Published online Nov 26, 2019. doi: 10.4252/wjsc.v11.i11.990
Peer-review started: March 15, 2019
First decision: July 31, 2019
Revised: September 2, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: November 26, 2019
Processing time: 238 Days and 1.4 Hours
Recently, the exclusive use of mesenchymal stem cell (MSC)-secreted molecules, called secretome, rather than cells, has been evaluated for overcoming the limitations of cell-based therapy, while maintaining its advantages. However, the use of naïve secretome may not fully satisfy the specificity of each disease. Therefore, it appears to be more advantageous to use the functionally reinforced secretome through a series of processes involving physico-chemical adjustments or genetic manipulation rather than to the use naïve secretome.
To determine the therapeutic potential of the secretome released from miR-122-transfected adipose-derived stromal cells (ASCs).
We collected secretory materials released from ASCs that had been transfected with antifibrotic miR-122 (MCM) and compared their antifibrotic effects with those of the naïve secretome (CM). MCM and CM were intravenously administered to the mouse model of thioacetamide-induced liver fibrosis, and their therapeutic potentials were compared.
MCM infusion provided higher therapeutic potential in terms of: (A) Reducing collagen content in the liver; (B) Inhibiting proinflammatory cytokines; and (C) Reducing abnormally elevated liver enzymes than the infusion of the naïve secretome. The proteomic analysis of MCM also indicated that the contents of antifibrotic proteins were significantly elevated compared to those in the naïve secretome.
We could, thus, conclude that the secretome released from miR-122-transfected ASCs has higher antifibrotic and anti-inflammatory properties than the naïve secretome. Because miR-122 transfection into ASCs provides a specific way of potentiating the antifibrotic properties of ASC secretome, it could be considered as an enhanced method for reinforcing secretome effectiveness.
Core tip: We herein intended to determine the antifibrotic effects of the secretome released from miR-122-transfected adipose-derived stromal cells (miR-122-secretome). miR122-secrectome and naïve secretome were intravenously administered to the mice with liver fibrosis, respectively. miR122-secrectome infusion provided higher therapeutic potential in terms of reducing collagen content in the liver, inhibiting proinflammatory cytokines, and reducing abnormally elevated liver enzymes than the infusion of the naïve secretome. Proteomic analysis of the miR122-secrectome indicated that the contents of antifibrotic proteins were significantly elevated compared to those in the naïve secretome. Our results demonstrate that miR122-secrectome has higher antifibrotic and anti-inflammatory properties than the naïve secretome.
- Citation: Kim KH, Lee JI, Kim OH, Hong HE, Kwak BJ, Choi HJ, Ahn J, Lee TY, Lee SC, Kim SJ. Ameliorating liver fibrosis in an animal model using the secretome released from miR-122-transfected adipose-derived stem cells. World J Stem Cells 2019; 11(11): 990-1004
- URL: https://www.wjgnet.com/1948-0210/full/v11/i11/990.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i11.990
Stem cell research is one of the promising areas of biomedical research. However, notwithstanding remarkable achievements in the field of mesenchymal stem cells (MSCs), their clinical applications are still challenging, especially due to safety concerns. To date, increasing evidence has been accumulating in support of the notion that the principal action mechanism of MSCs is secretome-mediated[1-5]. Thus, to overcome the limitations of cell-based therapy, numerous researchers have focused on the exclusive use of MSC-secreted molecules rather than the cells per se. The total set of molecules secreted or surface-shed by cells is generally referred to as secretome. The secretome includes bioactive peptides, such as cytokines, chemokines, and growth factors[1,4]. These soluble factors are released from MSCs either alone or in the form of extracellular vesicles.
The therapeutic potential of secretome can be potentiated by adjusting the conditions under which MSCs are incubated. Among these conditions, the genetic modification of MSCs can offer enormous and persistent reinforcements of the MSC secretome. Literature supports that microRNAs (miRNAs) play a substantial role in the process of liver fibrosis[6-8]. MicroRNAs are small non-coding RNA molecules (containing about 22 nucleotides) that alter gene expression at the posttranscriptional level, resulting in altered protein synthesis[9]. Hence, miRNAs can exquisitely adjust the expression of numerous genes particularly responsible for fundamental cellular processes, such as proliferation, development, and differentiation[10]. The miRNAs responsible for liver fibrosis can largely be divided into fibrotic and antifibrotic miRNAs. Of these, miR-122 is one of the representative antifibrotic miRNAs that negatively regulates collagen production in hepatic stellate cells (HSCs)[11,12]. Thus, harnessing MSCs to confer miR-122 to HSCs would be a potential novel therapeutic approach for reinforcing the antifibrotic effects of MSCs. In this study, we aimed to the determine the antifibrotic effects of the secretome released from miR-122-transfected ASCs in both in vitro and in vivo models of liver fibrosis.
Human adipose-derived stromal cells (ASCs) were obtained from lipoaspirated fat with inform consent of the volunteers. This research was approved by Institutional Review Board (IRB number 700069-201407-BR-002-01) of Hurim BioCell Co. Ltd. (Seoul, South Korea). ASCs were isolated and cultured according to previous reports[13]. Lipoaspirated fat was digested by 0.1% collagenase (Sigma-Aldrich, St. Louis, MO, United States) in saline and collected after centrifugation. Cells were plated into culture flask in low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific, Hemel Hempstead, United Kingdom) supplemented with 10% FBS (Thermo Fisher Scientific), 100 U/mL of penicillin (Thermo Fisher Scientific), and 0.1 mg/mL of streptomycin (Thermo Fisher Scientific). ASCs were incubated at 37 °C in humidified chamber containing 5% carbon dioxide and medium was changed every 3 d.
ASCs were transfected with miR-122 (Exiqon, Germatown, MD) per well mixed with the Lipofectamine RNAiMAX Reagent (Thermo). After 72hr of transfection, the cells were morphologically observed by the inverted microscope. The cell numbers of the experimental groups were counted automatic cell counter (Countess®, Invitrogen, San Diego, CA, United States) using trypan blue solution. Transfected cells were processed for cell phenotyping or differentiated into three-lineage induction.
ASCs with or without miR-122 transfection were grown in a 100 mm cell dishes (Corning Glass Works, Corning, NY, United States). After reaching 70%-80% confluence, 1.0 × 106 ASCs were cultured in 5 mL serum-free low-glucose DMEM for 48 h. Therefore, to obtain 0.2 mL amount of secretome from 1.0 × 106 ASCs, the conditioned media were concentrated 25-fold using ultra filtration units with a 3-kDa molecular weight cutoff (Amicon Ultra-PL 3; Millipore, Bedford, MA, United States). We then injected 0.1 mL amount of secretome per mouse. This means that one mouse is injected with the secretome obtained from 5 × 105 ASCs. In this study, NCM refers to the secretome shed from ASCs after 48 h of incubation, and MCM refers to the secretome shed from miR-122-transfected ASCs after 48 h of incubation.
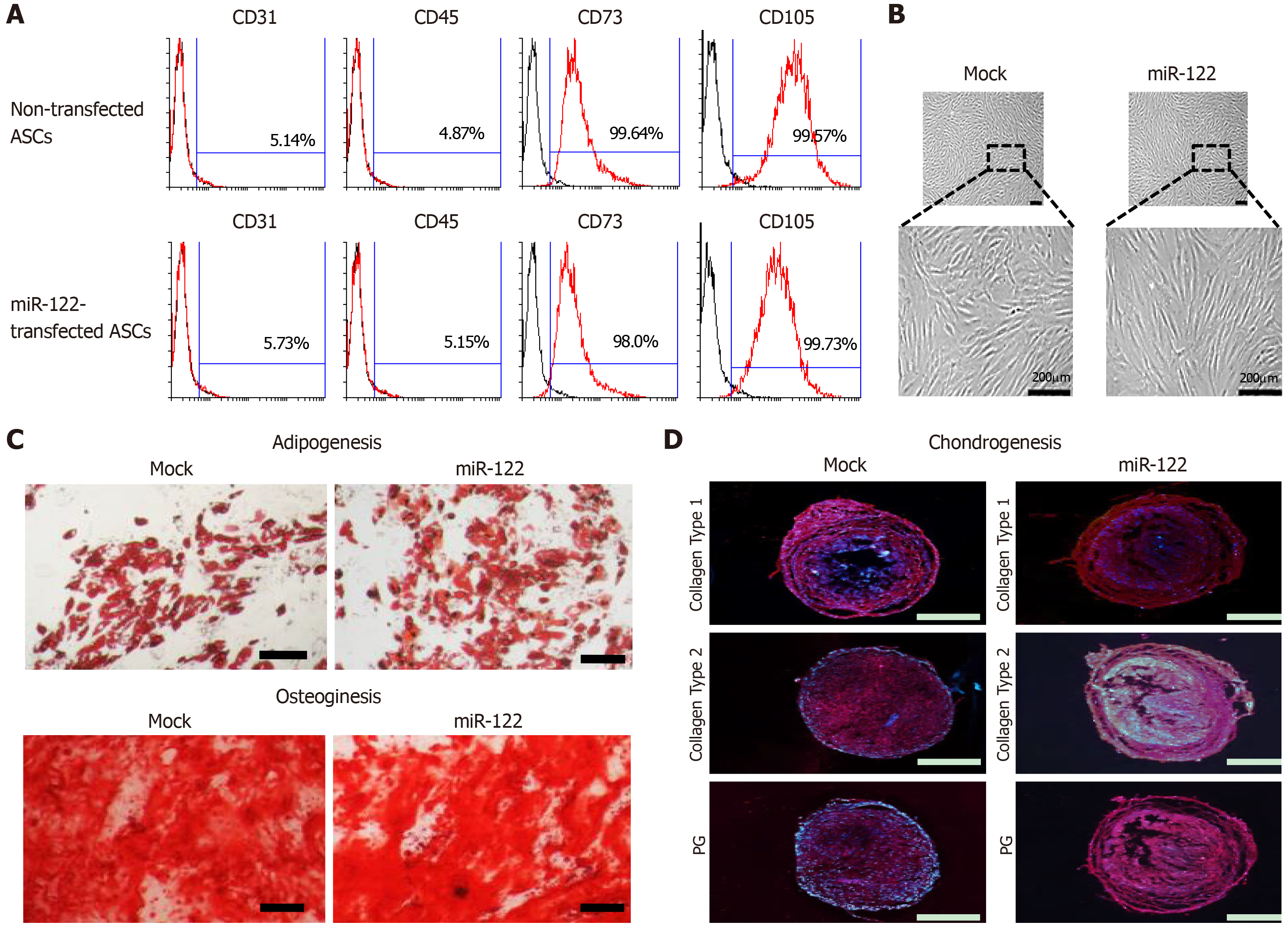
The immunophnotypes of the experimental groups were determined by flow cytometry analysis (Cytomics FC500 flow cytometer, Beckman Coulter, Fullerton, CA, United States) using FITC-conjugated CD31, CD45, and CD73 antibodies and PE-conjugated CD90 and CD105 antibodies (BD Pharmingen, San Jose, CA, United States). Isotype controls were performed with antibodies against IgG for samples.
Transfected cells were induced toward the three lineages for 21 d. The adipogenic, osteogenic and chondrogenic differentiation ability of MSCs was determined as previously described[14,15]. Briefly, the cells were plated at a density of 1 × 104 or 5 × 103 cells/cm2 in growth medium for 3 d, and then cultured in adipocyte and osteocyte differentiation medium (StemProTM, Gibco) for 3 wk. For chondrogenic induction, expansion medium containing 8 × 104 cells was cultured for 2 h. Then, chondrogenesis differentiation medium (StemProTM, Gibco) was added and cultured for 3 wk. After differentiation, Lipid vesicles and calcium deposition were observed by oil Red O and Alizarin Red staining. For chondrogenic induction, micromass cultures were plated by seeding 5 μL droplets of 8 × 104 cells into the center of 48-well plate. After incubating micromass cultures for 2 h at 37 °C, chondrogenic medium (StemPro, GIBCO) was added to 400 μL per culture wells and cultured for 3 wk. Chondrocyte induction was determined by immunohistochemical staining for collagen type I and II and proteoglycan[16]. Primary antibodies were purchased from Millipore (Millipore, CA, United States) and reacted with sections. After incubation with primary antibodies, sections were incubated with PE-conjugated goat anti-rabbit immunoglobulin G (Abcam, Cambridge, MA, United Kingdom) and rabbit anti-mouse immunoglobulin G (Abcam). Nuclei were counterstained with DAPI (4’,6-diamidino-2-phenylindole, Invitrogen).
The LX-2 human HSCs were obtained from were kindly donated by Dr. Won-il Jeong in KAIST Biomedical research of Korea. LX-2 cells were maintained in DMEM (Thermo, Carlsbad, CA, United States). The medium was supplemented with 10% FBS (GibcoBRL, Calsbad, CA, United States), 1% antibiotics (Thermo), at 37 °C.
LX-2 cells and liver specimens obtained from mice were lysed using the EzRIPA Lysis kit (ATTO Corporation; Tokyo, Japan), and quantified by Bradford reagent (Bio-RadHercules, CA, United States). Proteins were visualized by western analysis using the following primary antibodies (1:1000 dilution) at 4 °C overnight and then with HRP-conjugated secondary antibodies (1:2000 dilution) for 1 h at 25°C. From Cell Signaling Technology (Beverly, MA, United States), we obtained primary antibodies against Proliferating cell nuclear antigen (PCNA), transforming growth factor-β (TGF-β1), alpha-smooth muscle actin (α-SMA), metallopeptidase inhibitor 1 (TIMP-1), matrix metallopeptidase 2 (MMP2), collagen type- 1 alpha-1 (COL1A1), β-actin, and horseradish peroxidase (HRP)-conjugated secondary antibody. Specific immune complexes were detected using the Western Blotting Plus Chemiluminescence Reagent (Millipore, Bedford, MA, United States).
Five-week male BALB/c mice (Orient Bio, Seongnam, Korea) were used in this study. Animal studies were carried out in compliance with the guidelines of the Institute for Laboratory Animal Research, Korea (IRB No: CUMC-2017-0317-04). We then compared the effects of the MCM in an in vivo model of Thioacetamide (TAA)-induced hepatic fibrosis model. The in vivo model was generated by subcutaneous injection of TAA (200 mg/kg, three times a week for 8 wk) into experimental mice. Each group included 10 mice, and these were further divided into two subgroups: those for Control mice (n = 30), and those for TAA-treated mice (n = 30). Subsequently, control mice and TAA-treated mice were intravenously (using tail vein) infused with normal saline, CM, and MCM, respectively.
Blood samples were collected from each mouse, centrifuged for 10 min at 9500 g, and serum was collected. We measured the concentrations of markers for liver injury and kidney injury, such as aspartate transaminase (AST), alanine transaminase (ALT), and creatine, using an IDEXX VetTest Chemistry Analyzer (IDEXX Laboratories, Inc., Westbrook, ME, United States). The concentrations of mouse interleukin (IL)-6 and tumor necrosis factor (TNF)-α were measured by sandwich enzyme-linked immunosorbent assay (ELISA kits, Biolegend, San Diego, CA, United States) according to the manufacturer’s instructions.
For immunohistochemical analysis, formalin-fixed, paraffin-embedded tissue sections were deparaffinized, rehydrated in an ethanol series and subjected to epitope retrieval using standard procedures. Antibodies against of PCNA, TIMP-1, Albumin, α-SMA, TGF-β1, MMP-2, SOD, Catalase and GPx (all from Cell Signaling Technology, MA, United States) were used for immunochemical staining. The samples were then examined under a laser-scanning microscope (Eclipse TE300; Nikon, Tokyo, Japan) to analyze the expression of PCNA, TIMP-1, Albumin, a-SMA, TGF-β1, MMP-2, SOD, Catalase and GPx. Sirius red staining and Trichrome staining were performed using the Sirius red staining kit and Masson’s trichrome staining kit according to the manufacturer’s protocol (Polysciences, Warrington, PA, United Kingdom).
All data were analyzed with SPSS 11.0 software (SPSS Inc., Chicago, IL, United States) and SigmaPlot® ver. 12.0 (Systat Software Inc., Chicago, IL, United States). The data are presented as mean ± standard deviation (SD). Statistical comparison among groups was determined using Kruskal–Wallis test followed by Dunnett’s test as the post hoc analysis. Probability values of P < 0.05 were regarded as statistically significant.
We first determined whether miR-122 transfection impairs ASC functionality, especially their multilineage differentiation potential. Flow cytometric analysis showed that miR-122 transfection did not alter the expression of surface markers of ASCs (Figure 1A). Gross cell morphology was also identical regardless of miR-122 transfection (Figure 1B). In addition, transfecting miRNA did not affect multilineage differentiation potential of ASCs, including the potentials of differentiating adipocytic (Figure 1B) or osteogenic (Figure 1C) lineages, and the expression of collagens (type I and type II) and proteoglycan (Figure 1D).
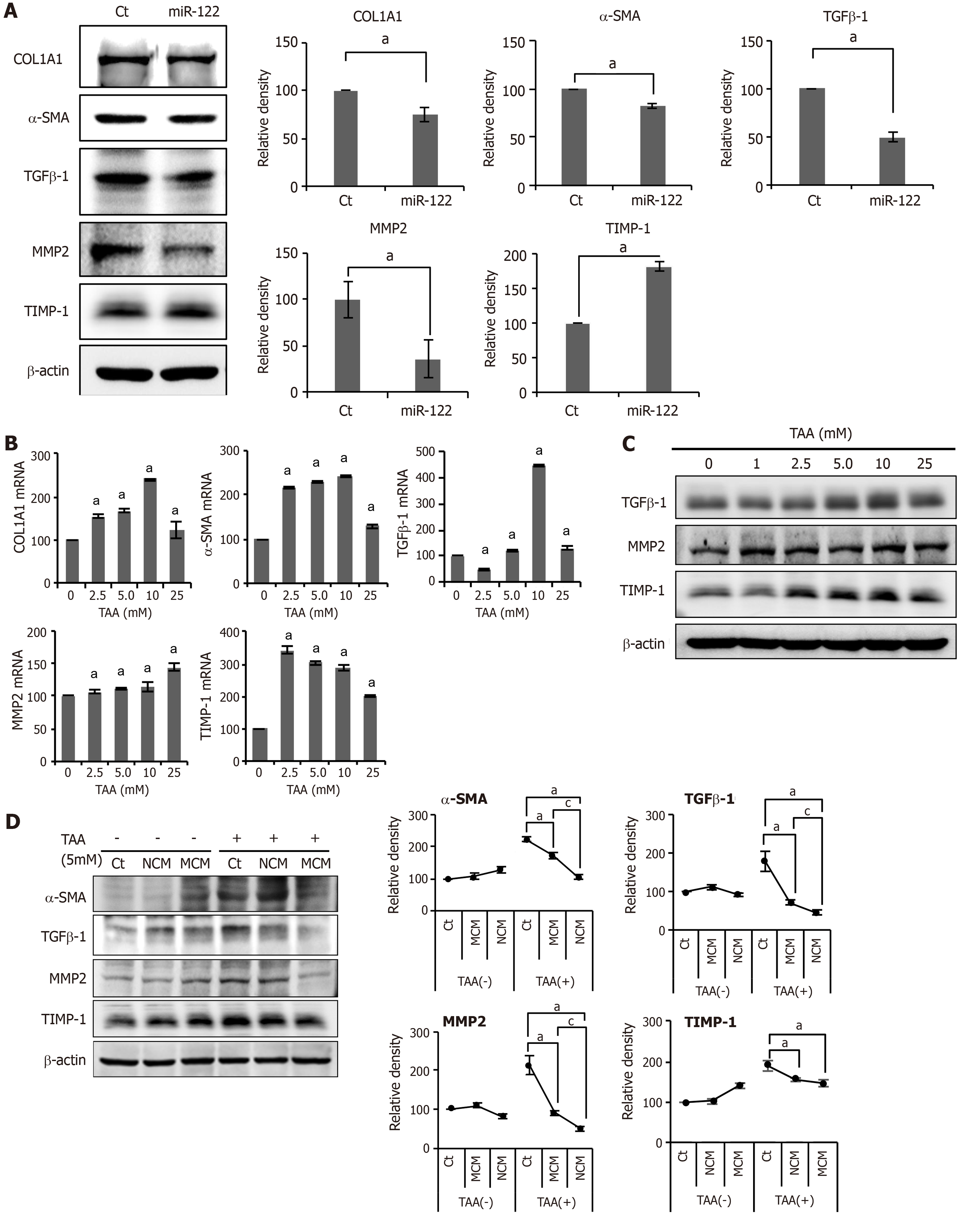
We investigated the expression of fibrosis-related markers in miR-122-transfected ASCs. miR-122-transfected ASCs showed a decreased expression of fibrosis-related proteins (TGF β1, MMP2, α-SMA, and TIMP) compared to control ASCs or ASCs transfected with miR-122 (Figure 2A). We obtained human HSCs (LX2 cells) and treated them with a varying concentration of TAA for determining in vitro model of liver fibrosis. TAA elicited a concentration-dependent increase of fibrosis markers to a certain extent, and we determined that 5.0 mmol TAA is appropriate for inducing fibrosis in LX2 cells (Figure 2B and C).
Next, we obtained the secretome from the CM of ASCs as described in the method. In this study, NCM refers to the secretome shed from ASCs after 48 h of incubation, and MCM refers to the secretome shed from miR-122-transfected ASCs after 48 h of incubation. The in vitro model of liver fibrosis was generated by treating human HSCs cells (LX2 cells) with a hepatotoxin (TAA). We then treated the TAA-treated LX2 cells with NCM or MCM, and investigated the expression of fibrosis-related markers using western blot analysis (Figure 2D). Overall, the addition of each secretome (NCM or MCM) to TAA-treated LX2 cells significantly decreased the expression of fibrotic markers (MMP2, TGF-β1, and α-SMA) (P < 0.05). When comparing the two kinds of secretome, MCM induced the more significant reduction of fibrotic markers than did NCM (P < 0.05).
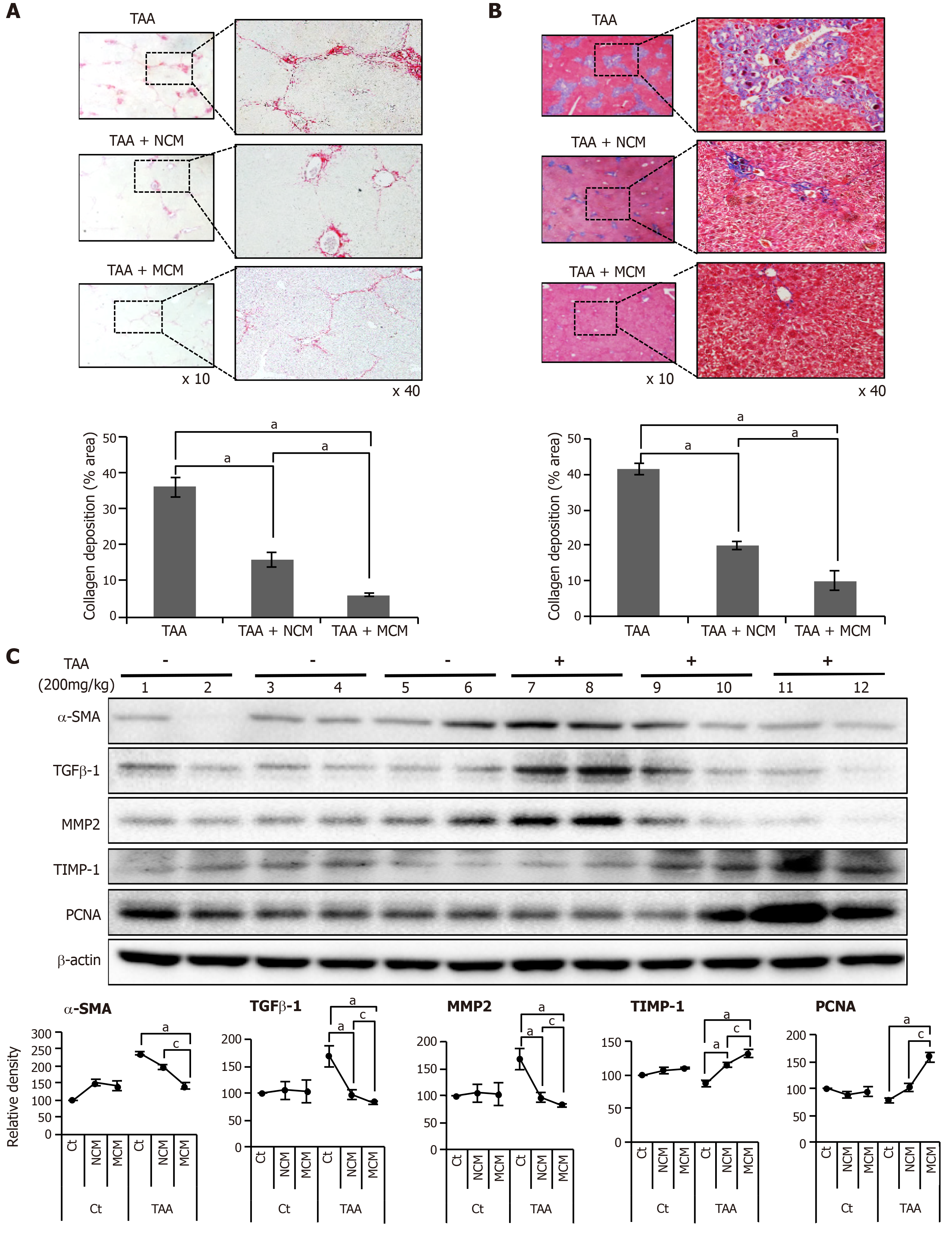
We generated an in vivo model of liver fibrosis in mouse by subcutaneous injection of TAA (200 mg/kg) three times a week for 5 wk and validated the effects of MCM in this model. The mice were divided into two groups: control (n = 30) and TAA-treated mice (n = 30), and the latter were intravenously infused normal saline (n = 10), NCM (n = 10), or MCM (n = 10) twice (200 mg/kg, three times a week for 8 wk). On the 7th d after infusion, the mice were euthanized and specimens were obtained for study. Sirius red and Masson trichrome stains were used for the estimation of fibrosis. These stains showed that, although both treatments (NCM and MCM) decreased the content of collagen, MCM significantly had the greatest effect (Figure 3A and B). In the western blot analysis of the liver specimens, MCM infusion significantly increased the expression of PCNA (a proliferation marker), and significantly decreased the expression of α-SMA, TGF-β1, and MMP1 (fibrotic markers) and increased an antifibrotic marker (TIMP-1) in the TAA-treated mice (Figure 3C).
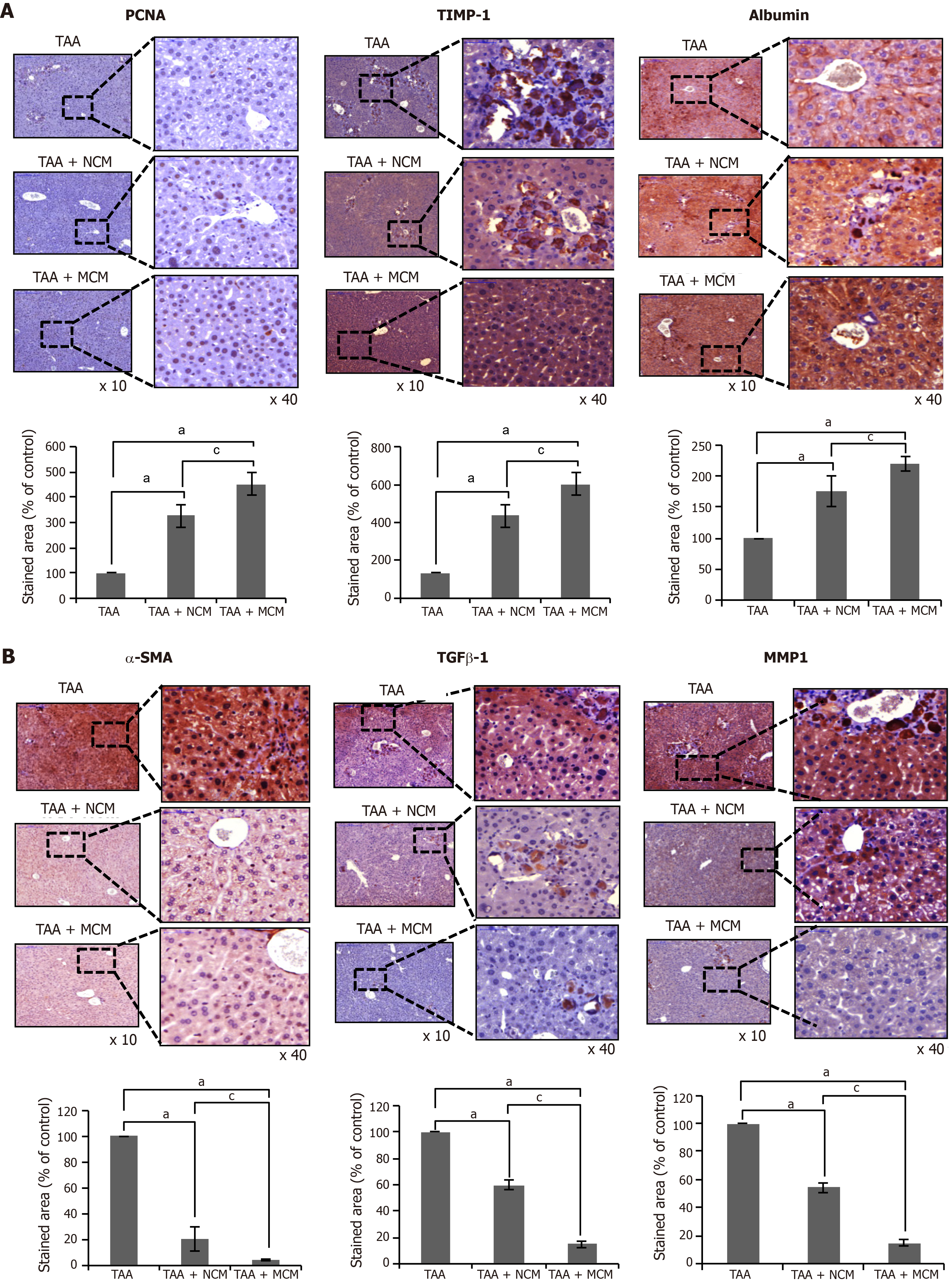
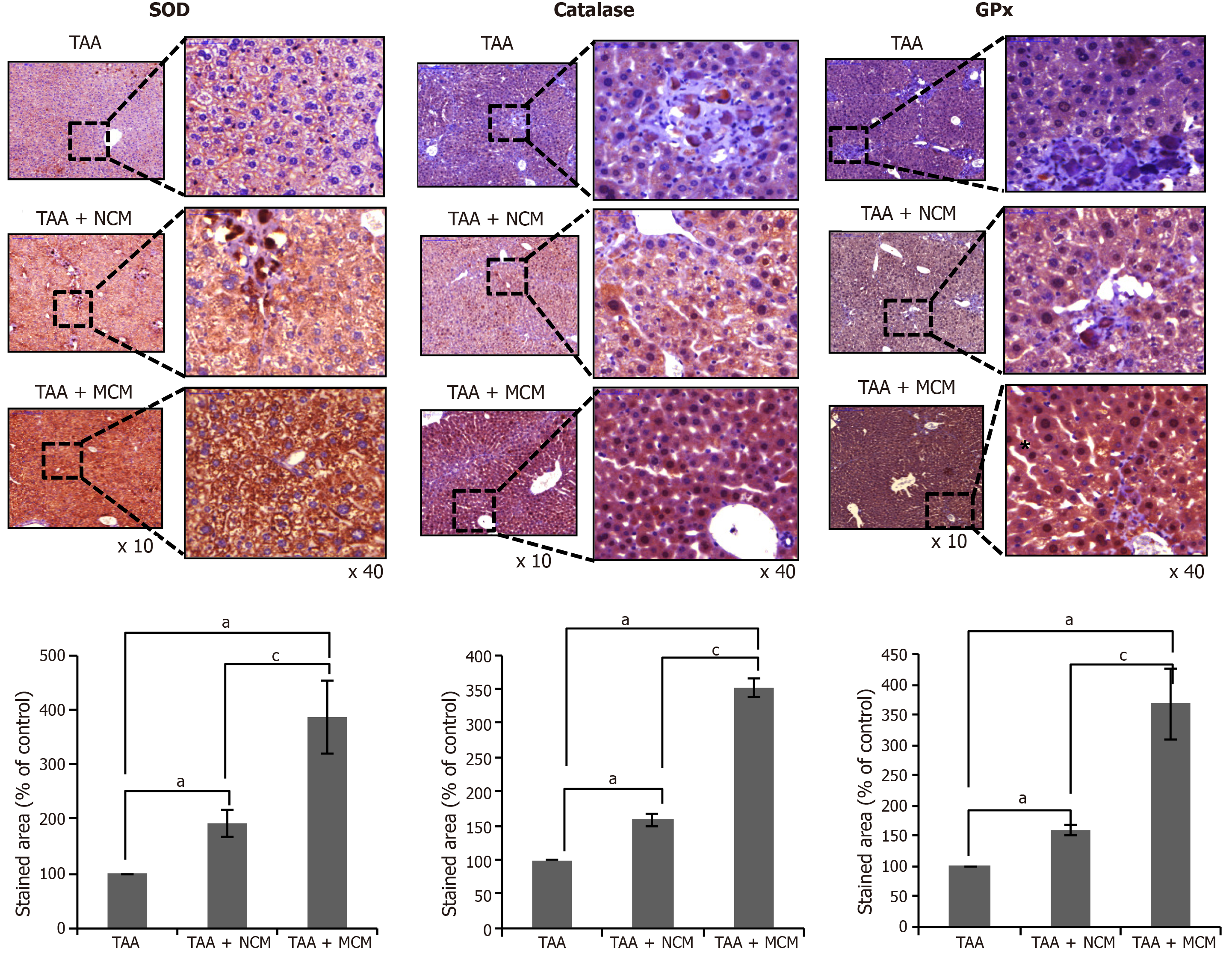
We compared the histological changes of the livers obtained from each mouse group. PCNA was used as the marker for hepatocyte proliferation; α-SMA, TGF-β1, and MMP1 for liver fibrosis; albumin for hepatic synthetic function; TIMP-1 for liver antifibrosis; and SOD, catalase, and GPx for liver antioxidant activity. Through immunohistochemical staining, the MCM group showed the highest expression of PCNA, albumin, and TIMP-1, and the lowest expression of α-SMA, TGF-β1, and MMP1 (Figure 4A and B). The MCM group also showed the highest expression of SOD, catalase, and GPx (Figure 5).
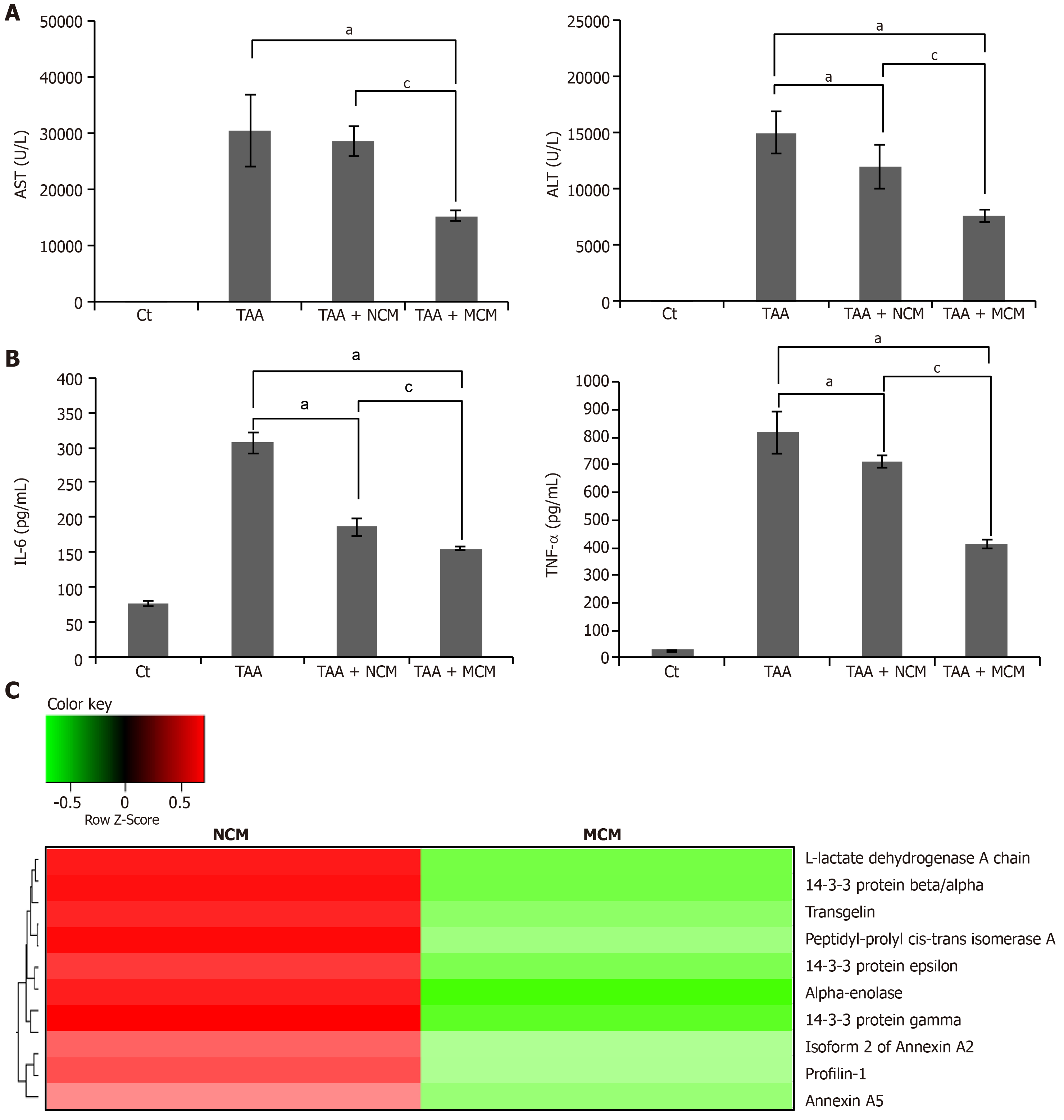
We compared the expression of systemic inflammatory markers, such as IL-6 and TNF-α, in the serum of each mouse group. Secretome infusions (NCM and MCM) significantly decreased the expression of these markers, and MCM decreased their expression in a higher degree than NCM (P < 0.05) (Figure 6A). Finally, we compared the serum levels of liver enzymes (AST and ALT) in each mouse group. Secretome infusions significantly decreased the elevated levels of liver enzymes, and MCM had a higher effect than NCM (P < 0.05) (Figure 6B).
Using liquid chromatography–mass spectrometry (LC/MS), we analyzed and compared the protein contents of NCM and MCM (Figure 6C). The protein constituents and concentrations of various important proteins varied widely between NCM and MCM, validating the effects of miR-125 transfection. Specifically, MCM exhibited a significantly decreased concentration of essential intermediates of the TGF-β/Smad signaling, such as transgelin, PIN1, and profilin-1, compared to NCM.
In this study, we have shown that the secretome released from miR-122 transfected ASCs was superior to the naïve secretome in improving liver fibrosis while minimizing inflammatory processes in mice with TAA-induced liver fibrosis. Specifically, infusion of the secretome from miR-122-transfected ASCs provided higher therapeutic potential in terms of: (A) Reducing collagen content in the liver; (B) Inhibiting proinflammatory cytokines; and (C) Reducing abnormally elevated liver enzymes than infusion of the naïve secretome. Thus, it can be postulated that miR-122 transfection into ASCs reconditions them to have higher antifibrotic properties and to release a secretome with higher antifibrotic components. In reality, our proteomic analysis of the secretome released from miR-122-transfected ASCs indicated that it had significantly lesser contents of essential intermediates of liver fibrosis compared to the naïve secretome. We could, thus, conclude that the secretome released from miR-122-transfected ASCs has higher antifibrotic and anti-inflammatory properties than the naïve secretome.
Accumulating evidence indicates that various miRNAs are essentially involved in the process of fibrosis, particularly related with the action of HSCs[17]. Fibrogenic injury of the liver prompts HSCs to undergo proliferation, migrate to injured sites, and transform into myofibroblast-like cells which apparently lose their lipid droplets[18-20]. Subsequently, the activated HSCs, named fibroblast-like cells, produce large amounts of extracellular matrix proteins, such as collagen I and II, finally leading to liver fibrosis[19,21-24]. Of various cytokines, TGF-β plays essential roles in the process of liver fibrosis[25-28].
A number of miRNAs are involved in the processes of liver fibrosis, by either promoting or preventing it. For instance, profibrotic miRNAs include miR-29b, miR-571, miR-199a, miR-200a, and miR-200b, and antifibrotic miRNAs include miR-122, miR-199, miR-200, miR-542, miR-652, and imR-181b[29-32]. Specifically, miR-29b exerts its antifibrotic properties by inhibiting activation of HSCs[31]. Increased serum level of miR-571 has been proposed as a potential biomarker of liver fibrosis, and serum levels of miR-542, miR-652, and imR-181b are decreased in cirrhosis. In addition, serum levels of miR-199a, miR-200a, and miR-200b were highly associated with progression of liver fibrosis in patients with chronic HCV infection[29].
miR-122 is highly expressed in liver, accounting for about 70% and 52% of total miRNAs in liver of adult mouse and human, respectively[33-35]. miR-122 is essentially involved in liver development, differentiation, homeostasis, and functions. Initially, investigators revealed the crucial role of miR-122 in the regulation of cholesterol and fatty acid metabolism in the adult liver[36-38]. Thereafter, anti-inflammatory and anti-fibrotic properties of miR-122 have been revealed by the generation of both germline knock-out (KO) mice and liver-specific KO[39-41]. Specifically, genetic deletion of miR-122 led to liver microsteatosis and inflammation, ultimately resulting in steatohepatitis and fibrosis[38,39]. Additionally, miR-122 expression was reduced in a carbon tetrachloride-induced liver fibrosis mouse model[11]. Interestingly, the restoration of miR-122 levels in miR-122 KO mice reversed the process of liver inflammation, by repressing two miR-122 targets, the chemokine Ccl2[39] and the pro-fibrogenic Krüppel-like factor 6 (KLF6)[40], demonstrating potential utility of miR-122 in therapeutics. We, thus, selected the delivery of miR-122 into ASCs as a mean of reinforcing the antifibrotic properties of ASCs in this study.
We have also shown that the expression of antioxidant enzymes in the liver specimens was significantly increased in the mice infused with the secretome released from miR-122 transfected ASCs compared with the mice infused with the naïve secretome. Although a variety of functional capacities of MSCs or their secretome have been reported, the protective effects against oxidative stress have rarely been reported. Kim et al[42] reported that incubation with secretomes derived from ASCs aided human dental fibroblast cells to resist free radicals, and increased antioxidant enzymes, such as SOD and glutathione peroxidase. Recently, Arslan et al[43] showed that MSC-derived exosome treatment decreased oxidative stress in the mouse model of ischemia/reperfusion.
It has been demonstrated that oxidative stress involves in both onset and progression of fibrosis arising from a variety origin, such as alcohol, viruses, iron or copper overload, or cholestasis[44]. Both expression and synthesis of this inflammatory and profibrogenic cytokines are mainly modulated through redox-sensitive reactions[45,46]. Further, redox-sensitive reactions also involve in other essential processes of liver fibrosis, such as activation of HSCs and expression of metalloproteinases and of their specific inhibitors[47-49]. We thus think that reduction of oxidative stress could be another way of antifibrotic mechanisms exerted by the secretome released from miR-122 transfected ASCs.
Here, we have focused on the effects of secretome and not those of the stem cells, on liver fibrosis. The term secretome was first mentioned by Black et al[50] to refer to all the factors secreted by a cell, along with the secretory pathway constituents. The main constituents of a secretome include secretory proteins and extracellular vesicles. The secreted proteins in humans account for 13%-20% of the entire proteome and include growth factors, cytokines, chemokines, adhesion molecules, proteases, and shed receptors[51]. Extracellular vesicles are typically 30-2000 nm in diameter and can be subdivided into exosomes, microvesicles, and apoptotic bodies, according to their size. Extracellular vesicles usually contain and, thus, carry non-protein components, such as lipids, DNAs, micro-RNAs, and mRNAs. In this study, we focused on the effects of the whole secretome, not its individual constituents, such as exosomes. Exosomes, for example, can be obtained by protracted, complex, and expansive processes[52]. We expect that our results will help eliminate the laborious and expensive process of obtaining exosomes.
The concept of using miRNAs for enhancing the therapeutic potential of the secretome released from stem cells is quite different from how they have been used before. Previously used methods for potentiating secretome, which include physical and chemical stimulation methods, such as hypoxic preconditioning[53,54] or the use of lipopolysaccharides[55], can be categorized as nonspecific stimulation. By contrast, the concept of using miRNAs can be categorized as liver-specific stimulation. In the future, the clinical application of secretome is expected to be tailored according to the needs of patients, combining nonspecific and specific stimulations.
In conclusion, we have shown that the secretome released from miR-122-transfected ASCs was superior to the naïve secretome in improving liver fibrosis, while minimizing inflammatory processes, in mice with TAA-induced liver fibrosis. Hence, it can be postulated that miR-122 transfection into ASCs reconditioned them to have higher antifibrotic properties and to release a secretome with higher antifibrotic components. We could, thus, conclude that the secretome released from miR-122 transfected ASCs has higher antifibrotic and anti-inflammatory properties than the naïve secretome. Because miR-122 transfection into ASCs provides a specific way of potentiating the antifibrotic properties of the ASC secretome, it could be considered as an enhanced method of reinforcing secretome effectiveness.
The therapeutic potential of mesenchymal stem cells (MSCs) is known to be mediated mainly by the secretome that refers to the total collection of secretory materials from MSCs. Basically, naïve secretome has anti-inflammatory, immunomodulatory, and tissue reparative properties. To increase the amount or to reinforce the potential of naïve secretome, researchers have attempted to adjust physico-chemical environment of MSCs or genetically manipulate MSCs. The former has the advantage of being simple but lacking persistence, while the latter has a strong persistence but has the disadvantage of a safety concern in the clinical application.
We have been considering genetic modification as a way of persistently potentiating the therapeutic potential of naïve secretome. In addition, contrasted by the use of genetically modified MSCs, we thought that the use of the secretome could significantly lower the safety concern. We also noted miRNAs as the materials to be used for genetic manipulation, because miRNA is critically involved in the process of liver fibrosis.
Our aim was to determine the antifibrotic potential of the secretome released from miR-122-transfected adipose-derived stromal cells (ASCs) in the model of liver fibrosis.
Secretory materials released from ASCs that had been transfected with antifibrotic miR-122 were collected and termed as miR122-secretome. The in vitro model of liver fibrosis was generated by treating human hepatic stellate cells (LX2 cells) with a hepatotoxin (thioacetamide; TAA), and the in vivo model of liver fibrosis was generated by subcutaneous injection of TAA (200 mg/kg, three times a week for 8 wk) into five-week male BALB/c mice. For determining in vivo effects of miR122-secretome, each secretome (miR122-secrectome and naïve secretome) was intravenously administered to the mice with liver fibrosis, respectively. The degree of liver fibrosis and other alternations in cells or tissues were determined using by molecular and histological investigations, including cell viability assay, western blotting, immunohistochemistry, serology tests, and sandwich enzyme-linked immunosorbent assays.
The addition of miR-122-secretome to fibrosis-induced LX2 cells significantly decreased the expression of fibrotic markers (MMP2, TGF-β1, TIMP-1, and α-SMA) and increased the expression of an antifibrotic marker (TIMP-1). The western blot analysis showed that miR122-secretome infusion significantly increased the expression of PCNA (a proliferation marker), significantly decreased the expression of α-SMA, TGF-β1, and MMP1 (fibrotic markers), and increased an antifibrotic marker (TIMP-1) in the livers of TAA-treated mice. In addition, miR122-secretome infusion significantly reduced collagen content in the livers, inhibited serum levels of proinflammatory cytokines, such as IL-6 and TNF-α, as well as serum levels of liver enzymes than infusion of the naïve secretome. Finally, our analysis of the components of miR-122-secretome showed that miR-122-secretome exhibited a significantly decreased concentration of essential intermediates of the TGF-β/Smad signaling, such as transgelin, PIN1, and profilin-1, compared to NCM.
miR-122-secretome was found to be superior to the naïve secretome in improving liver fibrosis while minimizing inflammatory processes in mice with TAA-induced liver fibrosis. Our proteomic analysis of the miR-122-secretome also validated that miR-122-secretome had significantly lesser contents of essential intermediates of liver fibrosis. Therefore, transfecting miR-122 into ASCs is worth considering as a way of reinforcing antifibrotic properties of the secretome from ASCs.
We thank Byung-Rok Do and Ji-Hyang Kim in Hurim BioCell Company for providing human ASCs and technical assistance. We would like to thank the Francis Sahngun Nahm (a professional statistician) for his devoted assistance of statistical analysis. We would like to thank Hye-Jung Kim for photoshop works that improved the figure quality. We also would like to thank Ji-Hye Park for data processing and statistical analysis. We would like to thank Drug and Disease Target Team (Ochang), Korea Basic Science Institute.
| 1. | Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 410] [Article Influence: 29.3] [Reference Citation Analysis (1)] |
| 2. | Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1645] [Cited by in RCA: 1568] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 3. | Hoch AI, Binder BY, Genetos DC, Leach JK. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7:e35579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 658] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 5. | Yang Z, Di Santo S, Kalka C. Current developments in the use of stem cell for therapeutic neovascularisation: is the future therapy "cell-free"? Swiss Med Wkly. 2010;140:w13130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Jiang XP, Ai WB, Wan LY, Zhang YQ, Wu JF. The roles of microRNA families in hepatic fibrosis. Cell Biosci. 2017;7:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Kitano M, Bloomston PM. Hepatic Stellate Cells and microRNAs in Pathogenesis of Liver Fibrosis. J Clin Med. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | O'Reilly S. MicroRNAs in fibrosis: opportunities and challenges. Arthritis Res Ther. 2016;18:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 9. | Chen SL, Zheng MH, Shi KQ, Yang T, Chen YP. A new strategy for treatment of liver fibrosis: letting MicroRNAs do the job. BioDrugs. 2013;27:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Hu J, Xu Y, Hao J, Wang S, Li C, Meng S. MiR-122 in hepatic function and liver diseases. Protein Cell. 2012;3:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Li J, Ghazwani M, Zhang Y, Lu J, Li J, Fan J, Gandhi CR, Li S. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J Hepatol. 2013;58:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Zeng C, Wang YL, Xie C, Sang Y, Li TJ, Zhang M, Wang R, Zhang Q, Zheng L, Zhuang SM. Identification of a novel TGF-β-miR-122-fibronectin 1/serum response factor signaling cascade and its implication in hepatic fibrogenesis. Oncotarget. 2015;6:12224-12233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5054] [Article Influence: 210.6] [Reference Citation Analysis (0)] |
| 14. | An SY, Han J, Lim HJ, Park SY, Kim JH, Do BR, Kim JH. Valproic acid promotes differentiation of hepatocyte-like cells from whole human umbilical cord-derived mesenchymal stem cells. Tissue Cell. 2014;46:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Swioklo S, Constantinescu A, Connon CJ. Alginate-Encapsulation for the Improved Hypothermic Preservation of Human Adipose-Derived Stem Cells. Stem Cells Transl Med. 2016;5:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Voga M, Drnovsek N, Novak S, Majdic G. Silk fibroin induces chondrogenic differentiation of canine adipose-derived multipotent mesenchymal stromal cells/mesenchymal stem cells. J Tissue Eng. 2019;10:2041731419835056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 18. | Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10:927-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 327] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 19. | Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728-1734. [PubMed] |
| 20. | Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Henderson NC, Iredale JP. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci (Lond). 2007;112:265-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Friedman SL. Stellate cell activation in alcoholic fibrosis--an overview. Alcohol Clin Exp Res. 1999;23:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2191] [Article Influence: 121.7] [Reference Citation Analysis (1)] |
| 24. | Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997;430:195-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 217] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, Schirmacher P, Biesterfeld S, Galle PR, Lohse AW, Kanzler S. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 611] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 27. | Liu Y, Wang Z, Kwong SQ, Lui ELH, Friedman SL, Li FR, Lam RWC, Zhang GC, Zhang H, Ye T. Inhibition of PDGF, TGF-β, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011;55:612-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Ogawa S, Ochi T, Shimada H, Inagaki K, Fujita I, Nii A, Moffat MA, Katragadda M, Violand BN, Arch RH, Masferrer JL. Anti-PDGF-B monoclonal antibody reduces liver fibrosis development. Hepatol Res. 2010;40:1128-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Murakami Y, Toyoda H, Tanaka M, Kuroda M, Harada Y, Matsuda F, Tajima A, Kosaka N, Ochiya T, Shimotohno K. The progression of liver fibrosis is related with overexpression of the miR-199 and 200 families. PLoS One. 2011;6:e16081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 30. | Roderburg C, Mollnow T, Bongaerts B, Elfimova N, Vargas Cardenas D, Berger K, Zimmermann H, Koch A, Vucur M, Luedde M, Hellerbrand C, Odenthal M, Trautwein C, Tacke F, Luedde T. Micro-RNA profiling in human serum reveals compartment-specific roles of miR-571 and miR-652 in liver cirrhosis. PLoS One. 2012;7:e32999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun. 2011;412:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Wang B, Li W, Guo K, Xiao Y, Wang Y, Fan J. miR-181b promotes hepatic stellate cells proliferation by targeting p27 and is elevated in the serum of cirrhosis patients. Biochem Biophys Res Commun. 2012;421:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Girard M, Jacquemin E, Munnich A, Lyonnet S, Henrion-Caude A. miR-122, a paradigm for the role of microRNAs in the liver. J Hepatol. 2008;48:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 300] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 34. | Hou W, Tian Q, Zheng J, Bonkovsky HL. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatology. 2010;51:1494-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2628] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 36. | Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjärn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1292] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 37. | Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1650] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 38. | Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3005] [Cited by in RCA: 3100] [Article Influence: 147.6] [Reference Citation Analysis (0)] |
| 39. | Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, Mao H, Wei M, Clark KR, Mendell JR, Caligiuri MA, Jacob ST, Mendell JT, Ghoshal K. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 636] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 40. | Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, Hsu MT, Wu JC, Huang HD, Shiao MS, Hsiao M, Tsou AP. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122:2884-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 677] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 41. | Zeisel MB, Pfeffer S, Baumert TF. miR-122 acts as a tumor suppressor in hepatocarcinogenesis in vivo. J Hepatol. 2013;58:821-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008;49:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 43. | Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 882] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 44. | Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 458] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 45. | Ahamed J, Laurence J. Role of Platelet-Derived Transforming Growth Factor-β1 and Reactive Oxygen Species in Radiation-Induced Organ Fibrosis. Antioxid Redox Signal. 2017;27:977-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Gonzalez-Gonzalez FJ, Chandel NS, Jain M, Budinger GRS. Reactive oxygen species as signaling molecules in the development of lung fibrosis. Transl Res. 2017;190:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 47. | Lee AY, Jang Y, Hong SH, Chang SH, Park S, Kim S, Kang KS, Kim JE, Cho MH. Ephedrine-induced mitophagy via oxidative stress in human hepatic stellate cells. J Toxicol Sci. 2017;42:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 2195] [Article Influence: 243.9] [Reference Citation Analysis (0)] |
| 49. | Zhang F, Jin H, Wu L, Shao J, Zhu X, Chen A, Zheng S. Diallyl Trisulfide Suppresses Oxidative Stress-Induced Activation of Hepatic Stellate Cells through Production of Hydrogen Sulfide. Oxid Med Cell Longev. 2017;2017:1406726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 365] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 51. | Agrawal GK, Jwa NS, Lebrun MH, Job D, Rakwal R. Plant secretome: unlocking secrets of the secreted proteins. Proteomics. 2010;10:799-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 52. | Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS One. 2017;12:e0170628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 498] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 53. | Lee SC, Jeong HJ, Lee SK, Kim SJ. Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling. Stem Cells Transl Med. 2016;5:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | Lee SC, Kim KH, Kim OH, Lee SK, Hong HE, Won SS, Jeon SJ, Choi BJ, Jeong W, Kim SJ. Determination of optimized oxygen partial pressure to maximize the liver regenerative potential of the secretome obtained from adipose-derived stem cells. Stem Cell Res Ther. 2017;8:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Lee SC, Jeong HJ, Lee SK, Kim SJ. Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome. Stem Cell Res Ther. 2015;6:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Labusca L S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ