©The Author(s) 2025.
World J Stem Cells. Aug 26, 2025; 17(8): 107639
Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.107639
Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.107639
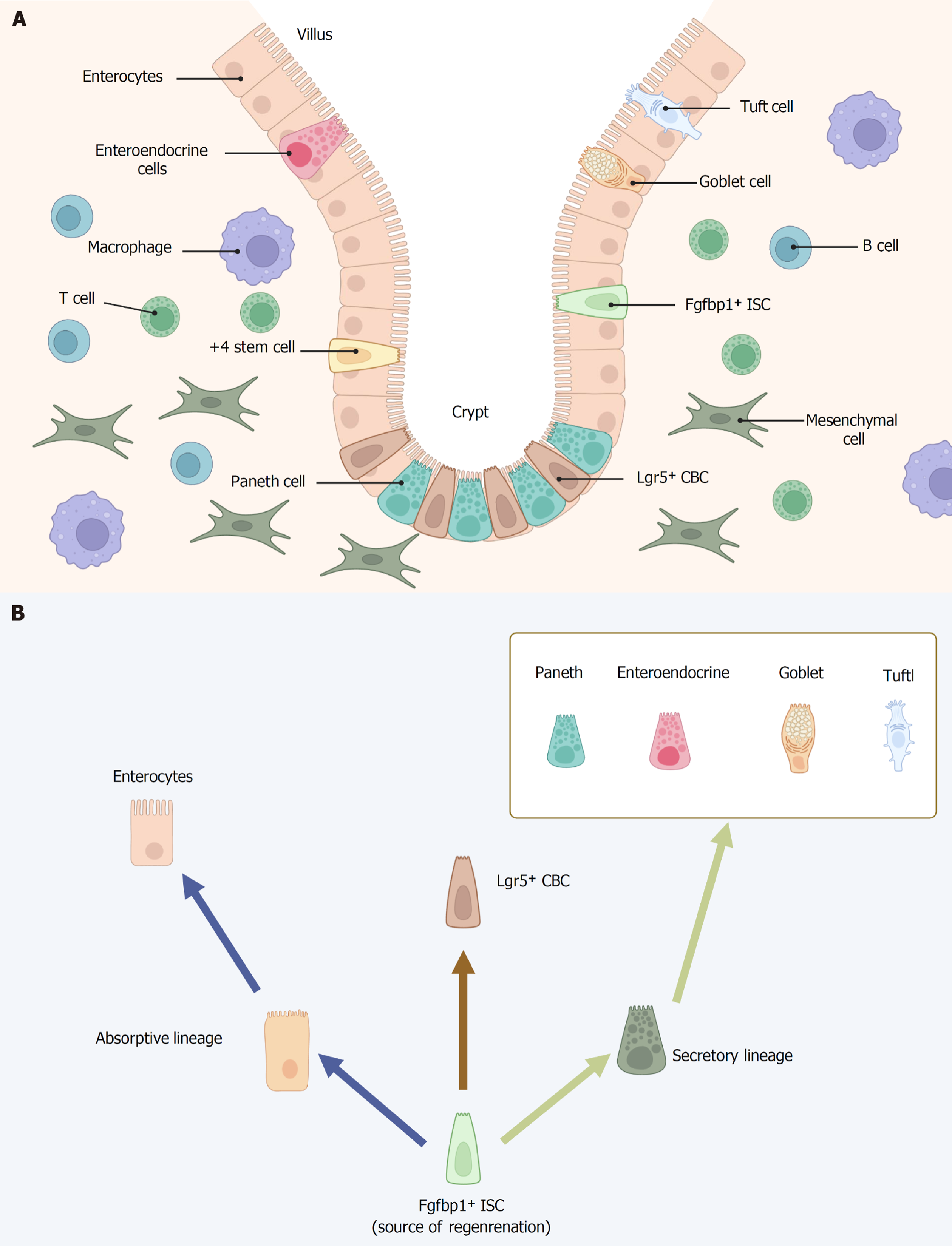
Figure 1 Intestinal stem cell niche and cell types in crypt-villus structures.
A: The intestinal crypt-villus axis and neighboring niche cells; B: Fibroblast growth factor binding protein 1 positive intestinal stem cells regenerate all lineages and leucine-rich repeat-containing G protein-coupled receptor 5 positive cells. Fibroblast growth factor binding protein 1 secreted by upper crypt intestinal stem cells is essential for intestinal epithelial regeneration. Images created with BioRender.com (Supplementary material). ISCs: Intestinal stem cells; Fgfbp1: Fibroblast growth factor binding protein 1; Lgr5: Leucine-rich repeat-containing G protein-coupled receptor 5; CBC: Crypt based columnar cell.
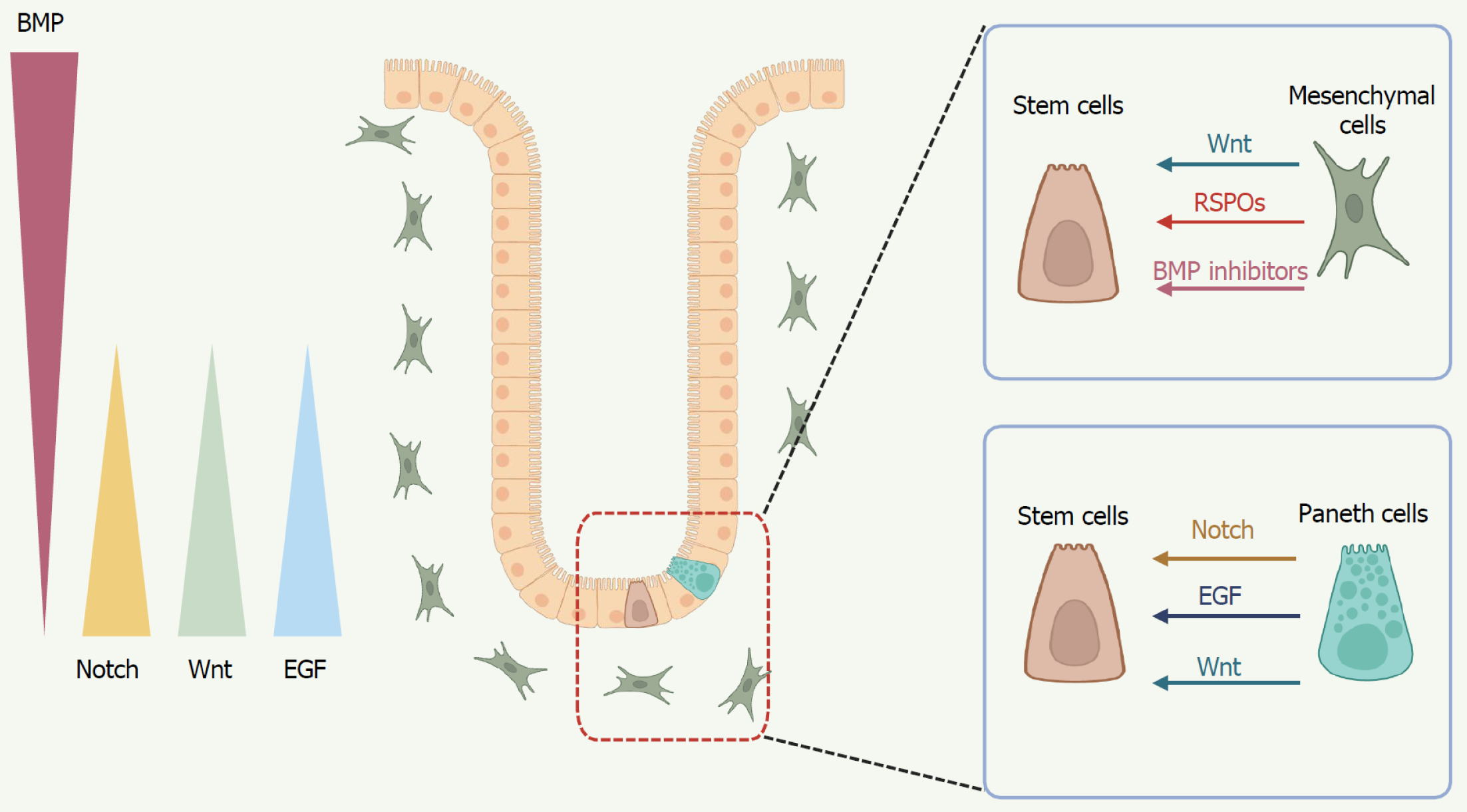
Figure 2 Key signaling pathways orchestrate stem cell fate regulation.
At the crypt base, mesenchymal cells and Paneth cells secrete Wnt ligands and R-spondins to sustain the stemness and proliferation of intestinal stem cells (ISCs). Mesenchymal cells also produce bone morphogenetic protein inhibitors, promoting the self-renewal of ISCs. Epidermal growth factor, derived from Paneth cells, promotes ISC proliferation, survival, and long-term organoid growth. In addition to Wnt and epidermal growth factor, Paneth cells provide critical Notch signals to ISCs, with Notch signaling orchestrating the balance between absorptive and secretory lineage commitment. Images created with BioRender.com (Supplementary material). BMP: Bone morphogenetic protein; EGF: Epidermal growth factor; RSPOs: R-spondins.
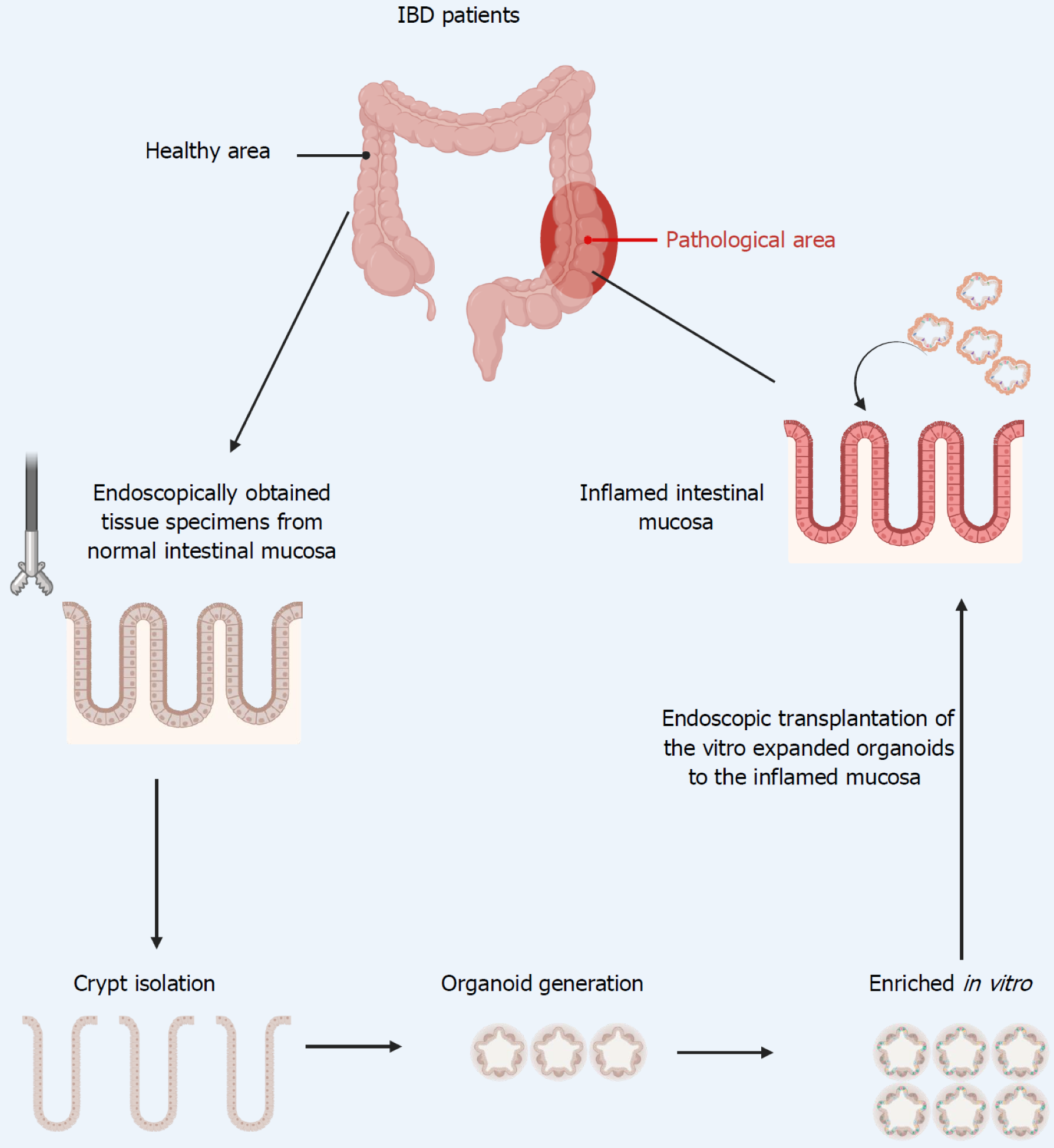
Figure 3 Endoscopic intestinal stem cell transplantation: Therapeutic potential in inflammatory bowel disease.
Images created with BioRender.com (Supplementary material). IBD: Inflammatory bowel disease.
- Citation: Zhang MJ, Chan SX, Jia ZG, Lv C, Chen JJ, Hong SC. Roles of intestinal stem cells in inflammatory bowel disease pathogenesis. World J Stem Cells 2025; 17(8): 107639
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/107639.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.107639