Published online Sep 14, 2025. doi: 10.3748/wjg.v31.i34.108623
Revised: June 18, 2025
Accepted: August 18, 2025
Published online: September 14, 2025
Processing time: 139 Days and 16.7 Hours
Ultrasound-guided percutaneous thermal ablation has gained popularity as treatment for malignant hepatic tumors. It was first introduced as ablation the
Core Tip: Intrahepatic cholangiocarcinoma is the second most common primary tumor in the liver. Its incidence is increasing in western countries. Ultrasound-guided percutaneous ablation techniques, initially used for the ablation of hepatocellular carcinoma, are now successfully used to treat intrahepatic cholangiocarcinoma. The most common thermal ablation techniques include radiofrequency and microwave ablation. A new non-thermal technique called irreversible electroporation has recently been introduced. This review describes the safety, efficacy, and clinical indications for these ablation techniques.
- Citation: Giorgio A, Ciracì E, De Luca M, Stella G, Rollo VC, Montesarchio L, Giorgio V. Ultrasound-guided percutaneous thermal and non-thermal ablation of intrahepatic cholangiocarcinoma. World J Gastroenterol 2025; 31(34): 108623
- URL: https://www.wjgnet.com/1007-9327/full/v31/i34/108623.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i34.108623
Biliary tract cancer includes epithelial tumors from both the intrahepatic and extrahepatic part of the biliary tract and the gallbladder. Cholangiocarcinoma (CCA) can be classified as intrahepatic CCA (iCCA), which originates from bile ductules and second-order bile ducts, perihilar CCA (pCCA), which originates from the right and/or left hepatic duct and/or their confluence, and distal CCA (dCCA), which originates from the common bile duct[1].
iCCA is the second most common primary liver cancer after hepatocellular carcinoma (HCC) and accounts for 3.0% of all gastrointestinal malignancies. Its incidence is 2.1 per 100000 and has increased globally in the past decade[2]. CCA has a poor prognosis with a high 5-year mortality rate of 95%[3]. iCCA represents 20% of all CCAs, and the remaining 80% are extrahepatic (including pCCA and dCCA). Compared to pCCA and dCCA, iCCA is frequently detected incidentally because it is most commonly asymptomatic. Therefore, iCCA has the worst prognosis of the three subtypes of CCA[4]. The main risk factors for CCA include choledochal cysts [odds ratio (OR) = 26.7], primary sclerosing cholangitis (OR = 22.0), and cirrhosis (OR = 15.3). Nevertheless, 60%-70% of patients have no risk factors[5,6].
iCCA diagnosis is often very difficult due to the absence of specific symptoms, unlike extrahepatic forms in which jaundice is the major symptom due to extrahepatic obstruction. Therefore, it is impossible to apply surveillance strategies to most patients. The current European Association for the Study of the Liver guidelines suggest annual magnetic resonance imaging (MRI) and/or magnetic resonance cholangiopancreatography for surveillance of patients with primary sclerosing cholangitis and ultrasound (US) every 6 months for patients with cirrhosis[1].
Currently, complete surgical resection with negative margins is the only potentially curative treatment for iCCA. Procedures to achieve this goal include hemihepatectomy, extended hepatectomy, segmentectomy, and occasionally excision of the bile duct bifurcation and extrahepatic bile duct. Associated chronic liver disease and function, the location of the tumor, and portal hypertension limit the applicability of liver resection even in patients with iCCA. Typically, liver resection is only applicable for a minority of patients due to late diagnosis. Most patients with iCCA present with large and unresectable tumors, and a multidisciplinary approach is needed to identify the best treatment option[1]. Liver transplantation is a potential treatment option for selected patients with iCCA who have an acceptable and prolonged response to neoadjuvant chemotherapy. However, more prospective data are required[7].
Percutaneous ablation techniques have gained popularity as an alternative treatment for liver lesions, most notably HCC. In 1986 percutaneous ethanol injection (PEI) was first reported in the literature[8]. US is a useful tool for both diagnosis and treatment. It was introduced to percutaneous ablation as a technique to guide the insertion of a fine or large needle, revolutionizing the treatment of malignant liver tumors. PEI was soon replaced by radiofrequency ablation (RFA), which induces a predictable volume of necrosis compared with PEI. RFA has been shown as capable of enlarging the area of necrosis to destroy satellite nodules responsible for frequent recurrences after PEI treatment[9]. Subsequently, additional thermal and non-thermal methods were created and implemented to treat HCC and other liver lesions including iCCA[10-12].
The aim of this minireview was to summarize safety, efficacy, and clinical indications of the most commonly used percutaneous techniques in clinical practice for the treatment of iCCA. We focused on the primary US-guided percuta
RFA was the first thermal ablation technique to be adopted for the ablation of iCCA in patients who were ineligible for resection. When RFA was first introduced, liver transplantation was not an option for the treatment of iCCA[13]. RFA destroys malignant tumor cells by inducing thermal damage to the tissue through electromagnetic energy deposition. In monopolar RFA there is a closed-loop circuit of the patient, the RF generator, the electrode needle, and a large dispersive electrode (grounding pads)[14]. This generates an alternating electric field within the neoplastic mass. The agitation of ions present in the target tissue surrounding the electrode produces friction (and heat) around the electrode. Due to the difference in the small surface area between the needle electrode and the large area of the grounding pads, the heat is concentrated around the needle electrode, while the grounding pads scatter energy to prevent skin burns[14].
In 2002, Slakey[15] first introduced RFA for the treatment of iCCA[15]. Following this report, there were fewer than 100 patients treated with this technique in the literature through 2011[16]. Furthermore, all studies were retrospective and had small sample sizes due to the low incidence[16-20]. However, in 2015 there was enough data to conduct a meta-analysis on the use of RFA for the treatment of iCCA[21]. Fu et al[18] showed complete necrosis even in large nodules. However, it was evident that smaller iCCA nodules led to better destruction of the tumor by RFA, leading to a better prognosis of the patients[16-20]. Carrafiello et al[22] conducted a study comprising 6 patients with iCCA. They observed complete necrosis of all four tumors < 4 cm. However, even when RFA transarterial embolization was added to increase the necrosis volume, they did not obtain the same results in 2 patients with tumors > 5 cm.
The largest series of primary unresectable iCCA treated with RFA was reported by Kim et al[20]. They treated 13 patients with 17 iCCA lesions including 9 patients with underlying cirrhosis and poor liver function reserve. Technical effectiveness (defined as the complete ablation of the tumor) was achieved in the 11 patients with nodules < 5 cm. However, in the patients with larger tumors complete ablation was not achieved. Median local progression-free survival (PFS) was 32.2%. The 1-year, 3-year, and 5-year overall survival (OS) rates were 85%, 51%, and 15%, respectively. The authors concluded that RFA can achieve successful local tumor control in patients with iCCA nodules < 5 cm but pre
In 2011 we reported our single-center experience with 10 patients with iCCA who were ineligible for surgery and were treated with RFA[16]. The iCCA was primary in 9 patients and recurrent after two previous resections in 1 patient. In this series 4 patients had underlying Child-Pugh A liver cirrhosis. RFA achieved 100% necrosis in iCCA lesions ≤ 3.4 cm and incomplete necrosis in lesions ≥ 4 cm. The 1-year, 3-year, and 5-year OS rates were very high: 100%, 83.3%, and 83.3%, respectively. The high cumulative OS rates were explained by repeated RFA procedures during a strict follow-up of the patients. Moreover, the patient with recurrent iCCA attained an 8-year survival. No major complications were encountered. Therefore, we concluded that RFA was a safe and effective treatment for unresectable iCCA in cases of small nodules (≤ 3.4 cm) even in patients with cirrhosis. RFA can be considered as a palliative treatment option for larger tumors[16].
The number of iCCA lesions developing on a background of cirrhosis is increasing[5,6]. From our experience and that described in the series by Kim et al[20], we hypothesize that RFA can be regarded as a safe and effective treatment for small iCCA nodules even in patients with cirrhosis. Extended survival can be expected when the hepatic functional reserve remains adequate, and RFA will have a reduced effectiveness in patients where the functional reserve is markedly diminished. Recently, Díaz-González et al[23] reported the effectiveness, safety, and OS in patients with iCCA and cirrhosis who were ineligible for resection. In their retrospective study, RFA was performed in 27 patients with Child-Pugh A cirrhosis and prevalent clinical portal hypertension from 2001 to 2017. Complete radiological necrosis was achieved in 92.6% of patients. In patients with a single ≤ 2 cm iCCA lesion, OS was 94.5 months. Furthermore, OS was statistically better in patients with a single lesion ≤ 2 cm compared with patients with a single lesion > 2 cm. Moreover, differences in OS were statistically significant between patients with a single lesion > 2 cm and patients with multino
In another study Kim et al[24] treated 20 patients with iCCA who had recurrent lesions after curative resection with RFA[24]. They obtained an effectiveness rate of 97% (28/29). Mean local tumor PFS was 39.8 months. The 1-year, 2-year, and 4-year OS rates were 93%, 74%, and 74%, respectively. They concluded that RFA is a safe and effective procedure that provides successful outcomes for patients experiencing recurrent iCCA lesions after curative resection.
Similarly, Zhang et al[25] retrospectively evaluated a relatively large cohort of patients with recurrent iCCA under
Xu et al[17] treated 18 patients with 25 iCCA nodules, comprised as follows: 8 patients were primary cases; And 10 patients were recurrent cases after curative resection. By univariate analysis, the type of tumor (primary or recurrent) was a significant prognostic factor for OS. The patient source (primary or recurrent after surgery) and the number of nodules were significant prognostic factors for recurrence-free survival. Recently, Chu et al[26] used multivariate analysis to determine the factors affecting survival in the treatment of recurrent iCCA. They confirmed that RFA is a safe and effec
In 2015 the first meta-analysis on using RFA for the treatment of patients with unresectable iCCA was published[21]. The analysis included 7 observational studies comprising 84 patients with unresectable iCCA. The 1-year, 3-year, and 5-year survival rates were 82%, 47%, and 24%, respectively. The authors concluded RFA is a valid locoregional tool prolonging survival. In that same year the results from the National Cancer Database of the United States on iCCA from 2004-2015 were presented[27]. The authors compared the prognosis of patients with iCCA who underwent surgical resection to those treated with RFA, external beam radiation, and radioactive implants. Only 29% of the 6140 patients were treated with surgery. The performance of RFA was inversely related to the clinical stage. The OS benefit in patients treated with RFA was significantly better than other local therapy in stage I disease.
Wu et al[28] observed similar results in a propensity-score matched study comparing RFA and radiochemotherapy in patients with a single, small (≤ 5 cm) iCCA lesion who were ineligible for surgery. The study revealed that patients treated with RFA had better survival compared with chemoradiotherapy in stage I disease. The 5-year OS was 17.6% vs 3.8% in patients treated with RFA and radiochemotherapy, respectively. After bivariate analysis RFA was statistically related to an OS benefit[28].
Another meta-analysis[29] revealed that no prospective randomized controlled trial to determine the safety and efficacy of RFA in the treatment of iCCA had been conducted. RFA was the most commonly used thermal ablation option with a low rate of complications (3.8%). The OS at 1 year, 3 years, and 5 years was 82%, 47%, and 24%, respectively, for patients receiving RFA treatment. This meta-analysis concluded that patients undergoing RFA with a single tumor ≤ 3 cm attained oncological efficacy and survival comparable with resection[29].
RFA is especially effective for small nodules because the temperature decreases as the distance to the electrode needle increases. RFA is affected by the “heat sink effect” in which nodules close to large vessels cannot be ablated due to the nearby blood flow. Therefore, the temperature must be reduced, preventing tumor cell necrosis. It became apparent that a new technique was required to overcome these limitations and obtain an ablation margin similar to resection.
MWA also uses heat for ablation and is routinely performed in clinical practice. Heat is generated by electromagnetic waves to induce the rotation of dipole water molecules, creating friction that results in an increased temperature in the tumor with subsequent coagulative necrosis[30,31]. MWA is a technique primarily utilized in China, and the majority of studies presented in the literature were conducted at Asian centers. There is a predominance of iCCA in Asian countries, but the incidence of iCCA is increasing in Western countries with a steady rise from 0.1 per 100000 cases to 0.6 per 100000 cases[32].
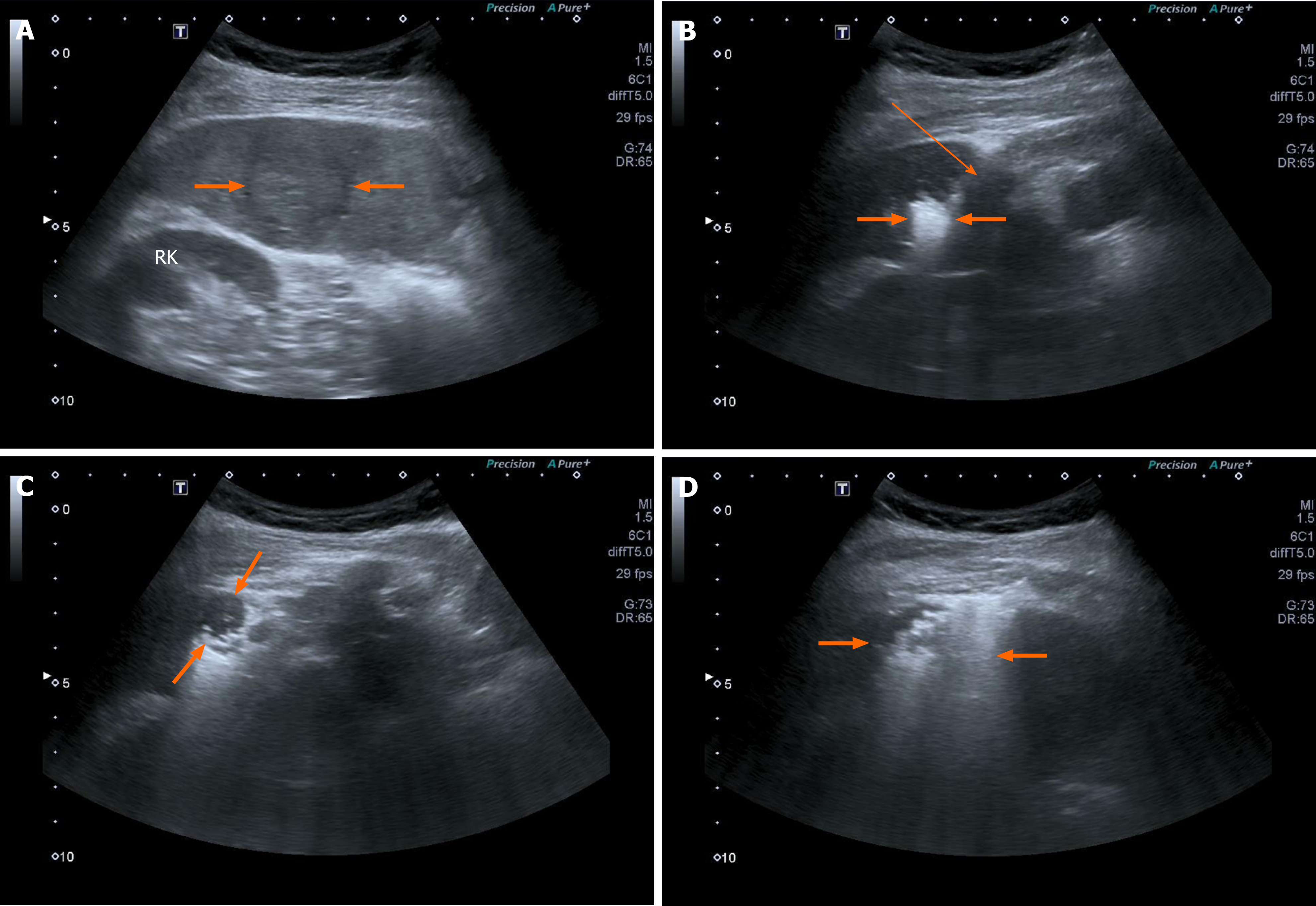
MWA has several advantages that theoretically render it superior to RFA. The first advantage of MWA is the greater power of microwaves over radio waves due to a higher frequency. MWA is subsequently capable of inducing a larger volume of necrosis in a shorter time than RFA (Figure 1). MWA induces an ablation margin of 0.5 cm-1.0 cm from the tip of the antenna like in a surgical resection. Another important advantage of MWA is the absence of the heat sink effect. MWA can also induce a spherical area of necrosis, whereas RFA induces an elliptical area of necrosis.
The first studies on MWA for the treatment of primary and secondary malignant liver tumors were performed using low-powered generators (20-60 watt)[33,34]. In the last two decades, high-powered microwave generators (100-240 watts) with cooled-tip antennas have been used. Many studies have used this technique to treat large-size malignant tumors of the liver, starting with HCC[35,36] and eventually testing the technique in iCCA.
Song et al[37] published a meta-analysis in 2025 and analyzed studies from 2011 to 2023 that treated patients with iCCA with MWA. They found eight eligible studies comprising 423 patients with iCCA treated with MWA who were ineligible for resection. All studies were retrospective, and all but one were conducted in Asia. The median OS was 22.0 months, and the 1-year, 3-year, and 5-year OS rates were 83.7%, 51.0%, and 33.3%, respectively. The median PFS was 12.5 months. Major complications were encountered in 2.8% of cases, but the technical success and efficacy of the procedure were 100% and 99%, respectively. The interesting finding of this meta-analysis was that patients with a serological value of carbohydrate antigen 19-9 > 37 U/mL had a poorer prognosis. The examined studies did not find any difference in OS and PFS between MWA and RFA in the seven Asian studies. However, our multicenter retrospective study revealed that OS and PFS were significantly better in patients treated with MWA compared with those treated with RFA[37].
MWA is generally well-tolerated and is safe for US-guided percutaneous treatment of iCCA. Currently, no deaths have been reported in the published studies. Major complication rates range from 2.8%-3.8%. One of the most reported complications is abscess formation. Kwak et al[38] analyzed 253 patients with hepatic malignancies undergoing MWA. The overall rate of complications was 1.1%, but patients with CCA had a statistically significant risk for development of abscess formation. Many factors lead to the occurrence of these types of complications, including sterilization procedures and the high degree of tumor necrosis due to prolonged exposure to high temperatures.
Contrast-enhanced US (CEUS) is a simple and effective tool available to study the blood supply of liver nodules. It is widely used in the differential diagnosis of benign and malignant liver lesions[39]. Recently, it was determined that CEUS findings in patients with iCCA can predict OS. Patients with iCCA with a preoperative feature of rim-enhancement of the nodule on CEUS have a much poorer prognosis after MWA treatment due to more frequent distant microvascular metastases[40].
MWA was also tested in patients with iCCA in a background of cirrhosis. These patients with an albumin-bilirubin (ALBI) grade of 1 had significantly better OS than patients with an ALBI grade of 2. The cumulative 1-year, 3-year, and 5-year OS rates were 95.5%, 72.4%, and 72.4% for patients with ALBI grade 1 and 62.5%, 40.6%, and 36.3% for patients with ALBI grade 2, respectively, showing a significant difference. On multivariate analysis tumor size ≥ 3 cm and ALBI grade 2 were predictors of poor OS[41].
A recent meta-analysis evaluated aggregated data from 20 studies (917 patients) on thermal ablation for the treatment of iCCA. All studies were observational and retrospective. The main conclusion was that thermal ablation is a successful alternative to surgical resection for the treatment of single iCCA lesions < 3 cm[42]. Currently, MWA exhibits greater thermal efficiency than RFA because of its intrinsic heating properties, suggesting that it may be a better therapeutic option for iCCA[43].
Our 10-year Italian multicenter study demonstrated that MWA was superior to RFA in treating unresectable iCCA[44]. The liver is a highly vascularized solid organ featuring many large blood vessels, which allows for the heat sink effect. Microwaves seem to be better at overcoming perfusion and large heat sinks than other heat-based ablation modalities. The heat sink effect reduces the efficacy of all thermal ablation techniques, leading to insufficient ablation and local tumor recurrence[45]. Wright et al[46] conducted direct comparisons of ablation regions produced by MWA and RFA. The heat sink effect resulted in a deviation of 3.5 ± 5.3% of the ablation zone in tissue treated with MWA, while RFA showed a deviation of 26.2 ± 27.9% (P < 0.05)[46].
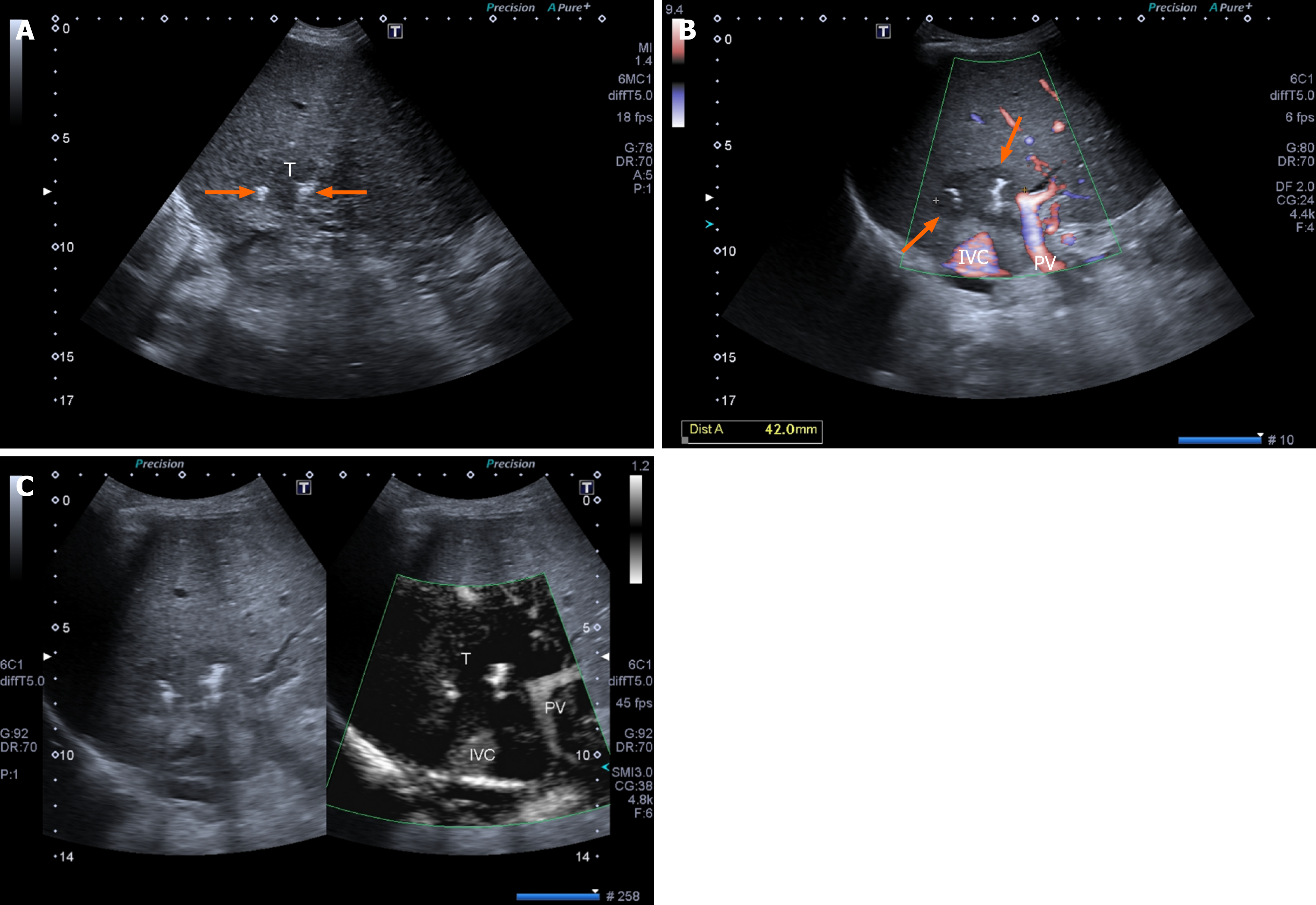
IRE is a relatively new non-thermal technique for the treatment of tumors that are not suitable for resection and/or thermal ablation with RFA or MWA. Although thermal ablative techniques are highly effective in treating liver tumors, there is still a significant number of patients who present with malignant nodules close to large vascular structures (> 3 mm) or major bile ducts where heat can be ineffective or dangerous[47] (Figure 2).
In contrast to thermal ablation techniques, IRE uses electric impulses (instead of heat) to cause an irreversible disruption of the cell membrane integrity, resulting in cell death in the ablated region[47]. IRE uses high-voltage electric fields (up to 3000 V) between two to six probes (preferably at a distance of 2 cm) and low-energy direct current, also known as a pulsed electric field. Multiple, rapid electrical impulses are applied to the region of interest, creating very small pores in the cell membrane (phospholipid bilayer), thereby altering the permeability of target cells and leading to apoptosis and cell death[48-50].
The main advantage of IRE over RFA and MWA is that vascular or bile structures are not affected by the destruction process of the tumor and their integrity is completely preserved[48]. The vessel-preserving effect of IRE is due to the vessel wall containing a higher proportion of collagenous connective tissue and elastic fiber and lacking a normal cellular membrane[51]. IRE can also be performed under US guidance for target insertion and needle placement[52]. IRE is often utilized during open surgery and occasionally under computed tomography (CT) guidance[53]. A recent prospective study on 15 patients with unresectable CCA (8 patients with iCCA) determined the efficacy of treatment with IRE under CT guidance. No major complications were reported, and the iCCA group was followed up for 6-36 months, showing an average survival of 18 months[53].
An important limitation of IRE is that high voltage causes muscle contraction, which can lead to a potential risk of cardiac arrhythmia. Therefore, IRE must be performed under general anesthesia with complete muscle block and electrocardiographic synchronization. In addition, the cost of the procedure (the IRE machine and probes) is very high (7-10 times) compared with RFA or MWA. Patients with heart arrhythmia, prohemorrhagic hematological alterations, or a biliary metallic stent[52] are excluded from receiving IRE as treatment[54]. Because of these limitations, IRE has been employed extensively for the treatment of pCCA[55-57].
In a recent meta-analysis estimating mean OS, PFS, and adverse event rate in patients undergoing IRE, a mean OS of 25.49 months and an adverse events rate of 12% was revealed. However, the studies included in the meta-analysis showed significant heterogeneity. Therefore, the authors concluded that there is insufficient evidence on the efficacy of IRE. Although it is an effective and relatively safe procedure for unresectable pCCA, future prospective and/or rando
There is a paucity of data on the treatment of CCA by IRE. Niessen et al[59] treated 71 primary malignant tumors with IRE, but only 3.9% of them were CCAs. We published a series of US-guided IRE-treated primary and secondary malig
Finally, although IRE is technically more complex than US-guided RFA and MWA, the percutaneous approach is feasible and safe, and US guidance can be easily utilized even in the intercostal space[52]. The hands-free technique should be used by interventional physicians and interventional radiologists when possible, and PEI may be needed[61]. Similarly to RFA and MWA, the outcomes of patients treated with IRE will be better in cases of small tumor lesions, a small number of lesions, and no metastatic lesions[50].
Percutaneous cryoablation therapy is based on the use of an ice ball at very cold temperatures (down to -160 °C). Intracellular and extracellular ice crystals are produced, inducing osmotic pressure changes and consequent cellular dehydration. This causes significant damage to the membrane phospholipid bilayer and leads to cell death.
The advantage of cryoablation treatment of hepatic tumors is the ability to use US guidance, which is cheaper and faster than CT and MRI guidance. Cryoablation is useful in treating lesions close to vascular structures to avoid the heat sink effect. Furthermore, the application of cold has analgesic properties. However, the cryoablation technique can have severe and fatal complications, including cryoshock. Fortunately, these complications are rare and can be avoided by treating small tumor areas.
Few cases have been reported using this non-thermal technique. Therefore, no conclusions can be made on its clinical indications for iCCA[62]. Glazer et al[63] treated 299 primary and secondary malignant hepatic tumors with cryoablation, and only 6 patients had CCA. Helling[64] used cryoablation for the treatment of 39 hepatic malignant tumors. Of these, only three were CCA. The conclusions of both studies stated that cryoablation was a safe and effective tool for the treatment of malignant liver tumors, but no specific data on OS were reported. Obviously, based on these very scarce reports, no conclusions can be drawn.
RFA, MWA, and IRE show potential effectiveness in the treatment of liver lesions. However, they vary in their cost-effectiveness. RFA might be less cost-effective than MWA, especially in specific patient groups or in comparison with surgical resection[65,66]. MWA is frequently considered the most economical ablation method because of low procedure expenses and lower usage of healthcare resources[65]. Studies have shown MWA to be a cost-effective alternative to surgery and RFA, especially in intermediate resource settings. IRE is more expensive than both RFA and MWA due to the initial setup costs and specialized equipment.
Non-ablative locoregional treatments include external beam radiotherapy, transarterial chemoembolization, selective internal radiation therapy, and hepatic artery infusion. Currently, no comparative study of these techniques has been performed. An interesting treatment approach is a combination of methods that can lead to percutaneous thermal segmentectomy. This procedure consists of a combination of balloon-occluded MWA followed by balloon-occluded transarterial chemoembolization. This approach achieves a segmental necrotic area[67,68]. A recent multicentric Italian retrospective study concluded that this technique has promising oncological results in patients with tumors > 3 cm[69]. To demonstrate the value of a multidisciplinary team, Seidensticker et al[70] observed that treatment combining systemic with advanced image-guided local or locoregional therapies can improve the survival in unresectable or recurrent iCCA.
The current European Association for the Study of the Liver guidelines suggest the use of thermal ablation in patients with an unresectable single iCCA lesion < 2 cm as a good, feasible, and safe alternative to surgery. Evidence is still lacking on the benefits of thermal ablation in the treatment of a single lesion > 2 cm or multinodular disease[1]. Future prospective and randomized controlled trials are still required to determine the efficacy of thermal ablation, non-thermal ablation, and nonablative techniques for the treatment of iCCA.
| 1. | European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79:181-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 194] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 2. | Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 392] [Article Influence: 65.3] [Reference Citation Analysis (1)] |
| 4. | Brown KM, Parmar AD, Geller DA. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23:231-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 710] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 6. | Kratz JD, Klein AB, Gray CB, Märten A, Vilu HL, Knight JF, Kumichel A, Ueno M. The Epidemiology of Biliary Tract Cancer and Associated Prevalence of MDM2 Amplification: A Targeted Literature Review. Target Oncol. 2024;19:833-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Sapisochin G, Ivanics T, Heimbach J. Liver Transplantation for Intrahepatic Cholangiocarcinoma: Ready for Prime Time? Hepatology. 2022;75:455-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Livraghi T, Festi D, Monti F, Salmi A, Vettori C. US-guided percutaneous alcohol injection of small hepatic and abdominal tumors. Radiology. 1986;161:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 224] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Rossi S, Garbagnati F, Rosa L, Azzaretti A, Belloni G, Quaretti P. Radiofrequency thermal ablation for treatment of hepatocellular carcinoma. Int J Clin Oncol. 2002;7:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Edeline J, Lamarca A, McNamara MG, Jacobs T, Hubner RA, Palmer D, Groot Koerkamp B, Johnson P, Guiu B, Valle JW. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat Rev 2021; 99: 102258 . [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 11. | Zhang K, Yu J, Yu X, Han Z, Cheng Z, Liu F, Liang P. Clinical and survival outcomes of percutaneous microwave ablation for intrahepatic cholangiocarcinoma. Int J Hyperthermia. 2018;34:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Xu C, Li L, Xu W, Du C, Yang L, Tong J, Yi Y. Ultrasound-guided percutaneous microwave ablation versus surgical resection for recurrent intrahepatic cholangiocarcinoma: intermediate-term results. Int J Hyperthermia. 2019;36:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Nagata Y, Hiraoka M, Akuta K, Abe M, Takahashi M, Jo S, Nishimura Y, Masunaga S, Fukuda M, Imura H. Radiofrequency thermotherapy for malignant liver tumors. Cancer. 1990;65:1730-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | McDermott S, Gervais DA. Radiofrequency ablation of liver tumors. Semin Intervent Radiol. 2013;30:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Slakey DP. Radiofrequency ablation of recurrent cholangiocarcinoma. Am Surg. 2002;68:395-397. [PubMed] |
| 16. | Giorgio A, Calisti G, DE Stefano G, Farella N, DI Sarno A, Amendola F, Scognamiglio U, Giorgio V. Radiofrequency ablation for intrahepatic cholangiocarcinoma: retrospective analysis of a single centre experience. Anticancer Res. 2011;31:4575-4580. [PubMed] |
| 17. | Xu HX, Wang Y, Lu MD, Liu LN. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 2012;85:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Fu Y, Yang W, Wu W, Yan K, Xing BC, Chen MH. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2012;23:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Haidu M, Dobrozemsky G, Schullian P, Widmann G, Klaus A, Weiss H, Margreiter R, Bale R. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol. 2012;35:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196:W205-W209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol. 2015;26:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Carrafiello G, Laganà D, Cotta E, Mangini M, Fontana F, Bandiera F, Fugazzola C. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Díaz-González Á, Vilana R, Bianchi L, García-Criado Á, Rimola J, Rodríguez de Lope C, Ferrer J, Ayuso C, Da Fonseca LG, Reig M, Forner A. Thermal Ablation for Intrahepatic Cholangiocarcinoma in Cirrhosis: Safety and Efficacy in Non-Surgical Patients. J Vasc Interv Radiol. 2020;31:710-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Kim JH, Won HJ, Shin YM, Kim PN, Lee SG, Hwang S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol. 2011;80:e221-e225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Zhang SJ, Hu P, Wang N, Shen Q, Sun AX, Kuang M, Qian GJ. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2013;20:3596-3602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Chu HH, Kim JH, Shin YM, Won HJ, Kim PN. Percutaneous Radiofrequency Ablation for Recurrent Intrahepatic Cholangiocarcinoma After Curative Resection: Multivariable Analysis of Factors Predicting Survival Outcomes. AJR Am J Roentgenol. 2021;217:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Kolarich AR, Shah JL, George TJ Jr, Hughes SJ, Shaw CM, Geller BS, Grajo JR. Non-surgical management of patients with intrahepatic cholangiocarcinoma in the United States, 2004-2015: an NCDB analysis. J Gastrointest Oncol. 2018;9:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Wu L, Tsilimigras DI, Farooq A, Hyer JM, Merath K, Paredes AZ, Mehta R, Sahara K, Shen F, Pawlik TM. Potential survival benefit of radiofrequency ablation for small solitary intrahepatic cholangiocarcinoma in nonsurgically managed patients: A population-based analysis. J Surg Oncol. 2019;120:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Sommer CM, Kauczor HU, Pereira PL. Locoregional Therapies of Cholangiocarcinoma. Visc Med. 2016;32:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 579] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 31. | Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25 Suppl 1:S69-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 601] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 32. | Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 724] [Article Influence: 144.8] [Reference Citation Analysis (3)] |
| 33. | Yu MA, Liang P, Yu XL, Cheng ZG, Han ZY, Liu FY, Yu J. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol. 2011;80:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Livraghi T, Meloni F, Solbiati L, Zanus G; Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 35. | Zhang NN, Lu W, Cheng XJ, Liu JY, Zhou YH, Li F. High-powered microwave ablation of larger hepatocellular carcinoma: evaluation of recurrence rate and factors related to recurrence. Clin Radiol. 2015;70:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Giorgio A, Gatti P, Montesarchio L, Merola MG, Amendola F, Calvanese A, Iaquinto G, Fontana M, Ciracì E, Semeraro S, Santoro B, Coppola C, Matteucci P, Giorgio V. Microwave Ablation in Intermediate Hepatocellular Carcinoma in Cirrhosis: An Italian Multicenter Prospective Study. J Clin Transl Hepatol. 2018;6:251-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Song M, Li J, Li Y, Zhang C, Sigdel M, Hou R, Jiao D, Zhou X. Efficacy of microwave ablation for intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Quant Imaging Med Surg. 2025;15:760-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 38. | Kwak DH, Yu Q, Malavia M, Sellers E, Said A, Patel M, Kumari D, Ahmed O. Risk Factors for Abscess Development Following Percutaneous Microwave Ablation Therapy of Hepatic Tumors. Cardiovasc Intervent Radiol. 2023;46:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 39. | Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, Chammas MC, Chaubal N, Choi BI, Clevert DA, Cui X, Dong Y, D'Onofrio M, Fowlkes JB, Gilja OH, Huang P, Ignee A, Jenssen C, Kono Y, Kudo M, Lassau N, Lee WJ, Lee JY, Liang P, Lim A, Lyshchik A, Meloni MF, Correas JM, Minami Y, Moriyasu F, Nicolau C, Piscaglia F, Saftoiu A, Sidhu PS, Sporea I, Torzilli G, Xie X, Zheng R. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 328] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 40. | Wang X, Liang P, Yu J, Yao JD, Fan FY, Yu X, Cheng ZG, Han ZY, Liu FY, Dou JP. Contrast-enhanced ultrasound features predict the prognosis of percutaneous microwave ablation of intrahepatic cholangiocarcinoma. Br J Radiol. 2022;95:20211379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Yang H, Cheng Z, Han Z, Liu F, Yu X, Yu J, Liang P. Assessment of the Outcomes of Intrahepatic Cholangiocarcinoma After Ultrasound-Guided Percutaneous Microwave Ablation Based on Albumin-Bilirubin Grade. Cardiovasc Intervent Radiol. 2021;44:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Kim GH, Kim PH, Kim JH, Kim PN, Won HJ, Shin YM, Choi SH. Thermal ablation in the treatment of intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Eur Radiol. 2022;32:1205-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Giorgio A, Gatti P, Montesarchio L, Santoro B, Dell'Olio A, Crucinio N, Coppola C, Scarano F, Biase F, Ciracì E, Semeraro S, Giorgio V. Intrahepatic Cholangiocarcinoma and Thermal Ablation: Long-term Results of An Italian Retrospective Multicenter Study. J Clin Transl Hepatol. 2019;7:287-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 364] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 46. | Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 47. | Narayanan G, Froud T, Suthar R, Barbery K. Irreversible electroporation of hepatic malignancy. Semin Intervent Radiol. 2013;30:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 49. | Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, Roberts S, Evans P, Ball C, Haydon A. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 50. | Narayanan G, Koethe Y, Gentile N. Irreversible Electroporation of the Hepatobiliary System: Current Utilization and Future Avenues. Medicina (Kaunas). 2024;60:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 51. | Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality--clinical implications. Technol Cancer Res Treat. 2007;6:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 547] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 52. | Giorgio A, Amendola F, Calvanese A, Ingenito E, Santoro B, Gatti P, Ciracì E, Matteucci P, Giorgio V. Ultrasound-guided percutaneous irreversible electroporation of hepatic and abdominal tumors not eligible for surgery or thermal ablation: a western report on safety and efficacy. J Ultrasound. 2019;22:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Belfiore MP, Reginelli A, Maggialetti N, Carbone M, Giovine S, Laporta A, Urraro F, Nardone V, Grassi R, Cappabianca S, Brunese L. Preliminary results in unresectable cholangiocarcinoma treated by CT percutaneous irreversible electroporation: feasibility, safety and efficacy. Med Oncol. 2020;37:45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Cohen EI, Field D, Lynskey GE, Kim AY. Technology of irreversible electroporation and review of its clinical data on liver cancers. Expert Rev Med Devices. 2018;15:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Scrofani AR, Valvano M, Lancellotta V, Pezzulla D, Vinci A, Cornacchione P, Bonome P, Tagliaferri L, Iezzi R. Efficacy and safety of irreversible electroporation in unresectable perihilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2024;97:1413-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | N M, D W. Novel Therapy for Unresectable Hilar Cholangiocarcinoma ‘Klatskin Tumor’ Utilizing Percutaneous Irreversible Electroporation: A Case Report. OMICS J Radiol. 2017;06. [DOI] [Full Text] |
| 57. | Martin EK, Bhutiani N, Egger ME, Philips P, Scoggins CR, McMasters KM, Kelly LR, Vitale GC, Martin RCG. Safety and efficacy of irreversible electroporation in the treatment of obstructive jaundice in advanced hilar cholangiocarcinoma. HPB (Oxford). 2018;20:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Gupta P, Maralakunte M, Sagar S, Kumar-M P, Bhujade H, Chaluvashetty SB, Kalra N. Efficacy and safety of irreversible electroporation for malignant liver tumors: a systematic review and meta-analysis. Eur Radiol. 2021;31:6511-6521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 59. | Niessen C, Thumann S, Beyer L, Pregler B, Kramer J, Lang S, Teufel A, Jung EM, Stroszczynski C, Wiggermann P. Percutaneous Irreversible Electroporation: Long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci Rep. 2017;7:43687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Distelmaier M, Barabasch A, Heil P, Kraemer NA, Isfort P, Keil S, Kuhl CK, Bruners P. Midterm Safety and Efficacy of Irreversible Electroporation of Malignant Liver Tumors Located Close to Major Portal or Hepatic Veins. Radiology. 2017;285:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 61. | Giorgio A, Tarantino L, de Stefano G, Perrotta A, Aloisio V, del Viscovo L, Alaia A, Lettieri G. Ultrasound-guided percutaneous ethanol injection under general anesthesia for the treatment of hepatocellular carcinoma on cirrhosis: long-term results in 268 patients. Eur J Ultrasound. 2000;12:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Sweeney J, Parikh N, El-Haddad G, Kis B. Ablation of Intrahepatic Cholangiocarcinoma. Semin Intervent Radiol. 2019;36:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Glazer DI, Tatli S, Shyn PB, Vangel MG, Tuncali K, Silverman SG. Percutaneous Image-Guided Cryoablation of Hepatic Tumors: Single-Center Experience With Intermediate to Long-Term Outcomes. AJR Am J Roentgenol. 2017;209:1381-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Helling TS. Realistic expectations for cryoablation of liver tumors. J Hepatobiliary Pancreat Surg. 2000;7:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Froelich MF, Schnitzer ML, Rathmann N, Tollens F, Unterrainer M, Rennebaum S, Seidensticker M, Ricke J, Rübenthaler J, Kunz WG. Cost-Effectiveness Analysis of Local Ablation and Surgery for Liver Metastases of Oligometastatic Colorectal Cancer. Cancers (Basel). 2021;13:1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 66. | Yue WW, Wang SR, Li XL, Xu HX, Lu F, Sun LP, Guo LH, He YP, Wang D, Yin ZQ. Quality of Life and Cost-Effectiveness of Radiofrequency Ablation versus Open Surgery for Benign Thyroid Nodules: a retrospective cohort study. Sci Rep. 2016;6:37838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Lucatelli P, Argirò R, Crocetti L, Rocco B, Bozzi E, Gasparrini F, Tanzilli A, Catalano C, Iezzi R. Percutaneous Thermal Segmentectomy: Proof of Concept. Cardiovasc Intervent Radiol. 2022;45:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Iezzi R, Posa A, Tanzilli A, Carchesio F, Pompili M, Manfredi R. Balloon-Occluded MWA (b-MWA) Followed by Balloon-Occluded TACE (b-TACE): Technical Note on a New Combined Single-Step Therapy for Single Large HCC. Cardiovasc Intervent Radiol. 2020;43:1702-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Lucatelli P, Rocco B, Argirò R, Semeraro V, Lai Q, Bozzi E, Crociati S, Barone M, Posa A, Catalano C, Crocetti L, Iezzi R. Percutaneous thermal segmentectomy for liver malignancies over 3 cm: mid-term oncological performance and predictors of sustained complete response from a multicentric Italian retrospective study. Radiol Med. 2024;129:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 70. | Seidensticker R, Seidensticker M, Doegen K, Mohnike K, Schütte K, Stübs P, Kettner E, Pech M, Amthauer H, Ricke J. Extensive Use of Interventional Therapies Improves Survival in Unresectable or Recurrent Intrahepatic Cholangiocarcinoma. Gastroenterol Res Pract. 2016;2016:8732521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/