Published online Nov 7, 2025. doi: 10.3748/wjg.v31.i41.111256
Revised: August 13, 2025
Accepted: September 24, 2025
Published online: November 7, 2025
Processing time: 133 Days and 15.1 Hours
There is insufficient evidence on the evaluation of liver fibrosis in Asian indivi
To assess advanced fibrosis (AF) using liver stiffness measurement (LSM) in Chinese patients with PBC.
In total, 277 Chinese patients diagnosed with PBC who underwent liver biopsy and VCTE were retrospectively included and categorized into the derivation and validation cohorts. The areas under the receiver operating characteristic curves (AUROCs) with 95% confidence intervals (CIs) were used to estimate the dia
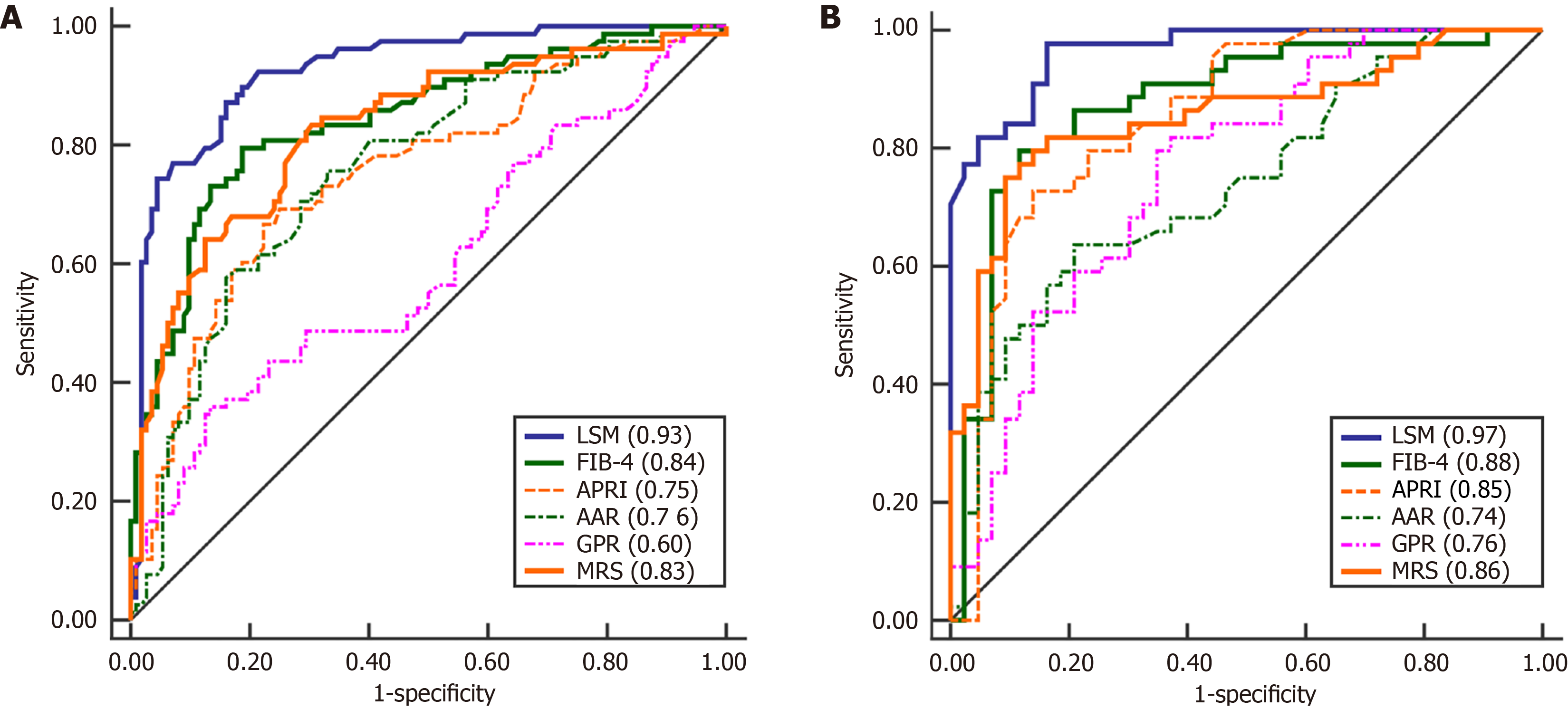
In the derivation cohort, VCTE accurately detected patients with AF, achieving an AUROC of 0.93 (95%CI: 0.88-0.96). AF was independently predicted by LSM according to multivariable analysis. AF can be excluded and confirmed using LSM cutoffs of ≤ 10.0 and > 14.5 kPa, respectively, with a sensitivity of 0.91, negative predictive value of 0.93, specificity of 0.96, positive predictive value of 0.92, and an error rate of 7.5%. The accuracy of these values was validated in an independent cohort, achieving an AUROC of 0.97 (95%CI: 0.90-0.99) for AF with a sensitivity of 0.89, negative predictive value of 0.88, specificity of 0.95, positive predictive value of 0.94, and error rate of 9.0%. Compared with serum fibrosis markers, the AUROC of LSM was significantly higher in both the derivation and validation cohorts.
VCTE has a high accuracy for assessing AF in Chinese patients with PBC in a real-world setting.
Core Tip: This study retrospectively used vibration-controlled transient elastography (VCTE) to assess advanced fibrosis using liver stiffness measurement (LSM) in Chinese patients with histologically verified primary biliary cholangitis (PBC). To our knowledge, this study is currently the largest sample size among similar studies exploring VCTE for evaluating PBC fibrosis. Furthermore, this study provides a dual cut-off approach utilizing LSM through VCTE to categorize individuals with PBC into three categories of risk: Early-stage (LSM ≤ 10.0 kPa), advanced-stage (LSM > 14.5 kPa), and a grey area of inaccurate discrimination. This non-invasive method of individual risk stratification for PBC will aid in the selection of clinical decisions.
- Citation: Chen JL, Hou YX, Liu Y, Jiang YY, Wang XB. Real-world performance of transient elastography in assessing advanced fibrosis in Chinese patients with primary biliary cholangitis. World J Gastroenterol 2025; 31(41): 111256
- URL: https://www.wjgnet.com/1007-9327/full/v31/i41/111256.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i41.111256
Primary biliary cholangitis (PBC), previously known as primary biliary cirrhosis, is a chronic autoimmune liver disease. It is characterized by non-suppurative, destructive intrahepatic cholangitis, leading to fibrosis, cirrhosis, and its associated complications[1]. PBC can affect individuals of all ethnicities with significant regional differences, and predominantly affects middle-aged females[2]. Ursodeoxycholic acid (UDCA) is currently the primary therapy for patients with PBC. However, approximately 40% of these patients exhibit an inadequate response to UDCA treatment[3]. PBC relies on the UDCA response and fibrosis stage as crucial factors for prognostic assessment and risk categorization[3,4]. Two recent studies conducted by the Globe PBC and UK-PBC research teams demonstrated that identifying the fibrosis stage at diagnosis holds greater significance for prognostic assessment than biochemical responses[4,5]. Due to the strong link between advanced histological stages and poor prognosis in PBC, diagnosing and staging liver fibrosis are crucial for guiding treatment strategies and predicting long-term outcomes.
Currently, liver biopsy (LB) is the definitive method for assessing fibrosis severity. Nevertheless, owing to its invasive nature, high expenses, potential complications, and the risk of sampling error, it is not commonly advised for fibrosis staging during the diagnosis of PBC[6-8]. Consequently, various non-invasive methods, including serum markers and imaging techniques, have been developed and validated for assessing liver fibrosis[9-13]. Vibration-controlled transient elastography (VCTE) determines the velocity of shear waves produced mechanically throughout the liver to calculate liver stiffness measurement (LSM). This method has been proven as a straightforward and reliable indicator of fibrosis in various chronic liver conditions[14]. Multiple studies[11,12,15] conducted on cohorts from France, Spain, and Italy have validated that VCTE exhibits superior precision in identifying severe fibrosis or cirrhosis in PBC compared to serum fibrosis markers such as the fibrosis-4 score (FIB-4)[16] and aspartate aminotransferase (AST) to platelet ratio index (APRI)[17]. In addition, some studies have indicated that LSM using VCTE can predict the long-term prognosis of PBC[18].
However, the increasing literature regarding the diagnostic accuracy of LSM for assessing histological fibrosis in PBC primarily originates from Europe and the United States, with only a limited number of studies assessing the accuracy of LSM in the Asian PBC cohort[19,20]. Due to differences in race and body mass index (BMI), the cut-off values of LSM for diagnosing fibrosis in PBC in European and American populations may not be applicable to Asian populations. To the best of our knowledge, only two studies conducted in Japan[21,22] and one in China[23] in the Asia-Pacific region have documented the utilization of this method in patients diagnosed with PBC. In addition, a study conducted in China examined the effectiveness of LSM using VCTE in identifying fibrosis in individuals with the overlap syndrome of autoimmune hepatitis (AIH)-PBC[24]. Furthermore, the uncertain diagnostic efficacy of LSM using VCTE for fibrosis staging in Chinese and Asian patients with PBC persists, primarily because of the limited sample size in prior studies. Hence, we aimed to investigate and confirm the diagnostic accuracy and LSM thresholds of VCTE for advanced fibrosis (AF) in Chinese individuals with PBC by conducting a real-world study at a single center in China, using LB as a benchmark.
We conduct a retrospective search to identify derivation and external validation cohorts for diagnosing PBC hepatic fibrosis using the electronic medical records in our institution. The following criteria were used for inclusion: (1) Patients aged 18 years or older; (2) PBC diagnosis confirmed by histological evidence of interlobular bile duct inflammation and damage, elevated alkaline phosphatase (ALP) levels, or serum antimitochondrial antibody (AMA) at a minimum titer of 1:40, or positive antinuclear antibodies (ANA) in AMA-negative cases via enzyme-linked immunosorbent assay (anti-gp210, anti-sp100); (3) Patients who had both VCTE and percutaneous LB within a maximum interval of 6 months from January 2011 to December 2021; and (4) Patients with complete clinical data. We excluded individuals with other concomitant liver diseases, such as hepatitis B or C, liver injury caused by alcohol or drugs, overlap syndrome with AIH, non-alcoholic steatohepatitis (NASH), or any other causes of liver disease. Individuals diagnosed and tested with both VCTE and LB between January 2011 and December 2019 were included in the derivation cohort. Similarly, individuals meeting the same criteria between January 2020 and December 2021 were included in the separate validation cohort to validate the diagnostic accuracy of the cut-off values obtained from the derivation cohort. The research protocol adhered to the Declaration of Helsinki guidelines and received approval from the Ethics Committees of Beijing Ditan Hospital, Capital Medical University (No. DTEC-KT2022-010-01). The study used anonymized, pre-existing data, thus waiving the need for patient-informed consent.
Information regarding the clinical, biochemical, serological, and histological characteristics and LSM measurements was collected retrospectively into a custom-made digital database using an electronic case report form. Baseline data were collected during the initial diagnosis upon admission. The primary data collected included the following variables: Age at diagnosis, gender, LB and VCTE dates, diagnosis date, LSM measurement, BMI, history of UDCA treatment at diagnosis (categorized as UDCA treatment-naïve and UDCA treated), presence of diabetes, hypertension, Sjogren’s syndrome, Hashimoto’s thyroiditis, as well as blood tests performed on the same day as the LB, such as serum total bilirubin, albumin, ALP, gamma-glutamyl-transferase, alanine aminotransferase, AST, immunoglobulin G (IgG), immunoglobulin M, platelet count (PLT), serum hyaluronic acid level, antinuclear antibodies, AMA, anti-gp210, and anti-sp100. In both cohorts, serum non-invasive liver fibrosis models were calculated at diagnosis using age, liver biochemical index, and PLT based on published literature[13,16,17,25]. These models or markers included the FIB-4, APRI, AST/alanine aminotransferase ratio (AAR), gamma-glutamyl transpeptidase to platelet ratio (GPR), and Mayo risk score (MRS).
According to the local standard procedure, an LB was carried out in the right lobe of the liver using a 16-G needle under ultrasound guidance. The liver tissue samples were preserved in formalin and embedded in paraffin. All samples were stained with hematoxylin-eosin-saffron, Masson’s trichrome stain, cytokeratin-7, and cytokeratin-19. Two experienced pathologists who were unaware of the LSM results and clinical data examined the biopsies. The histological fibrosis stage was determined according to Ludwig’s classification[26]. Stage I corresponds to portal hepatitis with minimal or no periportal inflammation or piecemeal necrosis. Stage II is characterized by periportal hepatitis with piecemeal necrosis, without bridging necrosis or septal fibrosis. Stage III indicates the presence of septal fibrosis or bridging necrosis, or both, without cirrhosis. Stage IV represents cirrhosis. To conduct this study, patients were classified into two groups: Those with early stage (Ludwig = I or II) and those with advanced stage (Ludwig = III or IV)[11]. This study included only liver specimens with a minimum of ten intact portal tracts.
Physicians who received training and certification from the manufacturer conducted LSM by VCTE examination using Fibroscan (EchoSens, Paris, France) following a standardized protocol. At least 350 Liver stiffness evaluations had been conducted before the start of this study. Scanning of the right liver lobe through the intercostal space was conducted for all LSMs included in the study after a minimum of 4 hours of fasting. LSMs were measured in kilopascals (kPa). A total of 10 valid measurements were performed in each patient. Only patients with a success rate > 60% and an interquartile range (IQR)/median ratio < 30% were included in this study[11].
As most continuous variables exhibited skewed distributions, these variables were characterized as median (IQR) and assessed using the Mann-Whitney U test. Additionally, the correlation between LSM and ordinate histological stages was determined using Spearman’s coefficient (r). Graphical comparison of LSM distributions at various histological stages were performed using box-and-whisker plots. The frequencies and percentages of categorical variables were described and compared using χ2d or Fisher’s exact tests. A logistic regression model was utilized to conduct multivariable analysis. Receiver operating characteristic (ROC) curves were utilized to assess diagnostic accuracy. The 95% confidence interval (CI) was reported, along with the area under the ROC curve (AUROC). DeLong’s test was used to compare the AUROC of the LSM and serum fibrosis models. Sensitivity, specificity, positive and negative predictive values [positive predictive value (PPV) and negative predictive value (NPV), respectively], and positive and negative likelihood ratios [platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR), respectively] were reported. A single cut-off value was determined using the maximum Youden index. The dual approach involved selecting cut-off values based on the following criteria: A high confirmatory cutoff required specificity and PPV greater than 0.90, whereas a low exclusionary cut-off required sensitivity and NPV greater than 0.90. If there were more than two cut-offs that satisfied these conditions, the further condition was to reduce the grey area. Calibration curves were used to describe the difference between predicted probabilities and actual probabilities while evaluating the model’s goodness of fit. The Hosmer-Lemeshow goodness-of-fit test was used to assess the degree of calibration. If P > 0.05, this indicated that there was no significant difference between the predicted values and the observed values, and the model had a good fitting degree. Statistical analyses were performed using SPSS (version 20.0, SPSS Inc., Chicago, IL, United States) and R software (version 3.4; R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was determined by a P value less than 0.05, using a two-tailed approach.
The 318 consecutively enrolled patients who had both VCTE and LB, 41 (12.9%) were excluded due to the presence of concomitant NASH (n = 6), AIH overlap (n = 25), or unsuitable LB (n = 10) (Supplementary Figure 1). The remaining 277 patients were included in the final analysis; 190 (68.6%) in the derivation cohort and 87 (31.4%) in the external validation cohort. The median interval between LB and VCTE was 1.5 days (IQR: 0.0-6.0) in the derivation cohort and 2.0 days (IQR: 0.0-7.0) in the validation cohort, with no statistically significant difference between the two groups. The median length of LB tissue in the finally enrolled patients (n = 277) was 1.5 cm (IQR: 1.2-1.6 cm), and the median number of portal tracts was 11 (IQR: 10-14), both meeting the quality control requirements. The patient characteristics are presented in Table 1. In the derivation cohort, there were 60 patients (31.6%) with histological stage I, 52 (27.4%) with stage II, 19 (10.0%) with stage III, and 59 (31.0%) with stage IV. Of the total patients, 112 (58.9%) were classified as early stage (Ludwig stage I-II), while 78 patients (41.1%) were categorized as advanced stage (Ludwig stage III-IV). The LSM varied between 2.8 and 75.0 kPa, with a median of 10.0 kPa. Supplementary Figure 2 presents the LSMs based on Ludwig’s fibrosis stage. There was a strong positive correlation between LSM and histological stage (r = 0.728, P < 0.001) (Supplementary Figure 2A). Table 2 presents the characteristics of the patients at both early and advanced stages. Patients in the advanced stage exhibited a higher median age at diagnosis, total bilirubin, AST, IgG, LSM, FIB-4, APRI, AAR, GPR, and MRS, and decreased median levels of albumin and PLT (all P < 0.05) compared with patients in the early stage. Furthermore, the proportion of patients not receiving UDCA treatment was lower (P = 0.016), whereas the proportion of patients with concomitant diabetes was higher (P = 0.022) in patients with advanced stage disease than in those with early stage disease.
| Variables | Derivation cohort (n = 190) | Validation cohort (n = 87) | P value |
| Year of diagnosis | 2011-2019 | 2020-2021 | - |
| Female gender | 171 (90.0) | 74 (85.1) | 0.232 |
| Age at diagnosis (years) | 51 (45-58) | 53 (47-61) | 0.127 |
| UDCA-naïve | 126 (66.3) | 54 (62.1) | 0.492 |
| Comorbidity | |||
| Sjogren’s syndrome | 8 (4.2) | 5 (5.7) | 0.575 |
| Hashimoto’s thyroiditis | 38 (20.0) | 21 (24.1) | 0.435 |
| Diabetes | 22 (11.6) | 13 (14.9) | 0.434 |
| Hypertension | 34 (17.9) | 20 (23.0) | 0.321 |
| Total bilirubin (mg/dL) | 0.9 (0.6-1.4) | 0.9 (0.6-1.4) | 0.944 |
| Albumin (g/dL) | 4.1 (3.7-4.4) | 4.1 (3.6-4.5) | 0.947 |
| ALT (× ULN) | 1.0 (0.5-1.8) | 0.7 (0.4-1.6) | 0.086 |
| AST (× ULN) | 1.3 (0.8-2.1) | 1.1 (0.7-1.8) | 0.121 |
| ALP (× ULN) | 1.4 (0.8-2.3) | 1.2 (0.7-1.8) | 0.038 |
| GGT (× ULN) | 2.7 (1.1-5.9) | 2.6 (1.1-2.8) | 0.627 |
| IgG (g/L) | 15.2 (12.6-18.6) | 14.7 (12.0-17.9) | 0.396 |
| IgM (g/L) | 3.15 (1.93-4.45) | 2.65 (1.42-4.19) | 0.103 |
| Platelet count (× 109/L) | 187 (121-258) | 190 (127-253) | 0.900 |
| ANA+ | 113 (59.5) | 54 (62.1) | 0.682 |
| AMA/AMA-M2+ | 117 (61.6) | 53 (60.9) | 0.917 |
| Anti-gp210+ | 55 (33.3)1 | 31 (35.6) | 0.714 |
| Anti-sp100+ | 25 (15.2)1 | 11 (12.6) | 0.589 |
| Hyaluronic acid (ng/mL) | 90.0 (66.4-126.1) | 84.1 (49.4-144.4) | 0.759 |
| Serum fibrotic markers | |||
| FIB-4 | 1.97 (1.23-4.30) | 1.98 (1.13-4.61) | 0.889 |
| APRI | 0.73 (0.44-1.44) | 0.69 (0.29-1.33) | 0.373 |
| AAR | 1.07 (0.79-1.44) | 1.10 (0.78-1.47) | 0.750 |
| GPR | 2.09 (0.94-4.24) | 2.07 (0.62-4.49) | 0.846 |
| MRS | 4.16 (3.62-4.80) | 4.25 (3.65-4.96) | 0.455 |
| Ludwig stage | 0.060 | ||
| Stage I | 60 (31.6) | 29 (33.3) | |
| Stage II | 52 (27.4) | 14 (16.1) | |
| Stage III | 19 (10.0) | 17 (19.5) | |
| Stage IV | 59 (31.0) | 27 (31.0) | |
| LSM (kPa) | 10.0 (6.6-18.6) | 10.2 (6.2-24.0) | 0.587 |
| Mix-Max | 2.8-75.0 | 3.2-75.0 | |
| IQR/Median | 0.15 (0.11-0.20) | 0.16 (0.11-0.23) | 0.124 |
| IQR/Median | 0.647 | ||
| Very reliable (≤ 0.1) | 44 (23.2) | 18 (20.7) | |
| Reliable (0.1-0.3) | 146 (76.8) | 69 (79.3) | |
| Poorly reliable (> 0.3) | 0 (0) | 0 (0) | |
| BMI (kg/m2) | 22.9 (20.3-25.2) | 22.6 (20.5-23.4) | 0.239 |
| Derivation cohort | Validation cohort | |||||
| Variables | Early stage (n = 112) | Advanced stage (n = 78) | P value | Early stage (n = 43) | Advanced stage (n = 44) | P value |
| Female | 99 (88.4) | 72 (92.3) | 0.376 | 37 (86.0) | 37 (84.1) | 0.798 |
| Age at diagnosis (years) | 48 (44-55) | 56 (49-61) | < 0.001 | 51 (46-59) | 54 (50-64) | 0.049 |
| UDCA-naïve, | 82 (73.2) | 44 (56.4) | 0.016 | 28(65.1) | 26 (59.1) | 0.563 |
| Comorbidity | ||||||
| Sjogren’s syndrome | 5 (4.5) | 3 (3.8) | 1.000 | 2 (4.7) | 3 (6.8) | 1.000 |
| Hashimoto’s thyroiditis | 21 (18.8) | 17 (21.8) | 0.606 | 10 (23.3) | 11 (25.0) | 0.849 |
| Diabetes | 8 (7.1) | 14 (17.9) | 0.022 | 6 (14.0) | 7 (15.9) | 0.798 |
| Hypertension | 16 (14.3) | 18 (23.1) | 0.120 | 9 (20.9) | 11 (25.0) | 0.652 |
| Total bilirubin (mg/dL) | 0.8 (0.6-1.2) | 1.1 (0.8-2.1) | < 0.001 | 0.7 (0.6-0.9) | 1.3 (0.8-2.4) | < 0.001 |
| Albumin (g/dL) | 4.2 (4.0-4.5) | 3.7 (3.4-4.2) | < 0.001 | 4.4 (4.1-4.7) | 3.8 (3.2-4.0) | < 0.001 |
| ALT (× ULN) | 1.1 (0.6-1.9) | 0.8 (0.5-1.6) | 0.118 | 0.6 (0.4-1.4) | 0.9 (0.5-1.6) | 0.209 |
| AST (× ULN) | 1.2 (0.8-1.9) | 1.4 (0.9-2.9) | 0.035 | 0.7 (0.5-1.5) | 1.5 (0.9-2.5) | < 0.001 |
| ALP (× ULN) | 1.4 (0.8-2.2) | 1.3 (0.8-2.4) | 0.761 | 0.8 (0.6-1.3) | 1.6 (0.9-2.0) | 0.002 |
| GGT (× ULN) | 3.1 (1.4-5.7) | 2.1 (0.9-6.2) | 0.484 | 1.9 (0.7-3.6) | 3.4 (1.7-6.8) | 0.012 |
| IgG (g/L) | 14.5 (12.3-16.9) | 17.2 (13.7-22.2) | < 0.001 | 12.6 (11.3-15.3) | 16.8 (13.9-23.9) | < 0.001 |
| IgM (g/L) | 3.19 (2.07-4.63) | 3.01 (1.66-4.20) | 0.734 | 2.2 (1.3-3.8) | 2.9 (1.7-5.3) | 0.094 |
| Platelet count (× 109/L) | 223 (173-285) | 121 (88-165) | < 0.001 | 242 (195-293) | 130 (93-189) | < 0.001 |
| ANA | 61 (54.5) | 52 (66.7) | 0.092 | 24 (55.8) | 30 (68.2) | 0.235 |
| AMA/AMA-M2 | 68 (60.7) | 49 (62.8) | 0.769 | 23 (53.5) | 30 (68.2) | 0.160 |
| Anti-gp210 | 28 (29.8)1 | 27 (38.0)2 | 0.266 | 12 (27.9) | 19 (43.2) | 0.137 |
| Anti-sp100 | 16 (17.0)1 | 9 (12.7)2 | 0.441 | 4 (9.3) | 7 (15.9) | 0.354 |
| Hyaluronic acid (ng/mL) | 82.6 (62.5-122.1) | 102.5 (78.5-132.1) | 0.095 | 60.4 (31.0-87.4) | 107.3 (77.2-276.6) | 0.008 |
| Serum fibrotic markers | ||||||
| FIB-4 | 1.49 (1.06-2.13) | 4.17 (2.49-6.88) | < 0.001 | 1.20 (0.78-1.81) | 4.27 (2.49-6.80) | < 0.001 |
| APRI | 0.59 (0.33-0.91) | 1.25 (0.69-2.33) | < 0.001 | 0.30 (0.18-0.68) | 1.23 (0.70-2.08) | < 0.001 |
| AAR | 0.91 (0.70-1.15) | 1.29 (1.05-1.91) | < 0.001 | 0.90 (0.70-1.14) | 1.32 (0.89-1.86) | < 0.001 |
| GPR | 1.98 (0.75-3.38) | 2.28 (1.16-5.74) | 0.019 | 1.02 (0.35-2.65) | 3.59 (1.72-6.40) | < 0.001 |
| MRS | 3.82 (3.39-4.24) | 4.81 (4.19-5.55) | < 0.001 | 3.71 (3.44-4.14) | 4.90 (4.38-5.83) | < 0.001 |
| LSM (kPa) | 7.0 (5.7-9.1) | 21.3 (14.1-30.8) | < 0.001 | 6.2 (5.1-7.8) | 23.2 (16.5-43.6) | < 0.001 |
| BMI (kg/m2) | 22.9 (20.3-25.2) | 22.0 (20.3-25.3) | 0.653 | 22.9 (22.6-24.7) | 21.4 (19.5-23.3) | 0.008 |
In the validation group, there were 29 patients (33.3%) with histological stage I, 14 patients (16.1%) with stage II, 17 patients (19.5%) with stage III, and 27 patients (31.0%) with stage IV. Forty-three individuals (49.4%) were classified as being in the early stage (Ludwig stage I-II), whereas 44 (50.5%) were categorized as being in the advanced stage (Ludwig stage III-IV). The LSM varied between 3.2 and 75.0 kPa, with a median value of 10.2 kPa. Similar to the derivation cohort results, there was also a strong positive correlation between LSM and histological stage (r = 0.872, P < 0.001) (Sup
To predict AF, the logistic model was fitted to the observed data, taking into account LSM, BMI, biochemical parameters, IgG, immunoglobulin M, PLT, and AMA as potential predictors. Furthermore, the analysis was adjusted for age, gender, UDCA- naïve status, diabetes, and hypertension. The results of the multivariable logistic regression showed that LSM (OR = 1.158, 95%CI: 1.069-1.256, P < 0.001), being UDCA-naïve (OR = 0.264, 95%CI: 0.091-0.761, P = 0.014), IgG (OR = 1.164, 95%CI: 1.044-1.297, P = 0.006), and PLT (OR = 0.990, 95%CI: 0.983-0.998, P = 0.010) were independent predictors of AF (Supplementary Table 1). Regardless of the initial or previous UDCA treatment, LSM remained an independent predictor of AF (OR = 1.102, 95%CI: 1.013-1.199, P = 0.024; OR = 2.645, 95%CI: 1.180-5.928, P = 0.018). The data in Supplementary Table 1 show no significant additional predictive contribution to LSM from either the biochemical parameters or BMI.
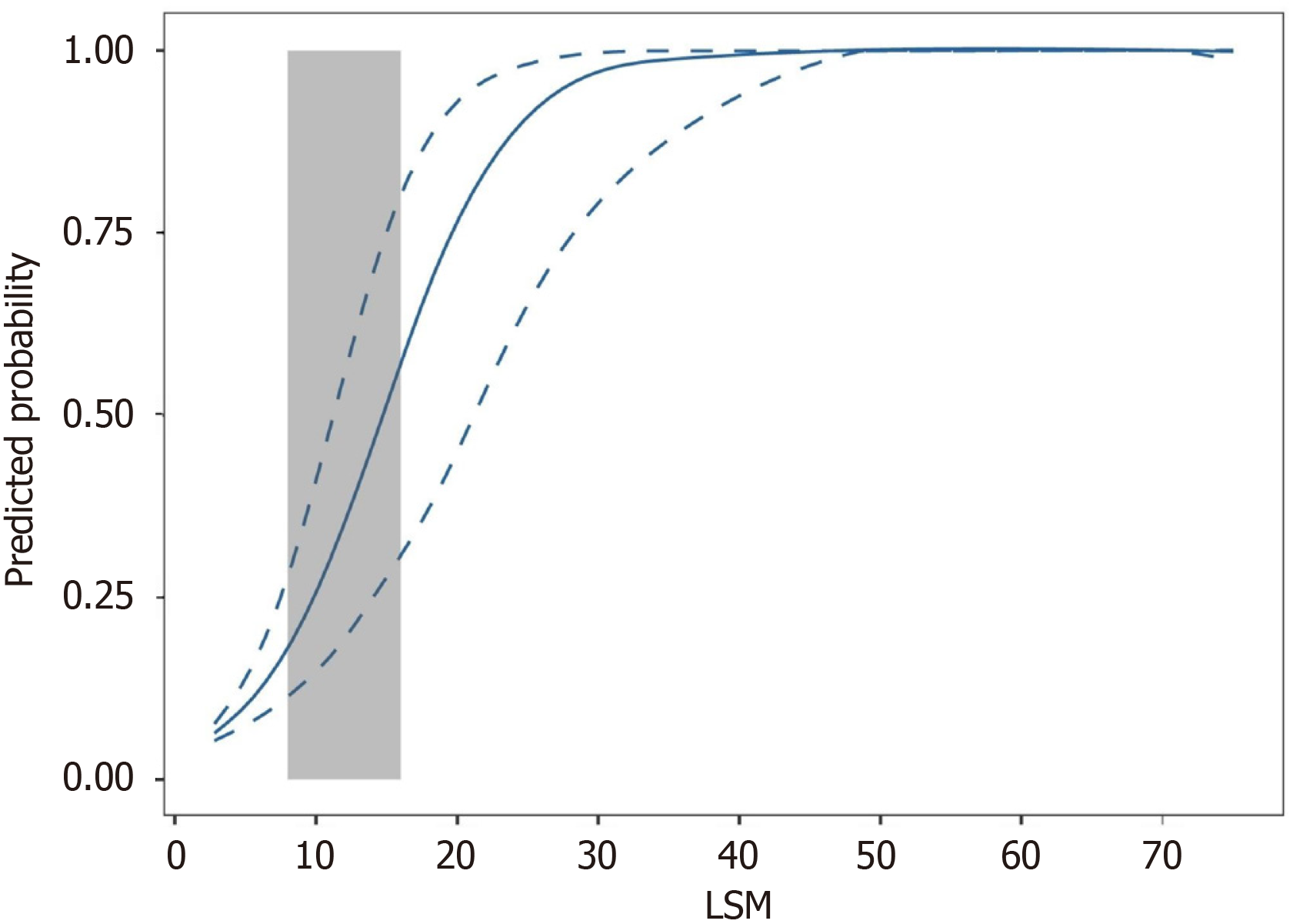
Furthermore, analysis of the ROC curve revealed that the AUROC of LSM for AF was 0.93 (95%CI: 0.88-0.96) (Figure 1A). At the maximum point of Youden’s index, the value was 11.0, accompanied by a sensitivity of 0.87 and a specificity of 0.84. However, while the NPV was 0.90, the PPV was only 0.79, resulting in the misclassification of 18 patients with advanced stage. The fitted logistic model illustrated the correlation between the predicted probabilities of AF and LSM values (Figure 2). Figure 2 shows a significant increase in the curve within the LSM range of 8-16 kPa, indicating the probability of AF between 0.18 and 0.57. Patients with Ludwig I-II and Ludwig III-IV exhibited overlapping LSM within this range. Therefore, although the LSM has a strong ability to predict AF, it is not feasible to reliably predict AF using a single-threshold method in this interval.
Consequently, we investigated the utilization of a dual cut-off strategy involving lower and higher thresholds to delineate regions of accurate prediction and a grey zone where VCTE might not offer a reliable prediction of AF. Supplementary Table 2 shows the reported diagnostic accuracies for various potentially high- and low-threshold values. At the optimal lower threshold of 10.0 kPa, which indicates the lack of AF, the sensitivity and NPV were 0.91 and 0.93, respectively. Due to this limitation, 96 individuals in the early stages were recognized, with 89 (92.7%) accurately predicted (Supplementary Table 2). The specificity and PPV for the optimal higher threshold of 14.5 kPa, which defines the presence of AF, were 0.96 and 0.92, respectively. This threshold detected 63 individuals at an advanced stage, with 58 (92.1%) accurately predicted. In the derivation cohort, employing this method of dual cut-off, the PLR and NLR were 20.4 and 0.09, respectively; the overall error rate was 7.5% (Table 3).
| Ludwig stage | Early stage (LSM ≤ 10) | Grey area (10 < LSM ≤ 14.5) | Advanced stage (LSM > 14.5) | Total | |
| Derivation cohort | Ludwig I | 55 (57.3) | 4 (12.9) | 1 (1.6) | 60 (31.6) |
| Ludwig II | 34 (35.4) | 14 (45.2) | 4 (6.3) | 52 (27.4) | |
| Ludwig III | 2 (2.1) | 4 (12.9) | 13 (20.6) | 19 (10.0) | |
| Ludwig IV | 5 (5.2) | 9 (29.0) | 45 (71.4) | 59 (31.1) | |
| Total | 96 | 31 | 63 | 190 | |
| Validation cohort | Ludwig I | 29 (69.0) | 0 (0) | 0 (0) | 29 (33.3) |
| Ludwig II | 8 (19.0) | 4 (44.4) | 2 (5.6) | 14 (16.1) | |
| Ludwig III | 4 (9.5) | 3 (33.3) | 10 (27.8) | 17 (19.5) | |
| Ludwig IV | 1 (2.4) | 2 (22.2) | 24 (66.7) | 27 (31.0) | |
| Total | 42 | 9 | 36 | 87 |
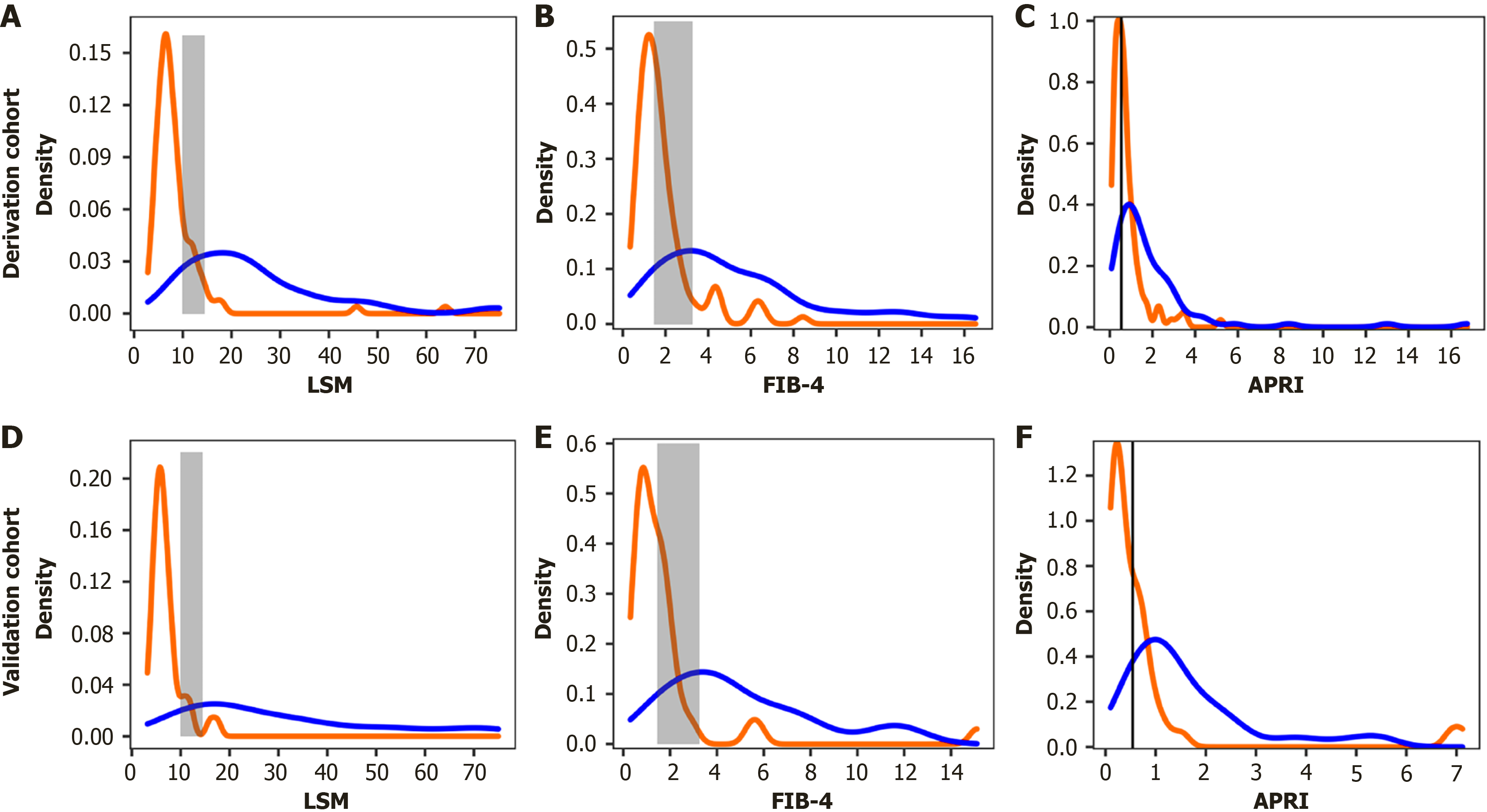
The AUROC for AF was 0.84 (95%CI: 0.78-0.89) when using FIB-4, 0.75 (95%CI: 0.68-0.81) using APRI, 0.76 (95%CI: 0.69-0.82) using AAR, 0.60 (95%CI: 0.53-0.67) using GPR, and 0.83 (95%CI: 0.76-0.88) when using MRS. LSM performed better than the other five tests (all P < 0.010; Figure 1A and Supplementary Table 3). We calculated the empirical distributions of FIB-4, APRI, and LSM to demonstrate their varying discriminatory abilities, categorized by the presence or absence of histological AF. The density distribution of FIB-4 and APRI overlaps moderately or more in early AF, LSM also exhibits a slight overlap, but was limited to the grey area (Figure 3A-C).
In addition, the dual cut-off method, which has been validated and is currently being used for FIB-4, correctly predicted 53 of 61 patients with FIB-4 < 1.45 (sensitivity = 0.90; NPV = 0.87). Similarly, among the 66 patients with a FIB-4 score > 3.25, 53 were accurately predicted (specificity = 0.88, PPV = 0.80). Of the 63 individuals who had FIB-4 measurements falling within the uncertain range (i.e., ≥ 1.45 and ≤ 3.25), 46 were classified as being in the early stage, while 17 were categorized as being in the advanced stage. Applying the validated cut-off of 0.54 for the APRI, we observed a specificity of 0.46, sensitivity of 0.82, NPV of 0.79, and PPV of 0.52. However, 61 patients were falsely classified as having an AF stage.
The two thresholds identified in the derivation cohort were verified in an external validation cohort of 87 patients with PBC. Table 3 shows that 42 patients in the early stages were correctly predicted with an accuracy of 88.1% using a lower threshold of 10.0 kPa. The sensitivity and NPV were 0.89 and 0.88, respectively. Of the 36 patients in advanced stages, 34 (94.4%), were accurately predicted using the higher threshold (14.5 kPa) (Table 3). The values for specificity and PPV were 0.95 and 0.94, respectively. When employing the dual cut-off in the validation cohort, the PLR and NLR values were 19.1 and 0.12, respectively, and the overall error rate was 9.0%.
The AUROC of LSM for AF in the validation cohort was 0.97 (95%CI: 0.90-0.99). In line with the findings from the derivation cohort, LSM demonstrated superior performance compared with FIB-4, APRI, AAR, GPR, and MRS (all P < 0.050; Figure 1B and Supplementary Table 3). In addition, we estimated the empirical distributions of LSM, FIB-4, and APRI based on the presence or absence of histological AF (Figure 3D-F). Similar to the derivation cohort, there was moderate or greater overlap in the density peaks of both groups in the FIB-4 and APRI plots, whereas the overlap in the LSM plot was slight. Furthermore, the calibration curve and results of the Hosmer-Lemeshow test showed no significant difference between the predicted values and observed values of LSM in evaluating AF, indicating that the model has a good goodness of fit (Supplementary Figure 4).
Among the 277 patients, 40 (14.4%) were in the grey area, with 22 (55.0%) in the early stage and 18 (45.0%) in the advanced stage. The median LSM value for patients classified as Ludwig stage I-II was 11.9 kPa (IQR: 10.9-12.8), whereas for patients classified as Ludwig stage III-IV, the median LSM value was 11.3 kPa (IQR: 10.8-12.2). Supplementary Table 4 demonstrates that patients in both the early and advanced stages exhibited similar clinical and laboratory parameters, along with non-invasive fibrosis models (e.g., FIB-4, APRI, GPR, and MRS), without any notable disparities.
The reported incidence and prevalence rates of PBC in Europe and North America surpass those in the Asia-Pacific region. Nevertheless, the prevalence of PBC in the Asia-Pacific region is increasing annually, particularly in Japan and China[27]. The degree of fibrosis is a significant determinant of PBC prognosis. A crucial clinical need in PBC is a non-invasive point-of-care test that aids in the detection and identification of AF during disease presentation. Although VCTE has the ability to accurately identify fibrosis, there is a scarcity of data defining the optimal utilization of VCTE in Chinese or other Asian populations. This study demonstrated the effectiveness of VCTE in detecting AF in a real-world Chinese population with histologically verified PBC. To the best of our knowledge, this study is the first real-world research with the largest sample size among similar studies exploring the evaluation of PBC fibrosis using VCTE. Additionally, we established externally validated dual cut-off values that exhibited exceptional sensitivity and specificity in confirming or ruling out AF at the time of diagnosis.
Furthermore, this study provides a dual cut-off approach utilizing LSM through VCTE to categorize individuals with PBC into three categories of risk: Early-stage (LSM ≤ 10.0 kPa), advanced-stage (LSM > 14.5 kPa), and a grey area of inaccurate discrimination (LSM > 10 kPa and ≤ 14.5 kPa). This non-invasive method of individual risk stratification for PBC will aid in clinical decision-making. Patients in the early stages, as indicated by VCTE, might be more likely to respond positively to UDCA treatment and experience a decreased risk of unfavorable outcomes, consequently leading to a reduction in the level of medical attention required. However, the early identification of AF using VCTE at baseline should enhance the intensity and timeliness of patient management, including timely second-line treatment. Despite its good sensitivity and specificity, the single cut-off approach results in the false classification of a significant number of patients. Although VCTE is not a definitive test, it can assist in identifying patients who may require further histological evaluation, thereby avoiding liver biopsies in those with little to no fibrosis. Our research shows that an LSM below 10.0 kPa has a sensitivity of 0.91 and an NPV of 0.93 in ruling out AF. This suggests that patients with an LSM below 10.0 kPa can opt for a less invasive approach since the presence of AF can be confidently excluded with at least 93% certainty. Higher LSM values provide greater specificity and can help identify individuals who may require further histological assessment for confirmation. Our study shows that an LSM exceeding 14.5 kPa has a specificity of 0.96 and a PPV of 0.92 for confirming AF. This implies that patients with LSM ≥ 14.5 kPa may not require additional LB because the presence of AF can be confirmed with at least 92% certainty.
In summary, the application of the dual cut-off value approach can decrease the need for LB in patients by 92.5%. However, for patients in the grey area, evaluating the presence of AF using LSM alone may be challenging. Within our research, 16.3% of the individuals in the derivation cohort and 10.3% in the validation cohort fell within the grey area. To accurately monitor and assess the presence of AF in this patient population, it is advisable to conduct LB in clinical practice. Viganò et al[28] proposed this dual cut-off method in patients with hepatitis B, while Cristoferi et al[11] recently suggested it for patients with PBC. Nevertheless, this study is the first attempt to utilize this approach for detecting fibrosis in patients with PBC in China and the broader Asian region. In a study conducted by Cristoferi et al[11], using a multicenter PBC cohort in Italy, the VCTE method with two cut-offs (LSM ≤ 6.5 and > 11.0 kPa) demonstrated an NPV of 0.94 and a PPV of 0.89 in distinguishing the absence or presence of AF (Ludwig III/IV) at the time of diagnosis. There is a stronger demand to solve the concern of finding new ways to correctly classify patients with PBC in the grey area. By defining the “grey area”, we quantified the range of uncertainty in VCTE-based staging of PBC (LSM 10.0-14.5 kPa). This itself provides critical risk prompts for clinical decision-making: Patients within this range require enhanced monitoring or multimodal assessment. Building on this study, we plan to explore the combination of serum biomarkers in future research to tackle the challenge of staging in the grey area. Supplementary Table 4 in this study shows that among patients in the grey area of the derivation cohort, the serum marker AAR exhibited significant heterogeneity between early and AF (median 0.91 vs 1.29, P = 0.016). Our preliminary analysis of this subgroup data revealed that combining AAR with VCTE could further improve staging accuracy (AUC increased from 0.72 to 0.79). The combination of VCTE and serological markers may thus be an effective approach to address the clinical challenge of difficult staging in the grey area.
Furthermore, the higher LSM thresholds observed in our study were likely due to racial differences and the lower BMI of PBC patients than those in the Italian cohort (22.9 kg/m2vs 24 kg/m2)[11]. Prior research has indicated that the presence of excess fat in overweight or obese individuals and the gap between the skin and the outer layer can potentially diminish the liver’s elastic waves detected by Fibroscan[29,30]. Consequently, this could result in an underestimation of the ultimate LSM value, as observed in previous studies. It is evident that the accuracy of LSM measurements appears to be influenced by BMI, consequently diminishing the reliability of the results. Therefore, the LSM cut-off values derived in our research may be more reliable and accurate due to the lower BMI. However, our results indicate that the evaluation of AF using LSM was not affected by BMI. By merging our study with an Italian cohort study that included more than 40% of patients who were overweight[11], we propose that LSM could serve as a dependable and consistent non-invasive substitute for assessing liver fibrosis in PBC patients with both high and low BMI. However, further confirmation is required in diverse ethnic populations.
In addition, it has been reported that liver inflammation, elevated transaminase levels, and cholestasis are additional factors that can potentially interfere with the accuracy of VCTE in evaluating liver fibrosis[31,32]. In our study, we also investigated the association between LSM and indicators (i.e., transaminase, ALP, gamma-glutamyl-transferase, and bilirubin) that serve as substitutes for liver inflammation and cholestasis, and no significant influence of their effect was observed. This aligns with a recent study on VCTE in PBC[11]. Notably, our multivariate analysis revealed that AF not only had an independent correlation with LSM, but also exhibited a connection with elevated IgG levels, decreased PLTs, and absence of prior UDCA treatment. In real-world clinical scenarios, it is not possible for every patient diagnosed with PBC to be initially treated with UDCA, and there are indeed some patients who may receive UDCA treatment before the diagnosis of PBC is confirmed due to the presence of cholestasis or other reasons. In our derivation cohort, there was a significantly higher proportion of UDCA-naïve individuals in the early stage than the advanced stage (73.2% vs 56.4%, P = 0.016). Likewise, our previous study demonstrated that patients with no prior exposure to UDCA treatment (OR = 2.543, 95%CI: 1.234-5.240) were identified as the independent variable influencing the biochemical response in individuals with PBC[33]. This could be due to the shorter duration of the disease in patients who had not previously received UDCA. Despite the established evidence of the ability of UDCA therapy to modify the histological progression of PBC, our subgroup analysis indicated that the association between LSM and fibrosis at diagnosis remained unaffected by prior UDCA treatment[1,8]. Thus, this may contribute to supporting LSM for the dynamic monitoring of changes in fibrosis staging in PBC patients.
At present, LB is not required for diagnosing the majority of PBC patients. Nevertheless, it remains essential for individuals lacking PBC-specific antibodies or those with suspected coexisting conditions such as AIH and NASH. Simultaneously, there are no recommendations advising the utilization of LB for disease staging upon diagnosis. Nevertheless, traditional histopathological characteristics, such as fibrosis staging, not only have a direct correlation with prognosis but also enhance the predictive capacity of the UDCA biochemical response for long-term consequences. As LB is an invasive procedure, numerous alternative methods have been suggested for assessing the fibrosis stage. Currently, VCTE using Fibroscan is the most extensively utilized and is regarded as an optimal substitute indicator for identifying severe fibrosis or cirrhosis in patients with PBC. VCTE offers several advantages over magnetic resonance elastography, which has been found to be highly accurate in evaluating liver fibrosis, including simpler and faster operation and lower price[30,34]. Numerous serum fibrosis models have been proposed to assess fibrosis in chronic liver conditions. The FIB-4 and APRI have been the focus of extensive research. Additionally, Nyblom et al[35] found that PBC-related cirrhosis could be predicted using an AAR of 1.1 as a cut-off value, with a sensitivity of 82% and specificity of 79%. According to a recent study[13], GPR demonstrated an AUROC of 0.84 with a sensitivity of 0.41% and specificity of 0.96, using a cut-off value of 4.81, in identifying AF in PBC individuals. However, compared with VCTE, these serum markers exhibit reduced accuracy when assessing AF and cirrhosis. Similar to previous studies, our study validated that VCTE is superior to serum fibrosis models for evaluating AF.
This study has several limitations. One major limitation of our study is its retrospective design, which may have led to selection bias. However, as LB is not essential for the diagnosis of PBC, conducting a prospective study is challenging. Second, there was a lack of complete synchronization between VCTE and LB. Nevertheless, considering the slow progression of fibrosis, it is improbable that the relatively short interval window between biopsy and VCTE greatly affects LSM. Third, we did not estimate inter- or intra-operator reproducibility of LSM. However, given the retrospective nature of the study, we have taken the following rigorous measures to maximize the quality and reliability of the VCTE data used in this study. Furthermore, not all subjects were initially administered UDCA upon enrollment. The LSM values and fibrosis staging evaluation may be influenced by the treatment history of certain patients who received informal UDCA treatment in other hospitals before being diagnosed with PBC in our hospital. However, our findings indicate that LSM remains useful for evaluating the extent of liver fibrosis at the time of diagnosis, even in patients who have previously received UDCA treatment.
In summary, VCTE using Fibroscan is a simple, reliable, rapid, and non-invasive approach for evaluating AF in individuals with PBC, surpassing the effectiveness of serum fibrosis markers. The findings of our study validated the usefulness and accuracy of VCTE in Chinese patients with PBC by employing a dual cut-off method. These results need to be verified in a larger prospective cohort study. Therefore, it is imperative to discover new alternative approaches to improve the recognition of disease staging in patients in the grey area.
The authors thank Dr. Lu of the Chinese Academy of Sciences for his technical support.
| 1. | Tanaka A, Ma X, Takahashi A, Vierling JM. Primary biliary cholangitis. Lancet. 2024;404:1053-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 76] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 2. | Lv T, Chen S, Li M, Zhang D, Kong Y, Jia J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1423-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 3. | Montano-Loza AJ, Corpechot C. Definition and Management of Patients With Primary Biliary Cholangitis and an Incomplete Response to Therapy. Clin Gastroenterol Hepatol. 2021;19:2241-2251.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Murillo Perez CF, Hirschfield GM, Corpechot C, Floreani A, Mayo MJ, van der Meer A, Ponsioen CY, Lammers WJ, Parés A, Invernizzi P, Carbone M, Maria Battezzati P, Nevens F, Kowdley KV, Thorburn D, Mason AL, Trivedi PJ, Lindor KD, Bruns T, Dalekos GN, Gatselis NK, Verhelst X, Janssen HLA, Hansen BE, Gulamhusein A; GLOBAL PBC Study Group. Fibrosis stage is an independent predictor of outcome in primary biliary cholangitis despite biochemical treatment response. Aliment Pharmacol Ther. 2019;50:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Carbone M, D’Amato D, Hirschfield GM, Jones DEJ, Mells GF. Letter: histology is relevant for risk stratification in primary biliary cholangitis. Aliment Pharmacol Ther. 2020;51:192-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 499] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 7. | Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hübscher S, Patanwala I, Pereira SP, Thain C, Thorburn D, Tiniakos D, Walmsley M, Webster G, Jones DEJ. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1013] [Article Influence: 112.6] [Reference Citation Analysis (1)] |
| 9. | Tu Z, Wang Y, Wang Y, Huang J, Han Y, Ji Q, Cao X, Wen X, Wang Y, Jin Q. TR score: A noninvasive model to predict histological stages in patients with primary biliary cholangitis. Front Immunol. 2023;14:1152294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Friedrich-Rust M, Müller C, Winckler A, Kriener S, Herrmann E, Holtmeier J, Poynard T, Vogl TJ, Zeuzem S, Hammerstingl R, Sarrazin C. Assessment of liver fibrosis and steatosis in PBC with FibroScan, MRI, MR-spectroscopy, and serum markers. J Clin Gastroenterol. 2010;44:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 11. | Cristoferi L, Calvaruso V, Overi D, Viganò M, Rigamonti C, Degasperi E, Cardinale V, Labanca S, Zucchini N, Fichera A, Di Marco V, Leutner M, Venere R, Picciotto A, Lucà M, Mulinacci G, Palermo A, Gerussi A, D’Amato D, Elisabeth O’Donnell S, Cerini F, De Benedittis C, Malinverno F, Ronca V, Mancuso C, Cazzagon N, Ciaccio A, Barisani D, Marzioni M, Floreani A, Alvaro D, Gaudio E, Invernizzi P, Carpino G, Nardi A, Carbone M; Italian PBC Registry. Accuracy of Transient Elastography in Assessing Fibrosis at Diagnosis in Naïve Patients With Primary Biliary Cholangitis: A Dual Cut-Off Approach. Hepatology. 2021;74:1496-1508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Corpechot C, Carrat F, Poujol-Robert A, Gaouar F, Wendum D, Chazouillères O, Poupon R. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 13. | Avcioğlu U, Eruzun H, Ustaoğlu M. The gamma-glutamyl transferase to platelet ratio for noninvasive evaluation of liver fibrosis in patients with primary biliary cholangitis. Medicine (Baltimore). 2022;101:e30626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Wilder J, Choi SS, Moylan CA. Vibration-Controlled Transient Elastography for Diagnosing Cirrhosis and Staging Hepatic Fibrosis. JAMA. 2018;320:2031-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Gómez-Dominguez E, Mendoza J, García-Buey L, Trapero M, Gisbert JP, Jones EA, Moreno-Otero R. Transient elastography to assess hepatic fibrosis in primary biliary cirrhosis. Aliment Pharmacol Ther. 2008;27:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3817] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 17. | Trivedi PJ, Bruns T, Cheung A, Li KK, Kittler C, Kumagi T, Shah H, Corbett C, Al-Harthy N, Acarsu U, Coltescu C, Tripathi D, Stallmach A, Neuberger J, Janssen HL, Hirschfield GM. Optimising risk stratification in primary biliary cirrhosis: AST/platelet ratio index predicts outcome independent of ursodeoxycholic acid response. J Hepatol. 2014;60:1249-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Corpechot C, Carrat F, Gaouar F, Chau F, Hirschfield G, Gulamhusein A, Montano-Loza AJ, Lytvyak E, Schramm C, Pares A, Olivas I, Eaton JE, Osman KT, Dalekos G, Gatselis N, Nevens F, Cazzagon N, Zago A, Russo FP, Abbas N, Trivedi P, Thorburn D, Saffioti F, Barkai L, Roccarina D, Calvaruso V, Fichera A, Delamarre A, Medina-Morales E, Bonder A, Patwardhan V, Rigamonti C, Carbone M, Invernizzi P, Cristoferi L, van der Meer A, de Veer R, Zigmond E, Yehezkel E, Kremer AE, Deibel A, Dumortier J, Bruns T, Große K, Pageaux GP, Wetten A, Dyson J, Jones D, Chazouillères O, Hansen B, de Lédinghen V; Global & ERN Rare-Liver PBC Study Groups. Liver stiffness measurement by vibration-controlled transient elastography improves outcome prediction in primary biliary cholangitis. J Hepatol. 2022;77:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 19. | Manzo-Francisco LA, Aquino-Matus J, Vidaña-Pérez D, Uribe M, Chavez-Tapia N. Systematic review and meta-analysis: Transient elastography compared to liver biopsy for staging of liver fibrosis in primary biliary cholangitis. Ann Hepatol. 2023;28:101107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Osman KT, Maselli DB, Idilman IS, Rowan DJ, Viehman JK, Harmsen WS, Harnois DM, Carey EJ, Gossard AA, LaRusso NF, Lindor KD, Venkatesh SK, Eaton JE. Liver Stiffness Measured by Either Magnetic Resonance or Transient Elastography Is Associated With Liver Fibrosis and Is an Independent Predictor of Outcomes Among Patients With Primary Biliary Cholangitis. J Clin Gastroenterol. 2021;55:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Joshita S, Yamashita Y, Sugiura A, Uehara T, Usami Y, Yamazaki T, Fujimori N, Matsumoto A, Tanaka E, Umemura T. Clinical utility of FibroScan as a non-invasive diagnostic test for primary biliary cholangitis. J Gastroenterol Hepatol. 2020;35:1208-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Koizumi Y, Hirooka M, Abe M, Tokumoto Y, Yoshida O, Watanabe T, Nakamura Y, Imai Y, Yukimoto A, Kumagi T, Takeshita E, Ikeda Y, Hiasa Y. Comparison between real-time tissue elastography and vibration-controlled transient elastography for the assessment of liver fibrosis and disease progression in patients with primary biliary cholangitis. Hepatol Res. 2017;47:1252-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Zhang YG, Zhao SX, Zhou GD, Li WC, Ren WG, Du HJ, Wang RQ, Nan YM. [Correlation of FibroTouch and FibroScan with the stage of primary biliary cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2016;24:902-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Wu HM, Sheng L, Wang Q, Bao H, Miao Q, Xiao X, Guo CJ, Li H, Ma X, Qiu DK, Hua J. Performance of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis-primary biliary cholangitis overlap syndrome. World J Gastroenterol. 2018;24:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Floreani A, Cazzagon N, Martines D, Cavalletto L, Baldo V, Chemello L. Performance and utility of transient elastography and noninvasive markers of liver fibrosis in primary biliary cirrhosis. Dig Liver Dis. 2011;43:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 538] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 27. | Zeng N, Duan W, Chen S, Wu S, Ma H, Ou X, You H, Kong Y, Jia J. Epidemiology and clinical course of primary biliary cholangitis in the Asia-Pacific region: a systematic review and meta-analysis. Hepatol Int. 2019;13:788-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Viganò M, Paggi S, Lampertico P, Fraquelli M, Massironi S, Ronchi G, Rigamonti C, Conte D, Colombo M. Dual cut-off transient elastography to assess liver fibrosis in chronic hepatitis B: a cohort study with internal validation. Aliment Pharmacol Ther. 2011;34:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | de Lédinghen V, Vergniol J, Foucher J, El-Hajbi F, Merrouche W, Rigalleau V. Feasibility of liver transient elastography with FibroScan using a new probe for obese patients. Liver Int. 2010;30:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Chen J, Yin M, Talwalkar JA, Oudry J, Glaser KJ, Smyrk TC, Miette V, Sandrin L, Ehman RL. Diagnostic Performance of MR Elastography and Vibration-controlled Transient Elastography in the Detection of Hepatic Fibrosis in Patients with Severe to Morbid Obesity. Radiology. 2017;283:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 391] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 32. | Tapper EB, Cohen EB, Patel K, Bacon B, Gordon S, Lawitz E, Nelson D, Nasser IA, Challies T, Afdhal N. Levels of alanine aminotransferase confound use of transient elastography to diagnose fibrosis in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2012;10:932-937.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Chen J, Xue D, Gao F, Tao L, Li Y, Zhang Q, Wang R, Sun L, Yang X, Liu Y, Zhu B, Niu S, Wang X. Influence factors and a predictive scoring model for measuring the biochemical response of primary biliary cholangitis to ursodeoxycholic acid treatment. Eur J Gastroenterol Hepatol. 2018;30:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Ozturk A, Olson MC, Samir AE, Venkatesh SK. Liver fibrosis assessment: MR and US elastography. Abdom Radiol (NY). 2022;47:3037-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 35. | Nyblom H, Björnsson E, Simrén M, Aldenborg F, Almer S, Olsson R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006;26:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/