Published online Sep 26, 2015. doi: 10.4252/wjsc.v7.i8.1137
Peer-review started: April 12, 2015
First decision: May 14, 2015
Revised: June 30, 2015
Accepted: August 4, 2015
Article in press: August 7, 2015
Published online: September 26, 2015
Processing time: 168 Days and 6.5 Hours
AIM: To establish an easily-handled method to isolate mesenchymal stem cells (MSCs) from coagulated human bone marrow samples.
METHODS: Thrombin was added to aliquots of seven heparinized human bone marrow samples to mimic marrow coagulation. The clots were untreated, treated with urokinase or mechanically cut into pieces before culture for MSCs. The un-coagulated samples and the clots were also stored at 4 °C for 8 or 16 h before the treatment. The numbers of colony-forming unit-fibroblast (CFU-F) in the different samples were determined. The adherent cells from different groups were passaged and their surface profile was analyzed with flow cytometry. Their capacities of in vitro osteogenesis and adipogenesis were observed after the cells were exposed to specific inductive agents.
RESULTS: The average CFU-F number of urokinase-treated samples (16.85 ± 11.77/106) was comparable to that of un-coagulated control samples (20.22 ± 10.65/106, P = 0.293), which was significantly higher than those of mechanically-cut clots (6.5 ± 5.32/106, P < 0.01) and untreated clots (1.95 ± 1.86/106, P < 0.01). The CFU-F numbers decreased after samples were stored, but those of control and urokinase-treated clots remained higher than the other two groups. Consistently, the numbers of the attached cells at passage 0 were higher in control and urokinase-treated clots than those of mechanically-cut clots and untreated clots. The attached cells were fibroblast-like in morphology and homogenously positive for CD44, CD73 and CD90, and negative for CD31 and CD45. Also, they could be induced to differentiate into osteoblasts and adipocytes in vitro.
CONCLUSION: Urokinase pretreatment is an optimal strategy to isolate MSCs from human bone marrow samples that are poorly aspirated and clotted.
Core tip: It is usually difficult to isolate mesenchymal stem cells (MSCs) from bone marrow samples that have been coagulated due to inappropriate marrow aspirations or in the setting of pathologically hypercoagulative state. In this study, the authors found that urokinase pretreatment could dissolve stem cells from the clots. The MSCs were fibroblat-like in morphology and homogenously positive for CD44, CD73 and CD90, and negative for CD31 and CD45. Also, they could be induced to differentiate into osteoblasts and adipocytes in vitro. Urokinase pretreatment is an optimal strategy to isolate MSCs from poorly aspirated and clotted human bone marrow samples.
- Citation: Wang HX, Li ZY, Guo ZK, Guo ZK. Easily-handled method to isolate mesenchymal stem cells from coagulated human bone marrow samples. World J Stem Cells 2015; 7(8): 1137-1144
- URL: https://www.wjgnet.com/1948-0210/full/v7/i8/1137.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i8.1137
Mesenchymal stromal cells (MSCs) are a kind of adult stem cells characterized by their hematopoiesis-supporting and immune regulatory activities, and have been clinically trialed in the treatment of various kinds of diseases including graft-versus-host disease, musculoskeletal defects, ischemic disorders, and neurological diseases[1-3]. Though MSCs are low in immunogenicity and allogeneic transplantation has been widely used, autologenous MSCs still remain an ideal option in some cases, such as in the repair of bone and cartilage defects. And in these settings, bone marrow is usually aspirated from the patients though adipose tissue might be another choice for MSC isolation[4]. In some cases, however, bone marrow samples are poorly aspirated and clotted promptly after the procedure, especially when the patients are in a hypercoagulative state[5-7]. This usually leads to the decrease of MSC output or even failure to culture-expand MSCs. To solve this practical problem, we have developed an easily-handled method to isolate MSCs from human marrow clots.
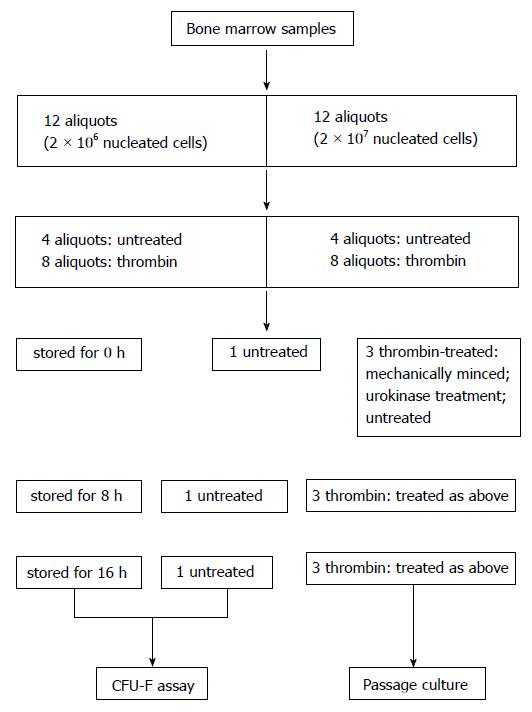
Heparinized bone marrow samples were aspirated from seven healthy adult donors after informed consent in accordance to the Ethical Guidelines for Research Involving Human Subjects or Human Tissue from General Hospital of Air Force. Nucleated cells were counted by trypan blue exclusion assay and the samples were diluted with PBS to a concentration of 2 × 106 or 2 × 107/mL. The samples were then treated as depicted in Figure 1. Briefly, one sample was divided into 12 aliquots containing 2 × 106 nucleated cells and 12 aliquots containing 2 × 107 nucleated cells. To mimic coagulation, eight aliquots of each group were treated with thrombin (Sigma) at a final concentration of 1 U/mL and maintained at 37 °C for 1 h. The thrombin-treated and untreated samples were stored at 4 °C for 0 (subgroup 0 h), 8 (subgroup 8 h) and 16 h (subgroup 16 h) for further treatment. Each subgroup contained 3 thrombin-treated samples and one un-coagulated sample. The three clotted samples were either untreated, mechanically minced into pieces or treated with urokinase. For urokinase treatment, urokinase (Urokinase for injection, Lizon Pharmaceutical Factory, Zhuhai, Guangdong) was added into the samples at a final concentration of 50000 U/mL and maintained at 37 °C with a horizontal rotation of 120 rpm for 20 min. All the samples were further treated with NH4Cl buffer to lyse the contaminated red blood cells, washed twice in PBS, and suspended in alpha-MEM containing 10% fetal bovine serum from selected lots (Stemcell Co. Canada)[8]. The cells were used in the experiments described below.
The samples which contained 2 × 106 nucleated cells each and stored at different times were separately seeded into culture dishes with a diameter of 100 mm. The culture was maintained for 12 d. The medium was then removed, and the cells were washed twice with PBS. Wright–Giemsa staining was performed to reveal the colonies that were more than 0.5 mm in diameter. The number of colonies was calculated with the software of Quantity One 4.4 as described previously[9].
When the confluence of the attached cells cultured from un-clotted samples reached to around 80%, the cells from all groups were harvested by trypsin digestion, counted and re-seeded into culture dishes at a density around 6000 cells/cm2. When the cell attachment from the control samples reached around 80%, the cells from all the counterpart groups were detached, the cell numbers were counted and the cells were plated for passage culture. The cells at passage 2 were also collected for phenotype analysis and in vitro differentiation assay as reported previously[9,10]. Colony-forming unit-fibroblast (CFU-F) was stained with Giemsa solution and counted as routinely described[9].
The attached cells from passage culture were detached by trypsin digestion, washed in PBS and reacted with PE-conjugated monoclonal antibodies (BD, United States) against human CD31 (endothelial cell marker), CD45 (hematopoietic cell marker), CD73 (positive on mesenchymal cell lineage) and CD90 (stem cell marker) for 30 min in dark at room temperature. The cells were washed twice in PBS and at least 10000 events were collected with FACSCalibur (BD, United States). The data were analyzed with WinMdi 2.9 software after the targeted events were gated according to the cell size and granularity.
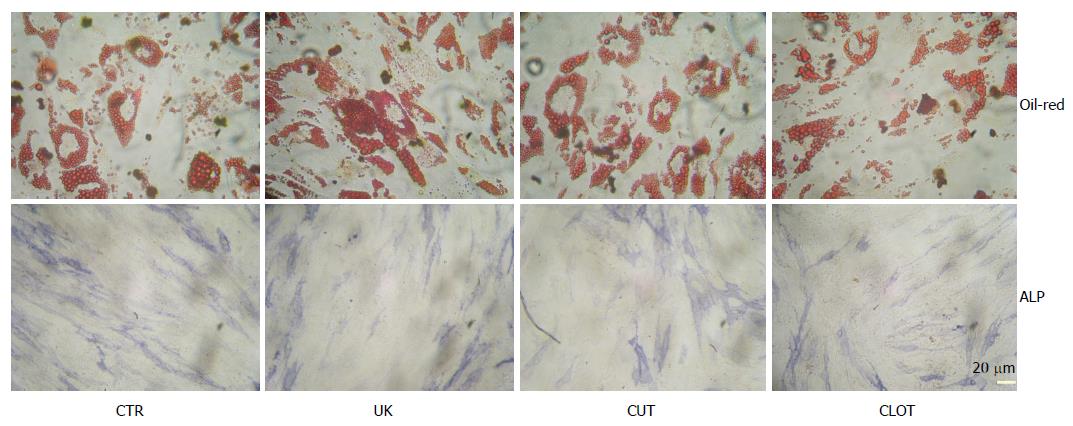
For in vitro osteogenic differentiation, the attached cells for passage culture were digested with 0.05% trypin, washed in PBS, and seeded into 24-well plates at a density of 5000 cells per well. The cells were allowed to attach overnight and then exposed to 0.1 μmol/L dexamethasone, 50 μmol/L ascorbic acid 2-phosphate, and 10 mmol/L b-glycerophosphate as previously described with a little modification[10,11]. After 12 d, cellular activity of alkaline phosphatase (ALP) was revealed with ALP immunohistochemistry kit (Sigma, United States) following the manufacture’s protocol.
For adipogenic differentiation, the cells were seeded a 24-well plate at a density of 10000 cells per well. Inductive agents containing 1 μmol/L dexamethasone, 0.5 mmol/L isobutylmethylxanthine (IBMX, Sigma, United States), and 100 μmol/L indomethacin (IDM, Sigma, United States) were supplemented. The culture was maintained for 7 d and intracellular lipid droplets were revealed by Oil-Red staining[9-11].
The data were expressed as mean values incorporating the standard deviation. Statistical significance was analyzed by Student’s t test. A P-value less than 0.05 was considered significant. The statistical analysis has been reviewed by Dr. Qing-Yi Zhang from Beijing Institute of Basic Medicine.
To establish a ready-use method to isolate MSCs from clots of human bone marrow aspirates, heparinized marrow samples were aliquoted according to the nucleated cell numbers and treated with thrombin to coagulate the samples. Then, the clots were treated with urokinase digestion, mechanically minced or untreated. The cells were plated to allow MSCs to attach and expand. The numbers of CFU-F and the attached cells were counted disparately.
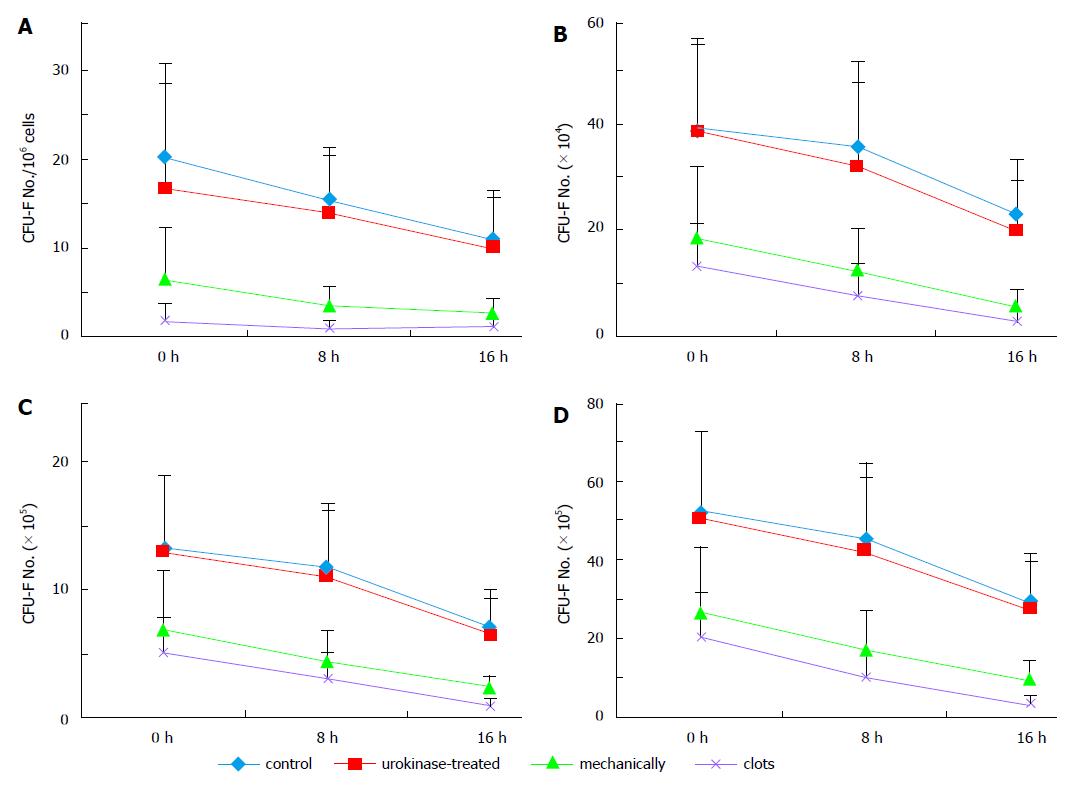
As expected, bone marrow coagulation did affect the output of MSCs. The numbers of CFU-F were greatly decreased from clotted samples compared with those from un-clotted samples (Figure 2A). However, the average CFU-F number of urokinase-treated samples (16.85 ± 11.77/106) was comparable to that of un-coagulated control samples (20.22 ± 10.65/106, P = 0.293), which was significantly higher than those of mechanically-cut clots (6.5 ± 5.32/106, P < 0.01) and untreated clots (1.95 ± 1.86/106, P < 0.01). Statistical analysis showed that the CFU-F numbers decreased after samples were stored for 8 h, and those were greatly decreased after the samples were stored for further 8 h compared with those from the same groups. However, the CUF-F numbers of control and urokinase-treated clots from stored samples were higher than those in the stored samples of the other two groups (Figure 2A).
Consistent with the results above, as shown in Figure 2B, the numbers of the attached cells at passage 0 were higher in control (average: 38.86 ± 17.4 × 104, n = 7) and urokinase-treated clots (38.57 ± 16.78 × 104, n = 7) than those of mechanically-cut clots (18.57 ± 13.69 × 104, n = 7, P = 0.016 compared with control) and untreated clots (13.14 ± 8.39 × 104, n = 7, P = 0.002 compared with control). These differences were also observed when cell counting was performed at passage 1 and 2 (Figure 2C and D). The results further confirm the advantages of urokinase treatment on bone marrow coagulated clots.
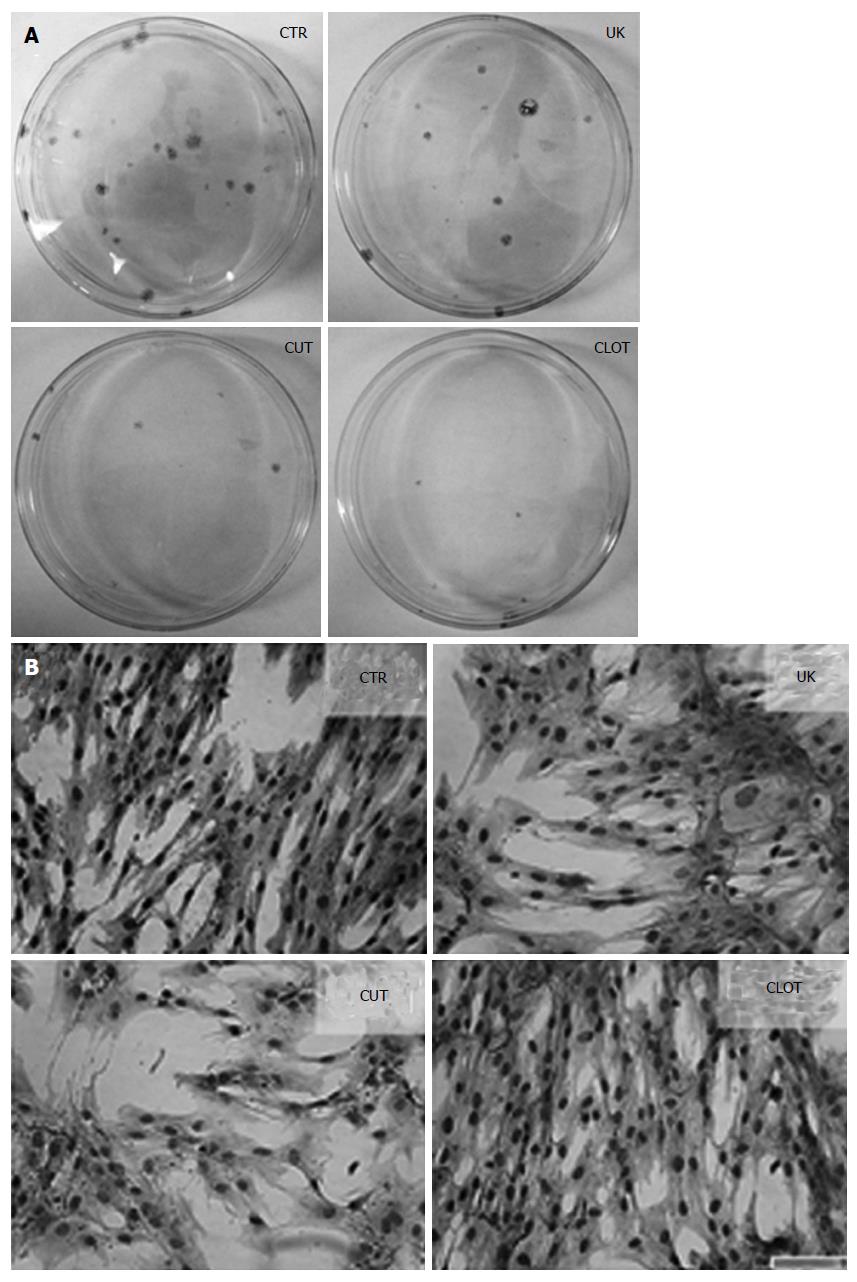
Marrow samples were treated by different protocols and the nucleated cells were cultured in media traditionally for MSC expansion after the contaminated red cells were lysed in NH4Cl. After 72 h, cells attached to the plastic were observed and colonies were evident after 12 d of culture. As shown in Figure 3, the attached cells appeared in all of the four groups and were fibroblast-like in morphology, suggestive of the features of the cells as MSCs.
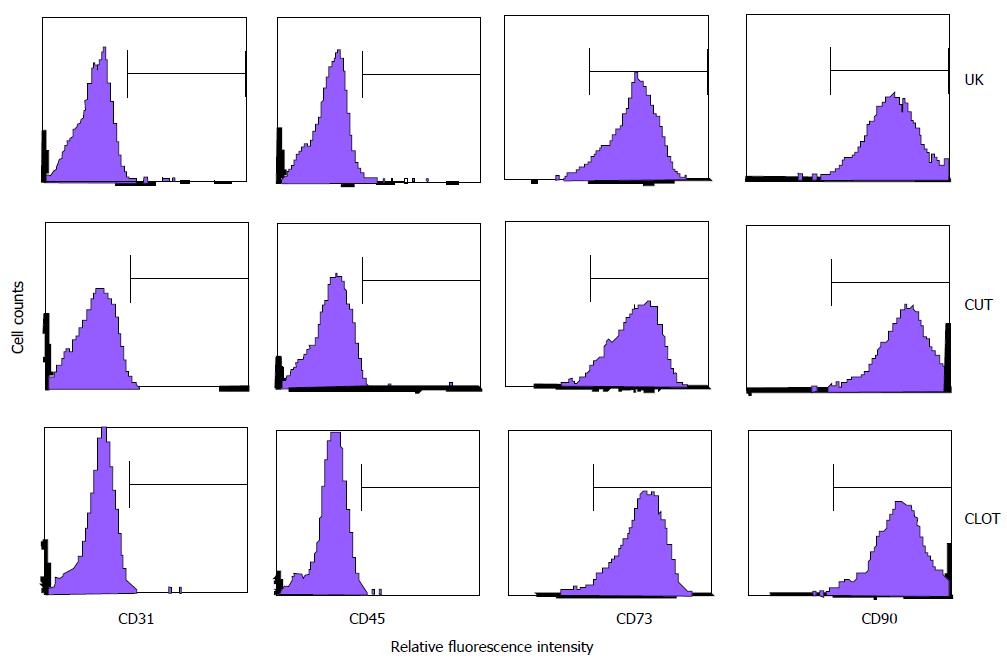
To further confirm that the attached cells from differently treated bone marrow samples were MSCs per se, some of the surface markers on several kinds of fibroblast-like cells were analyzed with flow cytometry. The results showed that the cells from the four groups were homogenously negative for CD31 and CD45, proving that the cells were devoid of endothelial and hematopoietic cells, two cell types that are commonly observed when bone marrow cells are cultured (Figure 4). Meanwhile, the cells were positive for CD73, a mesenchymal marker[12], and for CD90, a pan-marker for stem cells including MSCs[13].
Furthermore, when the attached cells were exposed to specific inductive conditions, they became ALP-positive and intracellular lipid droplets were observed (Figure 5). The results prove that the cells had the capacity to differentiate into osteoblasts and adipocytes. The fibroblast-like in morphology, the expression of unique surface markers and the differentiation capacity support the conclusion that the attached cells were MSCs per se[14].
Bone marrow sample coagulation is a common phenomenon in clinical practice and, it is indeed a problematic issue to isolate MSCs from the samples. To overcome this problem and establish an easily handled method, urokinase was used in this study to dissolve the clots and subsequently, the contaminant red blood cells in the pre-treated bone marrow samples were lysed with ammonium chloride, and the nucleated cells were seeded. The procedures described here seem practical in isolating MSCs from the bone marrow clots as the treated cells could form CFU-F and the attached cells had the capacity to differentiate osteoblasts and adipoblasts.
CFU-F is a well-recognized surrogate for MSCs[15-18]. The numbers of CFU-F in urokinase-treated clots were comparable to those in uncoagulated controls at three time points, suggesting that urokinase treatment could optimally release MSCs in the clots. Urokinase is not only able to dissolve the coagulated clots but also could migrate MSCs from the potentially existed undissolved small ones[19], resulting in the “liberation” of MSCs from the dense fibrin arrangement. Further, it is interesting that the numbers of CFU-F decreased sharply when the samples, pre-coagulated or uncoagulated, were stored at 4 °C for 8 or 16 h. It has been proven that prolonged storage time does decrease the viable cell numbers in bone marrow aspirates[20], which is also observed in this study. Meanwhile, the temperature is also an important issue for preservation of marrow samples. It is clear that room temperature is an optimal condition for marrow sample storage when the sample is designed to be used shortly afterwards[21]. Therefore, it is recommended that human bone marrow samples be used as soon as possible or be stored at room temperature when necessary.
The results above suggest that urokinase pretreatment is an optimal strategy to culture MSCs when human bone marrow are poorly aspirated and clotted. Urokinase might not only dissolve the coagulated clots but also mobilize MSCs wrapped in the fibrin structure[19]. Previous data have also shown that urokinase might affect the migration and differentiation[19,22]. However, urokinase treatment described in this protocol needs only four hours and the potential ligation of urokinase to the receptor on MSCs is transient. The differentiation properties of MSCs might be unaffected. Therefore, urokinase treatment should be a practical method to release MSCs in the coagulated clots.
Bone marrow is a commonly used origin for the isolation of mesenchymal stem cells (MSCs); however, in some cases, marrow samples are poorly collected and clotted promptly after aspiration, leading to the decrease of MSC output or even failure to culture-expand MSCs.
MSCs are adult stem cells with the capacities to differentiate several connective tissues, support hematopoiesis, promote angiogenesis, and regulate immune reactions. Therefore, several hundreds of clinical trials with MSCs are on-going and MSCs have officially approved to treat ischemic diseases and immune-associated disorders.
In this study, the authors found that urokinase treatment could release MSCs from clotted marrow samples and maintain the number of colony-forming units compared with the uncoagulated control. The isolated MSCs exhibited fibroblast in morphology, expressed the specific markers and could be induced to osteoblast and adipoblast.
Coagulation of human bone marrow samples is a common problem in practice. The study strongly suggests urokinase digestion is a good method to treat the clots to isolate MSCs in this setting.
Mesenchymal stromal cells: MSCs are adult stem cells characterized by their hematopoiesis-supporting, angiogenesis-promoting and immune regulatory activities, and have been officially approved by several countries to treat various kinds of diseases including graft-versus-host disease, musculoskeletal defects and ischemic heart disorders. Colony-forming unit-fibroblast: Colony-forming unit (CFU) is a unit commonly used to estimate the number of viable targeted cells in a sample. CFU-fibroblast (CFU-F) expresses the frequency of cells in a sample that can form colonies consisting of fibroblasts or fibroblast-like cells after culture for several days. The visual appearance of a colony in a cell culture requires significant growth, and when counting colonies it is uncertain if the colony arose from one cell or a group of cells. Expressing results as colony-forming units reflects this uncertainty. CFU-F is a well-recognized surrogate for MSCs and used to evaluate the potential proportion of MSCs in a sample. Urokinase: Urokinase is a serine protease that was originally isolated from human urine. The primary physiological substrate of urokinase is plasminogen. Clinically, urokinase is used in the treatment of deep venous thrombosis, pulmonary embolism, and myocardial infarction.
This is a well-designed study that clearly shows that urokinase-treated samples provides a superior method for obtaining useable mesenchymal stem cells. The methods of this study were sound and the conclusions followed logically from the data obtained. The study has significant relevance for stem cell therapies.
| 1. | Introna M, Rambaldi A. Mesenchymal stromal cells for prevention and treatment of graft-versus-host disease: successes and hurdles. Curr Opin Organ Transplant. 2015;20:72-78. [PubMed] |
| 2. | Labusca L, Zugun-Eloae F, Mashayekhi K. Stem cells for the treatment of musculoskeletal pain. World J Stem Cells. 2015;7:96-105. [PubMed] |
| 3. | Zack-Williams SD, Butler PE, Kalaskar DM. Current progress in use of adipose derived stem cells in peripheral nerve regeneration. World J Stem Cells. 2015;7:51-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 4. | Szpalski C, Barbaro M, Sagebin F, Warren SM. Bone tissue engineering: current strategies and techniques--part II: Cell types. Tissue Eng Part B Rev. 2012;18:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Lykissas MG, Gelalis ID, Kostas-Agnantis IP, Vozonelos G, Korompilias AV. The role of hypercoagulability in the development of osteonecrosis of the femoral head. Orthop Rev (Pavia). 2012;4:e17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Ghanny S, Ross C, Chan AK, Chan HH. Coagulopathy in a patient with nephrotic syndrome. Am J Hematol. 2010;85:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Parker RI. Thrombosis in the pediatric population. Crit Care Med. 2010;38:S71-S75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Horn P, Bork S, Diehlmann A, Walenda T, Eckstein V, Ho AD, Wagner W. Isolation of human mesenchymal stromal cells is more efficient by red blood cell lysis. Cytotherapy. 2008;10:676-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Jin JD, Wang HX, Xiao FJ, Wang JS, Lou X, Hu LD, Wang LS, Guo ZK. A novel rich source of human mesenchymal stem cells from the debris of bone marrow samples. Biochem Biophys Res Commun. 2008;376:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang PH, Mao N. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15369] [Article Influence: 569.2] [Reference Citation Analysis (2)] |
| 12. | Barry F, Boynton R, Murphy M, Haynesworth S, Zaia J. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun. 2001;289:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 204] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Lee TC, Lee TH, Huang YH, Chang NK, Lin YJ, Chien PW, Yang WH, Lin MH. Comparison of surface markers between human and rabbit mesenchymal stem cells. PLoS One. 2014;9:e111390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [PubMed] |
| 15. | Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, Lataillade JJ. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 507] [Article Influence: 24.1] [Reference Citation Analysis (4)] |
| 16. | Simmons DJ, Seitz P, Kidder L, Klein GL, Waeltz M, Gundberg CM, Tabuchi C, Yang C, Zhang RW. Partial characterization of rat marrow stromal cells. Calcif Tissue Int. 1991;48:326-334. [PubMed] |
| 17. | Wu X, Peters JM, Gonzalez FJ, Prasad HS, Rohrer MD, Gimble JM. Frequency of stromal lineage colony forming units in bone marrow of peroxisome proliferator-activated receptor-alpha-null mice. Bone. 2000;26:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Stenderup K, Justesen J, Eriksen EF, Rattan SI, Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res. 2001;16:1120-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Vallabhaneni KC, Tkachuk S, Kiyan Y, Shushakova N, Haller H, Dumler I, Eden G. Urokinase receptor mediates mobilization, migration, and differentiation of mesenchymal stem cells. Cardiovasc Res. 2011;90:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Badrinath R, Bohl DD, Hustedt JW, Webb ML, Grauer JN. Only prolonged time from abstraction found to affect viable nucleated cell concentrations in vertebral body bone marrow aspirate. Spine J. 2014;14:990-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Hahn S, Sireis W, Hourfar K, Karpova D, Dauber K, Kempf VA, Seifried E, Schmidt M, Bönig H. Effects of storage temperature on hematopoietic stability and microbial safety of BM aspirates. Bone Marrow Transplant. 2014;49:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kalbasi Anaraki P, Patecki M, Larmann J, Tkachuk S, Jurk K, Haller H, Theilmeier G, Dumler I. Urokinase receptor mediates osteogenic differentiation of mesenchymal stem cells and vascular calcification via the complement C5a receptor. Stem Cells Dev. 2014;23:352-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Dunbar GL, Phinney DG S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK