©The Author(s) 2015.
World J Stem Cells. Sep 26, 2015; 7(8): 1127-1136
Published online Sep 26, 2015. doi: 10.4252/wjsc.v7.i8.1127
Published online Sep 26, 2015. doi: 10.4252/wjsc.v7.i8.1127
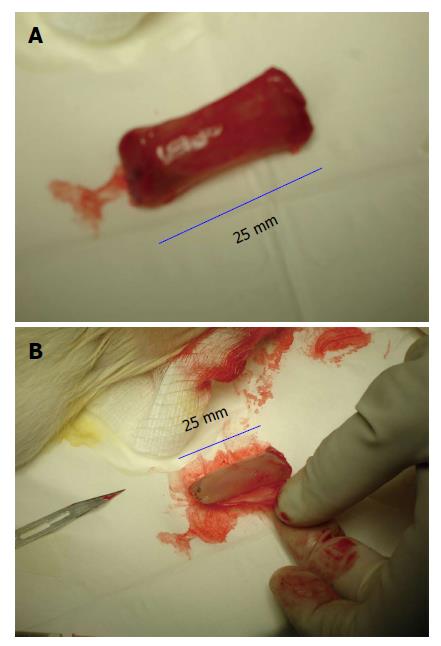
Figure 1 Gross appearance of tissue patch obtained by implanting a large-sized polyvinyl tube (L = 25 mm; ID = 7 mm) in the subcutaneous space of a rat and extracted after 4 d.
The implant was completely encapsulated by a de novo tissue (A) after tissue that could be conveniently stripped off the implant to obtain a flat piece of tissue patch (B) after patch. Note the high vascularity of the encapsulating tissue.
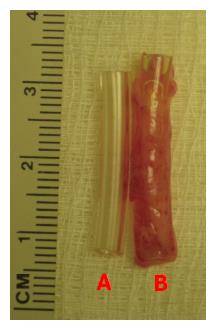
Figure 2 Gross appearance of the tissue patch obtained by implanting a fine polyvinyl tube (L = 25 mm; ID = 3 mm) in the subcutaneous space of a rat after 4 d.
A: Shows the construction of the polyvinyl tube; B: Shows the de novo tissue encapsulating the fine polyvinyl tube implant. The tissue could be gently slipped off the tube for creating a cylindrically shaped graft for tissue repair. Note the high vascularity of the tissue in (B). ID: Internal diameter.
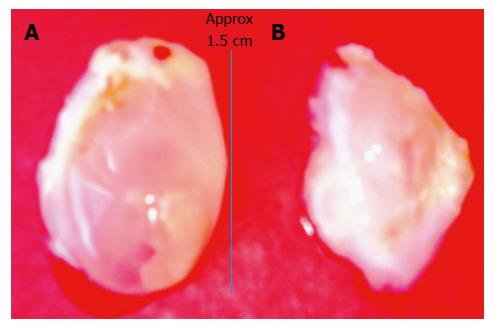
Figure 3 Bladder-like de novo tissue obtained 14 d after injecting a slurry of poldextran gel particles in the subcutaneous space of a rat.
A: The bladder-like tissue enclosed the injected gel particles; B: By piercing the balloon-like tissue the polydextran particles could be removed from the tissue to deflate the bladder. The tissue could then be used in a variety of ways including as a cusp for cardiac valve repair after cutting it appropriately to the required curvature.
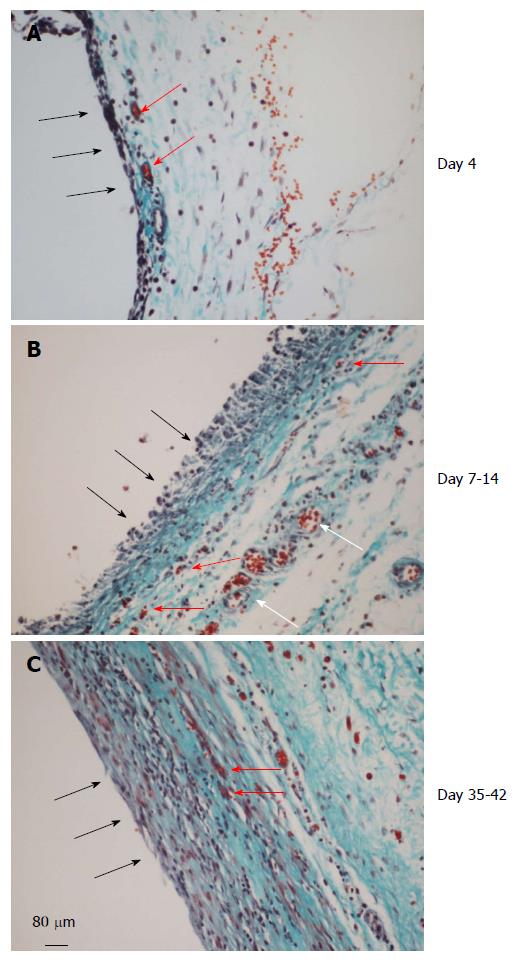
Figure 4 Histology of the de novo tissue patch at various times after implantation of the inert body in the subcutaneous space (trichrome stained).
Black arrows show the inner aspect of the tissue in contact with the inert body. A: The tissue at day 4 showed an inner layer consisting of one or two-cell thick mononuclear cells but devoid of blood vessels. The medial layer was 4-6 cell thick and contained microvessels (red arrows) and fibrous matrix (blue stain). The outer layer consisted of loose connective tissue; B: At day 7-14 the tissue was histologically better organized into inner, medial and outer layers. The inner layer in contact with the inert material was thicker, consisted of mononuclear cells but devoid of extracellular matrix and blood vessels. The medial layer was thicker than at day 4, consisting of fibroblastic cells, rich network of microvessels (shown in Figure 5) and extracellular matrix. The outer layer remained loose but contained large blood vessels (white arrows); C: Between day 35-42 the granulation tissue appeared slightly thicker and more compact than day 7-14 tissue with the inner layer becoming less distinguished than at day 7-14 and merging with the medial layer. Also, this layer showed reduced number of blood vessels and increased amounts of extracellular matrix arranged in parallel sheets. The outer layer remained unchanged.
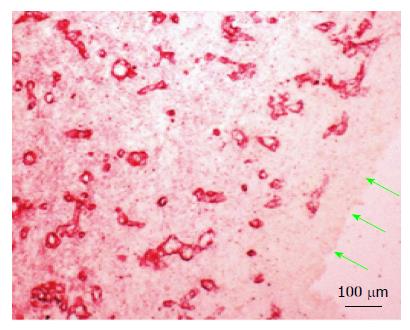
Figure 5 Vascularity of the medial layer of the subcutaneous tissue patch 14 d after implantation of the inert body.
Microvessels were revealed by immune-staining for collagen type IV, shown earlier to stain newly formed blood vessels[15]. Green arrows point to the inside aspect of the tissue patch in contact with the inert body. Note the absence of blood vessels in the inner layer.
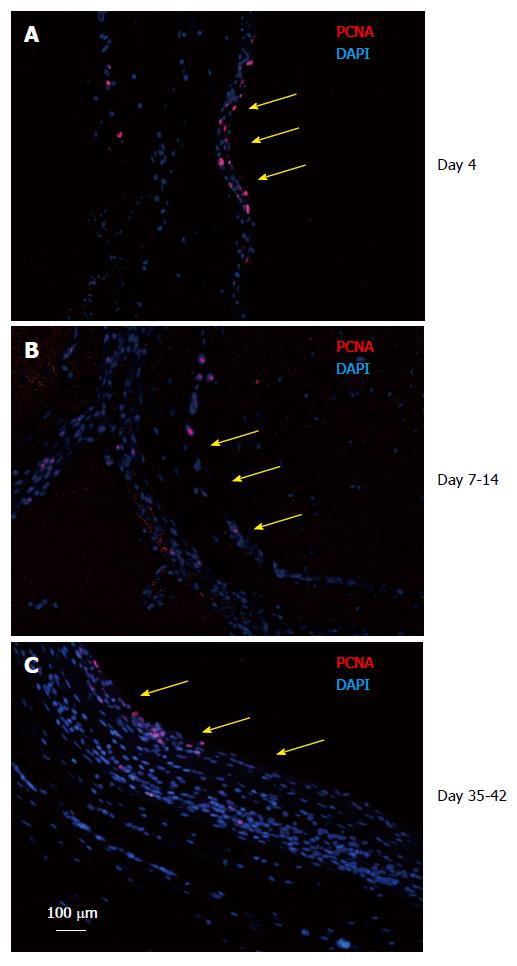
Figure 6 Tissue patch at various times immune-stained for proliferating cell nuclear antigen to determine the proliferating edge of the tissue.
We found that the patch was a continually growing tissue with the inner aspect (side in contact with the inert body shown by yellow arrows) and the medial layer staining positive for PCNA (red) at all times tested (A: Day 4; B: Day 7-14; C: Day 35-42), suggesting that the patch was growing from inside to outside. Sections were counterstained with DAPI to counterstain nuclei blue. PCNA: Proliferating cell nuclear antigen; DAPI: 4',6-diamidino-2-phenylindole.
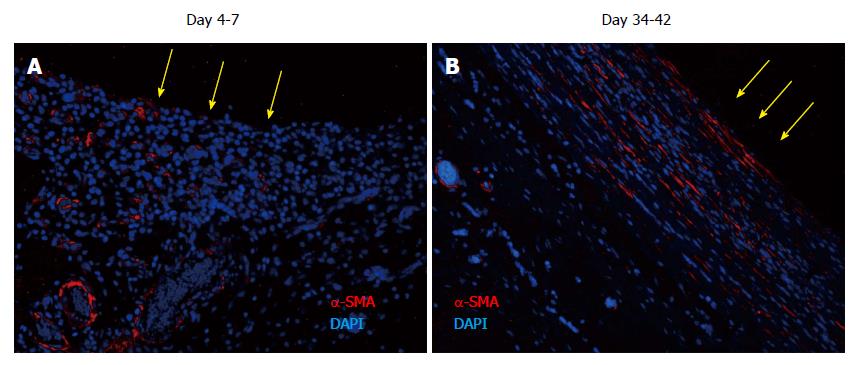
Figure 7 Tissue patch at various times immune-stained for ↑-smooth muscle antigen to determine the organization of the extracellular matrix in the patch.
At day 4-7, α-SMA was found to be associated with blood vessels as expected, but little extracellular α-SMA was observed at these times. At later time points there was more and more of α-SMA observed in the extracellular areas (not shown), which by day 35-42 had stratified and compacted itself in parallel to the length of the implant, consistent with the tissue being tougher and fibrous to touch. Sections were counterstained with DAPI to counterstain nuclei blue. Side in contact with the inert body is shown by yellow arrows. α-SMA: α-smooth muscle antigen; DAPI: 4',6-diamidino-2-phenylindole.
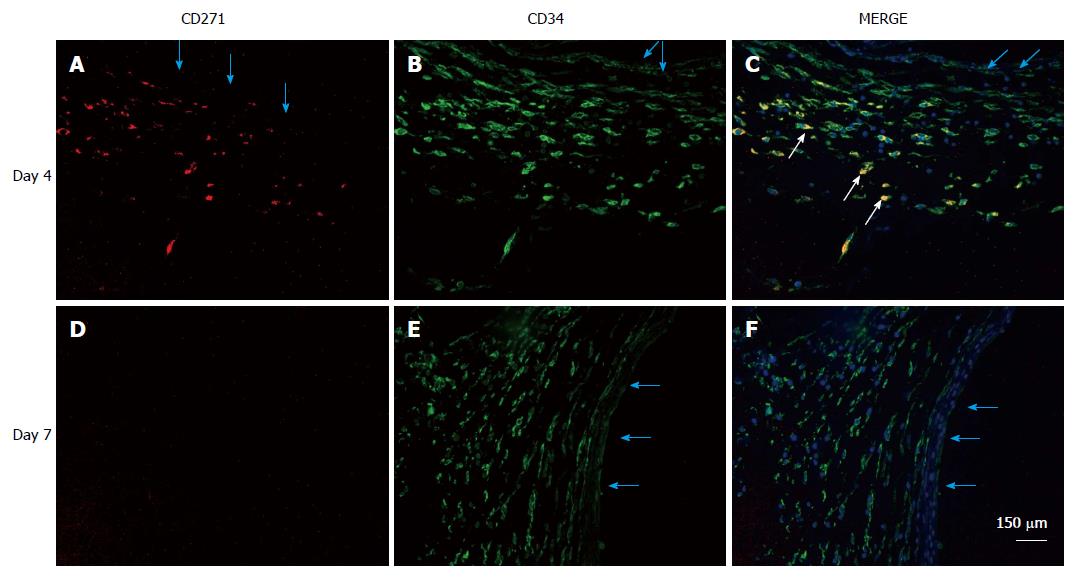
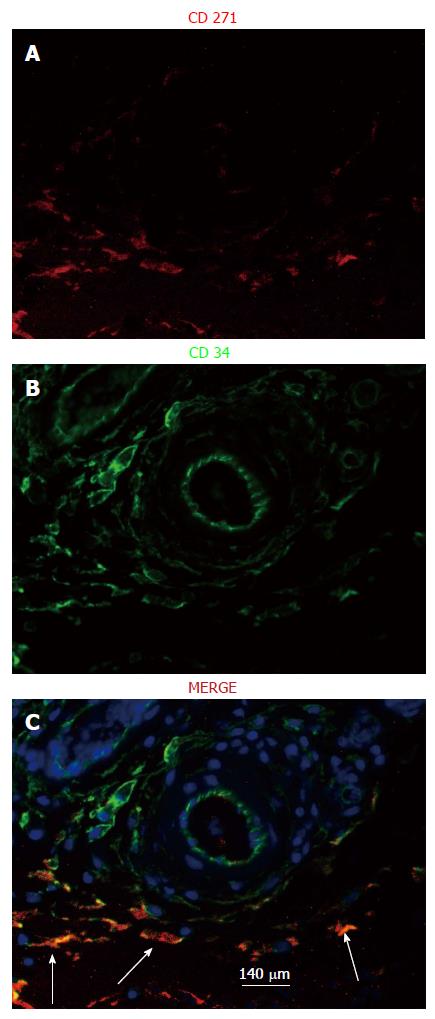
Figure 8 Localization of mesenchymal stem cells in the de novo tissue patch induced in the subcutaneous space by an inert body.
CD271 cells were immune-stained by a red fluorescent probe, CD34 cells by a green fluorescent probe and all nuclei (counterstained in the merge picture only) by DAPI. MSCs identified as CD271+CD34+ were clearly identified in the medial layer of the tissue patch at day 4 after the implantation of the inert body (white arrows in the merged picture), but rarely seen at day 7 or after. Light blue arrows indicate the inner aspect of the tissue patch. MSCs: Mesenchymal stem cells; DAPI: 4',6-diamidino-2-phenylindole.
Figure 9 Localization of mesenchymal stem cells in the vicinity of major vessels at day 4 after implantation of an inert body in the subcutaneous space.
MSCs, identified as CD34+CD271+ cells (white arrows in the merge picture), were found to be concentrated in the vicinity of major blood vessels in the medial layer at day 4 but never at day 7 or after (not shown). The merge section was counterstained with DAPI to counterstain nuclei blue. MSCs: Mesenchymal stem cells; DAPI: 4',6-diamidino-2-phenylindole.
- Citation: Garcia-Gomez I, Gudehithlu KP, Arruda JAL, Singh AK. Autologous tissue patch rich in stem cells created in the subcutaneous tissue. World J Stem Cells 2015; 7(8): 1127-1136
- URL: https://www.wjgnet.com/1948-0210/full/v7/i8/1127.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i8.1127