Published online Nov 7, 2025. doi: 10.3748/wjg.v31.i41.111361
Revised: August 19, 2025
Accepted: September 28, 2025
Published online: November 7, 2025
Processing time: 131 Days and 1 Hours
Acute variceal bleeding (AVB) in patients with cirrhosis remains life-threatening; moreover, the current risk stratification methods have certain limitations. Rebleeding and mortality after AVB remain major challenges. Although preemptive transjugular intrahepatic portosystemic shunt (p-TIPS) can improve outcomes, not all patients benefit equally. Accurate risk stratification is needed to guide treatment decisions and identify those most likely to benefit from p-TIPS.
To develop an artificial intelligence (AI)-driven model to guide AVB treatment decisions, and identify candidates eligible for p-TIPS.
Patients with cirrhosis and AVB, from two multicenter retrospective cohorts in China, who received endoscopic variceal ligation plus pharmacotherapy (n = 1227) or p-TIPS (n = 1863) were included. Baseline data within 24 hours of hospital admission were obtained. The AI-AVB model, based on the six-week failure and one-year mor
The AI-AVB model demonstrated superior predictive performance compared to traditional risk stratification methods. In the internal validation cohort, the model achieved an area under the curve (AUC) of 0.842 for predicting six-week treatment failure and 0.954 for one-year mortality. In the external validation cohort, the AUCs were 0.814 and 0.889, respectively. The model effectively identified patients at high risk of first-line treatment failure who may benefit from aggressive interventions such as p-TIPS. In contrast, advancing the treatment stra
The AI-AVB model can predict treatment outcomes, stratify the failure risk in cirrhotic patients with AVB, aid in clinical decisions, identify p-TIPS beneficiaries, and optimize personalized treatment strategies.
Core Tip: A novel deep learning model was developed to predict treatment outcomes in patients with acute variceal bleeding, a life-threatening condition that is often observed in patients with cirrhosis. By analyzing clinical data collected within 24 hours of hospital admission, the artificial intelligence model can effectively identify high-risk patients who may benefit from more aggressive treatments, such as preemptive transjugular intrahepatic portosystemic shunt, while also helping avoid unwarranted invasive procedures for low-risk patients.
- Citation: Xiang Y, Yang N, Zheng TL, Huang YF, Liu TY, Ma DQ, Hu SJ, Zhang WH, Xiang HL, Zhang LY, Yuan LL, Wang X, Dang T, Zhang G, Wu B, Peng LJ, Gao M, Xia DL, Liu ZB, Li J, Song Y, Zhou XQ, Qi XS, Zeng J, Tan XY, Deng MM, Fang HM, Qi SL, He S, He YF, Ye B, Wu W, Shao JB, Wei W, Hu JP, Yong X, He CH, Bao JL, Zhang YN, Ji R, Bo Y, Yan W, Li HJ, Li SL, Geng S, Zhao L, Liu B, Qi XL. Development of a deep learning model for guiding treatment decisions of acute variceal bleeding in patients with cirrhosis. World J Gastroenterol 2025; 31(41): 111361
- URL: https://www.wjgnet.com/1007-9327/full/v31/i41/111361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i41.111361
Acute variceal bleeding (AVB) is a severe and life-threatening complication of portal hypertension frequently observed in patients with cirrhosis[1-4]. Despite significant advances in the management of AVB over recent years, it continues to be associated with substantial mortality[5,6]. The primary treatment modalities for AVB include endoscopy combined with pharmacological treatment and transjugular intrahepatic portosystemic shunt (TIPS)[7]. Endoscopy, often involving band ligation, along with vasoactive drugs, is the first-line treatment for controlling bleeding[3,8]. However, TIPS is considered for patients who are at high risk of treatment failure with endoscopic and pharmacological interventions[9,10]. Owing to the varying severity of bleeding and survival prognosis among patients with AVB, clinicians typically tailor or optimize treatment strategies based on the expected risk[11,12]. High-risk patients, who have a poor prognosis, require more aggressive and invasive interventions, such as preemptive TIPS (p-TIPS), to improve their survival outcomes[13]. Conver
The most widely adopted risk stratification criteria for AVB management are those outlined in the Baveno VII consensus[15]. According to the Baveno VII criteria, patients with a hepatic venous pressure gradient (HVPG) of ≥ 20 mmHg, or a Child-Pugh class C (10-13 points) or B (8-9 points) with active bleeding are classified as high risk for treat
In recent years, several alternative scoring systems have been proposed to refine the early TIPS criteria for AVB in patients with cirrhosis. These include the model for end-stage liver disease (MELD) score[22] with a threshold of > 19, the recalibrated MELD model or MELD-HE score[23], and the chronic liver failure-consortium acute decompensation score[14]. Among these, the MELD score and its recalibrated versions have demonstrated better discriminative performances, aiding in the identification of patients with AVB who may benefit from p-TIPS[24]. Buckholz et al[25] reported that the MELD score achieved an AUC of 0.76 (95%CI: 0.70-0.82) for predicting six-week mortality, reflecting moderate discriminatory ability[25]. However, the MELD score components, bilirubin levels, international normalized ratio, and creatinine levels mainly reflect liver and renal functions, omitting other factors that may improve prediction. Consequently, the current scoring systems may inadvertently misclassify patients, leading to treatment failure in ostensibly ‘non-high-risk’ patients owing to insufficient intervention[7] and unnecessary p-TIPS in others, increasing the risk of overt HE without survival benefits[9,12,26]. These limitations highlight the need for a more objective, accessible, accurate, and cost-effective tool to guide treatment decisions in complex AVB cases.
With the rapid advancements in artificial intelligence (AI) and medical informatics, deep learning technology has emerged as a powerful data-centric approach, demonstrating remarkable potential in the healthcare sector[27]. Notably, deep learning shows significant promise in predicting treatment outcomes in patients with AVB[28]. The complex nature of AVB, coupled with the myriad factors that influence patient prognosis, often renders traditional predictive models inadequate. The ability of deep learning to analyze complex datasets and identify patterns offers a promising avenue for enhancing predictive accuracy in this field[29]. Specifically, deep learning algorithms can be trained on a variety of variables, including clinical parameters, laboratory results, and endoscopic findings[30], to generate models that predict the likelihood of various treatment outcomes, such as rebleeding, complications, or mortality[31].
This study aimed to develop and validate a deep learning model using baseline data from patients with cirrhosis-related AVB at initial admission to stratify the risks of rebleeding and mortality, thereby guiding treatment decisions and identifying candidates likely to benefit from p-TIPS.
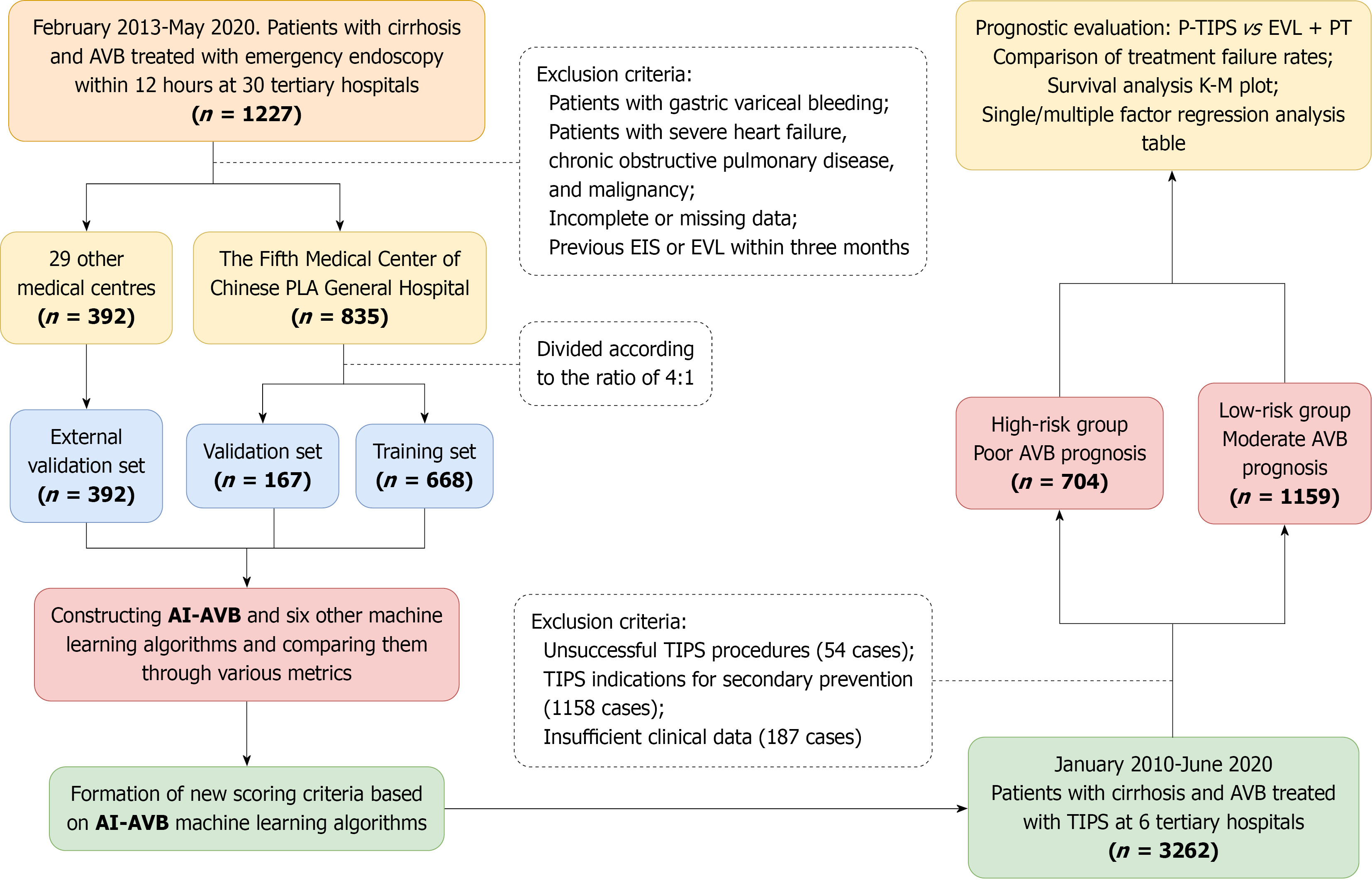
This study included two distinct cohorts of patients with cirrhosis-associated AVB. The first cohort was part of a multicenter retrospective study involving 30 tertiary hospitals across 18 provinces in China (Supplementary Table 1). This study included patients who received endoscopic variceal ligation (EVL) combined with pharmacotherapy (PT) between February 2013 and May 2020. Comprehensive demographic and clinical data were collected, and one-year treatment and survival outcomes were recorded. The second cohort included a multicenter retrospective study of patients with cirr
This study adhered to the ethical principles of the Declaration of Helsinki, and the study protocols were approved by the Ethics Committee of Zhongda Hospital, Southeast University. Given the retrospective nature of this study, the re
The inclusion criteria for both the training and validation cohorts were as follows: (1) Age between 18 and 80 years; (2) A confirmed diagnosis of cirrhosis based on liver biopsy or a combination of clinical, biochemical, and imaging findings; and (3) Endoscopic confirmation of AVB[8]. Exclusion criteria were: (1) Severe heart failure, chronic obstructive pul
As this study aimed to identify subgroups benefiting from p-TIPS, extremely high-risk patients who may not meet the criteria for p-TIPS treatment were excluded, i.e., those with uncontrollable initial bleeding, for whom TIPS may be ineffective, or even those for whom salvage TIPS becomes necessary (according to the Baveno criteria, such as patients with a Child-Pugh score ≥ 14 or a MELD score > 30)[33]. P-TIPS is defined as the placement of covered TIPS within 72 hours of admission as a preventive treatment before rebleeding occurs following EVL + PT therapy. Patients who had undergone p-TIPS were excluded during the model development and validation phases but were included in the evaluation of the model’s p-TIPS prediction efficiency. A flow diagram of the study population is illustrated in Figure 1.
The primary endpoint of the study was the rate of treatment failure within 6 weeks following either EVL + PT or p-TIPS treatment. Treatment failure was defined as rebleeding, including evidence of gastrointestinal hemorrhage such as hema
A total of 1227 patients with AVB receiving EVL + PT therapy were allocated to the training, internal validation, and external validation cohorts. Of these, 835 patients from the Fifth Medical Center of the Chinese PLA General Hospital were randomly assigned to either a training cohort (668 patients) or an internal validation cohort (167 patients) in a 4:1 ratio. The remaining 392 patients from 29 tertiary medical centers constituted the external validation cohort. Random assignment was performed using the scikit-learn package in Python with the ‘train test split’ function, to ensure balanced representation between the training and internal validation cohorts. The external validation cohort remained independent when assessing the generalizability of the model across different institutions.
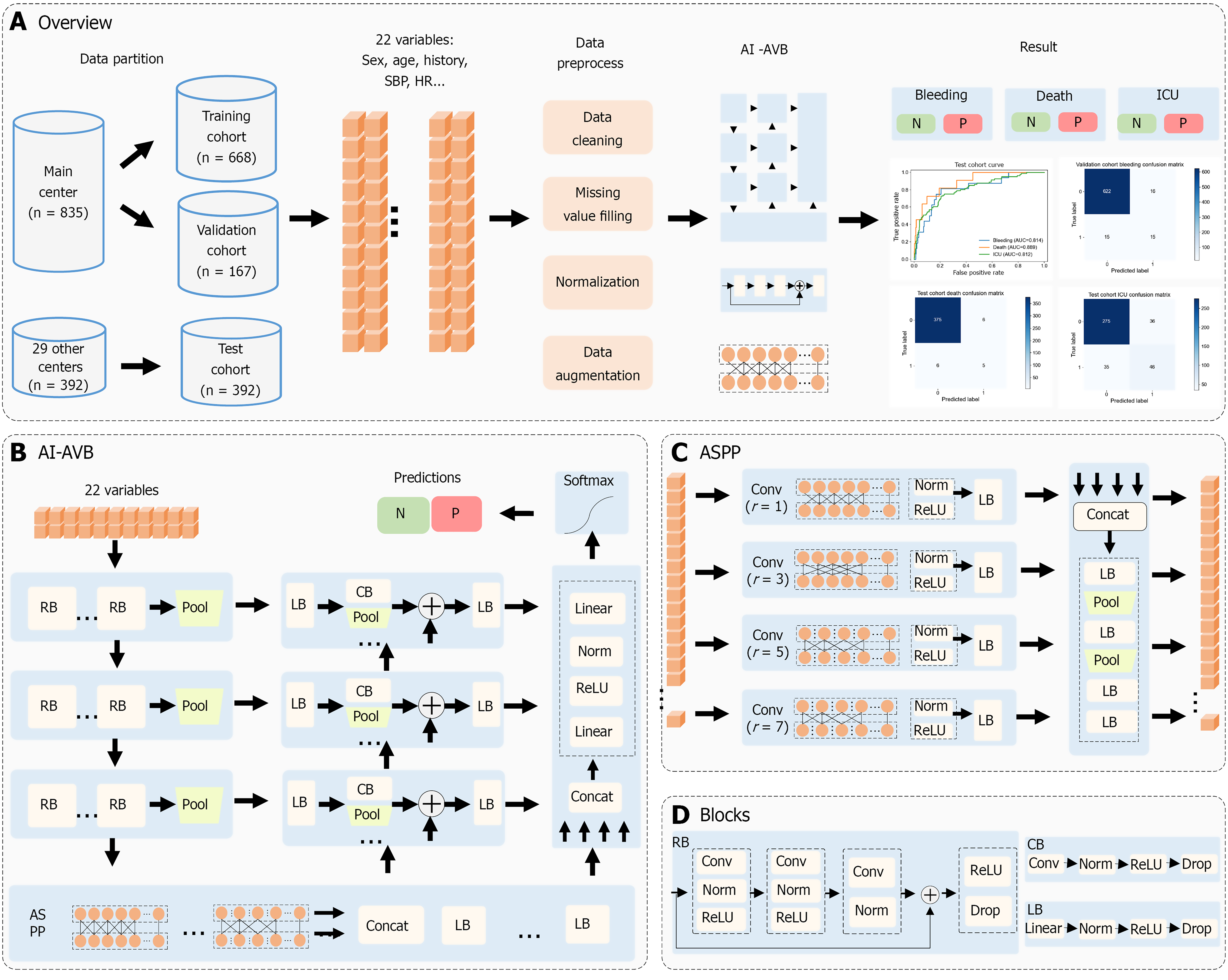
The proposed machine learning model, AI-AVB, is primarily based on the residual structure, pyramid architecture, and multilevel feature fusion strategy. It extracts the features of 22 input variables to generate prediction results for each endpoint; the overall architecture is shown in Figure 2A and B.
The initial input into the AI-AVB included 22 variables that were processed in three residual stages. Each residual stage was primarily comprised a complex one-dimensional residual block and a one-dimensional pooling layer. The three stages had two, three, and six residual blocks, respectively. The structure of the residual blocks is shown in Figure 2C and D. A shortcut was used to superimpose input features onto features generated by three one-dimensional convolution sets with kernel sizes of 1, 3, and 1. This cross-layered connection structure can limit gradient disappearance and network degradation in deep networks. At the end of each residual module, a dropout layer was appended to limit model over
After the residual-stage process, the third-stage features were input into the modified atrous spatial pyramid pooling (ASPP). The ASPP comprised four sets of one-dimensional atrous convolutions with dilation rates of 1, 3, 5, and 7, re
The multiscale features generated by the three residual stages and the ASPP features were fused using three sets of feature fusion blocks. Each set of feature fusion blocks included the corresponding residual stage and the last fusion features. Each fusion block adopted several convolution and pooling layers to down sample the residual-stage features. The down sampled features were superimposed with the last fusion feature and then integrated using a linear block to generate fusion features.
At the end of AI-AVB, the ASPP features and three groups of fusion features were concatenated and integrated using linear layers. A softmax layer was then used to generate the prediction probabilities for all classes of each endpoint.
To improve the anti-overfitting ability of the AI-AVB, we applied online data augmentation to the training cohort. The augmentation probability was initially 0.5 and was adjusted based on the decline curves of the training and validation cohorts. Augmentation methods primarily included random missing data, random variable vibration, and random label vibration.
To prevent AI-AVB from falling into a local optimum, cosine annealing was used to adjust the learning rate. The initial learning rate was 0.1, the minimum value was 0.0002, and the warm restart period was 8 epochs. We adopted an early stop strategy for training. The default number of epochs was 80. In terms of hyperparameters, the dropout rate was 0.4. The loss function was the crossing entropy loss, which is described as where is the number of classes, is the actual value of class, and is the predicted value. After calculating the loss value, chain derivation was performed according to the loss value, and the AdamW optimizer was used to update the model parameters.
To demonstrate the effectiveness and advancement of the proposed AI-AVB method, we compared and analyzed six frequently used machine learning methods based on our dataset with AI-AVB. These methods included support vector machine[34], logistic regression[35], decision tree[36], random forest[37], extreme gradient boosting[38], and light gra
Using the previously developed and validated AI-AVB model, all patients with cirrhosis and AVB who underwent EVL + PT and p-TIPS were stratified into high- and low-risk groups for EVL + PT failure. Within these high- and low-risk subgroups, we compared the outcomes and adverse effects associated with the two treatment strategies. The outcomes assessed were as follows: (1) The rate of treatment failure within 6 weeks; (2) One-year mortality; (3) ICU admission; and (4) The development or worsening of ascites and HE.
Further analysis involved both univariate and multivariate regression models to investigate the independent predictive value of baseline characteristics, Child-Pugh scores, MELD scores, and treatment modalities for six-week treatment failure and survival at discharge in both the high- and low-risk groups for standard therapy failure.
Normally distributed continuous variables are expressed as mean ± SD and compared using Student’s t-test, while the non-normally distributed continuous variables are presented as medians (interquartile ranges) and analyzed using the Mann-Whitney test. Categorical variables are represented as counts and percentages, and compared using the χ2 test or Fisher’s exact test. The performance metrics of the proposed and comparative methods were evaluated using a receiver operating characteristic curve, confusion matrix, and the following indices: AUC, accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The Delong test was used to compare the AUCs of the other models with that of AI-AVB.
A total of 1227 patients with cirrhosis and AVB were included in the study and underwent EVL + PT. Of these, 835 patients from the Fifth Medical Center of the Chinese PLA General Hospital were randomly allocated into a training cohort (668 patients) and an internal validation cohort (167 patients) in a 4:1 ratio. The remaining 392 patients from 29 tertiary medical centers were designated as the external validation cohort. The demographic characteristics, initial clinical conditions, laboratory results, liver-related risk scores, treatment modalities, and outcomes for the three cohorts are pre
| Characteristics | Training cohort (n = 668) | Internal validation cohort | P value | External validation cohort (n = 392) |
| Demographic characteristics | ||||
| Age (years) | 52.5 ± 11.8 | 52.6 ± 10.2 | 0.927 | 53.1 ± 11.8 |
| Sex | 0.273 | |||
| Male | 473 (70.8) | 126 (75.4) | 278 (70.9) | |
| Female | 195 (29.2) | 41 (24.6) | 114 (29.1) | |
| Etiology of cirrhosis | 0.394 | |||
| Chronic HBV infection | 401 (60.0) | 117 (70.1) | 246 (62.8) | |
| Chronic HCV infection | 41 (6.1) | 10 (6.0) | 28 (7.1) | |
| Alcohol | 73 (10.9) | 16 (9.6) | 56 (14.3) | |
| Others | 92 (13.8) | 14 (8.3) | 35 (8.9) | |
| Cryptogenic | 61 (9.1) | 10 (6.0) | 27 (6.9) | |
| Medical history | ||||
| Previous variceal bleeding | 240 (35.9) | 65 (38.9) | 0.407 | 163 (41.6) |
| Location of varices | 0.299 | |||
| Esophageal varices only | 388 (58.0) | 105 (62.9) | 214 (54.6) | |
| Esophageal and gastric varices | 280 (42.0) | 62 (37.1) | 178 (45.4) | |
| Hepatic encephalopathy | 65 (9.7) | 17 (10.2) | 0.437 | 33 (8.4) |
| Ascites | 0.502 | |||
| Mild | 237 (35.4) | 76 (45.5) | 142 (36.2) | |
| Moderate | 96 (14.4) | 31 (18.6) | 68 (17.4) | |
| Massive | 45 (6.7) | 13 (7.8) | 30 (7.7) | |
| Heart rate at admission (beats/minute) | 85.0 ± 15.6 | 85.7 ± 16.6 | 0.632 | 87.5 ± 15.5 |
| Systolic blood pressure at admission (mmHg) | 115.8 ± 17.5 | 116.5 ± 16.2 | 0.640 | 113.6 ± 19.8 |
| Diastolic blood pressure at admission (mmHg), median IQR | 71.0 (63-79) | 71.0 (62.5-79.5) | 0.746 | 64.0 (55.5-72.5) |
| Laboratory examination | ||||
| White blood cell (× 109 cell/L), median IQR | 7.3 (5.4-10.0) | 7.6 (6.2-9.0) | 0.052 | 7.6 (5.3-9.7) |
| Red blood cell (× 109 cell/L) | 2.7 ± 0.8 | 2.8 ± 0.7 | 0.483 | 2.3 ± 0.6 |
| Hemoglobin (g/L) | 77.3 ± 22.3 | 78.5 ± 23.4 | 0.521 | 70.8 ± 24.8 |
| Platelet count (× 109/L), median IQR | 84.0 (61.0-117.0) | 85.0 (62.5-128.0) | 0.867 | 94.5 (69.0-129.0) |
| NEC (× 109/L), median IQR | 5.7 (4.3-8.0) | 5.7 (4.3-8.2) | 0.674 | 6.3 (3.8-12.5) |
| AST (U/L), median IQR | 94.0 (83.7-118.2) | 104.0 (93.0-125.5) | 0.561 | 113.0 (103.5-133.0) |
| ALT (U/L), median IQR | 54.0 (46.0-67.0) | 53.0 (46.0-62.5) | 0.681 | 56.0 (49.0-70.0) |
| TBIL (μmol/L) | 38.0 ± 63.2 | 34.4 ± 38.0 | 0.577 | 33.0 ± 33.2 |
| Albumin (g/L) | 28.5 ± 5.4 | 29.0 ± 5.0 | 0.267 | 31.7 ± 8.8 |
| INR | 1.4 ± 2.7 | 1.3 ± 0.3 | 0.606 | 1.6 ± 1.5 |
| APTT (second) | 38.4 ± 16.3 | 36.6 ± 9.8 | 0.176 | 37.2 ± 13.4 |
| TT (second) | 19.9 ± 3.6 | 19.4 ± 3.2 | 0.124 | 19.8 ± 5.8 |
| PT (second), median IQR | 14.2 (12.9-15.8) | 13.9 (12.9-15.6) | 0.269 | 15.4 (13.9-17.9) |
| Creatinine (μmol/L), median IQR | 92.0 (81.0-107.0) | 90.0 (80.0-102.5) | 0.814 | 86.0 (75.0-105.0) |
| Risk stratification index | ||||
| MELD score (points) | 11.3 ± 3.2 | 12.1 ± 5.0 | 0.049 | 12.2 ± 3.7 |
| Child-Pugh score (points), median IQR | 8.0 (7.0-9.0) | 8.0 (7.0-9.1) | 0.797 | 7.0 (5.5-8.5) |
| Child-Pugh class | 0.395 | |||
| A (5-6) | 142 (21.3) | 29 (17.4) | 125 (31.9) | |
| B (7-9) | 407 (60.9) | 111 (66.5) | 180 (45.9) | |
| C (10-13) | 119 (17.8) | 27 (16.2) | 87 (22.2) | |
| Early TIPS criteria | 0.745 | |||
| Low risk | 534 (79.9) | 136 (81.4) | 280 (71.4) | |
| High risk | 134 (20.1) | 31 (18.6) | 112 (28.6) | |
| Treatment procedure | ||||
| Initial pharmacological therapy | 0.275 | |||
| Octreotide | 177 (26.5) | 47 (28.1) | 90 (22.9) | |
| Somatostatin | 403 (60.3) | 91 (54.5) | 255 (65.1) | |
| Terlipressin | 88 (13.2) | 29 (17.4) | 47 (12.0) | |
| Outcome measurements | ||||
| 6-week treatment failure to control bleeding | 78 (11.8) | 22 (13.2) | 0.113 | 55 (14.0) |
| ICU requirement | 155 (23.2) | 38 (22.8) | 0.785 | 81 (20.7) |
| 1-year mortality | 126 (18.8) | 30 (18.0) | 0.261 | 67 (17.1) |
| Treatment-related adverse events | ||||
| Hepatic encephalopathy | 86 (12.9) | 26 (15.6) | 0.093 | 74 (18.9) |
| New or worsening ascites | 18 (2.7) | 3 (1.8) | 0.194 | 10 (2.6) |
The mean age of patients in the training, internal validation, and external validation cohorts was 52.5 ± 11.8 years, 52.6 ± 10.2 years, and 53.1 ± 11.8 years, respectively. Most patients in the three cohorts were males (70.8%, 75.4%, and 70.9%, respectively). Chronic hepatitis B virus (HBV) infection was the most common etiology of cirrhosis in all cohorts.
The six-week treatment failure rates were 11.8%, 13.2%, and 14.0% in the training, internal validation, and external validation cohorts, respectively. The one-year mortality rates for the three cohorts were 18.8%, 18.0%, and 17.1%, respec
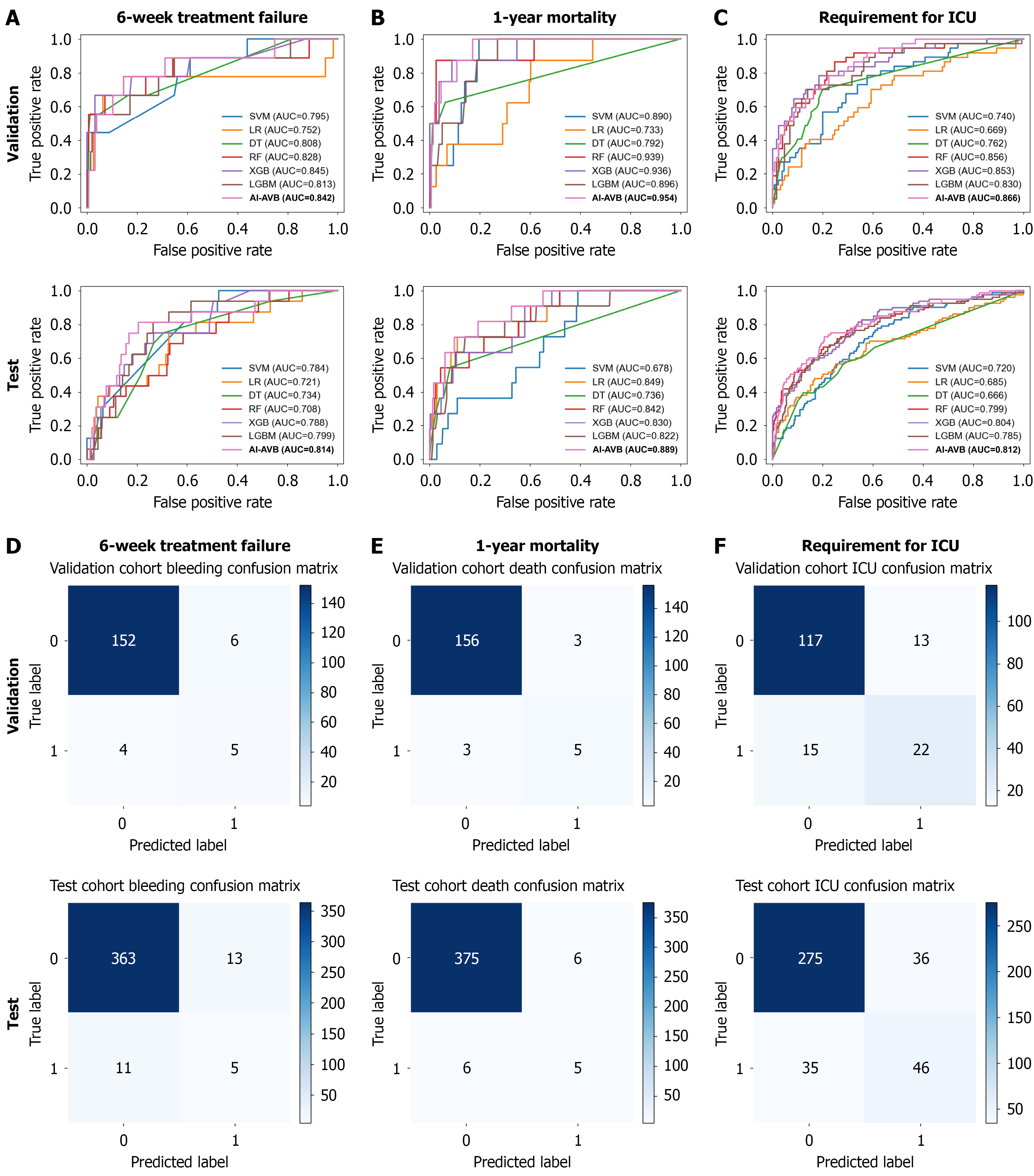
An AI-AVB model was developed to predict the likelihood of treatment failure within 6 weeks and mortality in 1 year following EVL + PT treatment in patients with cirrhosis and AVB. For the six-week treatment failure prediction in the internal validation cohort, the AI-AVB model achieved an AUC of 0.842, an accuracy of 0.940, a PPV of 0.455, and an NPV of 0.974. In the external validation cohort, the AI-AVB model demonstrated an AUC of 0.814, accuracy of 0.939, PPV of 0.278, and NPV of 0.971 (Figure 3 and Supplementary Table 2).
To predict one-year mortality in the internal validation cohort, the AI-AVB model achieved an AUC of 0.954, an accu
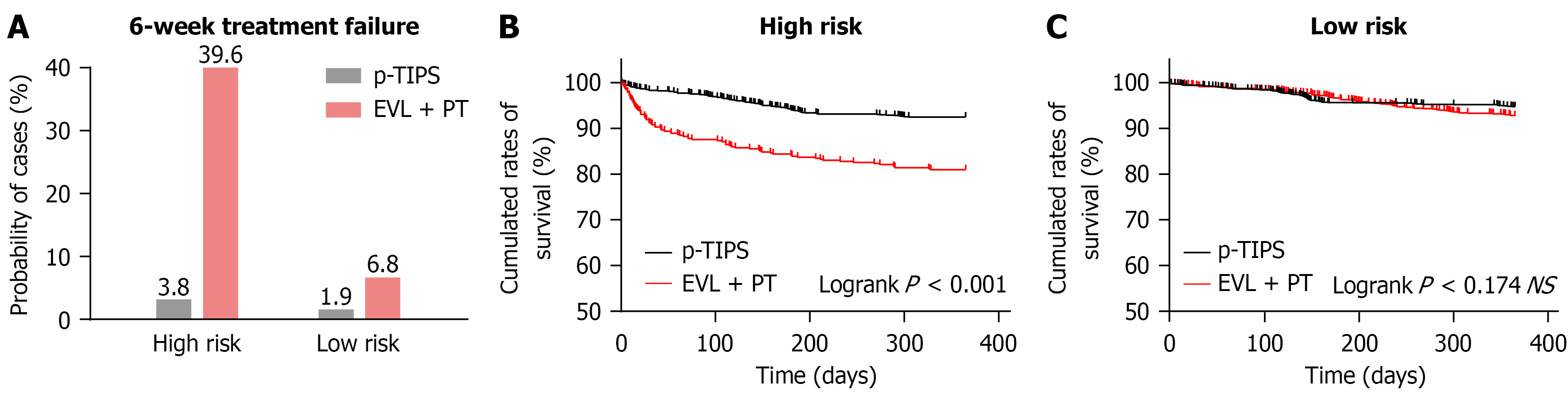
As shown in Table 2, for high-risk patients, the six-week treatment failure rate was significantly lower in the p-TIPS group (3.8%) than in the EVL + PT group (39.6%) (Table 2). Similarly, the one-year mortality rate was markedly lower in the p-TIPS group (14.6%) than in the EVL + PT group (37.3%) (Figure 4). These findings indicate that p-TIPS significantly improved short-term outcomes in high-risk patients.
| Characteristics | High-risk for EVL + PT failure | Low-risk for EVL + PT failure | ||||
| EVL + PT (n = 220) | p-TIPS (n = 704) | P value | EVL + PT (n = 1007) | p-TIPS (n = 1159) | P value | |
| Demographic characteristics | ||||||
| Age (years) | 52.4 ± 10.9 | 52.6 ± 11.7 | 0.167 | 53.3 ± 11.8 | 51.5 ± 12.5 | 0.042 |
| Sex | 0.011 | 0.020 | ||||
| Male | 162 (73.6) | 457 (64.9) | 715 (71.0) | 729 (62.9) | ||
| Female | 58 (26.4) | 247 (35.1) | 292 (29.0) | 430 (37.1) | ||
| Etiology of cirrhosis | 0.072 | 0.005 | ||||
| Chronic HBV infection | 154 (70.0) | 476 (67.6) | 610 (60.6) | 761 (65.7) | ||
| Chronic HCV infection | 6 (2.7) | 35 (5.0) | 73 (7.2) | 100 (8.6) | ||
| Alcohol | 23 (10.5) | 63 (8.9) | 122 (12.1) | 92 (7.9) | ||
| Others | 18 (8.1) | 92 (13.1) | 123 (12.2) | 133 (11.5) | ||
| Cryptogenic | 19 (8.6) | 38 (5.4) | 79 (7.8) | 73 (6.3) | ||
| Medical history | ||||||
| Previous variceal bleeding | 93 (42.3) | 319 (45.3) | 0.537 | 375 (37.2) | 447 (38.6) | 0.618 |
| Location of varices | 0.692 | 0.544 | ||||
| Esophageal varices only | 131 (59.5) | 432 (61.4) | 576 (57.2) | 679 (58.6) | ||
| Esophageal and gastric varices | 89 (40.5) | 272 (38.6) | 431 (42.8) | 480 (41.4) | ||
| Hepatic encephalopathy | 32 (14.5) | 116 (16.4) | 0.236 | 83 (8.2) | 105 (9.1) | 0.280 |
| Ascites | 0.021 | 0.066 | ||||
| Mild | 89 (40.5) | 263 (37.3) | 366 (36.3) | 395 (34.1) | ||
| Moderate | 42 (19.1) | 144 (20.4) | 153 (15.2) | 220 (19.0) | ||
| Massive | 9 (4.1) | 72 (10.2) | 79 (7.8) | 104 (9.0) | ||
| Heart rate at admission (beats/minute) | 84.4 ± 14.4 | 83.1 ± 15.3 | 0.384 | 87.8 ± 16.4 | 86.8 ± 15.7 | 0.033 |
| Systolic blood pressure at admission (mmHg) | 112.0 ± 12.9 | 113.8 ± 17.9 | 0.067 | 117.4 ± 14.5 | 115.8 ± 16.0 | 0.003 |
| Diastolic blood pressure at admission (mmHg) | 67.5 ± 10.8 | 70.6 ± 12.4 | 0.002 | 69.5 ± 9.9 | 68.1 ± 11.5 | 0.042 |
| Laboratory examination | ||||||
| White blood cell (× 109 cell/L) | 8.4 ± 5.9 | 8.0 ± 3.9 | 0.246 | 6.0 ± 3.9 | 6.3 ± 4.4 | 0.095 |
| Red blood cell (× 109 cell/L) | 2.5 ± 0.7 | 2.4 ± 0.6 | 0.387 | 2.8 ± 0.7 | 3.5 ± 7.3 | 0.003 |
| Hemoglobin (g/L) | 75.2 ± 22.8 | 72.6 ± 22.6 | 0.038 | 77.7 ± 22.3 | 82.5 ± 24.9 | < 0.001 |
| Platelet count (× 109/L) | 87.0 ± 57.8 | 86.4 ± 54.3 | 0.888 | 95.0 ± 89.0 | 97.6 ± 86.6 | 0.492 |
| NEC (× 109/L) | 7.6 ± 8.8 | 6.8 ± 5.7 | 0.115 | 5.1 ± 8.4 | 4.7 ± 5.9 | 0.196 |
| AST (U/L) | 124.9 ± 233.5 | 106.1 ± 167.5 | 0.189 | 76.1 ± 493.4 | 50.3 ± 99.0 | 0.082 |
| ALT (U/L) | 62.0 ± 101.9 | 55.6 ± 40.8 | 0.176 | 49.6 ± 214.2 | 34.9 ± 40.8 | 0.022 |
| TBIL (μmol/L) | 104.1 ± 120.8 | 60.2 ± 49.5 | < 0.001 | 23.7 ± 23.8 | 27.3 ± 25.4 | < 0.001 |
| Albumin (g/L) | 25.2 ± 4.6 | 25.6 ± 6.9 | 0.421 | 29.2 ± 5.3 | 32.9 ± 8.7 | < 0.001 |
| INR | 1.6 ± 0.5 | 2.1 ± 1.8 | < 0.001 | 1.4 ± 2.9 | 1.4 ± 1.4 | 0.898 |
| APTT (second) | 49.2 ± 19.1 | 46.2 ± 19.7 | 0.047 | 36.0 ± 14.6 | 35.3 ± 10.9 | 0.203 |
| TT (second) | 21.4 ± 3.8 | 22.1 ± 7.0 | 0.156 | 19.5 ± 3.5 | 19.3 ± 5.4 | 0.315 |
| PT (second) | 18.5 ± 6.7 | 23.9 ± 20.3 | 0.001 | 14.2 ± 3.5 | 15.6 ± 3.4 | < 0.001 |
| Creatinine (μmol/L) | 100 ± 82.9 | 86.8 ± 58.4 | 0.009 | 82.5 ± 70.1 | 73.6 ± 44.1 | < 0.001 |
| Risk stratification index | ||||||
| MELD score (points) | 13.5 ± 4.0 | 15.9 ± 6.5 | 0.002 | 11.2 ± 3.6 | 12.5 ± 3.8 | 0.034 |
| Child-Pugh score (points), median IQR | 7.9 (5.1-10.7) | 8.2 (6.1-10.3) | 0.068 | 7.4 (5.5-9.3) | 7.2 (5.6-8.8) | < 0.001 |
| Child-Pugh class | 0.167 | < 0.001 | ||||
| A (5-6) | 42 (19.2) | 101 (14.4) | 254 (25.2) | 336 (29.0) | ||
| B (7-9) | 131 (59.5) | 424 (60.3) | 567 (56.3) | 719 (62.1) | ||
| C (10-13) | 47 (21.4) | 179 (25.4) | 186 (18.5) | 104 (8.9) | ||
| Early TIPS criteria | < 0.001 | < 0.001 | ||||
| Low risk | 161 (73.2) | 390 (55.4) | 789 (78.4) | 707 (61.0) | ||
| High risk | 59 (26.8) | 314 (44.6) | 218 (21.6) | 452 (39.0) | ||
| Treatment procedure | ||||||
| Initial pharmacological therapy | 0.087 | < 0.001 | ||||
| Octreotide | 283 (28.1) | 314 (27.1) | 31 (14.1) | 140 (19.9) | ||
| Somatostatin | 597 (59.3) | 730 (63.0) | 152 (69.1) | 513 (72.9) | ||
| Terlipressin | 127 (12.6) | 115 (9.9) | 37 (16.8) | 51 (7.2) | ||
| p-TIPS stent diameter | ||||||
| < 8 mm | 137 (19.5) | 61 (5.3) | ||||
| 8 mm | 554 (78.2) | 1025 (88.4) | ||||
| 10 mm | 16 (2.3) | 73 (6.3) | ||||
| Outcome measurements | ||||||
| 6-week treatment failure to control bleeding | 87 (39.6) | 27 (3.8) | < 0.001 | 68 (6.8) | 22 (1.9) | 0.013 |
| ICU requirement | 102 (46.4) | 144 (20.5) | 0.005 | 172 (17.1) | 151 (13.0) | 0.049 |
| 1-year mortality | 82 (37.3) | 103 (14.6) | < 0.001 | 141 (14.0) | 121 (10.4) | 0.036 |
| Treatment-related adverse events | ||||||
| Hepatic encephalopathy | 36 (16.4) | 285 (30.5) | < 0.001 | 150 (14.9) | 314 (27.1) | < 0.001 |
| New or worsening ascites | 12 (5.5) | 10 (1.4) | < 0.001 | 19 (1.9) | 10 (0.9) | < 0.001 |
Regarding treatment-related adverse events, HE was more prevalent in the p-TIPS group, with an incidence of 30.5%, compared to 16.4% in the EVL + PT group. Conversely, the incidence of new or worsening ascites was lower in the p-TIPS group (1.4%) than that in the EVL + PT group (5.5%).
The univariate and multivariate regression analyses (Table 3) further supported these findings. In the high-risk sub
| Variables | High-risk patients for EVL + PT failure | Low-risk patients for EVL + PT failure | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| 1-year mortality | ||||||||
| Age (years, ≥ 60 vs < 60) | 1.125 (0.924-1.371) | 0.241 | 1.312 (1.009-1.706) | 0.043 | 1.189 (0.841-1.680) | 0.328 | ||
| Sex (male vs female) | 1.095 (0.909-1.320) | 0.337 | 0.981 (0.765-1.257) | 0.879 | ||||
| Etiology of cirrhosis | 1.123 (0.892-1.414) | 0.325 | 0.792 (0.587-1.068) | 0.127 | ||||
| Location of varices | 0.997 (0.808-1.229) | 0.974 | 0.802 (0.609-1.055) | 0.115 | ||||
| Hepatic encephalopathy (yes vs no) | 1.464 (1.204-1.781) | < 0.001 | 1.132 (0.875-1.465) | 0.345 | 1.369 (1.066-1.757) | 0.014 | 1.027 (0.738-1.429) | 0.876 |
| Ascites (yes vs no) | 1.784 (1.421-2.241) | < 0.001 | 1.409 (1.134-1.957) | < 0.001 | 2.282 (1.648-3.160) | < 0.001 | 2.088 (1.538-2.834) | < 0.001 |
| Systolic blood pressure at admission (≥ 120 mmHg vs < 120 mmHg) | 1.019 (0.847-1.227) | 0.84 | 1.054 (0.829-1.340) | 0.668 | ||||
| Diastolic blood pressure at admission (≥ 90 mmHg vs < 90 mmHg) | 1.353 (0.779-2.348) | 0.283 | 0.777 (0.458-1.316) | 0.348 | ||||
| MELD score (> 13 vs ≤ 13) | 4.968 (3.543-6.967) | < 0.001 | 1.320 (1.046-1.665) | 0.006 | 5.284 (3.331-8.381) | < 0.001 | 2.208 (1.155-3.504) | < 0.001 |
| Child-Pugh class (C vs A + B) | 4.137 (1.977-7.926) | < 0.001 | 3.309 (2.535-4.554) | < 0.001 | - | 0.996 | ||
| Different treatment strategies (EVL + PT vs p-TIPS) | 2.377 (1.855-2.842) | < 0.001 | 1.671 (1.096-2.259) | 0.029 | 0.954 (0.671-1.355) | 0.791 | ||
| 6-week treatment failure | ||||||||
| Age (years, ≥ 60 vs < 60) | 1.251 (1.170-1.363) | 0.009 | 1.090 (0.898-1.322) | 0.376 | 1.225 (1.075-1.526) | 0.028 | 1.128 (0.870-1.389) | 0.437 |
| Sex (male vs female) | 1.087 (0.917-1.272) | 0.346 | 0.997 (0.792-1.238) | 0.947 | ||||
| Etiology of cirrhosis | 1.134 (0.917-1.392) | 0.248 | 0.821 (0.631-1.068) | 0.144 | ||||
| Location of varices | 1.025 (0.839-1.240) | 0.839 | 0.852 (0.665-1.094) | 0.205 | ||||
| Hepatic encephalopathy (yes vs no) | 1.430 (1.211-1.704) | < 0.001 | 1.251 (1.010-1.548) | 0.040 | 1.378 (1.094-1.725) | 0.007 | 1.180 (0.984-1.548) | 0.230 |
| Ascites (yes vs no) | 1.750 (1.415-2.187) | < 0.001 | 1.256 (1.093-1.667) | < 0.001 | 2.001 (1.515-2.666) | < 0.001 | 1.875 (1.375-2.571) | < 0.001 |
| Systolic blood pressure at admission (≥ 120 mmHg vs < 120 mmHg) | 1.051 (0.875-1.260) | 0.591 | 1.079 (0.855-1.339) | 0.563 | ||||
| Diastolic blood pressure at admission (≥ 90 mmHg vs < 90 mmHg) | 1.303 (0.846-2.115) | 0.288 | 0.826 (0.540-1.280) | 0.353 | ||||
| MELD score (> 13 vs ≤ 13) | 5.020 (3.750-6.667) | < 0.001 | 1.516 (1.167-1.923) | 0.002 | 4.520 (3.697-6.750) | < 0.001 | 3.427 (2.167-4.154) | < 0.001 |
| Child-Pugh class (C vs A + B) | 6.125 (2.567-9.450) | < 0.001 | 4.231 (2.540-6.221) | < 0.001 | 0.995 | |||
| Different treatment strategies (EVL + PT vs p-TIPS) | 2.350 (1.903-2.999) | < 0.001 | 1.705 (1.300-2.250) | < 0.001 | 1.955 (1.700-2.300) | 0.018 | 1.051 (0.746-1.320) | 0.082 |
The six-week treatment failure rate was 6.8% in the EVL + PT group (68 patients) and 1.9% in the p-TIPS group (22 patients; P = 0.013). The one-year mortality rates were comparable in the EVL + PT (14.0%) and p-TIPS (10.4%) groups (P = 0.036). HE occurred in 14.9% and 27.1% of the EVL + PT and p-TIPS groups, respectively (P < 0.001), whereas new or worsening ascites was observed in 1.9% and 0.9% of the EVL + PT and p-TIPS groups, respectively (P < 0.001; Table 2).
Further multivariate regression analysis (Table 3) revealed that for patients with low-risk AVB, the presence of ascites and a MELD score > 13 were independent predictors of poor short-term prognosis. Specifically, ascites had an HR of 22.825 (95%CI: 16.485-31.604, P < 0.001), and MELD > 13 had an HR of 7.208 (95%CI: 4.155-12.504, P < 0.001). Conversely, the adjusted HR between the two treatment strategies (EVL + PT vs p-TIPS) was 0.954 (95%CI: 0.671-1.355, P = 0.791), indicating that the aggressive treatment strategy was not an independent factor directly contributing to the survival benefit of patients with low-risk AVB (Supplementary Tables 5-7).
This study developed and validated a novel AI-driven model using deep learning technologies based on clinical exami
Our findings corroborate those of previous studies, highlighting the key predictors of mortality and treatment failure in AVB. Advanced age, HE, and ascites are significant predictors of adverse outcomes[3,41,42]. Advanced age (≥ 60 years) was particularly influential in the low-risk subgroup, showing an HR of 1.312 in univariate analysis. HE (HR = 1.464) and ascites (HR = 17.848) were critical in the high-risk group. Moreover, elevated MELD scores (> 13) and higher Child-Pugh classifications were robustly associated with increased mortality, emphasizing the importance of liver and renal functions in determining prognosis[43]. Specifically, the MELD scores showed an HR of 4.968 (P < 0.001) and 7.208 (P < 0.001) in in high-and low-risk patients, respectively. These findings suggest these variables were more indicative of patient outcomes than the severity of the bleeding episodes. They can be attributed to advances in effective hemostatic treatments, such as endoscopy, PT, and the use of TIPS, along with improved general medical management practices, including restrictive transfusion strategies and prophylactic antibiotic use[44-46]. These insights underscore the need for early and aggressive management of the underlying liver dysfunction and related complications to improve outcomes in patients with AVB.
The superior performance of our decision-support model likely stems from its use of deep learning and AI tech
By leveraging comprehensive data collected within the first 24 hours of admission, clinicians can utilize the model’s predictions to identify high-risk patients who would benefit from more aggressive and invasive interventions, such as p-TIPS or liver transplantation, thereby reducing the likelihood of treatment failure[13,50]. Although these patients may incur additional treatment risks or adverse effects, the significant improvement in the success rates following six weeks of treatment and one-year survival justifies the approach[9]. Conversely, for patients identified as low-risk, although p-TIPS offers superior hemostatic efficacy, the increased incidence of HE in our study may ultimately negate the benefits, failing to improve prognosis independently. In such cases, the model can guide clinicians toward less-invasive treatments, minimizing unnecessary surgical interventions and their associated risks (such as puncture failure, HE, liver failure, secondary infections, and stent occlusion or displacement)[51-53]. This risk-stratified approach to post-treatment failure and mortality ensures more efficient resource allocation and tailored care according to each patient’s specific risk profile, ultimately improving the overall treatment outcomes and patient prognosis.
Beyond its clinical utility, the AI-AVB model may yield meaningful socioeconomic gains by aligning therapeutic inten
This study has some limitations. First, in our multicenter Chinese cohort, HBV-related cirrhosis was predominant and other etiologies were underrepresented, rendering underpowered etiology-specific analyses with limited generalizability, warranting further validation in etiology-balanced cohorts. Second, although AI-AVB demonstrated strong overall per
The AI-AVB model, developed using advanced deep learning techniques, excels in stratifying high- and low-risk patients for first-line treatment failure among patients with cirrhosis-associated AVB, accurately predicting the six-week treatment failure and one-year mortality rates. This model offers a promising tool for ensuring that patients with AVB receive personalized interventions to improve their overall prognosis and resource allocation.
The authors acknowledge all the clinical and research staff from the research centers.
| 1. | Guixé-Muntet S, Quesada-Vázquez S, Gracia-Sancho J. Pathophysiology and therapeutic options for cirrhotic portal hypertension. Lancet Gastroenterol Hepatol. 2024;9:646-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Allaire M, Thabut D. Portal hypertension and variceal bleeding in patients with liver cancer: Evidence gaps for prevention and management. Hepatology. 2024;79:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 3. | Ibrahim M, Mostafa I, Devière J. New Developments in Managing Variceal Bleeding. Gastroenterology. 2018;154:1964-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | O'Brien J, Triantos C, Burroughs AK. Management of varices in patients with cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:402-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Abraldes JG, Caraceni P, Ghabril M, Garcia-Tsao G. Update in the Treatment of the Complications of Cirrhosis. Clin Gastroenterol Hepatol. 2023;21:2100-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Ardevol A, Ibañez-Sanz G, Profitos J, Aracil C, Castellvi JM, Alvarado E, Cachero A, Horta D, Miñana J, Gomez-Pastrana B, Pavel O, Dueñas E, Casas M, Planella M, Castellote J, Villanueva C. Survival of patients with cirrhosis and acute peptic ulcer bleeding compared with variceal bleeding using current first-line therapies. Hepatology. 2018;67:1458-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Balcar L, Mandorfer M, Hernández-Gea V, Procopet B, Meyer EL, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Samaniego LI, Silva-Junior G, Martinez J, Genescà J, Bureau C, Trebicka J, Herrera EL, Laleman W, Palazón Azorín JM, Alonso JC, Gluud LL, Ferreira CN, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Grønbæk H, Hernandez Guerra MN, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Krag A, Nevens F, Calleja JL, Jansen C, Catalina MV, Albillos A, Rudler M, Tapias EA, Guardascione MA, Tantau M, Schwarzer R, Reiberger T, Laursen SB, Lopez-Gomez M, Cachero A, Ferrarese A, Ripoll C, La Mura V, Bosch J, García-Pagán JC; International Variceal Bleeding Observational Study Group by the Baveno Cooperation: an EASL consortium. Predicting survival in patients with 'non-high-risk' acute variceal bleeding receiving β-blockers+ligation to prevent re-bleeding. J Hepatol. 2024;80:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Guo CLT, Wong SH, Lau LHS, Lui RNS, Mak JWY, Tang RSY, Yip TCF, Wu WKK, Wong GLH, Chan FKL, Lau JYW, Sung JJY. Timing of endoscopy for acute upper gastrointestinal bleeding: a territory-wide cohort study. Gut. 2022;71:1544-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Hernández-Gea V, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Ibañez-Samaniego L, Silva-Junior G, Martinez J, Genescà J, Bureau C, Trebicka J, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud LL, Noronha Ferreira C, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Krag A, Nevens F, Calleja JL, Jansen C, Robic MA, Conejo I, Catalina MV, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Garcia-Pagán JC; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Preemptive-TIPS Improves Outcome in High-Risk Variceal Bleeding: An Observational Study. Hepatology. 2019;69:282-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 10. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 868] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 11. | Nicoară-Farcău O, Han G, Rudler M, Angrisani D, Monescillo A, Torres F, Casanovas G, Bosch J, Lv Y, Dunne PDJ, Hayes PC, Thabut D, Fan D, Hernández-Gea V, García-Pagán JC; pre-emptive TIPS individual data metanalysis, International Variceal Bleeding Study and Baveno Cooperation Study groups. Pre-emptive TIPS in high-risk acute variceal bleeding. An updated and revised individual patient data meta-analysis. Hepatology. 2024;79:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Lv Y, Zuo L, Zhu X, Zhao J, Xue H, Jiang Z, Zhuge Y, Zhang C, Sun J, Ding P, Ren W, Li Y, Zhang K, Zhang W, He C, Zhong J, Peng Q, Ma F, Luo J, Zhang M, Wang G, Sun M, Dong J, Bai W, Guo W, Wang Q, Yuan X, Wang Z, Yu T, Luo B, Li X, Yuan J, Han N, Zhu Y, Niu J, Li K, Yin Z, Nie Y, Fan D, Han G. Identifying optimal candidates for early TIPS among patients with cirrhosis and acute variceal bleeding: a multicentre observational study. Gut. 2019;68:1297-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Larrue H, D'Amico G, Olivas P, Lv Y, Bucsics T, Rudler M, Sauerbruch T, Hernandez-Gea V, Han G, Reiberger T, Thabut D, Vinel JP, Péron JM, García-Pagán JC, Bureau C. TIPS prevents further decompensation and improves survival in patients with cirrhosis and portal hypertension in an individual patient data meta-analysis. J Hepatol. 2023;79:692-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 14. | Lv Y, Bai W, Zhu X, Xue H, Zhao J, Zhuge Y, Sun J, Zhang C, Ding P, Jiang Z, Zhu X, Ren W, Li Y, Zhang K, Zhang W, Li K, Wang Z, Luo B, Li X, Yang Z, Wang Q, Guo W, Xia D, Yang C, Pan Y, Yin Z, Fan D, Han G. CLIF-C AD score predicts survival benefit from pre-emptive TIPS in individuals with Child-Pugh B cirrhosis and acute variceal bleeding. JHEP Rep. 2022;4:100621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1862] [Article Influence: 465.5] [Reference Citation Analysis (3)] |
| 16. | Dajti E, Villanueva C, Berzigotti A, Brujats A, Albillos A, Genescà J, García-Pagán JC, Colecchia A, Bosch J; PREDESCI trial investigators; A study by the Baveno Cooperation, an EASL Consortium. Exploring algorithms to select candidates for non-selective beta-blockers in cirrhosis: A post hoc analysis of the PREDESCI trial. J Hepatol. 2025;82:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Huang Y, Wang X, Li X, Sun S, Xie Y, Yin X. Comparative efficacy of early TIPS, Non-early TIPS, and Standard treatment in patients with cirrhosis and acute variceal bleeding: a network meta-analysis. Int J Surg. 2024;110:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, Marrero JM, Buceta E, Sánchez J, Castellot A, Peñate M, Cruz A, Peña E. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 338] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 19. | Lv Y, Bai W, Zhu X, Xue H, Zhao J, Zhuge Y, Sun J, Zhang C, Ding P, Jiang Z, Zhu X, Ren W, Li Y, Zhang K, Zhang W, Li K, Wang Z, Luo B, Li X, Yang Z, Guo W, Xia D, Xie H, Pan Y, Yin Z, Fan D, Han G. Development and validation of a prognostic score to identify the optimal candidate for preemptive TIPS in patients with cirrhosis and acute variceal bleeding. Hepatology. 2024;79:118-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Jaspers TJM, Boers TGW, Kusters CHJ, Jong MR, Jukema JB, de Groof AJ, Bergman JJ, de With PHN, van der Sommen F. Robustness evaluation of deep neural networks for endoscopic image analysis: Insights and strategies. Med Image Anal. 2024;94:103157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 21. | Yoo JJ, Maeng SA, Chang Y, Lee SH, Jeong SW, Jang JY, Cheon GJ, Kim YS, Kim HS, Kim SG. Enhancing liver cirrhosis varices and CSPH risk prediction with spleen stiffness measurement using 100-Hz probe. Sci Rep. 2024;14:13674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, Pandya P, Sitaraman S, Shen J. Model for end-stage liver disease (MELD) for predicting mortality in patients with acute variceal bleeding. Hepatology. 2002;35:1282-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, Llop E, González A, Seijo S, Berzigotti A, Ma M, Genescà J, Bosch J, García-Pagán JC, Abraldes JG. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412-19.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 291] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 24. | Xu M, Liu Z, Li X, Wang X, Yuan X, Han C, Zhang Z. Three-dimensional structure of liver vessels and spatial distribution of hepatic immune cells. J Innov Opt Health Sci. 2023;16:2330006. [DOI] [Full Text] |
| 25. | Buckholz A, Wong R, Curry MP, Baffy G, Chak E, Rustagi T, Mohanty A, Fortune BE. MELD, MELD 3.0, versus Child score to predict mortality after acute variceal hemorrhage: A multicenter US cohort. Hepatol Commun. 2023;7:e0258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 26. | Khan S, Tudur Smith C, Williamson P, Sutton R. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database Syst Rev. 2006;2006:CD000553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Kim HY, Lampertico P, Nam JY, Lee HC, Kim SU, Sinn DH, Seo YS, Lee HA, Park SY, Lim YS, Jang ES, Yoon EL, Kim HS, Kim SE, Ahn SB, Shim JJ, Jeong SW, Jung YJ, Sohn JH, Cho YK, Jun DW, Dalekos GN, Idilman R, Sypsa V, Berg T, Buti M, Calleja JL, Goulis J, Manolakopoulos S, Janssen HLA, Jang MJ, Lee YB, Kim YJ, Yoon JH, Papatheodoridis GV, Lee JH. An artificial intelligence model to predict hepatocellular carcinoma risk in Korean and Caucasian patients with chronic hepatitis B. J Hepatol. 2022;76:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 28. | Le Berre C, Sandborn WJ, Aridhi S, Devignes MD, Fournier L, Smaïl-Tabbone M, Danese S, Peyrin-Biroulet L. Application of Artificial Intelligence to Gastroenterology and Hepatology. Gastroenterology. 2020;158:76-94.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 356] [Article Influence: 59.3] [Reference Citation Analysis (3)] |
| 29. | Gao Y, Yu Q, Li X, Xia C, Zhou J, Xia T, Zhao B, Qiu Y, Zha JH, Wang Y, Tang T, Lv Y, Ye J, Xu C, Ju S. An imaging-based machine learning model outperforms clinical risk scores for prognosis of cirrhotic variceal bleeding. Eur Radiol. 2023;33:8965-8973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 30. | Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78:1216-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 31. | Theodosiou AA, Read RC. Artificial intelligence, machine learning and deep learning: Potential resources for the infection clinician. J Infect. 2023;87:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 32. | Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B; Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 748] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 33. | Zhang X, Song J, Zhang Y, Wen B, Dai L, Xi R, Wu Q, Li Y, Luo X, Lan X, He Q, Luo W, Lai Q, Ji Y, Zhou L, Qi T, Liu M, Zhou F, Wen W, Li H, Liu Z, Chen Y, Zhu Y, Li J, Huang J, Cheng X, Tu M, Hou J, Wang H, Chen J. Baveno VII algorithm outperformed other models in ruling out high-risk varices in individuals with HBV-related cirrhosis. J Hepatol. 2023;78:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 34. | Noble WS. What is a support vector machine? Nat Biotechnol. 2006;24:1565-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1395] [Cited by in RCA: 1581] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 35. | LaValley MP. Logistic regression. Circulation. 2008;117:2395-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 361] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 36. | Myles AJ, Feudale RN, Liu Y, Woody NA, Brown SD. An introduction to decision tree modeling. J Chemom. 2004;18:275-285. [DOI] [Full Text] |
| 37. | Rigatti SJ. Random Forest. J Insur Med. 2017;47:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 38. | Augi EH, Sultan A. The Early Warning Signs of a Stroke: An Approach Using Machine Learning Predictions. J Comput Commun. 2024;12:59-71. [DOI] [Full Text] |
| 39. | Nagassou M, Mwangi RW, Nyarige E. A Hybrid Ensemble Learning Approach Utilizing Light Gradient Boosting Machine and Category Boosting Model for Lifestyle-Based Prediction of Type-II Diabetes Mellitus. JDAIP. 2023;11:480-511. [DOI] [Full Text] |
| 40. | Akiba T, Sano S, Yanase T, Ohta T, Koyama M. Optuna: A Next-generation Hyperparameter Optimization Framework. KDD '19: Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining; 2019 Aug 4-8; New York, NY, United States. Association for Computing Machinery, 2019: 2623-2631. |
| 41. | Kumar R, Kerbert AJC, Sheikh MF, Roth N, Calvao JAF, Mesquita MD, Barreira AI, Gurm HS, Ramsahye K, Mookerjee RP, Yu D, Davies NH, Mehta G, Agarwal B, Patch D, Jalan R. Determinants of mortality in patients with cirrhosis and uncontrolled variceal bleeding. J Hepatol. 2021;74:66-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Lv Y, Wang Z, Li K, Wang Q, Bai W, Yuan X, Yu T, Niu J, Yang Z, Zhu X, Zhao J, Xue H, Jiang Z, Zhuge Y, Zhang C, Sun J, Ding P, Ren W, Li Y, Zhang K, Zhang W, Guo W, Luo B, Li X, Yuan J, Han N, Zhu Y, He C, Yin Z, Fan D, Han G. Risk Stratification Based on Chronic Liver Failure Consortium Acute Decompensation Score in Patients With Child-Pugh B Cirrhosis and Acute Variceal Bleeding. Hepatology. 2021;73:1478-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Conejo I, Guardascione MA, Tandon P, Cachero A, Castellote J, Abraldes JG, Amitrano L, Genescà J, Augustin S. Multicenter External Validation of Risk Stratification Criteria for Patients With Variceal Bleeding. Clin Gastroenterol Hepatol. 2018;16:132-139.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:l536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (36)] |
| 45. | Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY, Lee SD. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology. 2004;39:746-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 46. | Tandon P, Abraldes JG, Keough A, Bastiampillai R, Jayakumar S, Carbonneau M, Wong E, Kao D, Bain VG, Ma M. Risk of Bacterial Infection in Patients With Cirrhosis and Acute Variceal Hemorrhage, Based on Child-Pugh Class, and Effects of Antibiotics. Clin Gastroenterol Hepatol. 2015;13:1189-96.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 47. | Chen X, Chen X, Zhang Y, Fu X, Zha ZJ. Laplacian Pyramid Neural Network for Dense Continuous-Value Regression for Complex Scenes. IEEE Trans Neural Netw Learn Syst. 2021;32:5034-5046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, Aldairem A, Alrashed M, Bin Saleh K, Badreldin HA, Al Yami MS, Al Harbi S, Albekairy AM. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ. 2023;23:689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 1056] [Article Influence: 352.0] [Reference Citation Analysis (1)] |
| 49. | Hatami B, Asadi F, Bayani A, Zali MR, Kavousi K. Machine learning-based system for prediction of ascites grades in patients with liver cirrhosis using laboratory and clinical data: design and implementation study. Clin Chem Lab Med. 2022;60:1946-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 50. | Bossuyt P, De Hertogh G, Eelbode T, Vermeire S, Bisschops R. Computer-Aided Diagnosis With Monochromatic Light Endoscopy for Scoring Histologic Remission in Ulcerative Colitis. Gastroenterology. 2021;160:23-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Lee HL, Lee SW. The role of transjugular intrahepatic portosystemic shunt in patients with portal hypertension: Advantages and pitfalls. Clin Mol Hepatol. 2022;28:121-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Holster IL, Tjwa ET, Moelker A, Wils A, Hansen BE, Vermeijden JR, Scholten P, van Hoek B, Nicolai JJ, Kuipers EJ, Pattynama PM, van Buuren HR. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + β-blocker for prevention of variceal rebleeding. Hepatology. 2016;63:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 53. | Bañares R, Casado M, Rodríguez-Láiz JM, Camúñez F, Matilla A, Echenagusía A, Simó G, Piqueras B, Clemente G, Cos E. Urgent transjugular intrahepatic portosystemic shunt for control of acute variceal bleeding. Am J Gastroenterol. 1998;93:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/