fetoprotein (AFP), pyruvate-kinase M2 isoenzyme, and the albumin functional marker in this study[13].
The markers CK18, CK19, and AFP were identified on differentiating hBSCs into hepatocytes on the 30th day of culture through immunocytochemistry analysis[16]. They also analyzed the expression of hepatocyte-specific genes, such as hepatocyte nuclear factor 4 (HNF4), albumin, CK19, cytochrome p450 family 2 subfamily B member 6 (CYP2B6), glucose 6-phosphate (G6P), and Claudin, which were detected in differentiated cells and may indicate the formation of bile canaliculi. Semi-quantitative RT-PCR showed that the amount of CK19, CYP2B6, and G6P was comparable to that of the positive control used. These cells showed the ability to store glycogen through indocyanine green clearance test, in which hepatocellular function was dynamically assessed, indicating that the differentiated hBSCs are functional in vitro. Therefore, these cells can differentiate into endoderm lineages.
Both MSCs obtained from BM[45] and adipose tissue[46] have the ability to differentiate into multilineage, including transdifferentiation in pancreatic beta cells. RT-PCR analysis revealed that hBSCs, when exposed to an environment that induces pancreatic differentiation, positively expressed the pancreatic and duodenal homeobox 1 marker and insulin marker[13]. These genes are associated with pancreatic development, as previously proven in the literature[47]. From these findings, the use of these cells could be considered an alternative in the treatment of type I diabetes, with the ultimate aim of restoring the patient's glycemic levels.
The hBSCs can also stimulate the formation of cardiospheres. These cells start to express troponin T after 4 wk of culture[13]. Remarkably, MSCs have been used for decades in cardiac regenerative therapy, with several sources of these cells such as the umbilical cord, placenta, and bone marrow. For example, MSC cord was seen to help in the recovery of motor function and increase strength of the cardiac muscle[48].
The ability of MSCs to differentiate into neural cells under specific culture conditions has also been reported. For instance, BM MSCs, when cultured in the presence of epidermal growth factor or brain-derived neurotrophic factor expressed glial fibrillary acidic protein (GFAP) and neuron-specific nuclear protein[49]. In a previous study[14], hBSCs were cultured in DMEM/F12 with B27 1% and N2 2% for 7-10 days. After this time, some bead-like cellular aggregations were formed, which were dissociated and conditioned on a different plate containing DMEM/F12, B27, N2, beta fibroblast growth factor, and epidermal growth factor. To analyze the differentiation induction process into neural cells, and in order to compare whether there were differences between the cells before and after this protocol, the authors performed immunocytochemistry techniques for the antibody Nestin and flow cytometry for the CD133 marker. They found that a subpopulation of MSCs derived from breastmilk expressed Nestin (7.4% ± 3.30%) and CD133 (2.76% ± 1.93%), but after exposure to neurogenic induction medium, the frequency of Nestin-positive cells (58.20% ± 6.71%) and those expressing positivity for CD133 (58.74% ± 3.36%) significantly increased. Since these are markers of neural stem cells, it can be inferred that hBSCs may exhibit behavior similar to that of neural stem cells. Recently, hBSCs were also observed to be prone to differentiate into the following three neural cell lines: neurons, astrocytes, and oligodendrocytes. The β-tubulin III marker was used to detect neurons, the O4 antibody for oligodendrocytes, and the GFAP antibody for astrocytes[13,14].
Mesenchymal breastmilk stem cells can differentiate in vitro into cell lines of all three germ layers. Some studies have already shown the in vitro differentiation of MSCs to cells with neuronal and glial morphology, which express neuronal and glial markers after stimulation with retinoic acid, nerve growth factor, beta fibroblast growth factor, valproic acid, forskolin, and glial growth factor[49-51], pointing out the transdifferentiation potential of primitive mesenchymal progenitors to cells derived from other embryonic leaflets.
A theoretical model of MSC differentiation was created, in which undifferentiated cells would go through two phases before acquiring a specific phenotype. In the first one, after asymmetric divisions, the MSCs originate a population of cells less undifferentiated and a population of precursor cells with restricted self-renewal and differentiation potential. The transition from the stem cell compartment to the compromised compartment would occur with the symmetric division of tri- or bi-potent progenitor cells, which would originate the unipotent progenitors. This process is well controlled and involves a change in the secretion profile of cytokines and growth factors, as well as a modification in the three-dimensional structure of the extracellular matrix, besides the activation and inactivation of transcriptional factors and consequent expression of genes related to this differentiation pathway[52].
Injecting MSCs into immunodeficient animals showed that they can respond to the different stimuli in vivo, differentiating themselves in a disorganized way and leading to the formation of cysts, teratomas, and tumors present in different types of tissues originating from each of the three embryonic leaflets[53]. It is noteworthy that one of the studies performed teratoma formation testing, where freshly isolated hBSCs and cultured sphered milk cells were injected subcutaneously into SCID mice (n = 15). After 9 wk of the procedure, the mice were examined, and no tumor formation was observed[13]. SCID lineage mice have great immunological compromises since they are deficient in T lymphocytes, B lymphocytes, and natural killer cells, and the use of these mice for teratoma formation tests is the most appropriate[54]. In fact, the existence of pluripotent ASCs that do not form teratomas was previously described in BM, considering that these cells have unique patterns of DNA methylation in some genes and it is suggested that the non-formation of teratomas would be associated with these epigenetic characteristics[55].
Even though ESCs originate from the internal mass of the blastocysts, it is also possible to find them in adult tissues in specialized niches. Recent evidence has suggested that certain subpopulations of cells within ASC compartments can differentiate into cell types outside their dermal origin under specific microenvironments in vitro and in vivo[32]. These results indicate that hBSCs possibly have pluripotency characteristics, with many characteristics to be still explored.
In conclusion, human breastmilk stem cells have high cellular plasticity, exhibiting the ability to generate cells from all three germ layers as demonstrated by their stemness. Thus, further studies are needed to elucidate the implication of these cells in regenerative medicine and bioengineering as well to explore these ASCs obtained from no invasive sources.
Mesenchymal stem cells are pluripotent cells that have the ability to generate cells from a cell line or in other cell types from different tissues but from the same origin; although those cells have a more limited differentiation capacity than embryonic stem cells, they are easily obtained from the somatic tissue and can be grown in large quantities. This characteristic of undifferentiated stem cells differentiating into different cell lines arouses strategies in regenerative medicine for the treatment of different diseases, such as neurodegenerative diseases.
Mammary epithelium has been the focus of studies due to its remarkable population of human breastmilk stem cells (hBSCs). It will be important to evaluate the scientific literature as to the potential for differentiation of these cells for regenerative medicine.
The main objective was to evaluate the cell differentiation capacity of hBSCs for the three germ layers through a systematic review.
The searched databases were PubMed, EMBASE, OVID, and COCHRANE LIBRARY; the inclusion criteria were all papers that: (1) addressed the plasticity of stem cells derived from human breastmilk, that is, the ability of the cell to differentiate into more than one cell line; (2) published between 2007 and 2018 in the English language; and (3) were in vitro studies for the analysis of the "cell differentiation potential" in the literature using the keywords "HUMAN BREASTMILK," "STEM CELLS," and keywords combined with the Boolean operator "NOT" used to exclude those articles that had the word "CANCER" and their respective synonyms, which were previously consulted according to the medical subject heading terms. PRISMA 2009 guidelines were followed in this study.
A total of 315 titles and abstracts of articles were examined. From these, 21 were in common with more than one database; remaining 294 articles. Out of that total, 5 publications met the inclusion criteria. When analyzing the publications, it was demonstrated that human breastmilk stem cells have a high cellular plasticity, exhibiting the ability to generate cells of all three germ layers: endoderm, mesoderm and ectoderm, demonstrating their stemness. Those cells expressed the genes, TRA-1-60/81, octamer-binding transcription factor 4, and NANOG; NANOG was the gene most highly expressed, which is a regulator for self-renewing and its maintenance. Those cells have the ability to differentiate in vitro: adipocytes, chondrocytes, osteocytes, oligodendrocytes, astrocytes, neurons as well hepatocytes, β-pancreatic cells, and cardiomyocytes.
The hBSCs expressed the genes, TRA-1-60/81, NANOG, and Oct4. The pluripotentiality of hBSCs has been demonstrated by its NANOG expression, which is a regulator for self-renewal and its maintenance. This study opens the possibilities to use this source of adult stem cells for tissue engineering and cellular therapy. hBSCs demonstrated their stemness by high cellular plasticity and exhibiting the ability to generate cells of all three germ layers.
There will be great potential of hBSCs for tissue engineering and cellular therapy, but more studies for a safe translation will be needed. The single cell analysis seems to be the best method for the next step to evaluate the genetic stability of hBSCs.
| 1. | Bianco P. "Mesenchymal" stem cells. Annu Rev Cell Dev Biol. 2014;30:677-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 319] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 2. | Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 1104] [Article Influence: 100.4] [Reference Citation Analysis (1)] |
| 4. | Tsai CC, Hung SC. Functional roles of pluripotency transcription factors in mesenchymal stem cells. Cell Cycle. 2012;11:3711-3712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Indumathi S, Dhanasekaran M, Rajkumar JS, Sudarsanam D. Exploring the stem cell and non-stem cell constituents of human breast milk. Cytotechnology. 2013;65:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 550] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 8. | Cregan MD, Fan Y, Appelbee A, Brown ML, Klopcic B, Koppen J, Mitoulas LR, Piper KM, Choolani MA, Chong YS, Hartmann PE. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res. 2007;329:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Petersen OW, Gudjonsson T, Villadsen R, Bissell MJ, Rønnov-Jessen L. Epithelial progenitor cell lines as models of normal breast morphogenesis and neoplasia. Cell Prolif. 2003;36 Suppl 1:33-44. [PubMed] |
| 10. | Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011;. |
| 12. | Patki S, Kadam S, Chandra V, Bhonde R. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum Cell. 2010;23:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger AJ, Metzger P, Trengove N, Lai CT, Filgueira L, Blancafort P, Hartmann PE. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells. 2012;30:2164-2174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Hosseini SM, Talaei-Khozani T, Sani M, Owrangi B. Differentiation of human breast-milk stem cells to neural stem cells and neurons. Neurol Res Int. 2014;2014:807896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Sani M, Hosseini SM, Salmannejad M, Aleahmad F, Ebrahimi S, Jahanshahi S, Talaei-Khozani T. Origins of the breast milk-derived cells; an endeavor to find the cell sources. Cell Biol Int. 2015;39:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Sani M, Ebrahimi S, Aleahmad F, Salmannejad M, Hosseini SM, Mazarei G, Talaei-Khozani T. Differentiation Potential of Breast Milk-Derived Mesenchymal Stem Cells into Hepatocyte-Like Cells. Tissue Eng Regen Med. 2017;14:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Anjos-Afonso F, Bonnet D. Flexible and dynamic organization of bone marrow stromal compartment. Br J Haematol. 2007;139:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 638] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 19. | Testa U, Pelosi E, Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res. 2014;2:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 20. | Dejana E, Giampietro C. Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol. 2012;19:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, Peschle C, Valtieri M. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp Hematol. 2008;36:1035-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 759] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 23. | Schopperle WM, DeWolf WC. The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells. 2007;25:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1130] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 25. | Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 721] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 26. | Twigger AJ, Hepworth AR, Lai CT, Chetwynd E, Stuebe AM, Blancafort P, Hartmann PE, Geddes DT, Kakulas F. Gene expression in breastmilk cells is associated with maternal and infant characteristics. Sci Rep. 2015;5:12933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Klopp AH, Lacerda L, Gupta A, Debeb BG, Solley T, Li L, Spaeth E, Xu W, Zhang X, Lewis MT, Reuben JM, Krishnamurthy S, Ferrari M, Gaspar R, Buchholz TA, Cristofanilli M, Marini F, Andreeff M, Woodward WA. Mesenchymal stem cells promote mammosphere formation and decrease E-cadherin in normal and malignant breast cells. PLoS One. 2010;5:e12180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, Muceniece R, Ancans J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009;5:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Kapitein LC, Hoogenraad CC. Which way to go? Cytoskeletal organization and polarized transport in neurons. Mol Cell Neurosci. 2011;46:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Girolamo F, Strippoli M, Errede M, Benagiano V, Roncali L, Ambrosi G, Virgintino D. Characterization of oligodendrocyte lineage precursor cells in the mouse cerebral cortex: a confocal microscopy approach to demyelinating diseases. Ital J Anat Embryol. 2010;115:95-102. [PubMed] |
| 31. | Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 662] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 32. | Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1023] [Cited by in RCA: 938] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 33. | Thomas E, Lee-Pullen T, Rigby P, Hartmann P, Xu J, Zeps N. Receptor activator of NF-κB ligand promotes proliferation of a putative mammary stem cell unique to the lactating epithelium. Stem Cells. 2012;30:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Li J, Zheng H, Wang J, Yu F, Morris RJ, Wang TC, Huang S, Ai W. Expression of Kruppel-like factor KLF4 in mouse hair follicle stem cells contributes to cutaneous wound healing. PLoS One. 2012;7:e39663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 316] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 36. | Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-390. [PubMed] |
| 37. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13033] [Article Influence: 685.9] [Reference Citation Analysis (12)] |
| 38. | Ding J, Xu H, Faiola F, Ma'ayan A, Wang J. Oct4 links multiple epigenetic pathways to the pluripotency network. Cell Res. 2012;22:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 39. | Suila H, Pitkänen V, Hirvonen T, Heiskanen A, Anderson H, Laitinen A, Natunen S, Miller-Podraza H, Satomaa T, Natunen J, Laitinen S, Valmu L. Are globoseries glycosphingolipids SSEA-3 and -4 markers for stem cells derived from human umbilical cord blood? J Mol Cell Biol. 2011;3:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Barinaga M. Cells exchanged during pregnancy live on. Science. 2002;296:2169-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 670] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 42. | Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, Park HK, Han H, Kim H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 398] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 45. | Sun Y, Chen L, Hou XG, Hou WK, Dong JJ, Sun L, Tang KX, Wang B, Song J, Li H, Wang KX. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl). 2007;120:771-776. [PubMed] |
| 46. | Amer MG, Embaby AS, Karam RA, Amer MG. Role of adipose tissue derived stem cells differentiated into insulin producing cells in the treatment of type I diabetes mellitus. Gene. 2018;654:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Marappagounder D, Somasundaram I, Dorairaj S, Sankaran RJ. Differentiation of mesenchymal stem cells derived from human bone marrow and subcutaneous adipose tissue into pancreatic islet-like clusters in vitro. Cell Mol Biol Lett. 2013;18:75-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010-2015). Stem Cell Res Ther. 2016;7:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 49. | Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1133] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 50. | Qian L, Saltzman WM. Improving the expansion and neuronal differentiation of mesenchymal stem cells through culture surface modification. Biomaterials. 2004;25:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Safford KM, Safford SD, Gimble JM, Shetty AK, Rice HE. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 746] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 53. | Müller FJ, Goldmann J, Löser P, Loring JF. A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell. 2010;6:412-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Kuroda T, Yasuda S, Sato Y. Tumorigenicity studies for human pluripotent stem cell-derived products. Biol Pharm Bull. 2013;36:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ, Kucia M. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia. 2009;23:2042-2051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bragança J, Grawish ME, Scarfì S S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Wu YXJ