Published online Dec 28, 2025. doi: 10.3748/wjg.v31.i48.112004
Revised: September 20, 2025
Accepted: November 10, 2025
Published online: December 28, 2025
Processing time: 157 Days and 18.1 Hours
Sepsis-induced intestinal injury disrupts barrier function and exacerbates sys
To investigate the mechanism by which sphinganine protects against sepsis-induced intestinal injury, focusing on macrophage polarization and toll like receptor 2 (TLR2)/nuclear factor kappa-B (NF-κB) signaling.
A cecal ligation and puncture sepsis model was established in mice (n = 20/group). Treatments included sphinganine (15 mg/kg) and a TLR2 agonist [fibroblast-stimulating lipopeptide-1 (FSL-1), 10 μg/kg]. Serum markers [diamine oxidase (DAO), interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6] were measured by enzyme-linked immunosorbent assay. Intestinal injury and ma
Sphinganine significantly reduced serum levels of DAO (P < 0.05), IL-1β, TNF-α, and IL-6 (P < 0.05), preserved intestinal crypt structure, and enhanced tight junction protein zonula occludens-1 expression. It promoted a shift from M1 (CD86+) to M2 (CD206+) macrophages. Proteomics identified TLR2 as the most differentially expressed protein, and molecular docking confirmed strong binding between sphinganine and TLR2 (-4.3 kcal/mol). Sphinganine downregulated TLR2 and phosphorylated-NF-κB p65 expression (P < 0.05), effects reversed by FSL-1. Total NF-κB p65 levels remained unchanged.
Sphinganine protects against sepsis-induced intestinal injury by inhibiting TLR2/NF-κB signaling, modulating macrophage polarization toward the M2 phenotype, and preserving intestinal barrier integrity.
Core Tip: This study reveals that sphinganine protects against sepsis-induced intestinal injury by inhibiting toll like receptor 2/nuclear factor kappa-B signaling, modulating macrophage polarization toward the M2 phenotype, and preserving intestinal barrier integrity.
- Citation: Chen YF, Wang ZT, Zhao J, Tang JG, Zhang BY. Sphinganine inhibits macrophage polarization and protects against sepsis-induced intestinal injury. World J Gastroenterol 2025; 31(48): 112004
- URL: https://www.wjgnet.com/1007-9327/full/v31/i48/112004.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i48.112004
Sepsis can induce intestinal injury that impairs the structural integrity of the normal intestinal barrier. This disruption allows pathogens and toxins to translocate into the systemic circulation, thereby increasing the risk of mortality[1]. Additionally, such intestinal damage can further exacerbate systemic inflammatory responses, promote the development of organ failure, and ultimately elevate the overall mortality risk in patients with sepsis[2]. Therefore, the implementation of effective sepsis management and early intervention strategies is crucial for mitigating or controlling sepsis-associated intestinal damage, as well as for improving patient prognosis[3].
The sphingolipid metabolite sphinganine can be phosphorylated to form sphinganine-1-phosphate[4]. Our team previously showed that circulating sphinganine is detectable in the serum of septic patients and that it reduces lactate dehydrogenase release from lipopolysaccharide (LPS)-treated Caco-2 cells. Further studies using both cellular and animal models confirmed that sphinganine exerts robust protective effects against sepsis-induced intestinal barrier damage[5]. However, the specific mechanisms underlying sphinganine’s protective action against sepsis-mediated intestinal injury remain to be elucidated.
During sepsis, intestinal pathogenic microorganisms, LPS, and endogenous danger-associated molecular patterns activate pattern recognition receptors on intestinal macrophages, triggering phenotypic transformation and functional dysregulation[6]. These macrophages polarize toward the pro-inflammatory M1 phenotype and secrete pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and interferon-γ. These cytokines disrupt the expression and localization of tight junction proteins [e.g., zonula occludens-1 (ZO-1), occludin], thereby impairing intestinal barrier integrity and promoting bacterial translocation[7]. Additionally, the reactive oxygen species released by activated macrophages directly damage intestinal epithelial cells, inducing cell apoptosis and intestinal tissue destruction[8]. Meanwhile, the aberrant activation of intestinal macrophages perturbs intestinal immune homeostasis: Under physiological conditions, these macrophages exhibit hypo-responsiveness to commensal bacteria to maintain immune tolerance; However, during sepsis, they initiate an excessive immune response, which further drives gut microbiota dysbiosis[9]. Metabolites derived from the dysregulated gut microbiota subsequently amplify macrophage-mediated inflammatory responses, forming a vicious cycle that exacerbates sepsis-induced intestinal injury[10]. Thus, clarifying the regulatory mechanisms governing intestinal macrophage activation and developing targeted interventions to modulate their functions may provide innovative therapeutic approaches for sepsis-associated intestinal injury.
This study aims to clarify the mechanism by which sphinganine protects against sepsis-induced intestinal injury, with a focus on its effects on intestinal macrophage activation including regulating the transformation of M1 macrophages to the anti-inflammatory phenotype, modulating cytokine secretion, and preserving tight junction integrity while exploring its role in restoring intestinal immune homeostasis and gut microbiota balance.
Mouse were housed in a controlled environment (20 °C-22 °C, 12 hours light/dark cycle) in plastic cages with food and water access. All animal studies were performed at the Animal Experiment Center of East China Normal University with approval from the Experimental Animal Ethical Review Committee at East China Normal University (No. R20211202) (Shanghai, China). Mice were randomized (n = 20/group) into control, sepsis, sphinganine, sepsis + sphinganine (15 mg/kg)[5], and sepsis + sphinganine + toll like receptor 2 (TLR2) agonist [fibroblast-stimulating lipopeptide-1 (FSL-1); 10 μg/kg, Abcam, United States] groups. Sphinganine or FSL-1 was intraperitoneally administered to appropriate mice at 3 hours and 8 hours post-surgery. Dimethyl sulfoxide (10% in saline) was used to dissolve sphinganine. Sepsis induction method: Clearly described the cecal ligation and puncture (CLP) procedure: Mice were anesthetized with 1% pentobarbital sodium (50 mg/kg), a 1 cm midline abdominal incision was made, the cecum was exposed, ligated at 1/3 of the distal end with 4-0 silk suture, punctured twice with a 22G needle, and a small amount of cecal content was extruded to ensure patency. The control group only underwent laparotomy and suture without ligation/puncture. The animals were sacrificed at 24 hours post-surgery, and fresh stool, blood, and major organ samples were immediately harvested.
After using 4% neutral formalin to fix samples of intestinal tissue, they were dehydrated via ethanol gradient, paraffin-embedded, and cut to produce sections that were 5 mm thick. After mounting these sections on slides, they were cleaned, rehydrated, and subjected to hematoxylin and eosin (HE) staining. Three experienced pathologists independently evaluated the degree of intestinal tissue injury. The scoring indicators included four categories, as detailed below: Four categories were evaluated to establish these histopathological scores, including: (1) Dysregulation of the epithelia; (2) Reductions in goblet cell numbers; (3) Inflammatory cell infiltration; and (4) Stiffness of the submucosa. The total intestinal injury score was the sum of the four indicators, ranging from 0 to 12 points. A higher score indicated more severe intestinal tissue damage.
The mouse blood was centrifuged (10 minutes, 3000 rpm, 4 °C), and the serum fraction was stored at -80 °C. Levels of serum IL-6, TNF-α, diamine oxidase (DAO), and IL-1β were measured with appropriate enzyme-linked immunosorbent assay (ELISA) kits (88-7064, Thermo Fisher, Austria; EK280/3-01, MuLTI SCIENCE, Shanghai) used as directed.
Paraffin-embedded tissue sections were dewaxed and rehydrated, and antigen retrieval was performed using a pressure cooker with citrate buffer. After cooling to room temperature, the sections were incubated with 3% hydrogen peroxide for 15 minutes and washed three times with phosphate buffered saline with tween 20 (PBST). They were then blocked at room temperature for 20 minutes with 5% bovine serum albumin (BSA). The following primary antibodies were applied for overnight staining: TLR2 (1:1000, EPR20302-119, Abcam, United States) at 4 °C. The next day, the sections were washed three times with PBST and incubated at room temperature for 1 hour with horseradish peroxidase (HRP)-conjugated secondary antibodies. After washing three times, the sections were stained with diaminobenzidine (Maxim) and counterstained with hematoxylin. They were then dehydrated and mounted with neutral resin. A pathology slide scanner was used to scan and image the tissue.
Colonic tissues embedded in optimal cutting temperature compound were sectioned into 5 μm slices using a cryostat. After allowing the sections to reach room temperature, they were fixed in 4% paraformaldehyde for 10 minutes at room temperature. The sections were then washed three times with PBST. Blocking was performed at room temperature for 30 minutes with 10% goat serum and 5% BSA. The sections were subsequently incubated overnight with the following primary antibodies: ZO-1 (Affinity, AF5145, 1:1000); Cluster of differentiation (CD) 206 (Cell Signaling Technology, E6T5J, 1:1000); CD86 (wanlei, WL05184, 1:1000). The next day, after washing three times with PBST, the sections were incubated at room temperature for 1 hour with Alexa Fluor 488-conjugated secondary antibodies. After washing three times with PBST, the sections were mounted using an antifade mounting medium containing 4’,6-diamidino-2-phenylindole. Images were captured using a confocal microscope (LAX DMi 3000).
Intestinal tissues were lysed in lysis buffer, and their protein content was quantified via bicinchoninic acid assay (Beyotime, China). After the use of a Bio-Rad Mini-PROTEAN system for the sodium dodecyl sulfate-polyacrylamide gel electrophoresis separation of these proteins, they were electrophoretically transferred to membranes composed of polyvinylidene difluoride (Bio-Rad, France), which were subsequently blocked for 60 minutes using 5% skim milk at room temperature, followed by probing overnight with 1:1000 dilutions of antibodies specific for TLR2 (EPR20302-119, Abcam); Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (60004-1-Ig; Proteintech, United States); Nuclear factor kappa-B (NF-κB) p65 (E379, Abcam); and phosphorylated-NF-κB p65 (E379, Abcam) at 4 °C. After incubating the blots with secondary HRP-linked goat anti-rabbit IgG (111-035-144) or HRP-linked goat anti-mouse IgG (115-035-003) from Jackson ImmunoResearch, Enhanced chemiluminescence was used to image bands, with quantification then being performed with ImageJ (v1.50i; NIH, MD, United States). GAPDH was used to normalize total protein levels (target protein/GAPDH).
Protein extraction and digestion in intestinal samples: Harvested intestinal tissues from CLP-operated mice (n = 4) and those administered with CLP + sphinganine (n = 4). Employed the filter-aided sample preparation technique for protein extraction and digestion: Precipitated proteins using a solvent mix (50% ethanol, 50% acetone, 0.1% acetic acid). Solubilized pellets in 6 mol/L guanidine hydrochloride and denatured at -20 °C. Reduced with 2 μL dithiothreitol at 60 °C for 1 hour, followed by alkylation with 10 μL indole-3-acetic acid in the dark at 4 °C for 40 minutes. Concentrated proteins using a 10-kDa molecular weight cut-off filter post-centrifugation at 12000 g. Digested with Trypsin (Promega, United States) at an enzyme: Protein ratio of 1:20. Purified and concentrated peptides using C18 cartridges after enzymatic desalting.
Peptides were resuspended in 0.1% formic acid to a final concentration of 0.5 mg/mL for mass spectrometer (MS) analysis using an Easy Nano-ultra performance liquid chromatography 1000 system coupled with a QE Plus MS. Separation employed a gradient elution with a flow rate of 300 nL/minute, increasing from 3% to 80% buffer B (0.1% trifluoroacetic acid in acetonitrile) over 75 minutes. MS acquisition was performed in data-dependent mode, selecting the top 20 precursors for MS/MS after each full scan, with an isolation window of 2.0 m/z and higher-energy collisional dissociation fragmentation at 35% energy. Dynamic exclusion was set for 60 seconds, with an automatic gain control target of 100000 and maximum injection times of 100 ms for MS and 35 ms for MS/MS.
Raw data were analyzed using MaxQuant v1.5.5.1 for protein quantitation and characterization. Search parameters included up to 2 missed trypsin cleavages, variable mods (oxidation, acetylation), 6 ppm mass error for precursors and fragments, and identification based on ≥ 2 unique peptides with 0.5 Da mass tolerance. Significance was set at P < 0.05 and false discovery rate (FDR) < 1%, with protein abundance assessed from at least 2 peptides. Peptides with P < 0.05 indicated significant group differences; proteins exhibited ≥ 2-fold up-regulation or ≤ 0.5-fold down-regulation. FDR, a key metric in hypothesis testing, estimates false positive rate among significant hits.
The data are presented as the mean ± SE of the mean. The one-way analysis of variance was used to compare the differences across multiple subgroups, while the t-test was used to analyze the differences between two groups. aP < 0.05, bP < 0.01, cP < 0.001.was established as the significance threshold.
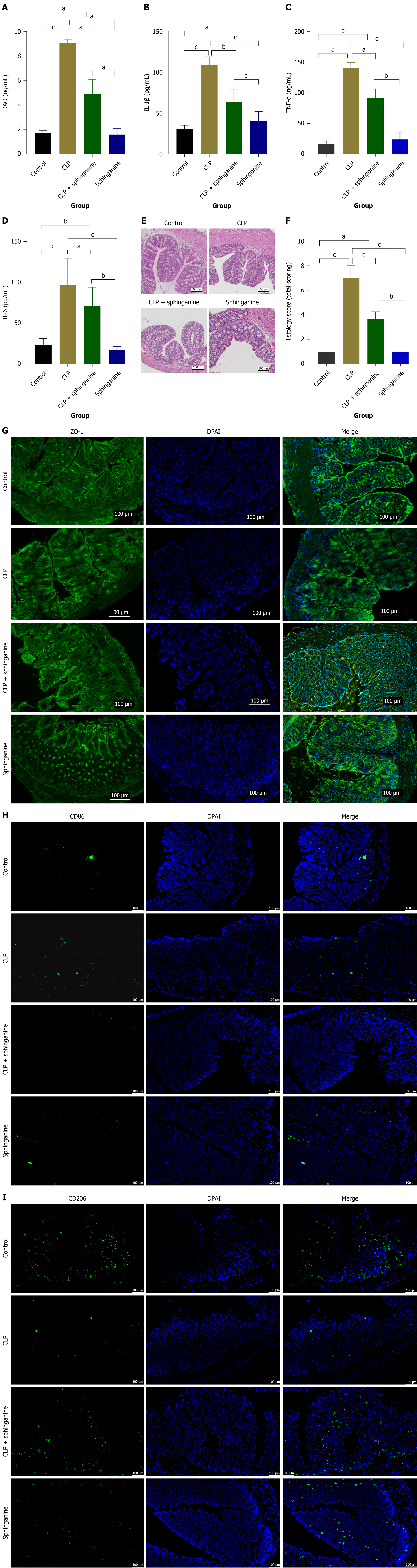
To elucidate the protective function of sphinganine against sepsis-induced intestinal injury, a mouse model study was performed. ELISA demonstrated a significant reduction in serum levels of DAO, IL-1β, TNF-α, and IL-6 following sphinganine administration (n = 6/group) (Figure 1A-D). Histopathological analysis of HE-stained colon tissue sections further validated sphinganine’s protective effects. Sepsis model mice exhibited severe intestinal damage characterized by near-complete loss of crypt architecture, pronounced dilation of the intestinal lumen, and extensive disruption of the brush border with fragmented microvilli. These findings collectively reflected a significant breakdown of the intestinal epithelial barrier, a key feature of sepsis-induced gut injury. In contrast, sphinganine treatment led to a notable attenuation of these pathological changes, preserving intact crypts, reducing lumen dilation, and restoring the continuity of the brush border microvilli, thereby reinforcing the integrity of the intestinal epithelial barrier (n = 3/group) (Figure 1E and F). Immunofluorescent staining further highlighted sphinganine’s protective role by demonstrating an increase in the expression of ZO-1, a critical tight junction protein essential for maintaining barrier function (n = 3/group) (Figure 1G). Regarding macrophage polarization, immunofluorescence analysis revealed that the expression of CD86, a marker of pro-inflammatory M1 macrophages, was upregulated in the CLP group but significantly decreased in the sphinganine-treated CLP group. Conversely, the expression of CD206, a marker of anti-inflammatory M2 macrophages, was downregulated in the CLP group but increased following sphinganine treatment (n = 3/group) (Figure 1H and I). These results indicate that sphinganine modulates macrophage polarization, shifting the balance from a pro-inflammatory to an anti-inflammatory state, thereby contributing to its protective effects against sepsis-induced intestinal injury.
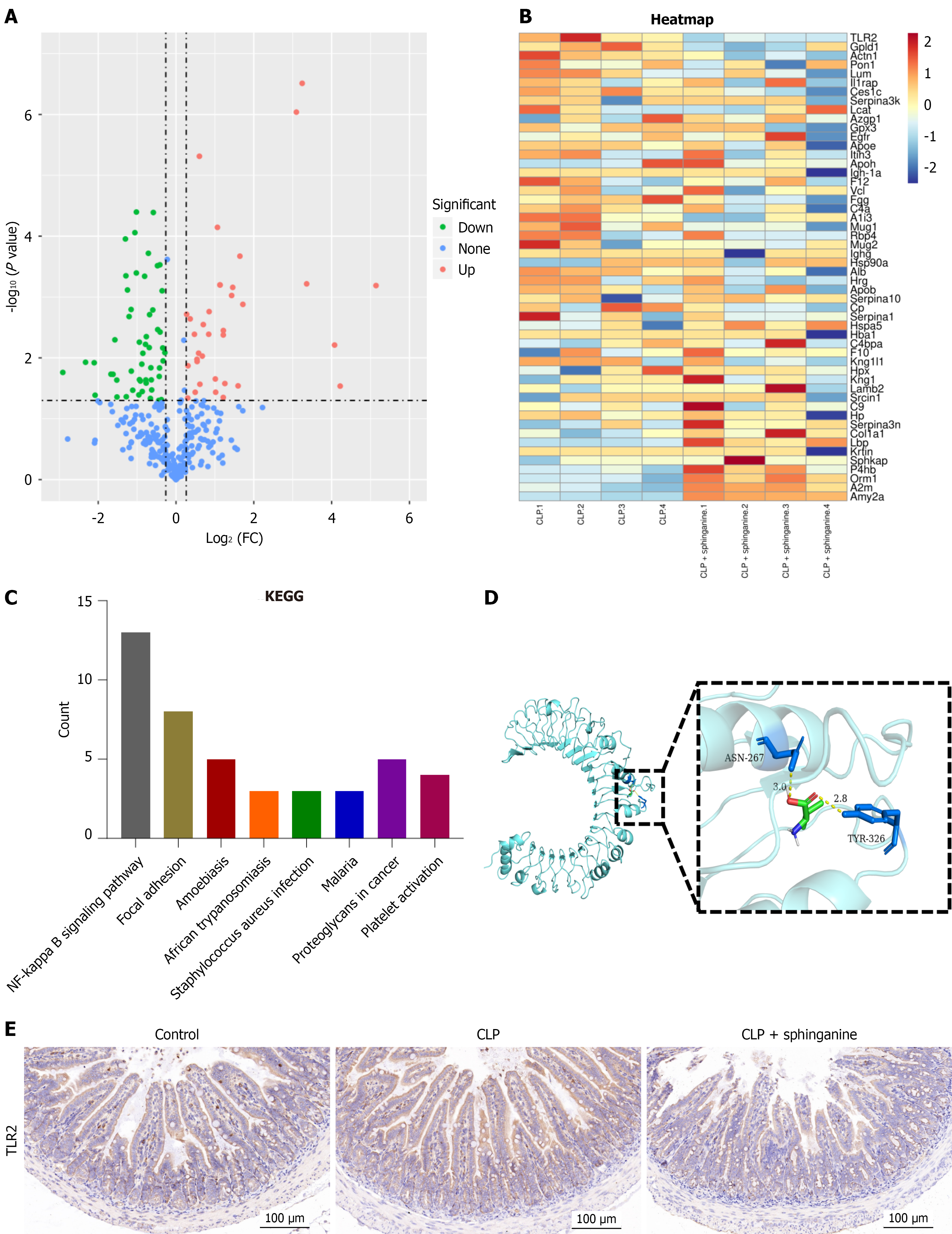
To analyze intestinal samples from the CLP (n = 4) and CLP + sphinganine (n = 4) groups, label-free proteomics was utilized. The differentially expressed proteins were analyzed and the results are shown in a volcano plot (Figure 2A). The relative abundance of each protein was summarized using a heatmap showing different levels of abundance (Figure 2B), and it was found that the expression of TLR2 differed most significantly between the two groups. Furthermore, Kyoto Encyclopedia of Genes and Genomes analysis also showed that NF-κB signaling pathway was the most significant compared with other pathways (Figure 2C). The docking results indicated a binding energy of -4.3 kcal/mol between sphinganine and TLR2 protein, indicating a potential interaction between sphinganine and the TLR2 (Figure 2D). TLR2 is a key pattern recognition receptor that plays a central role in coordinating of innate and adaptive immunity. Immunohistochemical analysis revealed increased TLR2 levels in the colon tissue samples from sepsis model mice, whereas sphinganine administration significantly reduced this upregulation (n = 3/group) (Figure 2E).
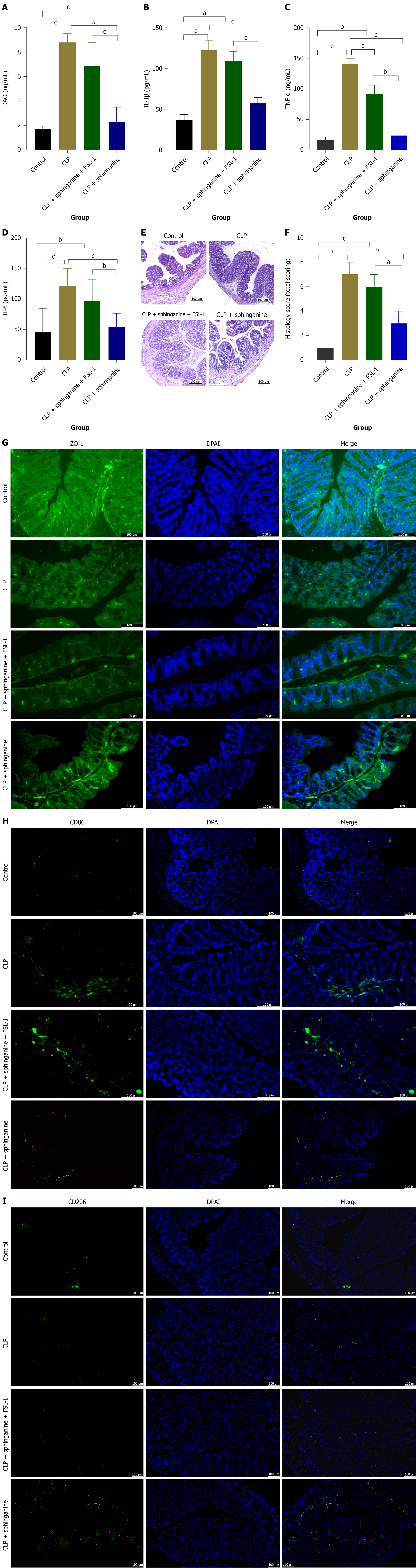
To elucidate the function of TLR2 in sphinganine protecting against sepsis-induced intestinal injury, a mouse model study was performed. ELISA demonstrated a significant reduction in serum levels of DAO, IL-1β, TNF-α, and IL-6 following sphinganine administration, however, this reduction was reversed by treatment with FSL-1 (a TLR2 agonist) (n = 6/group) (Figure 3A-D). HE staining revealed that FSL-1-mediated activation of TLR2 significantly aggravated sepsis-related intestinal damage (Figure 3E and F). Immunofluorescent staining further revealed a significant decrease in colon tissue ZO-1 expression in response to FSL-1 treatment (Figure 3G). With respect to macrophage polarization, immunofluorescence analysis demonstrated that the sphinganine-induced significant decrease in CD86 expression (a marker of pro-inflammatory M1 macrophages) in the CLP group was reversed by FSL-1 treatment. Conversely, the sphinganine-induced increase in CD206 expression (a marker of anti-inflammatory M2 macrophages) was also reversed by FSL-1 administration (n = 3/group) (Figure 3H and I).
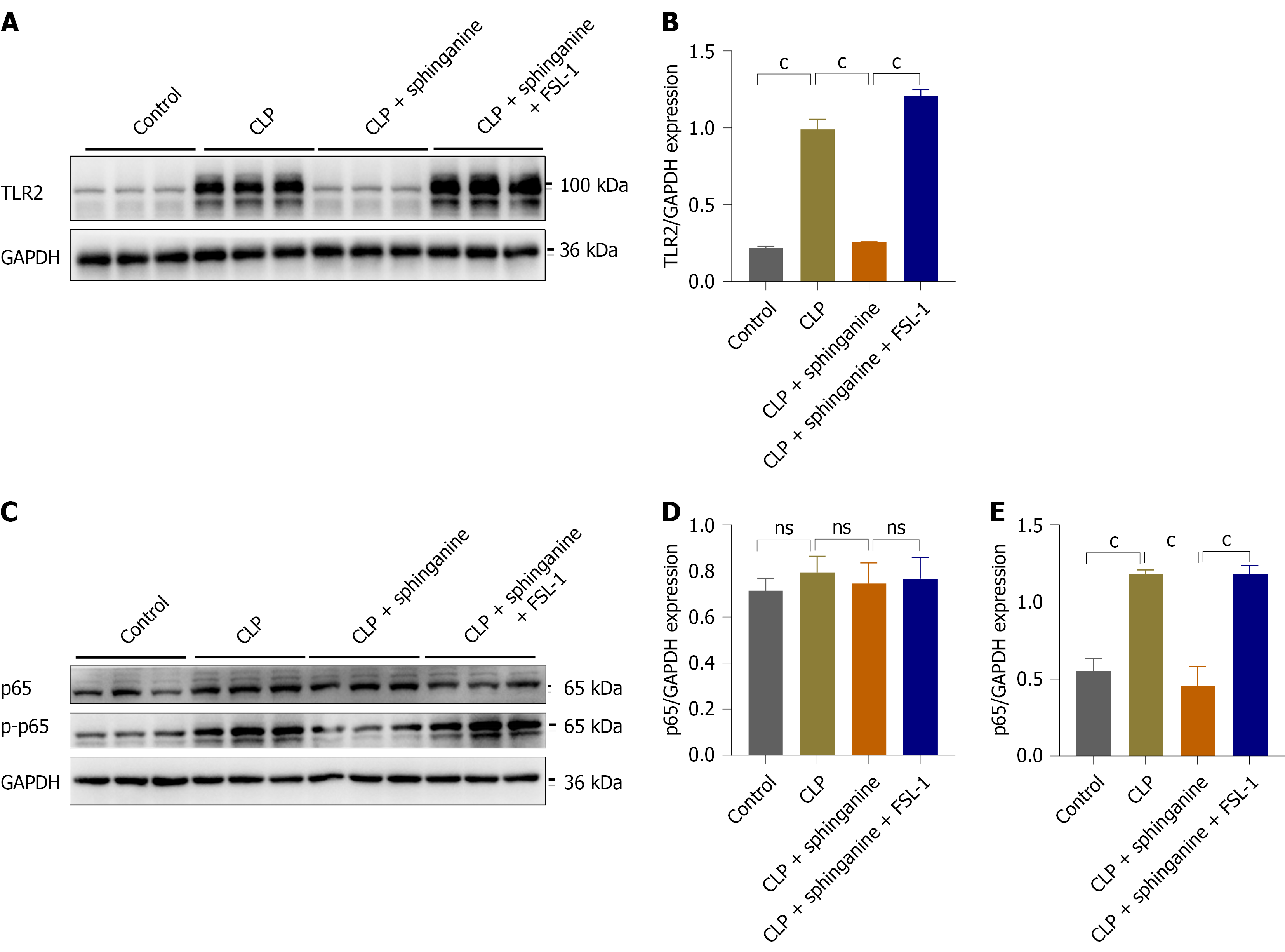
TLR2 signaling activation induces nuclear translocation of NF-κB[11]. In this study, colon tissue samples from sepsis model mice exhibited elevated levels of phosphorylated p65 (p-p65). However, sphinganine treatment reduced the expression of p-p65 protein, and this reduction was reversed when treated with FSL-1(n = 3/group). Additionally, total p65 protein was detected by Western blot, and the results showed no significant difference in total p65 expression among the groups (Figure 4 and Supplementary Figure 1). This is consistent with the known fact that NF-κB activation does not depend on changes in total p65 expression, indicating that sphinganine targets the TLR2/NF-κB axis to inhibit macrophage polarization, thereby protecting against sepsis-induced intestinal damages.
The pathogenesis of sepsis-induced intestinal injury is a complex process centered on the catastrophic breakdown of intestinal barrier function, which perpetuates a vicious cycle of inflammation, bacterial translocation, and multi-organ failure[12-14]. In this study, we identify the sphingolipid metabolite sphinganine as a potent protective agent against this pathology and delineate a novel mechanistic pathway through which it exerts its effects. Our principal finding is that sphinganine ameliorates intestinal injury by directly targeting the TLR2/NF-κB signaling axis in intestinal macrophages, thereby reprogramming their polarization from a pro-inflammatory (M1) to an anti-inflammatory (M2) phenotype, which in turn preserves epithelial barrier integrity and dampens systemic inflammation.
The integrity of the intestinal barrier is paramount in sepsis outcomes[15,16]. Our data corroborate previous findings that sepsis leads to a profound loss of tight junction proteins like ZO-1, and we demonstrate that sphinganine effectively reverses this defect[17]. This action is critical, as a compromised barrier is the primary gateway for pathogen translocation. The concomitant reduction in serum DAO, a highly sensitive marker of intestinal mucosal damage, further solidifies the conclusion that sphinganine’s primary site of action is the stabilization of the gut barrier. This aligns with a growing appreciation of the gut as a “motor” of sepsis and highlights the therapeutic potential of barrier-focused strategies.
The most significant and novel insight from this study is the identification of TLR2 as a key molecular target of sphinganine. Our integrative approach, combining label-free proteomics, in silico docking, and functional validation, provides a compelling chain of evidence. The proteomic analysis not only identified TLR2 as the most differentially expressed protein but also pointed to the NF-κB pathway as the dominant downstream mechanism. This is highly plausible, as TLR2 activation by gram-positive bacteria and fungal components is a major driver of immune dysregulation in polymicrobial sepsis[18]. The proposed direct interaction, with a favorable binding energy of -4.3 kcal/mol, suggests sphinganine may act as a functional TLR2 antagonist. This places sphinganine within an emerging class of endogenous lipid mediators that fine-tune innate immune responses, akin to how certain oxysterols modulate TLR3/4 signaling[19]. However, it is crucial to note that docking predictions require empirical validation; thus, sphinganine might also inhibit TLR2 signaling indirectly through upstream or parallel regulators.
The phenotypic shift in macrophages from CD86+ M1 to CD206+ M2 cells is likely the central cellular mechanism underpinning the improved outcomes[20]. Sepsis creates a pathological microenvironment that traps macrophages in a hyperactivated M1 state, unleashing a torrent of IL-1β, TNF-α, and IL-6 that directly damages the epithelium and recruits other immune cells[21]. By suppressing TLR2/NF-κB, sphinganine interrupts this positive feedback loop of inflammation. The subsequent promotion of M2 macrophages, which are adept at phagocytosing debris, producing anti-inflammatory IL-10, and facilitating tissue repair, would be essential for healing[22]. This is consistent with recent studies demonstrating that adoptive transfer of M2 macrophages or treatment with agents like L-malic acid that promote M2 polarization can significantly alleviate intestinal injury[5]. Our work positions sphinganine as an endogenous metabolite capable of orchestrating this beneficial reprogramming.
The use of the TLR2 agonist FSL-1 provides robust functional evidence for the necessity of this pathway. The fact that FSL-1 completely abrogated all of sphinganine’s benefits re-inflaming cytokine levels, reversing macrophage polarization, and disrupting the barrier powerfully argues that TLR2 inhibition is non-redundant and central to sphinganine’s mechanism of action.
In summary, this study unveils a novel and sophisticated mechanism by which the endogenous metabolite sphinganine safeguards the intestinal barrier in sepsis. It functions as a likely TLR2 signaling inhibitor, taming the hyperactive innate immune response by shifting macrophage polarization towards a pro-repair M2 phenotype. This work not only advances our understanding of sphingolipid immunology but also introduces sphinganine as a promising prototype for a new class of host-directed therapy for sepsis. By targeting a master regulator of inflammation (TLR2) to restore immune ho
The authors sincerely thank the staff of the Animal Experiment Center of East China Normal University for their expert technical support and assistance during the animal experiments. We also gratefully acknowledge our colleagues from the Department of Trauma-Emergency and Critical Care Medicine for their valuable discussions and insightful suggestions throughout this study.
| 1. | Zhou B, Zhang W, Yan Z, Zhao B, Zhao J, Feng W, Chen X, Li C, Liu KX. MicroRNA-26b-5p Targets DAPK1 to Reduce Intestinal Ischemia/Reperfusion Injury via Inhibition of Intestinal Mucosal Cell Apoptosis. Dig Dis Sci. 2022;67:1794-1805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Weis JA, Rauh JL, Ellison MA, Cruz-Diaz N, Yamaleyeva LM, Welch CD, Zeller KA, Weis VG. Photoacoustic Imaging for Non-Invasive Assessment of Physiological Biomarkers of Intestinal Injury in Experimental Necrotizing Enterocolitis. bioRxiv. 2023;2023.10.20.563296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Probst L, Schalk E, Liebregts T, Zeremski V, Tzalavras A, von Bergwelt-Baildon M, Hesse N, Prinz J, Vehreschild JJ, Shimabukuro-Vornhagen A, Eichenauer DA, Garcia Borrega J, Kochanek M, Böll B; Working Party on Intensive Care Medicine in Hematologic and Oncologic Patients (iCHOP) of the German Society of Hematology and Medical Oncology (DGHO). Prognostic accuracy of SOFA, qSOFA and SIRS criteria in hematological cancer patients: a retrospective multicenter study. J Intensive Care. 2019;7:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Klemetti MM, Alahari S, Post M, Caniggia I. Distinct Changes in Placental Ceramide Metabolism Characterize Type 1 and 2 Diabetic Pregnancies with Fetal Macrosomia or Preeclampsia. Biomedicines. 2023;11:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Zhang FL, Hu Z, Wang YF, Zhang WJ, Zhou BW, Sun QS, Lin ZB, Liu KX. Organoids transplantation attenuates intestinal ischemia/reperfusion injury in mice through L-Malic acid-mediated M2 macrophage polarization. Nat Commun. 2023;14:6779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 6. | Nierhaus A, Berlot G, Kindgen-Milles D, Müller E, Girardis M. Best-practice IgM- and IgA-enriched immunoglobulin use in patients with sepsis. Ann Intensive Care. 2020;10:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Scott E, De Paepe K, Van de Wiele T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules. 2022;12:1640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
| 8. | Xu M, Xin W, Xu J, Wang A, Ma S, Dai D, Wang Y, Yang D, Zhao L, Li H. Biosilicification-mimicking chiral nanostructures for targeted treatment of inflammatory bowel disease. Nat Commun. 2025;16:2551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Mei Y, Li W, Wang B, Chen Z, Wu X, Lin Y, Wang M. Gut microbiota: an emerging target connecting polycystic ovarian syndrome and insulin resistance. Front Cell Infect Microbiol. 2025;15:1508893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Liu M, Sheng Y, He Y, Wu S, Jin C, Shen L. Progresses in Questing for the Truth of Opioid-Related Constipation in Cancer Patients. J Cell Mol Med. 2025;29:e70553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Liu YX, Wang GD, Wang X, Zhang YL, Zhang TL. Effects of TLR-2/NF-κB signaling pathway on the occurrence of degenerative knee osteoarthritis: an in vivo and in vitro study. Oncotarget. 2017;8:38602-38617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Liu L, Yue Q, Chen J, Liu H, Zeng X. Intestinal injury signaling pathway in sepsis. Front Immunol. 2025;16:1620965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 466] [Cited by in RCA: 457] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 14. | Liu K, Sun H, Wang H, Zheng J, Wang X, Wang X, Hou Y, Ye Q, Li J. Ellagic acid alleviates sepsis-induced intestinal injury by modulating gut microbiota and NF-κB-mediated MLCK/MLC signaling pathway. Microb Pathog. 2025;208:108026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Yan Z, Niu L, Wang S, Gao C, Pan S. Intestinal Piezo1 aggravates intestinal barrier dysfunction during sepsis by mediating Ca(2+) influx. J Transl Med. 2024;22:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Kumar M, Leon Coria A, Cornick S, Petri B, Mayengbam S, Jijon HB, Moreau F, Shearer J, Chadee K. Increased intestinal permeability exacerbates sepsis through reduced hepatic SCD-1 activity and dysregulated iron recycling. Nat Commun. 2020;11:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 17. | Wang Z, Qi Y, Wang F, Zhang B, Jianguo T. Circulating sepsis-related metabolite sphinganine could protect against intestinal damage during sepsis. Front Immunol. 2023;14:1151728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Oliveira-Nascimento L, Massari P, Wetzler LM. The Role of TLR2 in Infection and Immunity. Front Immunol. 2012;3:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 592] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 19. | Hering M, Madi A, Sandhoff R, Ma S, Wu J, Mieg A, Richter K, Mohr K, Knabe N, Stichling D, Poschet G, Bestvater F, Frank L, Utikal J, Umansky V, Cui G. Sphinganine recruits TLR4 adaptors in macrophages and promotes inflammation in murine models of sepsis and melanoma. Nat Commun. 2024;15:6067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 20. | Fan Y, Hao Y, Gao D, Li G, Zhang Z. Phenotype and function of macrophage polarization in monocrotaline-induced pulmonary arterial hypertension rat model. Physiol Res. 2021;70:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 21. | Xing L, Zhongqian L, Chunmei S, Pingfa C, Lei H, Qin J, Genhua M, Yijun D. Activation of M1 macrophages in sepsis-induced acute kidney injury in response to heparin-binding protein. PLoS One. 2018;13:e0196423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Wang L, Yang K, Xie X, Wang S, Gan H, Wang X, Wei H. Macrophages as Multifaceted Orchestrators of Tissue Repair: Bridging Inflammation, Regeneration, and Therapeutic Innovation. J Inflamm Res. 2025;18:8945-8959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/