Published online Oct 21, 2025. doi: 10.3748/wjg.v31.i39.110751
Revised: August 22, 2025
Accepted: September 26, 2025
Published online: October 21, 2025
Processing time: 91 Days and 1.3 Hours
Perioperative blood transfusion is common in gastric cancer surgery, yet its im
To investigate the effect of perioperative blood transfusion on postoperative infla
A retrospective analysis was conducted on 200 patients who underwent gastric cancer surgery, divided into a non-transfusion group (n = 108) and a transfusion group (n = 92). Baseline characteristics, pathological features, postoperative inflammatory and stress markers, complications, and long-term survival were compared between the two groups. Statistical analyses were performed using
The transfusion group had a lower T-stage distribution and higher intraoperative blood loss than the non-transfusion group (P < 0.05). Postoperative inflammatory markers such as white blood cell count, neutrophil/lymphocyte ratio, C-reactive protein, interleukin-6, and stress markers like cortisol and adrenaline were sig
Perioperative blood transfusion is associated with increased postoperative inflammation, stress reactions, complication rates, and adverse prognosis in patients with gastric cancer. Reducing unnecessary transfusions can improve postoperative recovery and long-term prognosis.
Core Tip: This study aimed to investigate the impact of blood transfusion on postoperative inflammation and stress markers in gastric cancer patients undergoing radical gastrectomy, and its correlation with prognosis. Perioperative blood transfusion is associated with increased postoperative inflammation, stress reactions, complication rates, and adverse prognosis in gastric cancer patients. Reducing unnecessary transfusions can improve postoperative recovery and long-term prognosis.
- Citation: Chen YQ, Chen JH, Zhang YX, Fu LL, Luo XS. Influence of blood transfusion on postoperative inflammation, stress markers, and prognosis in patients with gastric cancer. World J Gastroenterol 2025; 31(39): 110751
- URL: https://www.wjgnet.com/1007-9327/full/v31/i39/110751.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i39.110751
Gastric cancer is one of the leading causes of cancer-related deaths worldwide. The cumulative risk of gastric cancer in Eastern Asia is markedly elevated at 2.64%[1]. Although surgical resection remains the primary treatment option for locally advanced gastric cancer, postoperative complications and their effects on long-term survival are critical concerns in clinical practice[2,3]. In recent years, the relationship between perioperative blood transfusion and patient prognosis has garnered significant attention[4]. Studies have shown that perioperative blood transfusion can lead to immune suppression, increase infection risk, and indirectly affect long-term survival by promoting tumor growth[5].
For instance, research has found that perioperative blood transfusion significantly increases the incidence of postoperative infections, especially when using non-leukocyte-filtered blood products[6]. Furthermore, perioperative transfusion is strongly associated with adverse outcomes in patients with gastric cancer, including increased recurrence rates and reduced overall survival (OS)[7]. These findings suggest that perioperative blood transfusion may have a detrimental effect on patients’ long-term prognosis. Further studies have pointed out that perioperative blood transfusion exacerbates postoperative inflammatory responses, which may further impact patient recovery and long-term survival[8]. The increase in inflammatory and stress markers indicates a heightened inflammatory response, which may elevate the risk of postoperative complications and prolong hospital stays, ultimately affecting long-term prognosis[9].
Moreover, the influence of perioperative blood transfusion on patient stress responses has received considerable attention. Patients who received transfusion exhibited significantly elevated levels of cortisol, adrenaline, noradrenaline, and blood glucose during the early postoperative period, thereby confirming that these individuals experienced more intense stress responses than their counterparts. High levels of stress hormones not only affect immune system function but also increase cardiovascular burden and the incidence of postoperative complications[10]. Therefore, rational management and reduction of transfusion requirements may be crucial strategies for improving overall recovery.
Despite these insights, there is limited comprehensive data on the specific effects of blood transfusion on postoperative inflammation and stress markers in patients with gastric cancer, as well as their correlation with long-term outcomes. Therefore, this study aimed to investigate the influence of blood transfusion on postoperative inflammatory and stress marker levels in patients with gastric cancer undergoing radical gastrectomy. Furthermore, we sought to assess the relationship between these biomarkers and patient prognosis, specifically OS and disease-free survival (DFS). By elucidating the potential mechanisms linking blood transfusion to adverse outcomes, our findings could provide valuable insights for optimizing perioperative management strategies and improving long-term survival rates in patients with gastric cancer. This research not only addresses a critical gap in current medical knowledge but also aims to guide clinical practices toward personalized and effective care.
This retrospective cohort study included 200 patients with gastric cancer who underwent radical gastrectomy (including standard D2 Lymph node dissection) at Yiwu Central Hospital between January 2017 and January 2020 as the research subjects. Demographic information of patients was obtained using the case system. All executed procedures adhered to the ethical standards of the responsible committees (institutions and countries) for human experiments, as well as the 1964 Helsinki Declaration and its subsequent versions. This study was approved by Yiwu Central Hospital’s Institutional Review Board. Given that we only used de-identified patient data, our hospital’s institutional review committee and ethics committee waived informed consent for this retrospective study, which poses no potential harm or impact on patient care.
Inclusion criteria: (1) Age > 18 years old; (2) Diagnosed with primary gastric adenocarcinoma through histopathology[11]; (3) Consent to radical gastrectomy (including standard D2 Lymph node dissection)[12]; (4) Preoperative imaging assessment showed no evidence of liver, lung, or abdominal metastasis; and (5) The clinical data during the perioperative period were complete and traceable.
Exclusion criteria: (1) Pre-existing infections or inflammatory conditions (such as vasculitis or rheumatoid arthritis); (2) Patients receiving long-term corticosteroid treatment; (3) Patients with preoperative severe anemia [hemoglobin (Hb)
Blood transfusion refers to the transfusion of any > 2 units of packed red cells during surgery. All units of packed red cells underwent leucodepletion following screening for blood-borne viruses (human immunodeficiency virus, human
Fasting venous blood (6 mL) was collected from the patient at 8:00 before surgery, 1 day, 3 days, and 7 days after surgery. The remaining amount was added to a test tube without anticoagulant and maintained in a stationary position until the serum was separated. The precipitated serum was precipitated via centrifugation at 4000 rpm for 10 minutes at 4 °C. The supernatant was aspirated and placed in a -80 °C low-temperature refrigerator for the following tests. Blood testing included the measurement of inflammatory markers and stress markers.
Inflammation markers: C-reactive protein (CRP) was measured by turbidimetry. Interleukin (IL)-6 and procalcitonin (PCT) were measured via chemiluminescence on a fully automatic chemiluminescence analyzer (AutoLumo A2000Plus, Autobio, China). Tumor necrosis factor-alpha (TNF-α) was measured by flow cytometry (BriCyte E6, Mindray, China). A Sysmex XN-1000 analyzer (Sysmex, Japan) was used to detect neutrophil, lymphocyte, and white blood cell (WBC) counts in ethylene diamine tetraacetic acid (ab93684, Abcam, United States) and anticoagulant blood samples. The neutrophil/Lymphocyte ratio (NLR) for each patient was calculated.
Stress markers: Enzyme-linked immunosorbent assay was used to detect the levels of stress markers, including adrenaline, norepinephrine, and cortisol. The reagent kits used in this process were adrenaline (ab260058, Abcam, United States), norepinephrine (ab287789, Abcam, United States), and cortisol (ab108665, Abcam, United States). The glucose concentration in plasma was measured using Beckman Glucose Analyzer II (Beckman, United States).
Follow-up evaluation began after the end of treatment and was conducted every 1-3 months. The postoperative complications of the patient within 1 month of treatment were recorded. The follow-up period of this study was limited to 5 years after surgery. Follow-up data were collected through outpatient or inpatient systems, external hospital records, and regular follow ups to monitor the patient’s survival status and disease condition. The follow-up deadline is March 20, 2025.
Data analysis was conducted using SPSS version 29.0 statistical software (SPSS Inc., Chicago, IL, United States). Categorical data were represented in the format [n (%)]. The χ2 test was utilized, with the test statistic denoted as χ2. For normally distributed continuous data, results were presented as (mean ± SD), and a t-test was employed for comparison. To evaluate the impact of blood transfusion status on postoperative inflammation and stress markers in patients with gastric cancer, as well as determine their association with prognosis, we conducted univariate and multivariate logistic regression analyses. Univariate logistic regression analysis was used to evaluate the independent impact of each parameter on prognosis. Variables that showed statistical significance in univariate analysis were subsequently included in a multiple Cox regression model to adjust for potential confounding factors and determine independent predictors of treatment response. The results of logistic regression analysis are expressed as the ratio of the 95% confidence interval (CI) to the corresponding P value (odds ratio). Statistical significance was set at P < 0.05.
In our study comparing the demographics characteristics between the non-transfusion group (n = 108) and the transfusion group (n = 92), no significant differences were observed (Table 1). Specifically, we found no significant difference in age (P = 0.682), sex distribution (P = 0.641), body mass index (P = 0.124), Eastern Cooperative Oncology Group (ECOG) performance status (P = 0.378), weight loss (P = 0.165), history of gastric surgery (P = 0.565), smoking habits (P = 0.861), drinking habits (P = 0.995), employment status (P = 0.924), marital status (P = 0.990), and current residence (P = 0.721). Overall, these results suggested that the two groups were well balanced with respect to baseline demographic and clinical characteristics.
| Parameters | Non-transfusion group (n = 108) | Transfusion group (n = 92) | t/χ2 value | P value |
| Age (years) | 60.54 ± 10.12 | 61.14 ± 10.79 | 0.410 | 0.682 |
| Sex | 0.217 | 0.641 | ||
| Male | 73 (67.59) | 65 (70.65) | ||
| Female | 35 (32.41) | 27 (29.35) | ||
| BMI (kg/m2) | 24.16 ± 2.73 | 23.61 ± 2.25 | 1.545 | 0.124 |
| ECOG | 0.779 | 0.378 | ||
| 0 | 93 (86.11) | 75 (81.52) | ||
| 1 | 15 (13.89) | 17 (18.48) | ||
| Weight loss | 3.607 | 0.165 | ||
| 0%-5% | 71 (65.74) | 59 (64.13) | ||
| 6%-10% | 24 (22.22) | 14 (15.22) | ||
| > 10% | 13 (12.04) | 19 (20.65) | ||
| History of gastric surgery | 0.332 | 0.565 | ||
| Yes | 21 (19.44) | 15 (16.30) | ||
| No | 87 (80.56) | 77 (83.70) | ||
| Smoking | 0.300 | 0.861 | ||
| Never | 23 (21.30) | 17 (18.48) | ||
| Former | 28 (25.93) | 26 (28.26) | ||
| Current | 57 (52.78) | 49 (53.26) | ||
| Drinking | 0.011 | 0.995 | ||
| Never | 8 (7.41) | 7 (7.61) | ||
| Former | 11 (10.19) | 9 (9.78) | ||
| Current | 89 (82.41) | 76 (82.61) | ||
| Employment status | 0.009 | 0.924 | ||
| Employed | 78 (72.22) | 67 (72.83) | ||
| Unemployed | 30 (27.78) | 25 (27.17) | ||
| Marital status | 0.020 | 0.990 | ||
| Married | 57 (52.78) | 49 (53.26) | ||
| Single | 35 (32.41) | 29 (31.52) | ||
| Divorced | 16 (14.81) | 14 (15.22) | ||
| Current residence | 0.127 | 0.721 | ||
| Urban | 69 (63.89) | 61 (66.30) | ||
| Rural | 39 (36.11) | 31 (33.70) |
Moreover, we found no significant difference in tumor size (P = 0.608) or node count P = 0.952) (Table 2). However, a significant difference in T stage distribution was observed (P = 0.017), indicating that patients in the transfusion group were more likely to have earlier stages of tumor invasion compared with those in the non-transfusion group. No sig
| Parameters | Non-transfusion group (n = 108) | Transfusion group (n = 92) | t/χ2 value | P value |
| Tumor size (cm) | 2.81 ± 1.02 | 2.89 ± 1.10 | 0.514 | 0.608 |
| Node count | 27.33 ± 11.43 | 27.24 ± 10.24 | 0.060 | 0.952 |
| Tumor location | 6.843 | 0.077 | ||
| Upper third | 10 (9.26) | 3 (3.26) | ||
| Middle third | 39 (36.11) | 24 (26.09) | ||
| Lower third | 57 (52.78) | 64 (69.57) | ||
| Entire | 2 (1.85) | 1 (1.09) | ||
| Histology | 0.406 | 0.816 | ||
| Differentiated | 48 (44.44) | 41 (44.57) | ||
| Undifferentiated | 56 (51.85) | 49 (53.26) | ||
| Others | 4 (3.70) | 2 (2.17) | ||
| T stage | 5.726 | 0.017 | ||
| ≤ T2 | 65 (60.19) | 70 (76.09) | ||
| > T2 | 43 (39.81) | 22 (23.91) | ||
| N stage | 0.436 | 0.509 | ||
| N0 | 56 (51.85) | 52 (56.52) | ||
| ≥ N1 | 52 (48.15) | 40 (43.48) | ||
| Gastrectomy | 0.061 | 0.805 | ||
| Distal subtotal | 71 (65.74) | 62 (67.39) | ||
| Total | 7 (34.26) | 30 (32.61) | ||
| Access | 2.787 | 0.095 | ||
| Open | 53 (49.07) | 56 (60.87) | ||
| Laparoscopic | 55 (50.93) | 36 (39.13) | ||
| Operation time (minute) | 243.55 ± 74.35 | 246.68 ± 77.33 | 0.292 | 0.771 |
| Intraoperative blood loss (mL) | 299.72 ± 36.72 | 322.03 ± 62.65 | 3.004 | 0.003 |
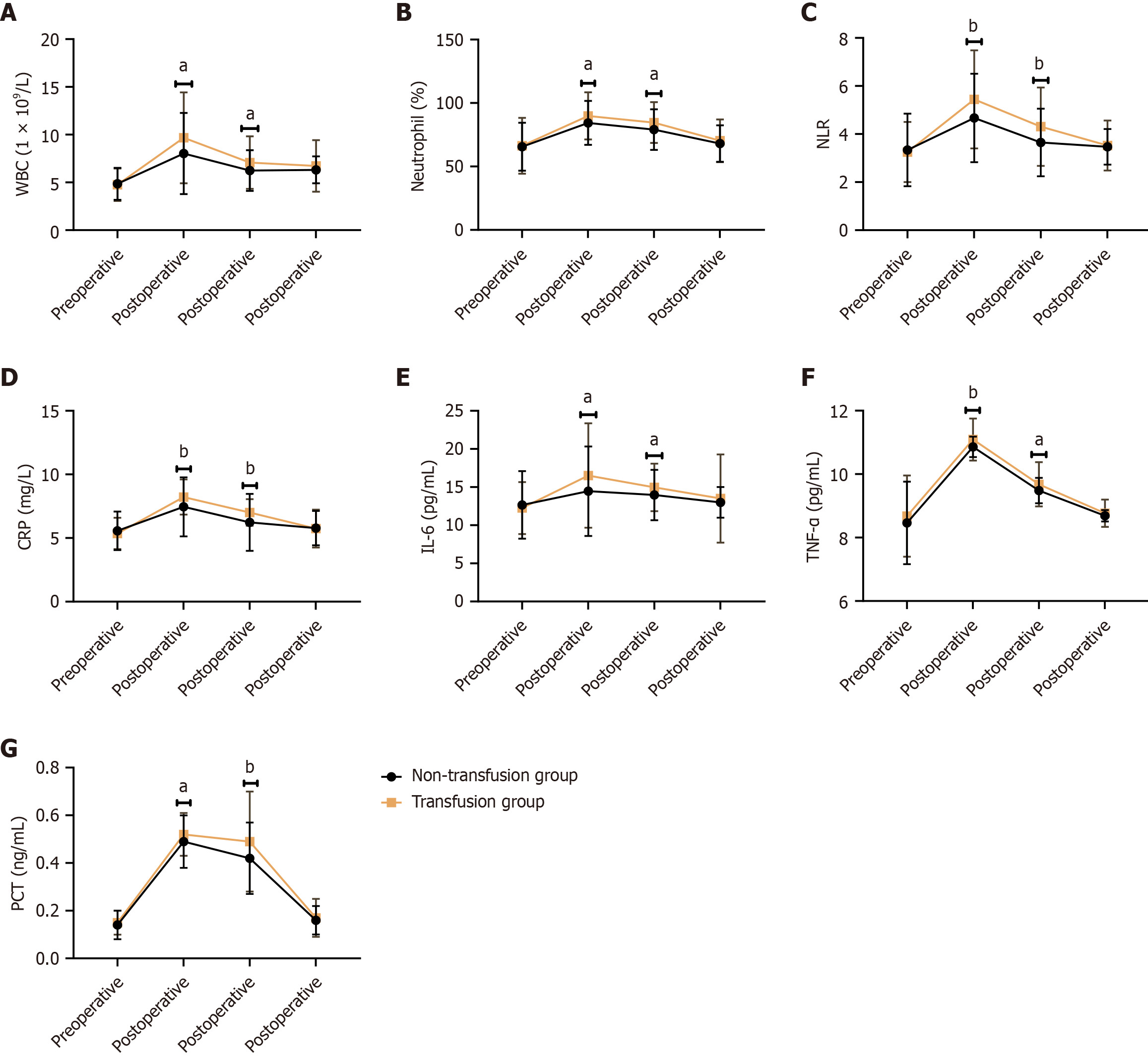
Figure 1 compares various inflammatory markers between the non-transfusion group and the transfusion group. Postoperative WBC counts were significantly higher in the transfusion group than in the non-transfusion group at postoperative day 1 (9.67 ± 4.76 vs 8.02 ± 4.25, t = 2.584, P = 0.010) and day 3 (7.08 ± 2.75 vs 6.24 ± 2.13, t = 2.406, P = 0.017). Neutrophil percentages also showed significant increases in the transfusion group at postoperative day 1 (89.87 ± 18.54 vs 84.36 ± 17.27, t = 2.177, P = 0.031) and day 3 (84.59 ± 16.05 vs 79.05 ± 15.96, t = 2.440, P = 0.016). The NLR was significantly elevated in the transfusion group at postoperative day 1 (5.44 ± 2.04 vs 4.67 ± 1.84, t = 2.808, P = 0.005) and day 3 (4.31 ± 1.63 vs 3.65 ± 1.41, t = 3.093, P = 0.002). CRP levels significantly increased in the transfusion group at postoperative day 1 (8.22 ± 1.38 vs 7.45 ± 2.32, t = 2.895, P = 0.004) and day 3 (7.01 ± 1.04 vs 6.23 ± 2.24, t = 3.224, P = 0.002). IL-6 Levels showed significant differences at postoperative day 1 (16.52 ± 6.84 vs 14.46 ± 5.87, t = 2.290, P = 0.023) and day 3 (14.96 ± 3.12 vs 13.96 ± 3.31, t = 2.185, P = 0.030). TNF-α levels were significantly elevated in the transfusion group at postoperative day 1 (11.09 ± 0.66 vs 10.86 ± 0.32, t = 3.068, P = 0.003) and day 3 (9.68 ± 0.70 vs 9.48 ± 0.40, t = 2.486, P = 0.014). PCT levels significantly increased in the transfusion group at postoperative day 1 (0.52 ± 0.09 vs 0.49 ± 0.11, t = 2.168, P = 0.031) and day 3 (0.49 ± 0.21 vs 0.42 ± 0.15, t = 2.925, P = 0.004). These results indicated that patients in the transfusion group experienced a pronounced inflammatory response and stress reaction following surgery, as evidenced by elevated levels of WBC, neutrophil percentage, NLR, CRP, IL-6, TNF-α, and PCT. These findings suggested that blood transfusions may contribute to increased postoperative inflammation and stress, which could have implications for patient recovery and overall prognosis.
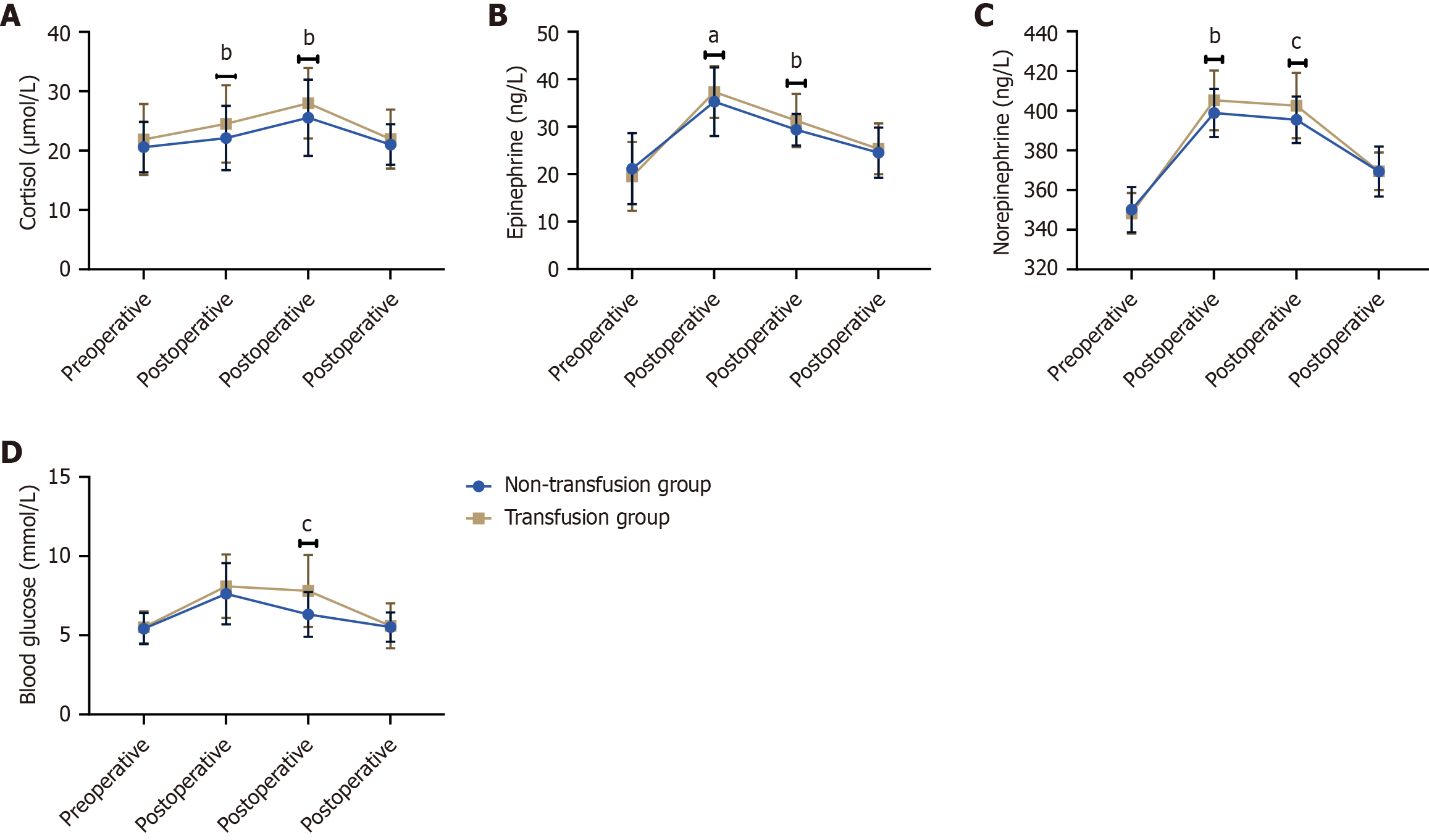
Figure 2 compares various stress markers between the non-transfusion group and the transfusion group. Postoperative cortisol levels were significantly higher in the transfusion group than in the non-transfusion group at postoperative day 1 (24.50 ± 6.52 vs 22.13 ± 5.42, t = 2.811, P = 0.005) and day 3 (27.98 ± 5.92 vs 25.54 ± 6.42, t = 2.780, P = 0.006). Epinephrine levels also showed significant increases in the transfusion group at postoperative day 1 (37.31 ± 5.45 vs 35.25 ± 7.21, t = 2.300, P = 0.022) and day 3 (31.29 ± 5.62 vs 29.36 ± 3.34, t = 2.893, P = 0.004). Norepinephrine levels were significantly elevated in the transfusion group at postoperative day 1 (405.21 ± 15.07 vs 398.89 ± 12.10, t = 3.233, P = 0.001) and day 3 (402.56 ± 16.46 vs 395.46 ± 11.67, t = 3.463, P < 0.001). Blood glucose levels significantly increased in the transfusion group at postoperative day 3 (7.80 ± 2.27 vs 6.32 ± 1.41, t = 5.427, P < 0.001). Preoperative levels of cortisol, epinephrine, norepinephrine, and blood glucose did not show significant differences between the two groups (P > 0.05 for all). Simi
Significant differences were observed in several complication rates between the two groups. Specifically, the incidence of bleeding was significantly higher in the transfusion group than in the non-transfusion group, with χ2 of 7.163 and P = 0.007 (Table 3). Similarly, anastomotic leak (P = 0.011) and dumping syndrome (P = 0.014) were more common in the transfusion group than in the non-transfusion group. Additionally, urinary infections were prevalent in the transfusion group, with χ2 of 4.169 and P = 0.041, and wound infections were exclusively reported in the transfusion group, contributing to a significant difference with χ2 of 3.997 and P = 0.046. Clostridium infections showed a trend toward being more frequent in the transfusion group than in the non-transfusion group, but this did not reach statistical significance
| Parameters | Non-transfusion group (n = 108) | Transfusion group (n = 92) | t/χ2 value | P value |
| Postoperative complications | ||||
| Bleeding | 2 (1.85) | 10 (10.87) | 7.163 | 0.007 |
| Anastomotic leak | 3 (2.78) | 11 (11.96) | 6.429 | 0.011 |
| Duodenal stump leak | 2 (1.85) | 3 (3.26) | 0.033 | 0.856 |
| Pancreatic fistula | 1 (0.93) | 2 (2.17) | 0.020 | 0.889 |
| Abdominal collection | 5 (4.63) | 7 (7.61) | 0.782 | 0.377 |
| Clostridium infection | 1 (0.93) | 6 (6.52) | 3.098 | 0.078 |
| Wound infection | 5 (5.43) | 3.997 | 0.046 | |
| Pneumonia | 5 (4.63) | 6 (6.52) | 0.342 | 0.559 |
| Catheter infection | 3 (2.78) | 5 (5.43) | 0.352 | 0.553 |
| Urinary infection | 1 (0.93) | 7 (7.61) | 4.169 | 0.041 |
| Dumping syndrome | 2 (1.85) | 9 (9.78) | 6.012 | 0.014 |
| Clavien-Dindo complication grade | ||||
| I | 5 (4.63) | 8 (8.70) | 1.351 | 0.245 |
| II | 11 (10.19) | 3 (3.26) | 3.659 | 0.056 |
| IIIa | 6 (5.56) | 4 (4.35) | 0.004 | 0.948 |
| IIIb | 2 (2.17) | 0.684 | 0.408 | |
| IVa | 2 (2.17) | 0.684 | 0.408 | |
| Length of stay (days) | 20.01 ± 0.98 | 19.96 ± 1.02 | 0.362 | 0.718 |
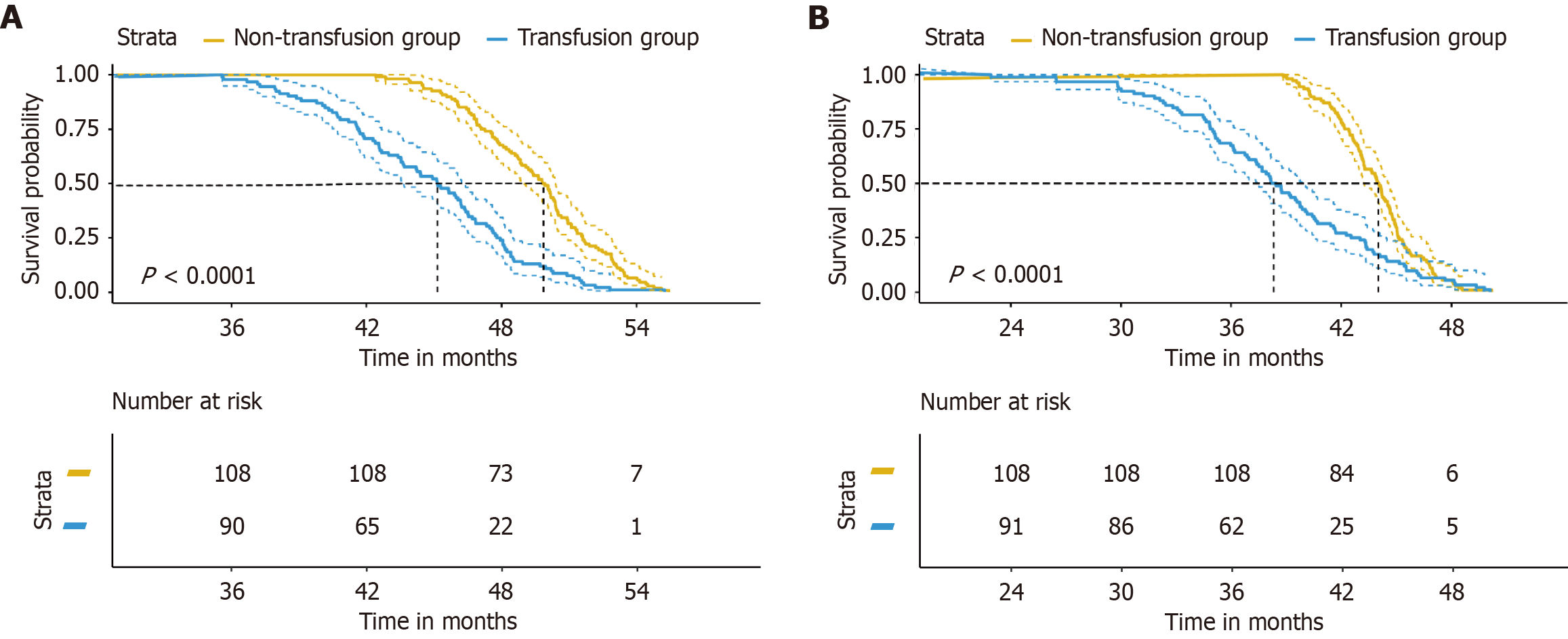
Figure 3 compares the postoperative survival between the non-transfusion group and the transfusion group. The results indicated a significant difference in OS between the two groups, with the non-transfusion group showing a higher mean OS of 49.52 ± 2.94 months compared with 44.74 ± 4.25 months in the transfusion group (t = 9.096, P < 0.001). Similarly, DFS significantly differed, where the non-transfusion group had a mean DFS of 43.85 ± 2.33 months, which was markedly longer than the 38.54 ± 5.54 months observed in the transfusion group (t = 8.558, P < 0.001). These findings suggested that avoiding blood transfusions may contribute to improved long-term outcomes in terms of OS and DFS following surgery. The statistically significant differences in survival times between the groups highlight the potential negative impact of blood transfusions on patient prognosis.
Table 4 presents the correlation analysis between various variables and poor prognosis. T stage (r = 0.169, P = 0.017), intraoperative blood loss (r = 0.230, P = 0.001), WBC at postoperative day 1 (r = 0.193, P = 0.006) and day 3 (r = 0.173, P = 0.014), neutrophil percentage at postoperative day 3 (r = 0.188, P = 0.008), NLR at postoperative day 1 (r = 0.207, P = 0.003) and day 3 (r = 0.219, P = 0.002), CRP at postoperative day 1 (r = 0.235, P < 0.001) and day 3 (r = 0.209, P = 0.003), IL-7 at postoperative day 1 (r = 0.147, P = 0.038), TNF-α at postoperative day 1 (r = 0.194, P = 0.006) and day 3 (r = 0.163, P = 0.021), PCT at postoperative day 1 (r = 0.139, P = 0.049) and day 3 (r = 0.187, P = 0.008), cortisol at postoperative day 1 (r = 0.181, P = 0.010) and day 3 (r = 0.205, P = 0.004), epinephrine at postoperative day 3 (r = 0.211, P = 0.003), norepinephrine at postoperative day 1 (r = 0.231, P = 0.001) and day 3 (r = 0.243, P < 0.001), blood glucose at postoperative day 3 (r = 0.377, P < 0.001), bleeding (r = 0.189, P = 0.007), anastomotic leak (r = 0.179, P = 0.011), wound infection (r = 0.173, P = 0.014), urinary infection (r = 0.170, P = 0.016), and dumping syndrome (r = 0.173, P = 0.014) showed a significant positive correlation with poor prognosis (P < 0.05), indicating their potential as prognostic markers. Overall, this table highlights multiple biomarkers that are significantly associated with patient prognosis following surgery, suggesting a complex interplay of inflammatory responses, stress hormones, and surgical complications in determining clinical outcomes.
| Variable | r value | P value | Variable | r value | P value |
| T stage | 0.169 | 0.017 | PCT (ng/mL) postoperative day 3 | 0.187 | 0.008 |
| Intraoperative blood loss (mL) | 0.230 | 0.001 | Cortisol (μmol/L) postoperative day 1 | 0.181 | 0.010 |
| WBC (1 × 109/L) postoperative day 1 | 0.193 | 0.006 | Cortisol (μmol/L) postoperative day 3 | 0.205 | 0.004 |
| WBC (1 × 109/L) postoperative day 3 | 0.173 | 0.014 | Epinephrine (ng/L) postoperative day 1 | 0.137 | 0.053 |
| Neutrophil (%) postoperative day 1 | 0.136 | 0.055 | Epinephrine (ng/L) postoperative day 3 | 0.211 | 0.003 |
| Neutrophil (%) postoperative day 3 | 0.188 | 0.008 | Norepinephrine (ng/L) postoperative day 1 | 0.231 | 0.001 |
| NLR postoperative day 1 | 0.207 | 0.003 | Norepinephrine (ng/L) postoperative day 3 | 0.243 | < 0.001 |
| NLR postoperative day 3 | 0.219 | 0.002 | Blood glucose(mmol/L) postoperative day 3 | 0.377 | < 0.001 |
| CRP (mg/L) postoperative day 1 | 0.235 | < 0.001 | Bleeding | 0.189 | 0.007 |
| CRP (mg/L) postoperative day 3 | 0.209 | 0.003 | Anastomotic leak | 0.179 | 0.011 |
| IL-6 (pg/mL) postoperative day 1 | 0.147 | 0.038 | Wound infection | 0.173 | 0.014 |
| IL-6 (pg/mL) postoperative day 3 | 0.141 | 0.047 | Urinary infection | 0.170 | 0.016 |
| TNF-α (pg/mL) postoperative day 1 | 0.194 | 0.006 | Dumping syndrome | 0.173 | 0.014 |
| TNF-α (pg/mL) postoperative day 3 | 0.163 | 0.021 | PCT (ng/mL) postoperative day 1 | 0.139 | 0.049 |
Table 5 presents the results of a multivariate Cox analysis assessing the effects of various indicators on OS and DFS. Blood transfusion significantly predicted poor OS [hazard ratio (HR) = 1.876, 95%CI: 1.293-2.723, P = 0.004] and DFS (HR = 1.644, 95%CI: 1.134-2.388, P = 0.014). Age was also identified as a significant factor for OS (HR = 1.034 per year, 95%CI: 1.004-1.064, P = 0.031), whereas ECOG performance status had a significant impact on OS (HR = 1.516, 95%CI: 1.055-2.175, P = 0.032) and showed a trend toward significance for DFS (HR = 1.425, 95%CI: 0.984-2.056, P = 0.063). Changes in CRP levels were significantly associated with OS (HR = 1.085 per mg/L, 95%CI: 1.022-1.145, P = 0.014) and DFS (HR = 1.073 per mg/L, 95%CI: 1.019-1.134, P = 0.034). Similarly, ΔIL-6 and ΔTNF-α were significant predictors for OS and DFS, with
| Parameters | Overall survival | Disease-free survival | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Blood transfusion | 1.876 (1.293-2.723) | 0.004 | 1.644 (1.134-2.388) | 0.014 |
| Age (per 1 year) | 1.034 (1.004-1.064) | 0.031 | 1.026 (0.997-1.046) | 0.157 |
| Sex (male/female) | 0.973 (0.705-1.342) | 0.884 | 0.956 (0.684-1.326) | 0.750 |
| BMI (per 1 kg/m²) | 0.985 (0.945-1.022) | 0.322 | 1.012 (0.975-1.056) | 0.606 |
| ECOG performance status (1/0) | 1.516 (1.055-2.175) | 0.032 | 1.425 (0.984-2.056) | 0.063 |
| ΔCRP (per 1 mg/L) | 1.085 (1.022-1.145) | 0.014 | 1.073 (1.019-1.134) | 0.034 |
| ΔIL-6 (per 1 pg/mL) | 1.063 (1.016-1.125) | 0.034 | 1.051 (1.007-1.104) | 0.052 |
| ΔTNF-α (per 1 pg/mL) | 1.106 (1.032-1.172) | 0.017 | 1.091 (1.028-1.165) | 0.024 |
| ΔCortisol (per 1 nmol/L) | 1.043 (0.993-1.095) | 0.128 | 1.037 (0.984-1.088) | 0.216 |
| ΔEpinephrine (per 1 ng/L) | 1.032 (0.985-1.083) | 0.232 | 1.027 (0.972-1.076) | 0.376 |
| Postoperative complication | ||||
| Bleeding (yes/no) | 1.925 (1.365-2.723) | 0.006 | 1.783 (1.284-2.482) | 0.007 |
| Anastomotic leak (yes/no) | 2.057 (1.402-3.005) | < 0.001 | 1.903 (1.305-2.792) | 0.004 |
| Duodenal stump leak (yes/no) | 1.882 (1.312-2.717) | 0.003 | 1.793 (1.253-2.567) | 0.006 |
| Pancreatic fistula (yes/no) | 2.108 (1.456-3.044) | < 0.001 | 1.955 (1.353-2.825) | < 0.001 |
| Abdominal collection (yes/no) | 1.752 (1.285-2.398) | 0.001 | 1.623 (1.205-2.196) | 0.008 |
| Clostridium infection (yes/no) | 1.685 (1.205-2.367) | 0.002 | 1.584 (1.132-2.217) | 0.007 |
| Wound infection (yes/no) | 1.624 (1.155-2.286) | 0.006 | 1.553 (1.104-2.197) | 0.013 |
| Pneumonia (yes/no) | 1.802 (1.254-2.587) | 0.001 | 1.703 (1.181-2.454) | 0.004 |
| Catheter infection (yes/no) | 1.553 (1.083-2.237) | 0.018 | 1.485 (1.027-2.142) | 0.037 |
| Urinary infection (yes/no) | 1.451 (1.002-2.105) | 0.052 | 1.405 (0.963-2.042) | 0.082 |
| Dumping syndrome (yes/no) | 1.304 (0.904-1.886) | 0.171 | 1.25 (0.863-1.815) | 0.242 |
This study aimed to investigate the influence of perioperative blood transfusion on postoperative inflammatory responses and stress indicators in patients with gastric cancer. Regarding pathological characteristics, although we found no significant differences in tumor size and lymph node count, intraoperative blood loss was significantly higher in the transfusion group than in the non-transfusion group. This suggested that increased blood loss may be a primary reason necessitating transfusions and indirectly indicates high surgical difficulty or poor intraoperative hemostasis[15,16]. Despite similarities in most baseline pathological characteristics, patients in the transfusion group exhibited higher risks in certain critical indicators than those in the non-transfusion group, posing new challenges for preoperative planning and postoperative management[17].
Regarding postoperative complications, our findings indicated that the rates of bleeding, anastomotic leakage, dumping syndrome, urinary tract infections, and wound infections were significantly higher in the transfusion group than in the non-transfusion group. Although other complications such as duodenal stump leakage, pancreatic fistula, ascites, pneumonia, and catheter-related infections did not show significant differences between the two groups, the aforementioned high-incidence complications are noteworthy. Concerning the dumping syndrome, although its occurrence was more frequent in the transfusion group than in the non-transfusion group, this condition is commonly associated with surgical techniques. Therefore, when analyzing these data, potential confounding effects of surgical types must be controlled to accurately assess the relationship between transfusion and dumping syndrome. Additionally, according to the Clavien-Dindo classification, grade II complications were more common in the non-transfusion group than in the transfusion group, but the difference was not significant[18]. In terms of hospital stay, we found no significant difference between the two groups, possibly due to timely and effective treatment measures that prevented a significant extension of hospital stays.
Subsequently, changes in postoperative inflammatory and stress indicators between the two groups were compared. On postoperative days 1 and 3, WBC counts, neutrophil percentages, NLR, CRP, IL-6, TNF-α, and PCT levels were significantly higher in the transfusion group than in the non-transfusion group. These results indicated that patients in the transfusion group experienced more pronounced inflammatory and stress responses than patients in the non-transfusion group. The reactive oxygen species and enzymes released by neutrophils can damage surrounding tissues, exacerbating local inflammation[19]. A high NLR indicates a sustained inflammatory response in the body, which is not only associated with poor prognosis but may also suppress immune system function and reduce the body’s ability to fight infections[20]. CRP can also exacerbate local inflammatory reactions by activating the complement system and promoting the expression of cell adhesion molecules[21]. The elevation of IL-6 and TNF-α can induce the release of other inflammatory mediators, forming a positive feedback loop and further amplifying the inflammatory response[22]. PCT is a biomarker for bacterial infections, but it can also be elevated in non-infectious inflammatory states[23]. Enhanced inflammatory responses might further increase the risk of postoperative complications, prolong hospital stays, and affect long-term prognosis[24]. Therefore, avoiding unnecessary transfusions may help reduce postoperative inflammatory responses and promote rapid recovery[25].
Regarding stress indicators, we observed that cortisol, adrenaline, noradrenaline, and blood glucose levels were significantly elevated in the transfusion group postoperatively. These changes further confirmed that patients in the transfusion group experienced more intense stress responses early after surgery than patients in the non-transfusion group. High cortisol levels can also lead to increased protein breakdown, fat redistribution, and insulin resistance, thereby affecting the patient’s recovery process[26]. Adrenaline and noradrenaline activate alpha and beta receptors, causing a series of physiological changes including increased heart rate, elevated blood pressure, vasoconstriction, and bronchiectasis. These hormones work together to cause an increase in blood sugar levels, known as “stress-induced hyperglycemia”[27]. High levels of stress hormones not only affect immune system function but may also increase cardiovascular burden and the incidence of postoperative complications[28,29]. Thus, reasonable management and reduction in transfusion needs may be important for improving the overall rehabilitation process.
In terms of postoperative complications, the incidence of bleeding, anastomotic leakage, dumping syndrome, urinary tract infections, and wound infections was significantly higher in the transfusion group than in the non-transfusion group. Although other complications such as duodenal stump leakage, pancreatic fistula, ascites, pneumonia, and catheter-related infections did not show significant differences, the high incidence of the aforementioned complications remains noteworthy. According to the Clavien-Dindo classification, grade II complications were more common in the non-transfusion group than in the transfusion group, although the difference was not statistically significant. Regarding hospital stay, we found no significant difference between the two groups, possibly due to timely and effective treatment measures that prevented significant extension of hospitalization.
In long-term survival analysis, we found that OS and DFS were significantly longer in the non-transfusion group than in the transfusion group. This result further underscores the potential benefits of avoiding unnecessary transfusions for improving long-term patient outcomes[30]. Multivariate Cox regression analysis revealed several independent prognostic predictors, including age, ECOG performance status score, CRP changes, IL-6 changes, TNF-α changes, and various postoperative complications. Among these, transfusion was identified as a significant negative factor for OS and DFS, indicating its effect not only on short-term complication risks but also on long-term survival.
Finally, through multivariate analysis, we identified several independent risk factors closely related to OS and DFS, such as transfusion, age, ECOG performance status score, CRP changes, IL-6 changes, TNF-α changes, and various postoperative complications. These results indicated that besides transfusion, other factors such as age, underlying health status, changes in inflammatory markers, and postoperative complications also play significant roles in long-term prognosis. Therefore, when developing individualized treatment plans, these factors should be comprehensively considered to optimize treatment outcomes and quality of life[31].
Although this study provides important information on the influence of blood transfusion on the postoperative inflammatory response, stress response, and long-term survival in patients with gastric cancer, several limitations persist. First, our study employed a single blood transfusion criterion (blood loss exceeding 15% of blood volume or systolic blood pressure below 90 mmHg) without establishing varying transfusion thresholds (such as Hb < 70 g/L vs < 80 g/L) for comparison. Future studies should consider the specific effects of different transfusion thresholds on clinical outcomes to precisely formulate transfusion strategies. Second, although we measured various inflammatory and stress markers, we did not comprehensively assess immune markers (such as cluster of differentiation 4 + T cell ratio) or tumor microenvironment markers (such as vascular endothelial growth factor). Future research needs to further explore changes in these markers and their effect on prognosis, thereby providing a comprehensive understanding. Notably, our study did not assess the preoperative nutritional status of patients. Previous studies have shown that preoperative malnutrition not only increases the demand for transfusion but also correlates with postoperative complications and poor long-term prognosis[32]. Malnutrition can lead to anemia, weakened immune function, and reduced healing capacity, which may indirectly result in elevated transfusion rates and poor clinical outcomes. However, our study did not measure or control for preoperative nutritional status, which represents a significant limitation. Additionally, the single-center retrospective design may lead to selection bias. For instance, transfusion decisions might be influenced by the surgeon’s experience rather than solely by blood loss volume. Such factors were not adequately controlled for in our study. Future studies should consider a multi-center design and employ methods such as propensity score matching to balance baseline characteristics between groups. Additionally, we only identified correlations between inflammatory and stress markers and clinical outcomes, without verifying causal relationships. For example, although we observed significantly elevated levels of inflammation and stress markers in the transfusion group and found these markers to be associated with adverse outcomes, we could not rule out the mediating effect where “complications lead to elevated inflammation, which in turn affects outcomes”. Therefore, future studies need to employ experimental designs or sophisticated statistical methods (such as path analysis or multivariate regression) to verify the direct causal effects of these markers on patient prognosis. Moreover, our study did not record specific recurrence sites and subsequent treatment details during follow up. This could affect the interpretation of differences in DFS, as recurrence at different sites and various treatment strategies may significantly influence patient survival outcomes. Future studies should incorporate detailed data collection on tumor recurrence sites and treatment status at the design stage to understand how these factors affect long-term prognosis.
In conclusion, this study demonstrated that perioperative blood transfusion is closely associated with enhanced postoperative inflammatory responses, exacerbated stress reactions, increased complications, and reduced long-term survival rates in patients with gastric cancer. To avoid these adverse effects, clinicians should strive to minimize un
Despite this study revealing the adverse effects of perioperative blood transfusion on postoperative inflammatory response, stress response, and long-term survival in patients with gastric cancer, further exploration of its mechanisms, through basic research and molecular biology to analyze the effect of red blood cell storage damage, is necessary. Preoperative assessments must be optimized and personalized treatment strategies that consider prognostic predictors like age, ECOG performance status score, CRP changes, IL-6 changes, TNF-α changes, and postoperative complications must be developed to formulate the optimal plan. Additionally, applying minimally invasive techniques and enhanced recovery after surgery concepts to optimize perioperative management can significantly reduce the need for blood transfusions and improve prognosis[33]. Multidisciplinary team collaboration is particularly important in clinical practice; close cooperation among gastrointestinal surgeons, anesthesiologists, and intensive care physicians provides comprehensive medical services and support, enhancing patient treatment outcomes and quality of life. Future high-quality studies are needed to validate these findings and explore how to decrease the demand for blood transfusions by improving surgical techniques and perioperative management strategies, ultimately enhancing overall patient prognosis.
The findings of this study are crucial for revising transfusion guidelines in gastric cancer surgery. Currently, several guidelines recommend transfusion when Hb levels fall below 70 g/L and propose individualized decision-making for patients with Hb levels between 70 and 100 g/L based on their clinical condition[34,35]. However, our study revealed that even at relatively high Hb levels (e.g., ≥ 80 g/L), avoiding unnecessary transfusions may help reduce postoperative inflammatory responses, alleviate stress reactions, and improve long-term prognosis. Our findings suggested that transfusions should be avoided in gastric cancer surgery, unless the patient exhibits clear symptoms of anemia or significant hemodynamic instability (such as systolic blood pressure < 90 mmHg). Furthermore, future studies should investigate the specific effects of different transfusion thresholds on clinical outcomes to provide a basis for developing precise transfusion strategies.
This study showed that perioperative blood transfusion is correlated with increased postoperative inflammatory re
| 1. | Mamun TI, Younus S, Rahman MH. Gastric cancer-Epidemiology, modifiable and non-modifiable risk factors, challenges and opportunities: An updated review. Cancer Treat Res Commun. 2024;41:100845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 2. | Ren LF, Xu YH, Long JG. Prognostic Value of Postoperative Complication for Gastric Cancer. J Laparoendosc Adv Surg Tech A. 2024;34:339-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Fujitani K, Kurokawa Y, Wada R, Takeno A, Kawabata R, Omori T, Imamura H, Hirao M, Endo S, Kawada J, Moon JH, Takiguchi S, Mori M, Eguchi H, Doki Y; Osaka University Clinical Research Group for Gastroenterological Surgery. Prospective single-arm multicenter interventional study of surgical resection for liver metastasis from gastric cancer; 3-year overall and recurrence-free survival. Eur J Cancer. 2024;213:115080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Erikstein BS, Alnæs MB, Apelseth TO. Blood transfusion-associated anaphylaxis in perioperative- and non-perioperative patients in Western Norway 2002-2021. Blood Transfus. 2024;22:502-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Shouman M, Goubran H, Seghatchian J, Burnouf T. Hematological toxicities of immune checkpoint inhibitors and the impact of blood transfusion and its microbiome on therapeutic efficacy and recipient's safety and survival outcome:A systematic narrative appraisal of where we are now! Transfus Apher Sci. 2023;62:103685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Li X, Xie H, Liu S, Wang J, Shi Z, Yao Q, Yang Q, Li Q, Bao L. Analysis of the incidence and risk factors of blood transfusion in total knee revision: a retrospective nationwide inpatient sample database study. BMC Musculoskelet Disord. 2024;25:225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 7. | Van den Broeck T, Oprea-Lager D, Moris L, Kailavasan M, Briers E, Cornford P, De Santis M, Gandaglia G, Gillessen Sommer S, Grummet JP, Grivas N, Lam TBL, Lardas M, Liew M, Mason M, O'Hanlon S, Pecanka J, Ploussard G, Rouviere O, Schoots IG, Tilki D, van den Bergh RCN, van der Poel H, Wiegel T, Willemse PP, Yuan CY, Mottet N. A Systematic Review of the Impact of Surgeon and Hospital Caseload Volume on Oncological and Nononcological Outcomes After Radical Prostatectomy for Nonmetastatic Prostate Cancer. Eur Urol. 2021;80:531-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Aguilar-Nascimento JE, Zampieri-Filho JP, Bordin JO. Implications of perioperative allogeneic red blood cell transfusion on the immune-inflammatory response. Hematol Transfus Cell Ther. 2021;43:58-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Zhang B, Chen ZY, Jiang Z, Huang S, Liu XH, Wang L. Nephroprotective Effects of Cardamonin on Renal Ischemia Reperfusion Injury/UUO-Induced Renal Fibrosis. J Agric Food Chem. 2023;71:13284-13303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Narasimhan A, Flores RR, Robbins PD, Niedernhofer LJ. Role of Cellular Senescence in Type II Diabetes. Endocrinology. 2021;162:bqab136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 11. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1382] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 12. | Leong CN, Chung HT, Lee KM, Shakespeare TP, Mukherjee RK, Wong LC, Lu JJ, Tey J, Lim R, So JB, Back MF. Outcomes of adjuvant chemoradiotherapy after a radical gastrectomy and a D2 node dissection for gastric adenocarcinoma. Cancer J. 2008;14:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | de Las Cuevas Allende R, Díaz de Entresotos L, Conde Díez S. Anaemia of chronic diseases: Pathophysiology, diagnosis and treatment. Med Clin (Barc). 2021;156:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:CD002042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 15. | Zhang M, Feng Q, Wang M. Impact of perioperative blood transfusion on elderly gastric cancer patients. J Gastrointest Oncol. 2024;15:2024-2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Wang W, Zhao L, Niu P, Zhang X, Luan X, Zhao D, Chen Y. Effects of perioperative blood transfusion in gastric cancer patients undergoing gastrectomy: A systematic review and meta-analysis. Front Surg. 2022;9:1011005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Wang W, Sun C, Zhao L, Han X, Luan X, Zhang X, Niu P, Zhao D, Chen Y. Blood transfusion might not be recommended for gastric cancer patients with pretransfusion minimum hemoglobin values higher than 90 g/l: a real-world study covering 20 years of 13 470 patients. Int J Surg. 2024;110:7020-7033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Parzer V, Resl M, Stechemesser L, Wakolbinger M, Itariu B, Brix JM. [Postoperative management]. Wien Klin Wochenschr. 2023;135:729-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Wu D, Wang Y, Shi Y, Shao Y, Zeng F, Spencer CB, Ortoga L, Wu D, Miao C. Ferritin-mediated neutrophil extracellular traps formation and cytokine storm via macrophage scavenger receptor in sepsis-associated lung injury. Cell Commun Signal. 2024;22:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Russo E, Guizzardi M, Canali L, Gaino F, Costantino A, Mazziotti G, Lania A, Uccella S, Di Tommaso L, Ferreli F, Malvezzi L, Spriano G, Mercante G. Preoperative systemic inflammatory markers as prognostic factors in differentiated thyroid cancer: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2023;24:1205-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Agrawal A, Wu Y. Editorial: Biology of C-reactive protein. Front Immunol. 2024;15:1445001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Behrens EM, de Benedetti F. Anti-Interferon-γ Therapy for Cytokine Storm Syndromes. Adv Exp Med Biol. 2024;1448:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Metersky ML, Kalil AC. Management of Ventilator-Associated Pneumonia: Guidelines. Infect Dis Clin North Am. 2024;38:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 24. | He L, Li J, Li X, Wang X, Yan Q. Inflammatory status predicts prognosis in patients with gastric cancer with early pyloric stenosis who underwent radical resection: A propensity score‑matching analysis. Oncol Lett. 2024;28:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Puértolas N, Osorio J, Jericó C, Miranda C, Santamaría M, Artigau E, Galofré G, Garsot E, Luna A, Aldeano A, Olona C, Molinas J, Pulido L, Gimeno M, Pera M. Effect of Perioperative Blood Transfusions and Infectious Complications on Inflammatory Activation and Long-Term Survival Following Gastric Cancer Resection. Cancers (Basel). 2022;15:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 26. | Bagheri A, Asoudeh F, Rezaei S, Babaei M, Esmaillzadeh A. The Effect of Mediterranean Diet on Body Composition, Inflammatory Factors, and Nutritional Status in Patients with Cachexia Induced by Colorectal Cancer: A Randomized Clinical Trial. Integr Cancer Ther. 2023;22:15347354231195322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 27. | Pirchio R, Graziadio C, Colao A, Pivonello R, Auriemma RS. Metabolic effects of prolactin. Front Endocrinol (Lausanne). 2022;13:1015520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 28. | Tiwari RK, Rawat SG, Rai S, Kumar A. Stress regulatory hormones and cancer: the contribution of epinephrine and cancer therapeutic value of beta blockers. Endocrine. 2025;88:359-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Hou Y, Lu J, Xie J, Zhu R, Wu M, Wang K, Zhou J, Li J. Effects of electroacupuncture on perioperative anxiety and stress response in patients undergoing surgery for gastric or colorectal cancer: Study protocol for a randomized controlled trial. Front Psychiatry. 2023;14:1095650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Kawakami LE, Bonomi PB, Pereira MA, Carvalho FO, Ribeiro U Jr, Zilberstein B, Sampaio LR, Carneiro-D'Albuquerque LA, Ramos MFKP. Risk factors for blood transfusion and its prognostic implications in curative gastrectomy for gastric cancer. World J Gastrointest Surg. 2023;15:643-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 583] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 32. | Lopez-Delgado JC, Patel JJ, Stoppe C, McClave SA. Considerations for medical nutrition therapy management of the critically ill patient with hematological malignancies: A narrative review. Nutr Clin Pract. 2024;39:800-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Gillis C, Ljungqvist O, Carli F. Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br J Anaesth. 2022;128:434-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 34. | Kietaibl S, Ahmed A, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, De Robertis E, Faraoni D, Filipescu DC, Fries D, Godier A, Haas T, Jacob M, Lancé MD, Llau JV, Meier J, Molnar Z, Mora L, Rahe-Meyer N, Samama CM, Scarlatescu E, Schlimp C, Wikkelsø AJ, Zacharowski K. Management of severe peri-operative bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second update 2022. Eur J Anaesthesiol. 2023;40:226-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 198] [Article Influence: 66.0] [Reference Citation Analysis (1)] |
| 35. | Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung MK, Grossman BJ, Mintz PD, O'Malley BA, Sesok-Pizzini DA, Shander A, Stack GE, Webert KE, Weinstein R, Welch BG, Whitman GJ, Wong EC, Tobian AA; AABB. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 676] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/