Published online Nov 28, 2025. doi: 10.3748/wjg.v31.i44.113017
Revised: September 18, 2025
Accepted: October 21, 2025
Published online: November 28, 2025
Processing time: 100 Days and 19.4 Hours
This review examines the distinct mechanisms of Tuina massage and topical herbal medicine in pediatric diarrhea management, focusing on their independent regulation of gastrointestinal metabolism and barrier function. Tuina therapy primarily enhances gastrointestinal motility through the stimulation of targeted acupoints such as Tianshu (ST25) and Shenque (CV8), activating vagal efferent pathways to normalize motility hormones including motilin while suppressing pro-inflammatory cytokines such interleukin-6 and tumor necrosis factor-alpha. It further reinforces mucosal defense by inhibiting mast cell degranulation and augmenting microcirculation via nitric oxide-mediated vasodilation. Herbal medicine containing bioactive compounds from Atractylodes macrocephala and Poria cocos directly restores intestinal barrier integrity by upregulating tight junction proteins occludin and zonula occludens-1, inhibiting epithelial apoptosis, and stimulating MUC2 production. These formulations additionally reprogram enterocyte metabolism by reactivating mitochondrial tricarboxylic acid cycle flux and modulating short-chain fatty acid profiles, with the independent prebiotic effects enhancing commensal butyrate production. Tuina primarily modulates neuroimmune pathways and motility, and herbal medicine directly targets epithelial repair and metabolism. These therapeutic pathways may address core diarrhea pathophysiology, providing holistic complementary therapies. Stan
Core Tip: This review elucidates the distinct mechanisms by which integrated traditional Chinese medicine therapies Tuina massage and topical herbal medicine ameliorate pediatric diarrhea. We highlight that Tuina primarily modulates neuro-immune pathways to regulate motility and inflammation, while herbal applications directly restore intestinal barrier integrity and reprogram enterocyte metabolism. Crucially, this work synthesizes evidence for their synergistic action in repairing the gut barrier, a core pathophysiological defect, providing a mechanistic foundation for non-pharmacological, holistic complementary strategies in children.
- Citation: Zhu Y, Dong HC, Zhang Y, Wang XA, Chen JY, Wang JJ. Application of traditional Chinese medicine integrated therapy based on Tuina and herbal medicine in pediatric diarrhea. World J Gastroenterol 2025; 31(44): 113017
- URL: https://www.wjgnet.com/1007-9327/full/v31/i44/113017.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i44.113017
Pediatric diarrhea remains a leading cause of morbidity and mortality in children under 5 years old, accounting for approximately 500000 annual deaths worldwide according to World Health Organization 2023 surveillance data[1-3]. Beyond acute mortality, recurrent episodes contribute to malnutrition, growth retardation, and compromised immune development[4-6]. Current first-line therapies primarily involve oral rehydration solutions (ORS), zinc supplementation, and antibiotics for bacterial pathogens[7-10]. However, significant limitations persist: ORS fails to reduce stool volume or duration[11,12], antibiotics induce gut dysbiosis and antimicrobial resistance, and symptomatic treatments such as loperamide are contraindicated in young children[13]. These gaps underscore the urgent need for safe, mechanism-targeted alternatives[14,15].
In traditional Chinese medicine (TCM), pediatric diarrhea is pathophysiologically characterized as Xiao’er Xiexie (pediatric diarrhea syndrome), a condition fundamentally attributed to spleen deficiency complicated by dampness accumulation. This paradigm postulates that compromised spleen-Qi impairs fluid and nutrient transportation, leading to dysfunctional digestion and intestinal barrier disruption. Contemporary TCM strategies employ external and internal interventions to address this pathomechanism. In particular, therapeutic massage (Tuina) modulates neuroimmunological pathways through stimulation of specific acupoints, especially Tianshu (ST25) and Shenque (CV8), thereby enhancing gastrointestinal motility and visceral sensitivity regulation[16]. Transdermal delivery of bioactive compounds from herbal applications, such as umbilical compresses containing Atractylodis Macrocephalae Rhizoma, directly targets intestinal mucosal metabolism[17-19].
TCM offers distinct nonpharmacological or adjunctive therapeutic strategies for pediatric diarrhea, primarily utilizing Tuina and topical herbal applications. While these modalities are frequently employed within a broad TCM management plan, either concurrently or sequentially depending on clinical judgment and presentation, they possess unique methodologies grounded in TCM theory and clinical practice. This section details the specific application procedures, core techniques, and key herbal formulations for Tuina and topical herbal therapy as documented in clinical research and practice for managing childhood diarrhea.
Pediatric Tuina, a specialized form of therapeutic massage adapted for the unique physiological and pathological characteristics of infants and children, constitutes a cornerstone of the integrated approach[20-22]. Its efficacy in pediatric diarrhea management is attributed to its ability to regulate gastrointestinal function through the precise stimulation of key acupoints and meridians using gentle, noninvasive manual techniques[23,24]. The selection of acupoints is guided by TCM syndrome differentiation, typically focusing on points known to fortify the spleen, harmonize the stomach, warm the middle jiao, arrest diarrhea, and regulate intestinal peristalsis[25].
Among the most clinically significant acupoints is ST25, bilaterally located adjacent to the umbilicus. Stimulation at ST25, primarily achieved through the application of Rou Fa (kneading technique), is fundamental for regulating intestinal movement, alleviating abdominal distension, and harmonizing the function of the large intestine[26]. CV8, the umbilicus itself, serves as a vital center for energy and is a primary site for Tuina manipulation and topical herbal application[27]. Gentle Mo Fu (circular rubbing) techniques centered on CV8 are routinely employed to warm the abdomen, strengthen the spleen yang, and dispel cold-dampness and are particularly relevant in deficiency or cold-type diarrhea presentations[28]. The lumbosacral region, specifically the Qijiegu (seven-segment bone, spanning from the lumbosacral depression to the coccyx tip) and Guwei (turtle tail, GV1, located at the tip of the coccyx), is another critical therapeutic focus. Manipulations in this area, particularly the technique of Tui Shang Qijiegu (pushing upward along the Qijiegu) and Rou Fa applied to GV1, are highly valued for their potent ability to warm and tonify the spleen and kidney yang, consolidate the intestines, and effectively stop chronic or deficiency-type diarrhea[29]. Furthermore, the manipulation of the spleen meridian (Pi Jing) on the radial aspect of the thumb, often using Bu Pi Jing (supplementing the spleen meridian technique) involving pushing toward the thumb tip, is essential for invigorating spleen qi, resolving dampness accumulation, and improving digestive and absorptive capacities[30]. Zusanli (ST36), located below the knee, is frequently stimulated with kneading techniques to broadly tonify the spleen and stomach qi, enhancing overall digestive function and nutrient assimilation[31,32].
The application of Tuina involves a repertoire of standardized, gentle manual techniques specifically adapted for pediatric patients[33,34]. Rou Fa (kneading) entails applying rhythmic, circular pressure using the pad of the thumb or the thenar eminence over specific acupoints such as ST25, ST36, or GV1 to generate a sensation that is soothing and regulatory[35]. Mo Fu (abdominal rubbing) involves broad, clockwise circular motions performed with the palm over the entire abdominal region, with particular emphasis around the umbilicus (CV8); this technique promotes overall abdominal warmth, regulates peristaltic activity, and alleviates discomfort[36]. Nie Ji (spine pinching) is a foundational pediatric Tuina technique where the skin and underlying tissue along the governor vessel (Du meridian, typically from the lower thoracic to the sacral region) are gently pinched and lifted sequentially; this powerful technique is believed to strengthen the spleen and lung, boost yang qi throughout the body, enhance overall resistance (Zheng Qi), and regulate autonomic nervous function influencing the gut[37]. Tui Shang Qijiegu (pushing upwards on the seven-segment bone) involves applying unidirectional, firm yet gentle strokes upward along the Qijiegu region, usually by using the radial side of the thumb or the pads of the index and middle fingers, specifically to warm yang and lift sinking qi to arrest diarrhea[38]. Treatment sessions are conducted with careful attention to the child’s comfort and response, with typical session durations falling within a clinically established range suitable for pediatric tolerance and administered at a frequency commonly employed in practice over a therapeutic course determined by the severity and chronicity of the condition[39,40].
Complementing Tuina manipulations, topical medicinal herbs, most frequently applied via the umbilical region, represents the second pillar of the integrated TCM approach for pediatric diarrhea[41]. This method leverages the unique anatomical and physiological properties of the umbilicus, recognized in TCM as a highly permeable gateway connected to the internal organs and in biomedicine as a site with relatively thin stratum corneum, rich vascularity, and absence of subcutaneous, all of which facilitate the efficient transdermal and transmucosal absorption of bioactive compounds[42,43]. This absorption efficiency may be further heightened in infants and young children because of their thinner stratum corneum and greater skin permeability compared with adults. While these features enhance the delivery of therapeutic compounds, they underscore the importance of careful dosage calibration and monitoring for potential local or systemic adverse effects in this vulnerable population. Topical herbal therapy circumvents the challenges of oral administration in young children, such as poor compliance, gastric irritation, or first-pass metabolism, allowing for the direct delivery of therapeutic agents to the affected abdominal region[44-46].
The selection of herbal formulas for umbilical compresses (Fu Qi Liao Fa) is meticulously based on TCM syndrome differentiation to address the core patterns underlying pediatric diarrhea, predominantly spleen deficiency, dampness encumbrance, yang deficiency, or food stagnation. Spleen-strengthening and dampness-resolving formulas are central to the treatment. These formulations typically incorporate herbs such as Baizhu (Rhizoma Atractylodis Macrocephalae and Atractylodes macrocephala) and Fuling (Poria and Poria cocos), renowned for their abilities to fortify the spleen, promote diuresis, and drain pathogenic dampness, which address a fundamental pathological factor in diarrhea[47-49]. Spleen function-regulating formulas might include components such as Taizishen (Radix Pseudostellariae and Pseudostellaria heterophylla) and Cangzhu (Rhizoma Atractylodis and Atractylodes lancea) to restore the spleen’s crucial role in transportation and transformation (Jian Yun Pi Wei), which are essential for normal digestion and stool formation[50,51]. Warm herbal compresses (Re Yan Bao) are often utilized for presentations involving cold patterns, abdominal pain, or yang deficiency[52]. These warm packs contain herbs with thermogenic and dispersing properties, such as Wuzhuyu (Fructus Evodiae and Evodia rutaecarpa) and Laifuzi (Semen Raphani and Raphanus sativus seeds), applied to warm the middle jiao, dispel cold pathogens, alleviate cramping pain, and promote the downward flow of qi to regulate bowel movements[53,54].
The preparation and application of these topical herbs follow specific protocols. Selected herbs are typically processed into fine powders or blended into thick, paste-like consistencies using appropriate excipients to enhance adhesion and transdermal penetration[55]. Before application, the umbilical region and sometimes the surrounding lower abdomen are meticulously cleansed. The prepared herbal mixture is then applied directly to the umbilicus, covered with a sterile gauze pad or a specialized adhesive patch designed for prolonged wear[56,57]. While generally safe, topical medicinal herbs may occasionally cause local skin reactions, such as erythema or pruritus, particularly in children with sensitive skin or herbal allergies. Contraindications include broken skin or known hypersensitivity to specific herbal components. The compress is typically retained for a duration considered optimal for therapeutic effect while minimizing potential skin irritation, applied daily over a treatment course aligned with clinical need and response monitoring. This method ensures the sustained contact and delivery of bioactive constituents through the highly vascularized umbilical area[58,59]. The core Tuina techniques and herbal formulations applied in clinical practice are systematically categorized in Table 1.
| Therapy modality | Specific technique/herbal formula type | Key components/target | Standard application | Primary proposed therapeutic mechanisms |
| Tuina | Rou Fa (kneading) | ST25, ST36, GV1 | Gentle rhythmic pressure applied to specific points during sessions | Local stimulation modulating neural reflexes influencing gut motility/secretion; promoting local Qi/blood flow |
| Tuina | Mo Fu (abdominal rubbing) | Abdomen centered on CV8 | Broad clockwise circular motions over abdomen | Regulating peristalsis, alleviating spasm/distension; warming the middle jiao; resolving abdominal Qi stagnation |
| Tuina | Nie Ji (spine pinching) | Skin along Du meridian (GV14-GV4 region) | Gentle sequential pinching and lifting | Regulating autonomic nervous system; strengthening overall Zheng Qi (spleen/Lung); influencing gut-brain axis |
| Tuina | Tui Shang Qijiegu | Qijiegu region (GV2-GV1) | Upward unidirectional pushing | Warming spleen/kidney Yang; lifting sinking Qi to consolidate intestines and stop diarrhea |
| Tuina | Bu Pi Jing (supp spleen meridian) | Radial side of thumb (spleen meridian) | Pushing towards thumb tip | Tonifying spleen Qi; resolving dampness; enhancing digestive and absorptive functions |
| Topical herbal (umbilical) | Spleen-fortifying and damp-resolving | Atractylodes macrocephala (Baizhu), Poria cocos (Fuling) | Paste applied to CV8 region daily | Transdermal absorption of compounds modulating intestinal fluid transport/ion channels; reducing inflammation; strengthening spleen function |
| Topical herbal (umbilical) | Spleen-transporting and stagnation-resolving | Pseudostellaria heterophylla (Taizishen), Atractylodes lancea (Cangzhu), Crataegus pinnatifida (Shanzha) | Paste applied to CV8 region daily | Enhancing digestive enzyme activity; promoting gastrointestinal motility; resolving food accumulation; regulating gut microbiota |
| Topical herbal (umbilical) | Warm compress (Re Yan Bao) for cold | Evodia rutaecarpa (Wuzhuyu), Raphanus sativus (Laifuzi) | Warmed paste applied to CV8 region daily | Dispelling cold pathogen; warming middle jiao; alleviating smooth muscle spasm/pain; promoting downward Qi movement |
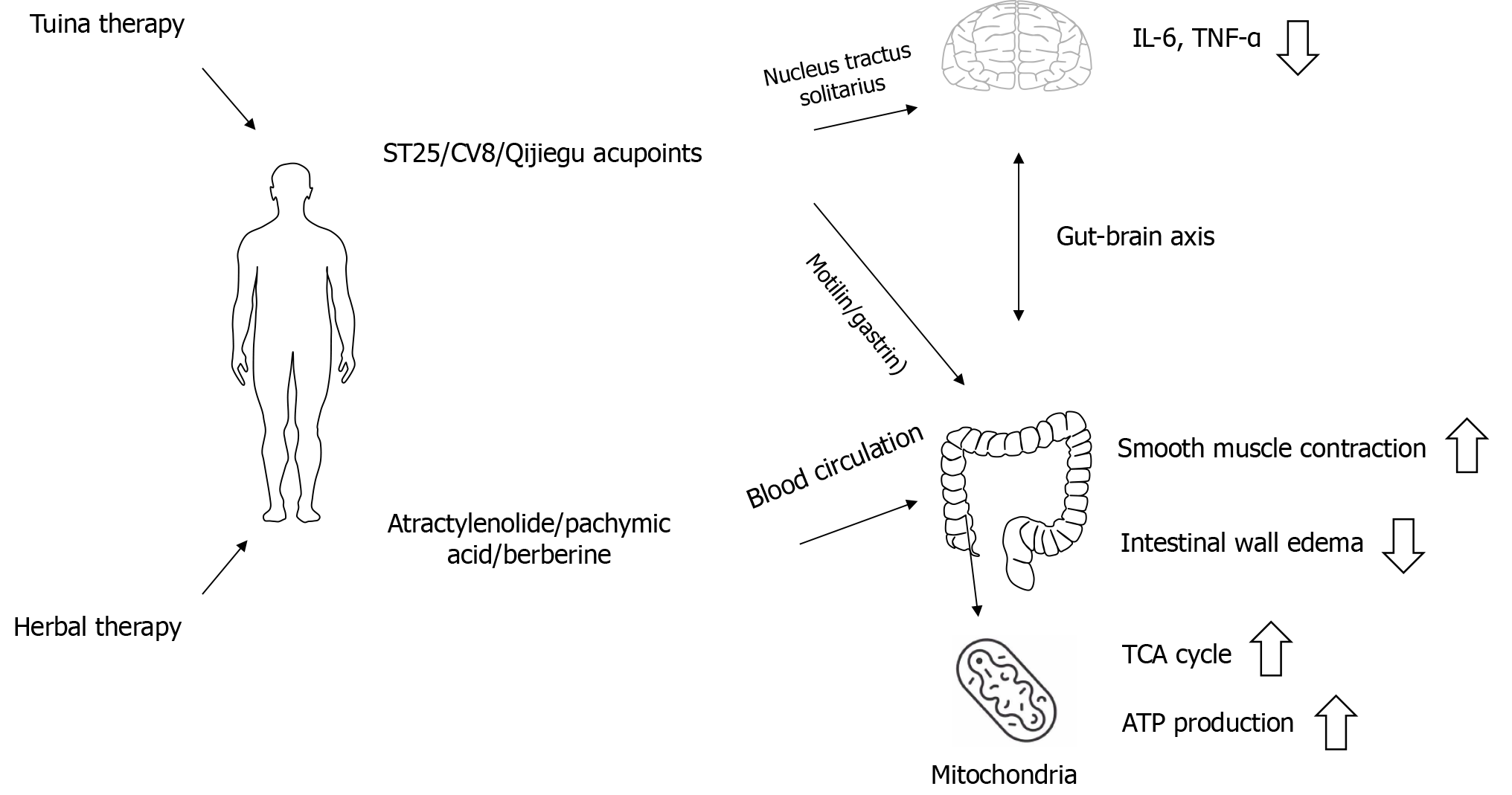
The pathophysiology of pediatric diarrhea involves the complex dysregulation of intestinal motor function and metabolic processes. TCM interventions exert therapeutic effects through distinct mechanistic pathways. Tuina therapy primarily modulates neuroimmunological circuits, whereas transdermal herbal applications directly influence epithelial metabolism and restore gastrointestinal homeostasis. Figure 1 illustrates the mechanisms through which Tuina therapy and transdermal herbal applications regulate gastrointestinal motility and metabolic homeostasis in the management of pediatric diarrhea, as detailed in the following subsections.
Pediatric Tuina exerts profound effects on gastrointestinal motility through somatovisceral neural reflex arcs[60]. The mechanical stimulation of specific abdominal and lumbosacral acupoints generates afferent signals transmitted via spinal cord pathways to central autonomic nuclei. ST25 and CV8 stimulation activates vagal efferent projections to the enteric nervous system[61,62]. This neural modulation enhances the synthesis and release of key gastrointestinal hormones including motilin and gastrin, which serve as critical regulators of intestinal contractile activity and migrating motor complex patterns[63]. The subsequent normalization of peristaltic rhythms facilitates efficient luminal content transit, reducing fluid sequestration in the intestinal lumen that characterizes diarrheal states[36]. Furthermore, afferent signals from spinal manipulation sites such as Qijiegu converge in the nucleus tractus solitarius, establishing bidirectional communication along the gut–brain axis that modulates the central processing of visceral sensitivity[64].
Concurrent with its neurological actions, Tuina demonstrates significant immunoregulatory properties relevant to diarrheal pathophysiology. Manual stimulation at key acupoints reduces the systemic and local intestinal concentrations of pro-inflammatory cytokines, particularly interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α)[65-67]. This cytokine downregulation attenuates the inflammatory cascade that disrupts epithelial tight junctions and increases vascular permeability[68]. Clinical studies further indicated measurable reductions in acute-phase reactants such as C-reactive protein following Tuina regimens, suggesting its systemic anti-inflammatory effects[66]. The amelioration of intestinal wall edema through these mechanisms contributes substantially to the restoration of normal mucosal barrier function and reduction in fluid exudation into the intestinal lumen[69].
Topical herbal applications directly affects intestinal epithelial metabolism through the systemic absorption and enterohepatic recirculation of bioactive constituents[70]. Pharmacokinetic studies demonstrated that lipophilic compounds from umbilical compresses, including sesquiterpenes from Atractylodes macrocephala and triterpenoids from Poria cocos, are significantly concentrated in intestinal tissues[71]. These phytochemicals enhance epithelial integrity through multiple pathways: Modulating the expression of tight junction proteins including occludin and zonula occludens-1 (ZO-1), reducing epithelial apoptosis rates, and stimulating mucin production from goblet cells[71,72]. From a clinical per
Beyond their structural effects, herb permeates significantly reprogram cellular metabolism within the intestinal epithelium[75]. Metabolomic analyses revealed that key herbal compounds influence mitochondrial function, particularly by restoring tricarboxylic acid cycle flux and enhancing fatty acid β oxidation[76,77]. This metabolic shift increases adenosine triphosphate production capacity in enterocytes, providing the energy substrate necessary for active nutrient transport and epithelial repair[77]. Certain herbal constituents demonstrate inhibitory effects on sodium glucose cotransporter overactivity, thereby reducing osmotically driven water secretion into the intestinal lumen. The net effect is a reestablishment of the normal absorptive-secretory balance in the intestinal epithelium[78].
Tuina and herbal therapies may indirectly influence host metabolism by modulating microbial-derived metabolites. Herbal compounds such as berberine undergo microbial biotransformation into active metabolites that inhibit bacterial pathogen virulence factors while stimulating the commensal production of short-chain fatty acids (SCFAs)[79]. These microbiota-derived SCFAs serve as preferential energy substrates for colonocytes and activate G-protein-coupled receptors (GPCRs) that regulate gastrointestinal motility reflexes[80,81]. The mechanical pressure from abdominal Tuina may further enhance microbial metabolite absorption through improved mucosal perfusion, creating a metabolic environment that supports epithelial repair[82].
These integrated effects collectively reverse the pathophysiological triad of diarrhea: Accelerated transit, reduced absorption, and increased secretion. While clinical studies often reported these outcomes as a whole, mechanistic research increasingly delineated the distinct contributions of neural modulation vs direct metabolic intervention, providing a scientific foundation for their application in pediatric diarrheal disorders.
The intestinal barrier constitutes a critical defense system comprising physical, immunological, and microbial components. Its dysfunction in pediatric diarrhea involves epithelial damage, impaired tight junctions, reduced mucus production, and dysregulated immune responses. TCM interventions target these pathological alterations through distinct mechanistic pathways, facilitating comprehensive barrier restoration.
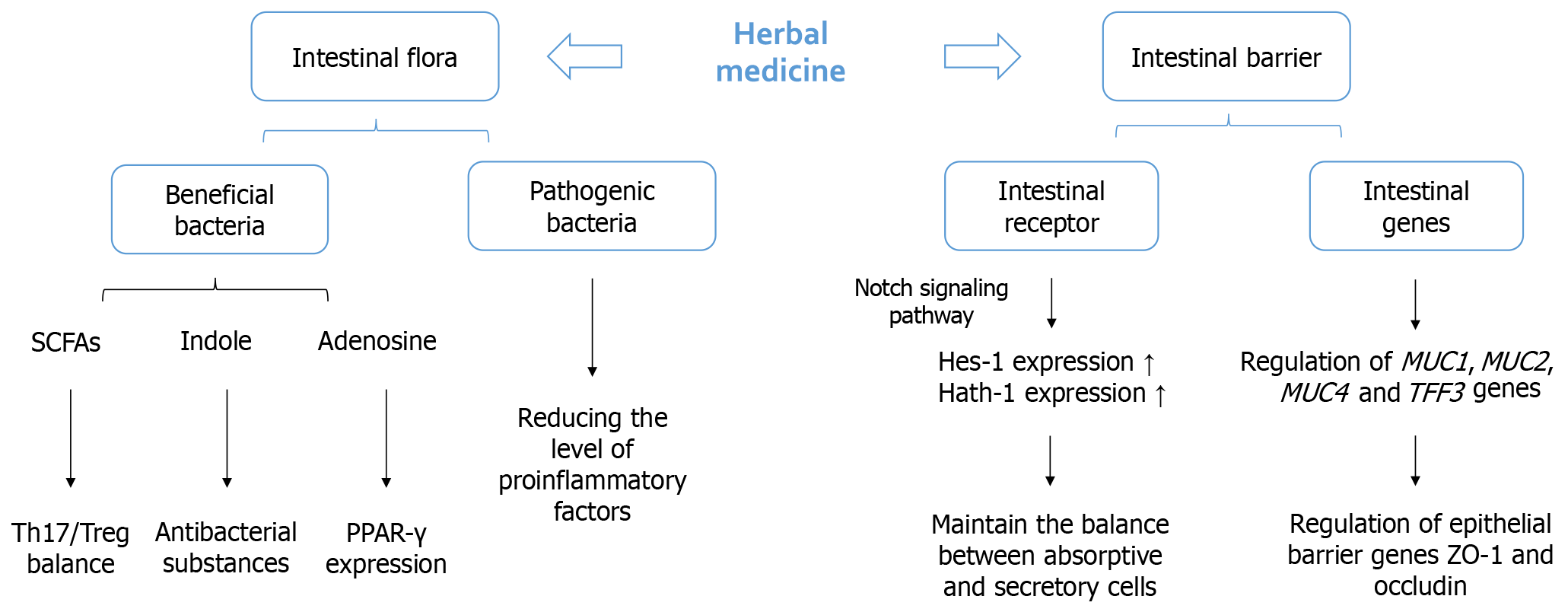
Herbal applications demonstrate significant efficacy in enhancing epithelial structural integrity. Bioactive compounds such as polysaccharides from Poria cocos and atractylenolides from Atractylodes macrocephala upregulate the expression of tight junction proteins, particularly occludin and ZO-1[83,84]. These proteins form the primary seal between adjacent enterocytes, regulating paracellular permeability. Experimental models confirmed that herbal permeates reduce intestinal permeability by reinforcing these protein complexes, thereby limiting the translocation of luminal antigens and endotoxins[85]. This effect correlates with decreased intestinal permeability, reflecting improved barrier competence[86]. The molecular mechanisms involve modulating the signaling pathways for actin cytoskeleton organization and suppressing myosin light chain kinase activation, which disrupts junctional complexes during inflammation[87].
The mucus layer serves as the primary chemical barrier against pathogens in the gut lumen[88]. Topical herbal formulations, which contain compounds such berberine from Coptis species, promote the differentiation of goblet cells and increase expression of the MUC2 gene[89]. MUC2 forms the primary structural component of the protective gel layer, trapping pathogens and facilitating their clearance[88]. The reduced activity of intestinal mast cells (MCs) observed with Tuina might indirectly aid mucus production. Activated MCs release histamine and TNF-α, which inhibit goblet cell function and deplete mucin reserves[90]. By inhibiting MC degranulation in the colon, Tuina mitigates this suppression and allows unimpeded herbal-induced mucin upregulation[91]. The central effect of Tuina on hippocampal MCs is postulated to be modulating the brain-gut axis via descending pathways. This regulation likely involves the vagus nerve, i.e., increasing acetylcholine release to promote intestinal motility and reduce inflammation and rebalancing serotonin signaling, which is crucial in gut secretion and sensation[90]. In addition, Tuina therapy provides protection through mechanotransduction pathways. Gentle manipulation of the abdomen lowers the rate of epithelial cell death by decreasing the levels of pro-apoptotic factors such as caspase-3 while boosting cell growth[92]. The mucus production induced by medicinal herbs and the cytoprotective effect of Tuina work together to thicken the mucus layer and maintain epithelial integrity, resulting in a strong barrier against harmful substances in the gut lumen.
Herbal-directed suppression operates primarily through phytochemical interactions, where compounds such as evodiamine from Evodia rutaecarpa inhibit nuclear factor-kappa B nuclear translocation, thereby suppressing the downstream expression of pro-inflammatory cytokines including IL-6 and TNF-α, which collectively reduce neutrophil infiltration and mitigate epithelial cytokine exposure[93]. By contrast, Tuina-mediated regulation leverages neuroimmunological pathways. Pediatric Tuina targeting spleen-meridian and large-intestine-meridian acupoints significantly reduces MC infiltration and tryptase release in colonic tissues, directly ameliorating mucosal inflammation and barrier disruption in spleen-deficiency diarrhea[94]. This finding aligns with the established role of MCs in rapidly responding to intestinal anomalies by triggering local inflammation[95]. Clinical studies further confirmed that heightened MC activation correlates with symptom severity in diarrheal conditions[96], supporting Tuina’s therapeutic mechanism. Abdominal massage similarly reduces colonic MC hypertrophy in irritable bowel syndrome models[97], validating the broad relevance of MC modulation. Furthermore, mechanical stimulation at acupoints such as ST25 can activate the vagal-adrenal anti-inflammatory axis to increase the systemic release of anti-inflammatory mediators such as IL-10, ultimately attenuating dendritic cell maturation and T-helper 17 cell polarization within the lamina propria[98,99]. The convergence of these interventions disrupts the inflammatory cascade responsible for tight junction degradation and elevated epithelial permeability, establishing a microenvironment conducive to mucosal repair through the coordinated resolution of immunopathological processes[100].
Intestinal barrier dysfunction involves microvascular compromise and ischemia-reperfusion injury. Tuina techniques, particularly abdominal Mo Fu, enhance local microcirculation through neurogenic vasodilation and nitric oxide release. Improved tissue perfusion alleviates hypoxia-induced epithelial damage and supports cellular repair[101]. Certain herbal components (e.g., paeoniflorin from Paeonia lactiflora) further stabilize endothelial tight junctions and reduce vascular permeability, limiting edema formation in the submucosa. This vascular normalization reduces hydrostatic pressure on the epithelial barrier and facilitates nutrient delivery for tissue regeneration[102].
Emerging evidence highlights the gut microbiota’s role in barrier maintenance through metabolite production. Herbal compounds serve as prebiotic substrates for commensal bacteria, stimulating the production of SCFAs, particularly butyrate[103]. Butyrate serves as the primary energy source for colonocytes, enhances mucin synthesis, and strengthens tight junctions via the activation of GPCRs[104]. Tuina may indirectly support this by improving luminal SCFA absorption through enhanced mucosal blood flow. Pediatric Tuina modulates the skin-brain-gut axis by suppressing hippocampal MC activation and tryptase release, implicating neuroimmune pathways in gut barrier restoration[94]. This systemic regulation is critical because brain–gut axis dysregulation exacerbates intestinal barrier failure in chronic diarrhea[105]. Both interventions reduce pathogenic bacterial adherence to the epithelium by downregulating host adhesion molecules.
The multidimensional barrier restoration mechanisms detailed above spanning epithelial reinforcement, mucus enhancement, immunomodulation, and microbiota regulation were empirically validated through preclinical and clinical studies of herbal medicine interventions. Table 2 systematically synthesizes key evidence demonstrating herbal-mediated repair across physical, chemical, and immunological barrier components. Meanwhile, Figure 2 illustrates the critical interplay between herbal-modulated gut microbiota and intestinal barrier subsystems[106]. Together, these visual summaries consolidate how TCM strategies collectively resolve barrier dysfunction to ameliorate pediatric diarrhea.
| Barrier component | Assessment marker | Intervention modality | Observed effect | Proposed mechanism | Ref. |
| Physical barrier | Intestinal permeability/tight junction proteins | TCM monomer (baicalein) | Reduced intestinal permeability/restored colonic tight junction integrity/attenuated gut inflammation | Activation of AhR in ILC3s-promotes IL-22 production-upregulates TJ proteins | Li et al[107] |
| Serum DAO/gastrointestinal dysfunction score/first exhaust and defecation time | Modified Huanglian Jiedu decoction + electroacupuncture | Reduced serum DAO levels, gastrointestinal dysfunction scores and mechanical ventilation time/accelerated first exhaust/defecation time | Purgation and detoxification reduced intestinal permeability and restored barrier integrity/DAO reduction indicates attenuated epithelial damage | Wang et al[108] | |
| Chemical barrier | Goblet cell density/MUC2 mRNA expression/mucin production | Gegen Qinlian decoction | Goblet cell differentiation/MUC2 synthesis/restored mucus layer/promoted mucosal healing | Bidirectional regulation of Notch signaling | Zhao et al[109] |
| Immune barrier | SIgA expression/plasma DAO/D-lactate/gut microbiota diversity | Hetiao Jianpi decoction | SIgA in colon tissue (increase)/beneficial bacteria (increase)/plasma DAO/D-lactate (decrease)/pathogenic bacteria (decrease) | Enhanced mucosal immunity via SIgA secretion-reduced intestinal permeability-modulated gut microbiota to restore immune homeostasis | Li et al[110] |
The mechanisms discussed above, particularly those involving the gut microbiota and immune development, may exhibit age-dependent variations. The majority of clinical evidence supporting the efficacy of TCM interventions, such as the Tuina trials in children aged 0-6 years[21,107] and herbal applications in children aged from 6 months to 12 years[52], comes from a broad pediatric age range. The infant gut microbiome is highly dynamic and undergoes sequential maturation, which may influence the response to herbal prebiotics and the anti-inflammatory effects of Tuina. Similarly, the development of the intestinal immune system and the integrity of the epithelial barrier are age-related processes. While the core pathophysiological mechanisms targeted by TCM therapies are consistent, the magnitude of response and the relative contribution of each pathway (e.g., microbial modulation vs neural-immune regulation) might differ between infants and older children. Future mechanistic studies should stratify participants by age to elucidate these developmental nuances, which will be crucial for optimizing personalized treatment protocols.
While the integrated application of Tuina and herbal medicine shows promise for pediatric diarrhea management, several key challenges must be addressed to solidify its evidence base and optimize its clinical implementation. Standardization represents a primary hurdle. Variations in Tuina techniques among practitioners and the individualized nature of TCM herbal prescriptions based on pattern differentiation complicate the design of rigorous, reproducible clinical trials and hinder the development of universally applicable protocols. Future efforts should prioritize establishing detailed, consensus-driven guidelines for Tuina manipulation specific to pediatric diarrhea and exploring strategies for standardizing core herbal components or formulations while respecting TCM principles to facilitate large-scale, high-quality studies.
The heterogeneity in Tuina techniques and herbal formulations across practitioners currently poses a significant limitation in interpreting clinical outcomes. This variability may affect the reproducibility and generalizability of study results, thereby underscoring the need for standardized protocols in future research.
To move past clinical observations, we need to deeply understand the specific biological pathways affected by these combined therapies. Sophisticated in vitro models such as patient-derived intestinal organoids and gut-on-a-chip systems provide a valuable tool for this investigation. They allow researchers to pinpoint how mechanical forces that mimic Tuina, along with individual herbal compounds or their combinations, influence key aspects of diarrhea, such as the epithelial barrier, cell metabolism, interactions with microbes, and immune signaling pathways.
Integrating new technologies also offers important benefits. Developing novel delivery systems may improve practicality and adherence, especially for children. This step involves creating wearable devices that deliver controlled, standardized physical stimulation similar to Tuina at specific acupoints. It also includes designing advanced transdermal patches for the sustained, localized release of optimized herbal extracts. Longitudinal multi-omics analyses, including metagenomics, metabolomics, and proteomics, within clinical groups are also crucial. This comprehensive approach will map the body’s and gut microbes’ responses to integrated TCM therapy. It will identify reliable biomarkers of effectiveness and further clarify the complex network of interactions responsible for its therapeutic benefits.
Ultimately, progress hinges on interdisciplinary collaboration. Bridging the expertise of TCM practitioners, pediatric gastroenterologists, microbiologists, immunologists, pharmacologists, and biomedical engineers is paramount. Such collaborative frameworks are necessary to rigorously validate the efficacy and safety of integrated TCM approaches, refine treatment protocols based on mechanistic insights, and effectively translate this knowledge into tangible im
Pediatric diarrhea remains a significant global health challenge, demanding effective and well-tolerated therapeutic strategies. This review synthesizes evidence supporting the application of integrated TCM therapies, specifically Tuina manipulation and herbal medicine, for managing this condition. The therapeutic rationale centers on their modulation of two fundamental pathophysiological aspects: Gastrointestinal metabolic dysfunction and impaired intestinal barrier integrity. Evidence indicates that Tuina, by applying physical stimulation to specific acupoints and abdominal areas, can improve gastrointestinal motility, help regulate fluid secretion and absorption, and enhance local microcirculation. The bioactive compounds found in TCM herbal formulas seem to help normalize enterocyte metabolism and energy production, supporting the digestion and absorption of nutrients. Tuina and herbal medicine demonstrate significant effects on restoring intestinal barrier function. Herbal components often aid in epithelial repair, strengthen tight junctions, and encourage mucus production. Tuina may additionally enhance mucosal defense mechanisms. Although not the main focus (i.e., metabolism and barrier function), the positive effects on gut microbiota composition and mucosal immune responses are also notable. Herbal medicine can modulate immune factors such as secretory immunoglobulin A secretion and immune cell balance, and Tuina may also influence these areas. Together, these actions support barrier restoration and help establish a resilient gut environment. The integrated TCM approach of Tuina and herbal medicine offers a promising strategy for pediatric diarrhea. Its strength lies in the potential role of physically stimulating gastrointestinal function and biochemically repairing barrier integrity and metabolic capacity, addressing core pathophysiological mechanisms in a holistic manner. Future research emphasizing rigorous standardization and mechanistic exploration using advanced tools is essential to further validate and optimize this multidimensional therapeutic paradigm for integration into comprehensive pediatric care.
| 1. | Cohen AL, Platts-Mills JA, Nakamura T, Operario DJ, Antoni S, Mwenda JM, Weldegebriel G, Rey-Benito G, de Oliveira LH, Ortiz C, Daniels DS, Videbaek D, Singh S, Njambe E, Sharifuzzaman M, Grabovac V, Nyambat B, Logronio J, Armah G, Dennis FE, Seheri ML, Magagula N, Mphahlele J, Fumian TM, Maciel ITA, Gagliardi Leite JP, Esona MD, Bowen MD, Samoilovich E, Semeiko G, Abraham D, Giri S, Praharaj I, Kang G, Thomas S, Bines J, Liu N, Kyu HH, Doxey M, Rogawski McQuade ET, McMurry TL, Liu J, Houpt ER, Tate JE, Parashar UD, Serhan F. Aetiology and incidence of diarrhoea requiring hospitalisation in children under 5 years of age in 28 low-income and middle-income countries: findings from the Global Pediatric Diarrhea Surveillance network. BMJ Glob Health. 2022;7:e009548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 2. | Fifi A, Raphael BP, Terreri B, Uddin S, Kaufman SS. Effects of Teduglutide on Diarrhea in Pediatric Patients with Short Bowel Syndrome-Associated Intestinal Failure. J Pediatr Gastroenterol Nutr. 2023;77:666-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Chen XQ, Zhang KL, Shan QW. Bloody Diarrhea Caused by Intestinal Myiasis in an Infant: A Case Report and Review of Pediatric Literature. J Trop Pediatr. 2021;67:fmaa037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Zambrana JV, Bustos Carrillo FA, Ojeda S, Lopez Mercado B, Latta K, Schiller A, Kuan G, Gordon A, Reingold A, Harris E. Epidemiologic Features of Acute Pediatric Diarrhea in Managua, Nicaragua, from 2011 to 2019. Am J Trop Med Hyg. 2022;106:1757-1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Vassilopoulou L, Spyromitrou-Xioufi P, Ladomenou F. Effectiveness of probiotics and synbiotics in reducing duration of acute infectious diarrhea in pediatric patients in developed countries: a systematic review and meta-analysis. Eur J Pediatr. 2021;180:2907-2920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Altcheh J, Carosella MV, Ceballos A, D'Andrea U, Jofre SM, Marotta C, Mugeri D, Sabbaj L, Soto A, Josse C, Montestruc F, McFarland LV. Randomized, direct comparison study of Saccharomyces boulardii CNCM I-745 versus multi-strained Bacillus clausii probiotics for the treatment of pediatric acute gastroenteritis. Medicine (Baltimore). 2022;101:e30500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Buccigrossi V, Lo Vecchio A, Bruzzese E, Russo C, Marano A, Terranova S, Cioffi V, Guarino A. Potency of Oral Rehydration Solution in Inducing Fluid Absorption is Related to Glucose Concentration. Sci Rep. 2020;10:7803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Karlsson O, Kim R, Subramanian SV. International Trends in Zinc Treatment for Diarrhea. Pediatrics. 2024;154:e2024066701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Pavlinac PB, Platts-Mills JA, Liu J, Atlas HE, Gratz J, Operario D, Rogawski McQuade ET, Ahmed D, Ahmed T, Alam T, Ashorn P, Badji H, Bahl R, Bar-Zeev N, Chisti MJ, Cornick J, Chauhan A, De Costa A, Deb S, Dhingra U, Dube Q, Duggan CP, Freyne B, Gumbi W, Hotwani A, Kabir M, Islam O, Kabir F, Kasumba I, Kibwana U, Kotloff KL, Khan SS, Maiden V, Manji K, Mehta A, Ndeketa L, Praharaj I, Qamar FN, Sazawal S, Simon J, Singa BO, Somji S, Sow SO, Tapia MD, Tigoi C, Toure A, Walson JL, Yousafzai MT, Houpt ER; AntiBiotics for Children with severe Diarrhea (ABCD) Study Group. Azithromycin for Bacterial Watery Diarrhea: A Reanalysis of the AntiBiotics for Children With Severe Diarrhea (ABCD) Trial Incorporating Molecular Diagnostics. J Infect Dis. 2024;229:988-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Lukasik J, Dierikx T, Besseling-Van der Vaart I, de Meij T, Szajewska H; Multispecies Probiotic in AAD Study Group. Multispecies Probiotic for the Prevention of Antibiotic-Associated Diarrhea in Children: A Randomized Clinical Trial. JAMA Pediatr. 2022;176:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Harrell JE, Cheng SX. Inability to reduce morbidity of diarrhea by ORS: can we design a better therapy? Pediatr Res. 2018;83:559-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Seifu BL, Legesse BT, Yehuala TZ, Kase BF, Asmare ZA, Mulaw GF, Tebeje TM, Mare KU. Factors associated with the co-utilization of oral rehydration solution and zinc for treating diarrhea among under-five children in 35 sub-saharan Africa countries: a generalized linear mixed effect modeling with robust error variance. BMC Public Health. 2024;24:1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Zwisler G, Simpson E, Moodley M. Treatment of diarrhea in young children: results from surveys on the perception and use of oral rehydration solutions, antibiotics, and other therapies in India and Kenya. J Glob Health. 2013;3:010403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1417] [Cited by in RCA: 1553] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 15. | Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE; Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2620] [Article Influence: 187.1] [Reference Citation Analysis (0)] |
| 16. | Yao Y, Zhao Y. Diseases spectrum study on pediatric tuina in recent 10 years. J Acupunct Tuina Sci. 2012;10:181-184. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Yang P, Qin LL, Yu M, Zou ZM. Rhizome of Atractylodes macrocephala alleviates spleen-deficiency constipation in rats by modulating gut microbiota and bile acid metabolism. J Ethnopharmacol. 2025;348:119884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Yang L, Yu H, Hou A, Man W, Wang S, Zhang J, Wang X, Zheng S, Jiang H, Kuang H. A Review of the Ethnopharmacology, Phytochemistry, Pharmacology, Application, Quality Control, Processing, Toxicology, and Pharmacokinetics of the Dried Rhizome of Atractylodes macrocephala. Front Pharmacol. 2021;12:727154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Liang SB, Cao HJ, Kong LY, Wei JL, Robinson N, Yang SH, Zhu SJ, Li YQ, Fei YT, Han M, Liu JP. Systematic review and meta-analysis of Chinese herbal formula Tongxie Yaofang for diarrhea-predominant irritable bowel syndrome: Evidence for clinical practice and future trials. Front Pharmacol. 2022;13:904657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Hu L, Li L, Wang Y, Zhang C, Su J, Di H, Gao Q, Tai X, Guo T. Pediatric Tuina for functional constipation in children: study protocol for a randomized controlled trail. Trials. 2022;23:750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Lu T, Zhang H, Yin L, Cai J, Li M, Dai L, Zhu C, Zhang Y, Xiang F, Wang L, Li L, Wang L, Wu D. Chinese pediatric Tuina on children with acute diarrhea: study protocol for a randomized sham-controlled trial. Trials. 2019;20:689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Li SS, Lin XY, Li X, Zhang YD, Wang LQ, Lai SX. Chinese pediatric Tuina can prevent premature infant feeding intolerance and is conducive to weight gain: a prospective randomized controlled study. Afr Health Sci. 2023;23:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Zhang X, Zhang XP, Jia CS, Wang JL, Xu J, Qin L, Xu XK, Zhang ML, Kang SG, Duan XD, Liu BB, Cai CY. [Basic rules and characteristics of acupoint application therapy based upon data mining]. Zhen Ci Yan Jiu. 2012;37:416-421. [PubMed] |
| 24. | Sun S, Lin X, Yang Y, Cen J, Luo F, Chen X. Acupoint application for rotavirus diarrhea in infants and children: A protocol for systematic review and meta analysis. Medicine (Baltimore). 2020;99:e22227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Chen XB, Luo JJ. [Controlled observation of efficacy on herb-partitioned moxibustion and western medicine in the treatment of persistent and chronic diarrhea of children]. Zhongguo Zhen Jiu. 2013;33:113-116. [PubMed] |
| 26. | Chen SC, Yu J, Yuen SC, Lam JC, Suen LK, Yeung WF. Massage therapy in infants and children under 5 years of age: protocol for an overview of systematic reviews. Syst Rev. 2021;10:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Tang Y, Liu M, Yang Q, Yu J, Yue Z, Chang X. Clinical observation of treatment of infantile diarrhea due to spleen deficiency using five-step pediatric tuina of Huxiang school. J Acupunct Tuina Sci. 2019;17:328-335. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Zhang J, Chen Y, Cao L, Zhang R, Ren R, Zhang Q. Tuina for children with upper respiratory tract infections: A protocol for a systematic review. Medicine (Baltimore). 2019;98:e16443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Li J. Clinical study on Tuina plus Shen Ling Bai Zhu San in treating children with diarrhea due to spleen deficiency. J Acupunct Tuina Sci. 2022;20:65-71. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Gan Q, Lian Z, Zheng L, Feng Q, Wei L, Wang Y. Effectiveness of Moxibustion Combined with Chinese Medicine in the Treatment of Spleen and Stomach Deficiency Cold-Type Gastroparesis: A Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med. 2022;2022:6552819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Yang X, He M, Tang Q, Cao J, Wei Z, Li T, Sun M. Metabolomics as a promising technology for investigating external therapy of traditional Chinese medicine: A review. Medicine (Baltimore). 2024;103:e40719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | He M, Lim XY, Li J, Li L, Zhang T. Mechanisms of acupuncture at Zusanli (ST36) and its combinational acupoints for stress gastric ulcer based on the correlation between Zang-fu and acupoints. J Integr Med. 2025;23:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Tian Y, Wang L, Wang Z, Ding L, Wei L, Guo L, Sun X, Wang L, Yang F, Sun L. Efficacy and safety of Tuina for treatment of pediatric recurrent respiratory tract infections: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e27939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Liu M, Li Y, Xian J, Yang W, Gao Q, Yu J. Pediatric Tuina (massage) for primary monosymptomatic nocturnal enuresis: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e23738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Wang YY, Liu XY. [Clinical observation on fuzhong (supporting the middle-jiao) manipulation of tuina for infantile anorexia]. Zhongguo Zhen Jiu. 2014;34:67-70. [PubMed] |
| 36. | Gao L, Jia C, Huang H. Paediatric massage for treatment of acute diarrhoea in children: a meta-analysis. BMC Complement Altern Med. 2018;18:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Liu Y, Hu L, Lai D, Duan W, Xu J, Wu Z, Li T. Abdomen-rubbing qigong exercise and health preservation concept of Fang Kai, a Xin’an medical physician. J Acupunct Tuina Sci. 2016;14:170-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Peng Y, Leng L, Chen Z, Zhang J, Wang DH, Ge YF, Wang HY. [Acute infantile diarrhea treated with infantile Tuina: a multicentre randomized controlled trial]. Zhongguo Zhen Jiu. 2011;31:1116-1120. [PubMed] |
| 39. | Bu FL, Han M, Lu CL, Liu XH, Wang WG, Lai JL, Qiu XH, He BX, Zhang H, Robinson N, Fei YT, Liu JP. A systematic review of Tuina for irritable bowel syndrome: Recommendations for future trials. Complement Ther Med. 2020;52:102504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Wang X, Lan Y, Zeng Z, Ge L. Therapeutic mechanism of steaming umbilical cord therapy with Chinese herbal medicine on a rat model of IBS-D via the PAR-2/TRVP1 pathway. Am J Transl Res. 2021;13:6288-6296. [PubMed] |
| 41. | Niu LQ, Xiao L, Cai QH, Wu YY, Hu SY, Guo SX, Tian YL, Wang QR. Comparative effectiveness of Chinese herbal injections treating for rotavirus enteritis in children: A systematic review and Bayesian network meta-analysis. Integr Med Res. 2023;12:100944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Anheyer D, Frawley J, Koch AK, Lauche R, Langhorst J, Dobos G, Cramer H. Herbal Medicines for Gastrointestinal Disorders in Children and Adolescents: A Systematic Review. Pediatrics. 2017;139:e20170062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Zhong L, Cao X, Li L, He Y, Liu Y, Chen W, Yang F, Xiao N, Zhang J, He H. Renzhu Ointment Regulates L-Type Voltage-Dependent Calcium Channel in Mice Model of Senna-Induced Diarrhea by Transdermal Administration. Drug Des Devel Ther. 2023;17:2355-2368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Yao Y, Habib M, Bajwa HF, Qureshi A, Fareed R, Altaf R, Ilyas U, Duan Y, Abbas M. Herbal therapies in gastrointestinal and hepatic disorders: An evidence-based clinical review. Front Pharmacol. 2022;13:962095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 45. | Pilch NA, Sell ML, McGhee W, Venkataramanan R. Important considerations for drugs, nutritional, and herbal supplements in pediatric solid organ transplant recipients. Pediatr Transplant. 2021;25:e13881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Zhao ZR, Wang YX, Xu FY, Zhang WC, Wang QY, Huang W. [Herbal-moxa plaster for diarrhea type irritable bowel syndrome of spleen and kidney yang deficiency: a randomized controlled trial]. Zhongguo Zhen Jiu. 2023;43:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Zhang YL, Wang YL, Yan K, Li H, Zhang X, Essola JM, Ding C, Chang K, Qing G, Zhang F, Tan Y, Peng T, Wang X, Jiang M, Liang XJ, Hua Q. Traditional Chinese Medicine Formulae QY305 Reducing Cutaneous Adverse Reaction and Diarrhea by its Nanostructure. Adv Sci (Weinh). 2024;11:e2306140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 48. | Wang H, Hou YN, Yang M, Feng Y, Zhang YL, Smith CM, Hou W, Mao JJ, Deng G. Herbal Formula Shenling Baizhu San for Chronic Diarrhea in Adults: A Systematic Review and Meta-analysis. Integr Cancer Ther. 2022;21:15347354221081214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Sun Y, Zhang Y, Wang Z, Liu Q, Mo J. Efficacy and safety of Chinese herbal medicine in treating postcholecystectomy diarrhea: A systematic review and meta-analysis. Medicine (Baltimore). 2024;103:e38046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Shao H, Wang L, Zhang H. Chinese herbal medicine, Tongxieyaofang, alleviates diarrhea via gut microbiota remodeling: evidence from network pharmacology and full-length 16S rRNA gene sequencing. Front Cell Infect Microbiol. 2024;14:1502373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Xu Y, Zheng X, Zhang X, Lan Y, Wang J, Shen J. Clinical efficacy of umbilical therapy with herbal cakes of different dosages for damp-heat diarrhea in young children. J Acupunct Tuina Sci. 2022;20:72-78. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Liu N, Li J, Wang Y, Zhang S. Different therapies of Chinese herbal medicine for diarrhea-predominant irritable bowel syndrome: A network meta-analysis of double-blinded, placebo-controlled trials. J Ethnopharmacol. 2023;317:116672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Lin X, Fang Y, Cheng Y, Wang Q. Chinese herbal medicine for irinotecan-induced diarrhea: A systematic review and meta-analysis. Explore (NY). 2024;20:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Liu B, Yan B, Jiang H, Zhao X, Wang L, Li T, Wang F. The effectiveness of herbal acupoint application for functional diarrhea: Protocol for a meta-analysis and data mining. Medicine (Baltimore). 2021;100:e27702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 55. | Leung WK, Wu JC, Liang SM, Chan LS, Chan FK, Xie H, Fung SS, Hui AJ, Wong VW, Che CT, Sung JJ. Treatment of diarrhea-predominant irritable bowel syndrome with traditional Chinese herbal medicine: a randomized placebo-controlled trial. Am J Gastroenterol. 2006;101:1574-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Chen MR, Zhao J, Fu SF, Yu JQ, Zhang X, Zhang QY, Zhou ZH. Clinical practice of Chinese medicine navel therapy for chronic diarrhea: A literature review. J Gastroenterol Hepatol. 2019;34:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Liang SB, Han M, Cheng HJ, Zhang QY, Zhang NW, Jia BY, Robinson N, Liu JP. Chinese herbal formula Tongxie Yaofang for diarrhea-predominant irritable bowel syndrome: study protocol for a randomized, multiple-blind, placebo-controlled trial. Trials. 2022;23:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Li Y, Chen Y, Liao Z, Liu Y, Liu C, Yang W, Bai J, Huang X, Hao Y, Liu S, Liu Y. WenTongGanPi decoction alleviates diarrhea-predominant irritable bowel syndrome by improving intestinal barrier. J Ethnopharmacol. 2024;334:118544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 59. | Yu Z. Neuromechanism of acupuncture regulating gastrointestinal motility. World J Gastroenterol. 2020;26:3182-3200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Hao YH, Liu ZZ, Zhao H, Wang L, Khan A, Mu JB, Wang YF, Yang LH, Zhou R, Xie J. Identification and characterization of murine adipose tissue-derived somatic stem cells of Shenque (CV8) acupoint. Chin Med J (Engl). 2021;134:2730-2737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 61. | Gao X, Zhao Y, Su Y, Liu K, Yu X, Cui C, Yang Z, Shi H, Jing X, Zhu B. β1/2 or M2/3 Receptors Are Required for Different Gastrointestinal Motility Responses Induced by Acupuncture at Heterotopic or Homotopic Acupoints. PLoS One. 2016;11:e0168200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Deloose E, Verbeure W, Depoortere I, Tack J. Motilin: from gastric motility stimulation to hunger signalling. Nat Rev Endocrinol. 2019;15:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Zhang H, Li H, Wang J, Bao A. Observation on the Efficacy of Abdominal Massage in Treating Generalized Anxiety Disorder: A Randomized Controlled Trial Study Protocol. Cureus. 2025;17:e77030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Liu ZF, Zhang Y, Liu J, Wang YY, Chen M, Liu EY, Guo JM, Wang YH, Weng ZW, Liu CX, Yu CH, Wang XY. Effect of Traditional Chinese Non-Pharmacological Therapies on Knee Osteoarthritis: A Narrative Review of Clinical Application and Mechanism. Orthop Res Rev. 2024;16:21-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 65. | Liu ZF, Wang HR, Yu TY, Zhang YQ, Jiao Y, Wang XY. Tuina for peripherally-induced neuropathic pain: A review of analgesic mechanism. Front Neurosci. 2022;16:1096734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 66. | Wang Z, Xu H, Zhou H, Li W, Yang T, Zhou Y. Current Status of Research on Tuina for Analgesia: A Bibliometric and Visual Analysis. J Pain Res. 2023;16:2955-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 67. | Molotla-Torres DE, Guzmán-Mejía F, Godínez-Victoria M, Drago-Serrano ME. Role of Stress on Driving the Intestinal Paracellular Permeability. Curr Issues Mol Biol. 2023;45:9284-9305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 68. | Wang H, Liu Z, Yu T, Zhang Y, Jiao Y, Wang X, Du H, Jiang R, Liu D, Xu Y, Guan Q, Lu M. The effect of tuina on ulcerative colitis model mice analyzed by gut microbiota and proteomics. Front Microbiol. 2022;13:976239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Wang L, Gou X, Ding Y, Liu J, Wang Y, Wang Y, Zhang J, Du L, Peng W, Fan G. The interplay between herbal medicines and gut microbiota in metabolic diseases. Front Pharmacol. 2023;14:1105405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 70. | Zheng L, Luo M, Zhou H, Chen J. Natural products from plants and microorganisms: Novel therapeutics for chronic kidney disease via gut microbiota regulation. Front Pharmacol. 2022;13:1068613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Li E, Li C, Horn N, Ajuwon KM. Quercetin attenuates deoxynivalenol-induced intestinal barrier dysfunction by activation of Nrf2 signaling pathway in IPEC-J2 cells and weaned piglets. Curr Res Toxicol. 2023;5:100122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 72. | Yang YJ, Kim MJ, Lee HJ, Lee WY, Yang JH, Kim HH, Shim MS, Heo JW, Son JD, Kim WH, Kim GS, Lee HJ, Kim YW, Kim KY, Park KI. Ziziphus jujuba Miller Ethanol Extract Restores Disrupted Intestinal Barrier Function via Tight Junction Recovery and Reduces Inflammation. Antioxidants (Basel). 2024;13:575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 73. | Jiang C, Lin W, Wang L, Lv Y, Song Y, Chen X, Yang H. Fushen Granule, A Traditional Chinese Medicine, ameliorates intestinal mucosal dysfunction in peritoneal dialysis rat model by regulating p38MAPK signaling pathway. J Ethnopharmacol. 2020;251:112501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Xu J, Chen HB, Li SL. Understanding the Molecular Mechanisms of the Interplay Between Herbal Medicines and Gut Microbiota. Med Res Rev. 2017;37:1140-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 261] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 75. | Li S, Xu Y, Guo W, Chen F, Zhang C, Tan HY, Wang N, Feng Y. The Impacts of Herbal Medicines and Natural Products on Regulating the Hepatic Lipid Metabolism. Front Pharmacol. 2020;11:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 76. | Belizário JE, Faintuch J, Garay-Malpartida M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm. 2018;2018:2037838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 77. | Wei X, Tao J, Xiao S, Jiang S, Shang E, Zhu Z, Qian D, Duan J. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci Rep. 2018;8:3685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 78. | Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 841] [Article Influence: 168.2] [Reference Citation Analysis (0)] |
| 79. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 4164] [Article Influence: 320.3] [Reference Citation Analysis (1)] |
| 80. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2951] [Cited by in RCA: 4056] [Article Influence: 312.0] [Reference Citation Analysis (0)] |
| 81. | Wu SE, Hashimoto-Hill S, Woo V, Eshleman EM, Whitt J, Engleman L, Karns R, Denson LA, Haslam DB, Alenghat T. Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature. 2020;586:108-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 82. | Dai TT, Fang W, Zhu WT, Han ZL, Sun NX, Yin G, Wang DL. Atractylenolide III ameliorates DSS-induced colitis by improving intestinal epithelial barrier via suppressing the NF-κB-Mediated MLCK-pMLC signaling pathway. Food Chem Toxicol. 2025;196:115158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 83. | Duan Y, Huang J, Sun M, Jiang Y, Wang S, Wang L, Yu N, Peng D, Wang Y, Chen W, Zhang Y. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int J Biol Macromol. 2023;249:125953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 80] [Reference Citation Analysis (0)] |
| 84. | Ji R, Wang A, Shang H, Chen L, Bao C, Wu L, Wu H, Shi Y. Herb-partitioned moxibustion upregulated the expression of colonic epithelial tight junction-related proteins in Crohn's disease model rats. Chin Med. 2016;11:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Zheng D, Liao H, Chen S, Liu X, Mao C, Zhang C, Meng M, Wang Z, Wang Y, Jiang Q, Xue Y, Zhou L, Chen Y. Elevated Levels of Circulating Biomarkers Related to Leaky Gut Syndrome and Bacterial Translocation Are Associated With Graves' Disease. Front Endocrinol (Lausanne). 2021;12:796212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 86. | Park J, Choi TJ, Kang KS, Choi SH. The Interrelationships between Intestinal Permeability and Phlegm Syndrome and Therapeutic Potential of Some Medicinal Herbs. Biomolecules. 2021;11:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Zierden HC, Josyula A, Shapiro RL, Hsueh HT, Hanes J, Ensign LM. Avoiding a Sticky Situation: Bypassing the Mucus Barrier for Improved Local Drug Delivery. Trends Mol Med. 2021;27:436-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 88. | Buentzel J, Bauer C, Buentzel J. How to bridge the gap? European medical plants used for treating oral mucositis: on the search for evidence. J Cancer Res Clin Oncol. 2020;146:985-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Chen HY, Liu J, Weng DZ, Yan L, Pan CS, Sun K, Guo X, Wang D, Anwaier G, Jiao YQ, Li ZX, Han JY. Ameliorative effect and mechanism of Si-Ni-San on chronic stress-induced diarrhea-irritable bowel syndrome in rats. Front Pharmacol. 2022;13:940463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 90. | Wang K, Wu LY, Dou CZ, Guan X, Wu HG, Liu HR. Research Advance in Intestinal Mucosal Barrier and Pathogenesis of Crohn's Disease. Gastroenterol Res Pract. 2016;2016:9686238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Lu Q, Su C, Liu H, Luo C, Yan B. Effect of constant compressive stress induced by imitating Tuina stimulation with various durations on the cell cycle, cellular secretion, apoptosis, and expression of myogenic differentiation and myogenic factor 5 of rat skeletal muscle cells in vitro. J Tradit Chin Med. 2020;40:550-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 92. | Meng T, Fu S, He D, Hu G, Gao X, Zhang Y, Huang B, Du J, Zhou A, Su Y, Liu D. Evodiamine Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in BV-2 Cells via Regulating AKT/Nrf2-HO-1/NF-κB Signaling Axis. Cell Mol Neurobiol. 2021;41:115-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 93. | Li Y, Fu S, Li F, Guo Y, Cao Y, Ren F, Li R, Wang Y, Luo M. Pediatric tuina treatment for spleen deficiency diarrhea regulated through the skin-brain-gut axis and mast cell degranulation. J Tradit Complement Med. 2025;15:205-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Albert-Bayo M, Paracuellos I, González-Castro AM, Rodríguez-Urrutia A, Rodríguez-Lagunas MJ, Alonso-Cotoner C, Santos J, Vicario M. Intestinal Mucosal Mast Cells: Key Modulators of Barrier Function and Homeostasis. Cells. 2019;8:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 95. | Katinios G, Casado-Bedmar M, Walter SA, Vicario M, González-Castro AM, Bednarska O, Söderholm JD, Hjortswang H, Keita ÅV. Increased Colonic Epithelial Permeability and Mucosal Eosinophilia in Ulcerative Colitis in Remission Compared With Irritable Bowel Syndrome and Health. Inflamm Bowel Dis. 2020;26:974-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Li H, Zhang W, Ma F, Zhang X, Wang Y, Wang J. Abdominal Massage Improves the Symptoms of Irritable Bowel Syndrome by Regulating Mast Cells via the Trypase-PAR2-PKCε Pathway in Rats. Pain Res Manag. 2022;2022:8331439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1115] [Article Influence: 74.3] [Reference Citation Analysis (1)] |
| 98. | Yu WL, Park JY, Park HJ, Kim SN. Changes of local microenvironment and systemic immunity after acupuncture stimulation during inflammation: A literature review of animal studies. Front Neurol. 2022;13:1086195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 99. | Maturo MG, Soligo M, Gibson G, Manni L, Nardini C. The greater inflammatory pathway-high clinical potential by innovative predictive, preventive, and personalized medical approach. EPMA J. 2020;11:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Jiang JZ, Li WQ, Kuang KL, Jiang YQ, He ZX, Zhang LJ, Cao JY, Wang D, Zhang XY, Tian ZL, Zhu J, Peng DZ. Long-term tuina can inhibit the occurrence of gastroparesis by protecting gastrointestinal function in diabetic rats. Front Endocrinol (Lausanne). 2025;16:1536567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 101. | Xu H, Song J, Gao X, Xu Z, Xu X, Xia Y, Dai Y. Paeoniflorin attenuates lipopolysaccharide-induced permeability of endothelial cells: involvements of F-actin expression and phosphorylations of PI3K/Akt and PKC. Inflammation. 2013;36:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Zhai L, Zheng Y, Lo CW, Xu S, Jiang X, Liu Q, Ching JY, Ning Z, Bao G, Yang W, Zhang Q, Cheng CW, Lam WC, Chan KL, Zhang X, Lam PY, Wu XY, Zhong LLD, Cao PH, Koh M, Cheong PK, Lin Z, Lin C, Zhao L, Wong XHL, Wu JC, Bian Z. Butyrate-producing commensal bacteria mediates the efficacy of herbal medicine JCM-16021 on abdominal pain in diarrhea-predominant irritable bowel syndrome: a randomized clinical trial. Phytomedicine. 2025;145:157040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 103. | Dang G, Wu W, Zhang H, Everaert N. A new paradigm for a new simple chemical: butyrate & immune regulation. Food Funct. 2021;12:12181-12193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 104. | Cangemi DJ, Lacy BE. Management of irritable bowel syndrome with diarrhea: a review of nonpharmacological and pharmacological interventions. Therap Adv Gastroenterol. 2019;12:1756284819878950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 105. | Che Q, Luo T, Shi J, He Y, Xu DL. Mechanisms by Which Traditional Chinese Medicines Influence the Intestinal Flora and Intestinal Barrier. Front Cell Infect Microbiol. 2022;12:863779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 106. | Lu T, Yin L, Chen R, Zhang H, Cai J, Li M, Dai L, Zhu C, Zhang Y, Xiang F, Wang L, Li L, Wang L, Wu D. Chinese pediatric Tuina on children with acute diarrhea: a randomized sham-controlled trial. Health Qual Life Outcomes. 2021;19:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 107. | Li YY, Wang XJ, Su YL, Wang Q, Huang SW, Pan ZF, Chen YP, Liang JJ, Zhang ML, Xie XQ, Wu ZY, Chen JY, Zhou L, Luo X. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol Sin. 2022;43:1495-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 108. | Wang L, Zhu HY, He JZ, Yin X, Guo LH. [Effect of Modified Huanglian Jiedu Decoction Purgation Combined Electroacupuncture in Intervening Gastrointestinal Dysfunction of Critically Ill Patients Undergoing Abdominal Surgery]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:966-970. [PubMed] |
| 109. | Zhao Y, Luan H, Gao H, Wu X, Zhang Y, Li R. Gegen Qinlian decoction maintains colonic mucosal homeostasis in acute/chronic ulcerative colitis via bidirectionally modulating dysregulated Notch signaling. Phytomedicine. 2020;68:153182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 110. | Li X, Wu Y, Xu Z, Chen J, Li Y, Xing H, Zhang X, Yuan J. Effects of Hetiao Jianpi Decoction on Intestinal Injury and Repair in Rats with Antibiotic-Associated Diarrhea. Med Sci Monit. 2020;26:e921745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/