Published online Sep 14, 2025. doi: 10.3748/wjg.v31.i34.108617
Revised: May 22, 2025
Accepted: August 13, 2025
Published online: September 14, 2025
Processing time: 135 Days and 18.5 Hours
Yes-associated protein 1 (YAP1), a downstream transcriptional coactivator regu
To investigate the therapeutic effect of levodopa and the downstream mechanism on carbon tetrachloride (CCl4)-induced liver fibrosis, including liver DRD1 ex
SD rats were intraperitoneally injected with 40% CCl4 for 8 weeks to induce liver fibrosis, followed by treatment with varying doses of levodopa for 2 weeks. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured, and liver pathology was assessed using hematoxylin and eosin and Masson's staining. Alpha-smooth muscle actin (α-SMA) content, along with the expressions of DRD1, YAP, and phosphorylated protein, was analyzed by Western blot, immunohistochemistry, and reverse transcription–quantitative real-time polymerase chain reaction.
Compared with the controls, levodopa-treated rats showed a decrease in the proportion of collagen in the liver and a recovery from liver fibrosis (P = 0.0007). Western blot and immunohistochemistry indicated that DRD1 was upregulated in the fibrotic liver of rats treated with levodopa, showing an increase in DRD1 Level (P < 0.0001). In addition, the upregulation of DRD1 activated the Hippo signaling pathway, manifested as increased YAP phos
This was the first study to demonstrate that levodopa attenuates CCl4-induced liver fibrosis by inhibiting the Hippo/YAP signaling pathways.
Core Tip: This study found that levodopa significantly alleviated carbon tetrachloride-induced liver fibrosis in rats by upregulating Gα s-coupled protein dopamine receptor D1 (DRD1) expression and enhancing Yes-associated protein 1 (YAP) phosphorylation through the activation of the Hippo signaling pathway. This study for the first time demonstrates levodopa's potential as a novel therapeutic strategy targeting the DRD1-Hippo/YAP axis in liver fibrosis.
- Citation: Wang HY, Qi MM, Zhang K, Zhu YZ, Zhang J. Dopamine receptor D1-mediated suppression of liver fibrosis via Hippo/Yes-associated protein 1 signaling in levodopa treatment. World J Gastroenterol 2025; 31(34): 108617
- URL: https://www.wjgnet.com/1007-9327/full/v31/i34/108617.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i34.108617
Liver fibrosis is a pathological condition characterized by the destruction of hepatocytes and the activation of hepatic stellate cells (HSCs) resulting from various chronic tissue injuries. Triggered by factors such as alcohol abuse, food poisoning, viral infections (e.g., hepatitis B and C), metabolic disorders (e.g., non-alcoholic steatohepatitis), and environmental toxins, these injuries can lead to abnormal distribution and excessive deposition of extracellular matrix com
The development of liver fibrosis is primarily driven by the activation of HSCs. The Hippo/Yes-associated protein 1 (YAP) signaling pathway serves as a central regulator of HSC activation following chronic liver injury. Both upstream kinases and downstream transcription coactivators, such as YAP, are essential for modulating downstream signals. In the Hippo signaling pathway, these components primarily exert regulatory effects through a kinase cascade[6]. Upon phosphorylation, YAP is unable to translocate from the cytoplasm to the nucleus, thereby inhibiting the expression of pro-fibrotic factors and consequently suppressing HSC activation, which help alleviate fibrosis[7,8]. Recent studies have shown that YAP activity is elevated in diet-induced liver injury models[9]. Bou Saleh et al[10] demonstrated that key liver functions, including bile duct differentiation and regenerative capacity, which were impaired in human liver fibrotic cells, were restored in fibrotic mice after treatment with YAP inhibitors, suggesting that inhibiting YAP expression in the Hippo signaling pathway might reduce liver fibrosis. Although evidence indicates that YAP activity in fibroblasts promotes fibrosis progression, RNA interference targeting YAP/transcriptional coactivator with PDZ-binding motif (TAZ) in a mouse model of pulmonary fibrosis led to increased lung injury and fibrosis[11]. Recent studies have focused on strategies that specifically inhibit YAP in fibroblasts.
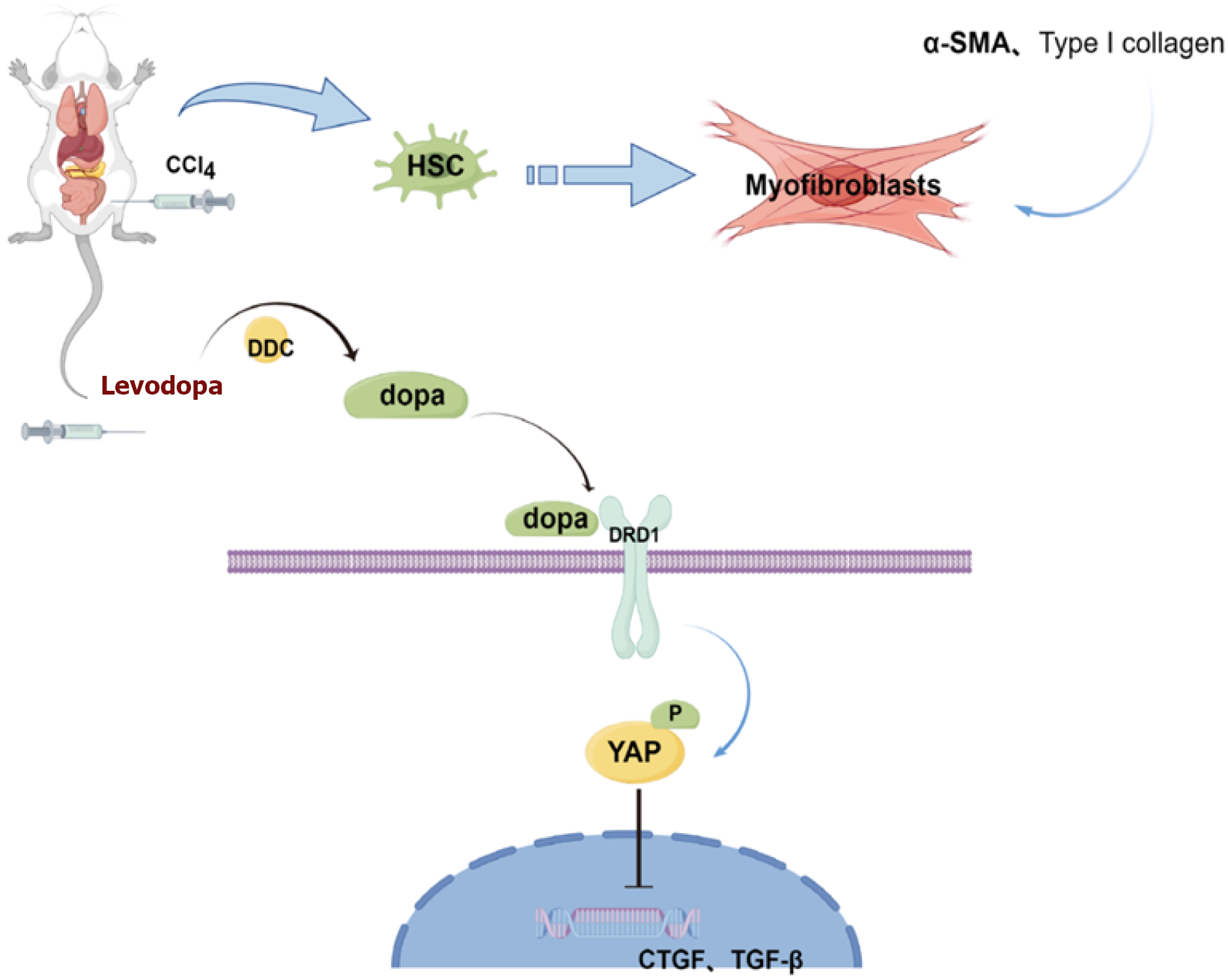
Dopamine receptors are part of the G-protein coupled receptors (GPCRs) superfamily, which includes five receptor members, including the Gα s-coupled protein dopamine D1 (DRD1). DRD1 is preferentially expressed in lung and liver mesenchymal cells, and the use of DRD1 agonists in vivo inhibits YAP nuclear translocation in these cells. Levodopa, an intermediate product of L-tyrosine in catecholamine synthesis, serves as a dopamine precursor and is currently the most effective and widely-used drug for the treatment of Parkinson's disease[12,13]. When injected into rodent and non-human primate models, levodopa is converted into dopamine by the dopa decarboxylase in vivo. The resulting dopamine binds to DRD1 on cell surface, activating the downstream signaling pathway of DRD1[14,15].
The present study for the first time investigated whether levodopa reduces the liver fibrosis index in rats, examining its effects and exploring whether DRD1, a member of GPCRs, could serve as a potential treatment for liver fibrosis.
Carbon tetrachloride (CCl4; C805329; Sigma), olive oil (0815211; Sigma), ascorbic acid (A800295; Sigma), and benserazide hydrochloride (B801922; Sigma) were purchased from Shanghai Macklin Biochemical Technology, Inc. Levodopa (72816; Sigma-Aldrich) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The anti-phospho-YAP1 (AF2371), anti-α-smooth muscle actin (anti-α-SMA; AG8004), and GAPDH (AF2819) antibodies were purchased from Beyotime Institute of Biotechnology, Shanghai, China.
Male SD rats aged 6 weeks and weighing 200-220 g were purchased from Shanghai Slack Laboratory Animal Center (Shanghai, China) and housed in the animal facility of Shanghai University under specific pathogen-free conditions with a 12-hour light/dark cycle and free access to food and water. All animals received humane care according to the criteria outline in the Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences. The experimental plan was approved by the Ethics Committee of Animal Experiments of the Shanghai University (Shanghai, China; approval No. ECSHU 2023-103). All rats were kept at a controlled temperature of 22-23 °C and maintained on a 12 hours light/12 hours dark cycle, with ad libitum food and water. All animals were acclimated to their new housing conditions for one week prior to the start of the experiments.
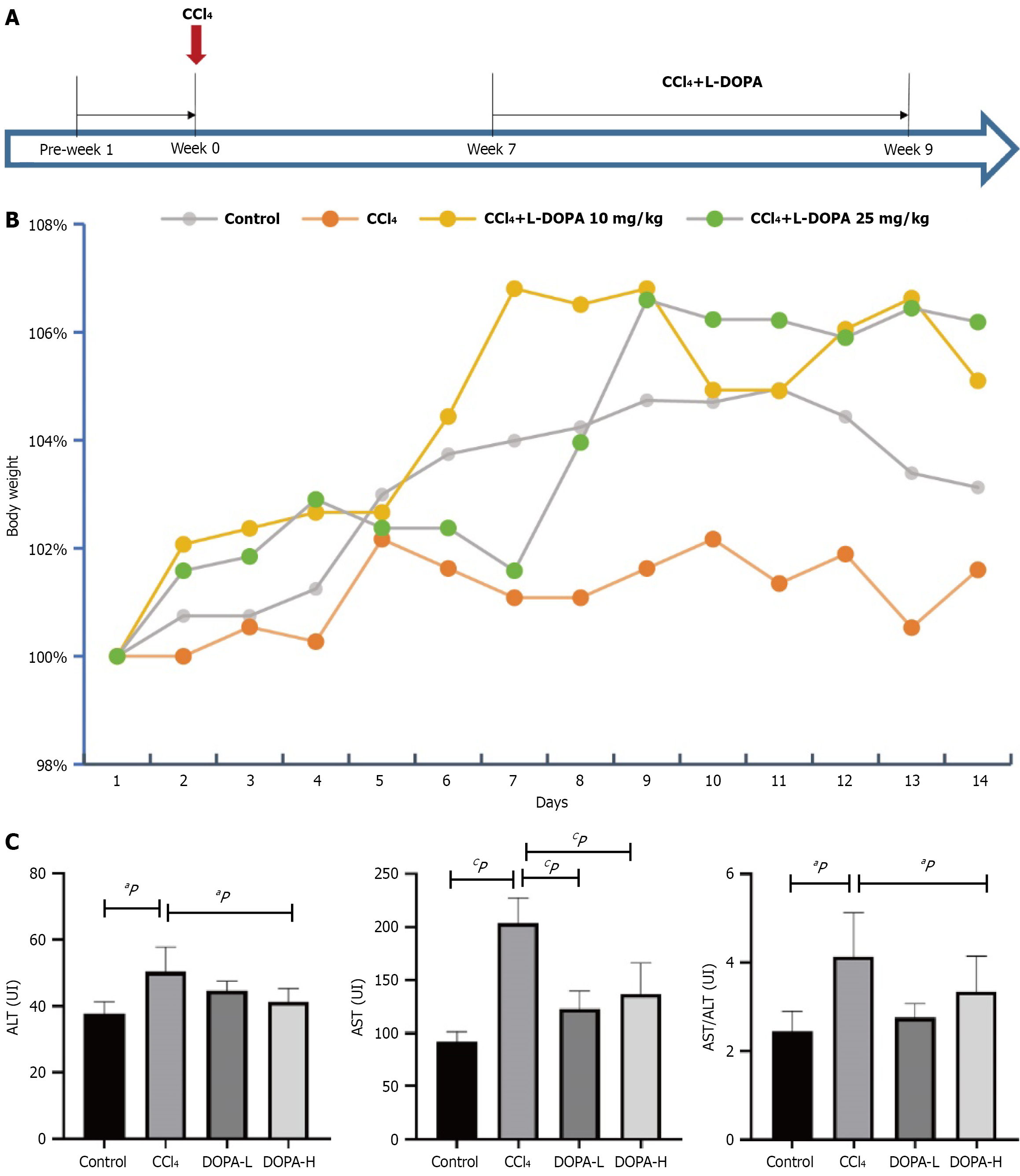
Euthanasia was performed under pentobarbital anesthesia followed by cervical dislocation to ensure painless procedures. Based on a previous study, rats received intraperitoneal injection of 1.5 mL/kg 40% CCl4 (dissolved in olive oil) twice a week for 8 weeks[16,17]. As shown in Figure 1A, the rats were randomly assigned into four groups (n = 8 in each group): CCl4 group, DOPA-L group, DOPA-H group, and blank control group. Rats in the CCl4, DOPA-L, and DOPA-H groups were administered with CCl4, while rats in the blank control group received 1.5 mL/kg normal saline. In week 7, rats in the DOPA-L and DOPA-H groups were additionally given levodopa (10 mg/kg and 25 mg/kg, respec
To evaluate potential histopathological changes and collagen deposition, liver sections (5 µm thick) were stained with hematoxylin and eosin (H&E) and Masson trichrome and then examined under a microscope. For quantitative histolo
Paraffin-embedded liver tissue sections were dewaxed and rehydrated, then antigen repair and peroxidase inhibition were performed in 3% H2O2 for 15 minutes. The sections were blocked with 10% normal goat serum (Beyotime, Shanghai, China, https://www.beyotime.com) at room temperature for 1 hour, then incubated overnight with primary antibody at 4 °C. Subsequently, the sections were incubated with horseradish peroxidase-labeled secondary antibody or fluorescently-labeled secondary antibody in phosphate-buffered saline. Finally, the tissue slides were covered with a cover glass and examined under an optical microscope.
Tissue lysate was prepared using RIPA lysis buffer (Beyotime Institute of Biotechnology). The lysate was centrifuged, and total protein was separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE0 and transferred onto a polyvinylidene fluoride membrane (Merck Millipore). After blocking with 5% BSA for 1 hour, the membranes were incubated with primary antibodies at 4 °C overnight, followed by incubation with secondary antibodies for 2 hours at room temperature. The protein bands were visualized using an enhanced chemiluminescence detection reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. The gray values of protein bands were analyzed using Image J software.
Total RNA was extracted from liver tissues using TRIzol® reagent (R711-01; Vazyme, Inc.). The RNA concentration was determined by measuring the absorbance at 260 nm using a SMA2000 spectrophotometer (Vazyme, Inc.). Reverse transcription was performed using HiScript III RT SuperMix for quantitative real-time polymerase chain reaction (qPCR) (+gDNA wiper; R323-01; Vazyme, Inc.), with the following conditions: 37 °C for 15 minutes and 98 °C for 5 minutes, followed by storage at 4 °C. qPCR was carried out using the SYBR Green PCR Master Mix-PLUS kit (Vazyme, Inc.) on the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.). Each 20 µL of reaction mixture contained 10 µL of SYBR Green Mix, 6 µL of nuclease-free H2O, 2 µL of cDNA, 1 µL of upstream primer, and 1 µL of downstream primer. The qPCR thermal cycling conditions were as follows: Initial denaturation at 95 °C for 60 seconds, followed by 40 cycles of 95 °C for 15 seconds, 60 °C for 15 seconds, and extension at 72 °C for 45 seconds. The primers were synthesized by Tsingke, Ltd. Relative mRNA expression levels were calculated using the 2-ΔΔCq method, with β-actin used as an internal reference gene. The primer sequences are listed in Table 1.
| Gene | Sequence (5'-3') | Product size, bp |
| β-actin | 375 | |
| Forward | CGTAAAGACCTCTATGCCAACA | |
| Reverse | TAGGAGCCAGGGCAGTAATC | |
| YAP1 | 162 | |
| Forward | TCCCGGGATGACTCAGGAAT | |
| Reverse | TCCACGCTGTTCAGGAAGTC |
Data are presented as mean ± SD. An unpaired two-tailed Student’s t-test and ANOVA analysis followed by Tukey’s post hoc test were used for single and multiple comparisons between two or more groups, respectively. Images were analyzed using Image J software. P < 0.05 was considered statistically significant.
Rat models of CCl4-induced liver fibrosis were successfully established (n = 34; Figure 1A). Animals in the modeling groups exhibited significant weight loss after CCl4 injection; however, levodopa treatment slowed the weight loss in rats after two weeks of administration (Figure 1B). Furthermore, animals in the DOPA-L group did not exhibit behavioral abnormalities, whereas rats in DOPA-H group displayed slight involuntary movements.
To evaluate the effect of levodopa on liver injury, the serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured (Figure 1C). Compared with the blank control group, the AST and ALT levels were significantly elevated in the model groups (P < 0.0001). However, compared to the CCl4 group, the AST levels in the levodopa treatment groups were significantly reduced (P < 0.0001 and P = 0.0009), indicating that CCl4 caused liver damage in SD rats and that levadopa treatment reduced the CCl4-induced liver injury. However, no significant difference in AST levels was observed between DOPA-L and DOPA-H groups (P = 0.7325). Additionally, multiple comparisons revealed that the AST/ALT ratios in the blank control and the treatment groups were significantly lower than that in the CCl4 group (P = 0.021 and P = 0.029; Figure 1C). These findings suggest that levodopa could attenuate CCl4-induced hepatic fibrosis in rats.
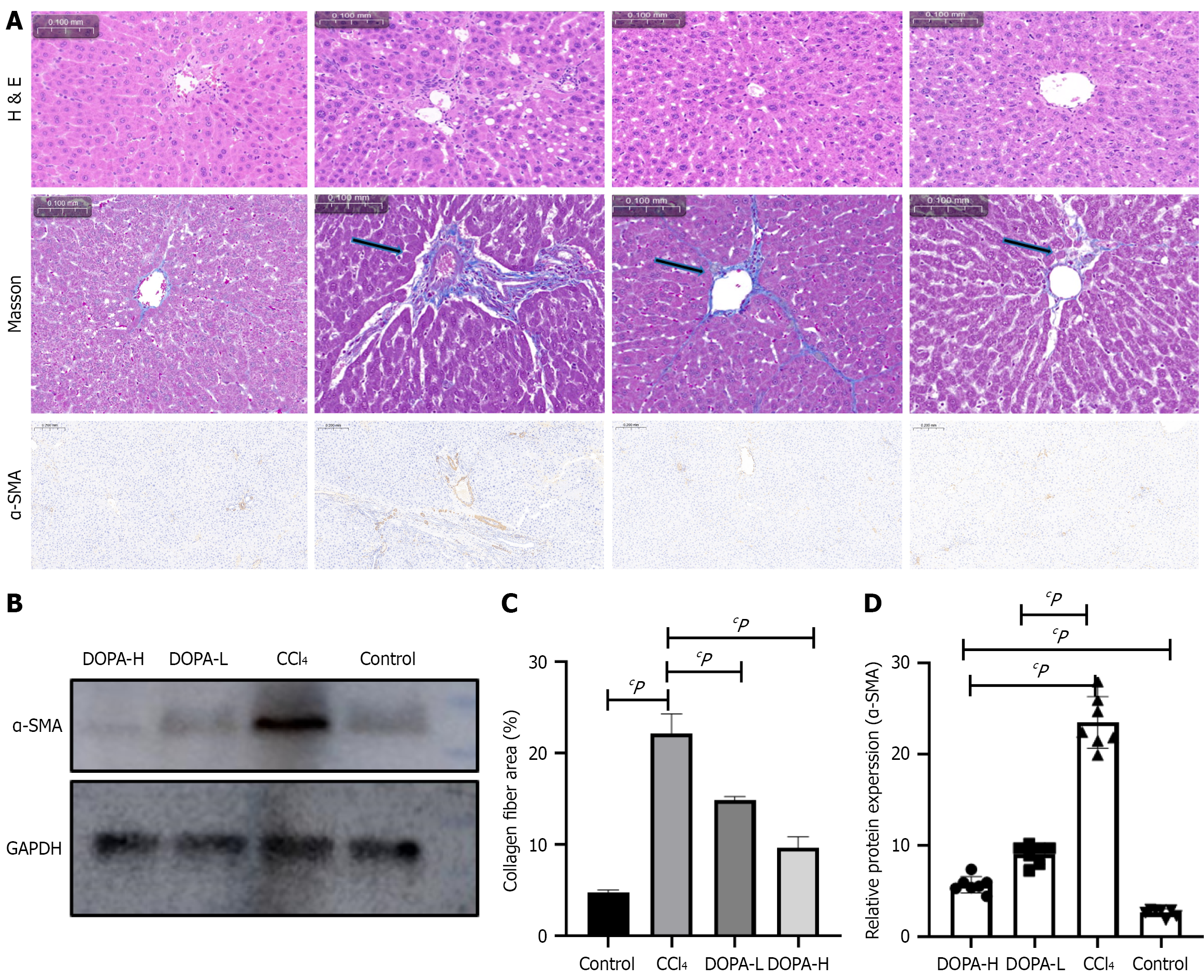
The liver tissue of rats in the model groups showed pathological changes, including hepatic steatosis and fat vacuoles. H&E staining showed that rats treated with CCl4 exhibited liver fibrosis with a small amount of fibrous septa, diffuse proliferation of fibers in hepatic lobules, and degeneration and necrosis of hepatocytes around hepatic lobules. Compared with the CCl4 group, the levodopa treatment groups had significantly reduced connective tissue hyperplasia and segmentation; however, the degree of degeneration and necrosis of liver cells was reduced to a lesser extent. Overall, liver tissue morphologies were improved in DOPA-L and DOPA-H groups; however, the difference was not significant between these two levodopa treatment groups, possibly due to the small difference in the levodopa doses.
It is worth noting that there was no significant difference in liver fibrosis between the low- and high-dose groups (Figure 2A), possibly due to the small difference in drug doses. Masson's trichrome staining revealed that collagen deposition in the CCl4 group was significantly increased, while the total collagen content in the levodopa treatment groups was lower than that in the blank control group (P < 0.0001 and P = 0.0007). However, there was no significant difference in collagen deposition between DOPA-L and DOPA-H groups (Figure 2B; P = 0.3903).
Additionally, potential variations in the protein levels of α-SMA in rat liver tissues were also investigated. Western blot analysis revealed that compared with the blank control group, the α-SMA protein level was upregulated in the CCl4 group and downregulated in the levodopa treatment groups. Furthermore, immunofluorescence staining of liver tissue paraffin sections showed that, compared with the blank control group, the α-SMA expression was increased in the CCl4 group (P < 0.0001) but decreased in levodopa treatment groups (P = 0.0076 and P < 0.0001; Figure 2B-D). These results suggest that levodopa significantly inhibits collagen deposition and fibrogenic protein production, thereby reducing CCl4-induced liver fibrosis in rats.
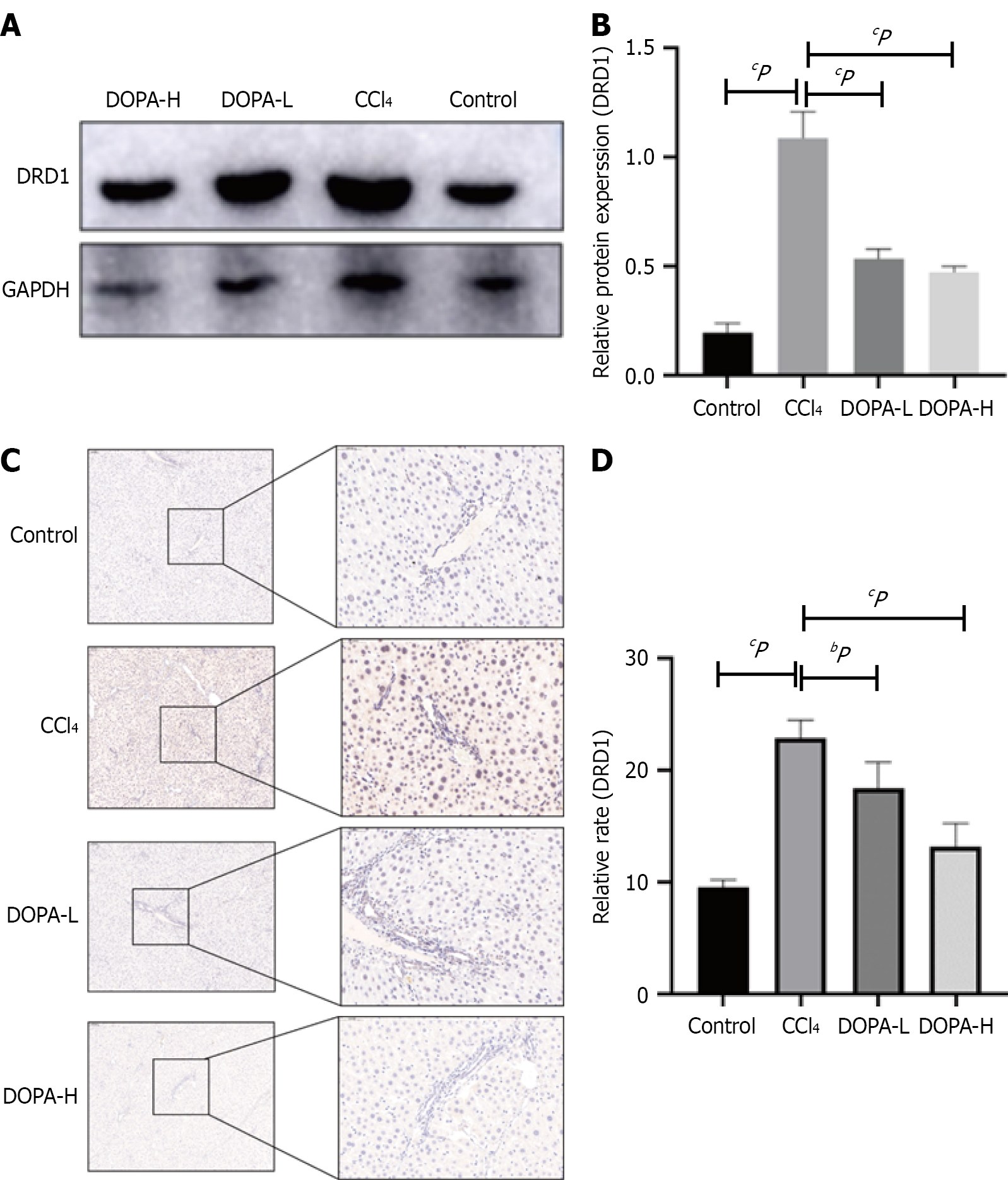
To explore the signaling pathway through which levodopa inhibits liver fibrosis, the DRD1 protein level was measured using Western blotting and immunohistochemistry (IHC). As shown in Figure 3A and B, compared with the blank control group, DRD1 content in the CCl4 group was significantly increased (P < 0.0001). Compared with the CCl4 group, the DRD1 content in the levodopa group was also significantly increased (P < 0.0001). However, no significant difference was observed between the two treatment groups (P = 0.5924). IHC results further confirmed that DRD1 Levels in the CCl4 group were significantly higher than in the levodopa treatment groups (P < 0.0001; Figure 3C and D) but was still higher than that in the blank control group (P = 0.0008). These findings are consistent with our previous study, which demon
Based on these results, it can be concluded that DRD1 Levels increase with the severity of liver fibrosis, and that levodopa administration reduces DRD1 Levels in vivo, thereby exerting its anti-liver fibrosis effects.
Previous studies have shown that the Hippo/YAP signaling pathway was involved in liver regeneration, and inhibition of the nuclear translocation of the core factor YAP led to reduced expression of pro-fibrotic factors in the nucleus[7]. Research has also demonstrated that the Hippo signaling pathway was regulated by GPCR signaling. Stimulatory GPCRs activate large tumor suppressor 1 and 2 (LATS1/2) kinase activity, which phosphorylates YAP and inhibits its nuclear transfer function. DRD1 is a stimulatory GCPR-α subtybe. Dopamine binds to DRD1 to regulate Hippo pathway signal transduction. Previous studies have indicated that non-specific knockdown of YAP in the liver promotes hepatocyte necrosis and inhibits the expression of downstream pro-hepatic fibrosis genes[20].
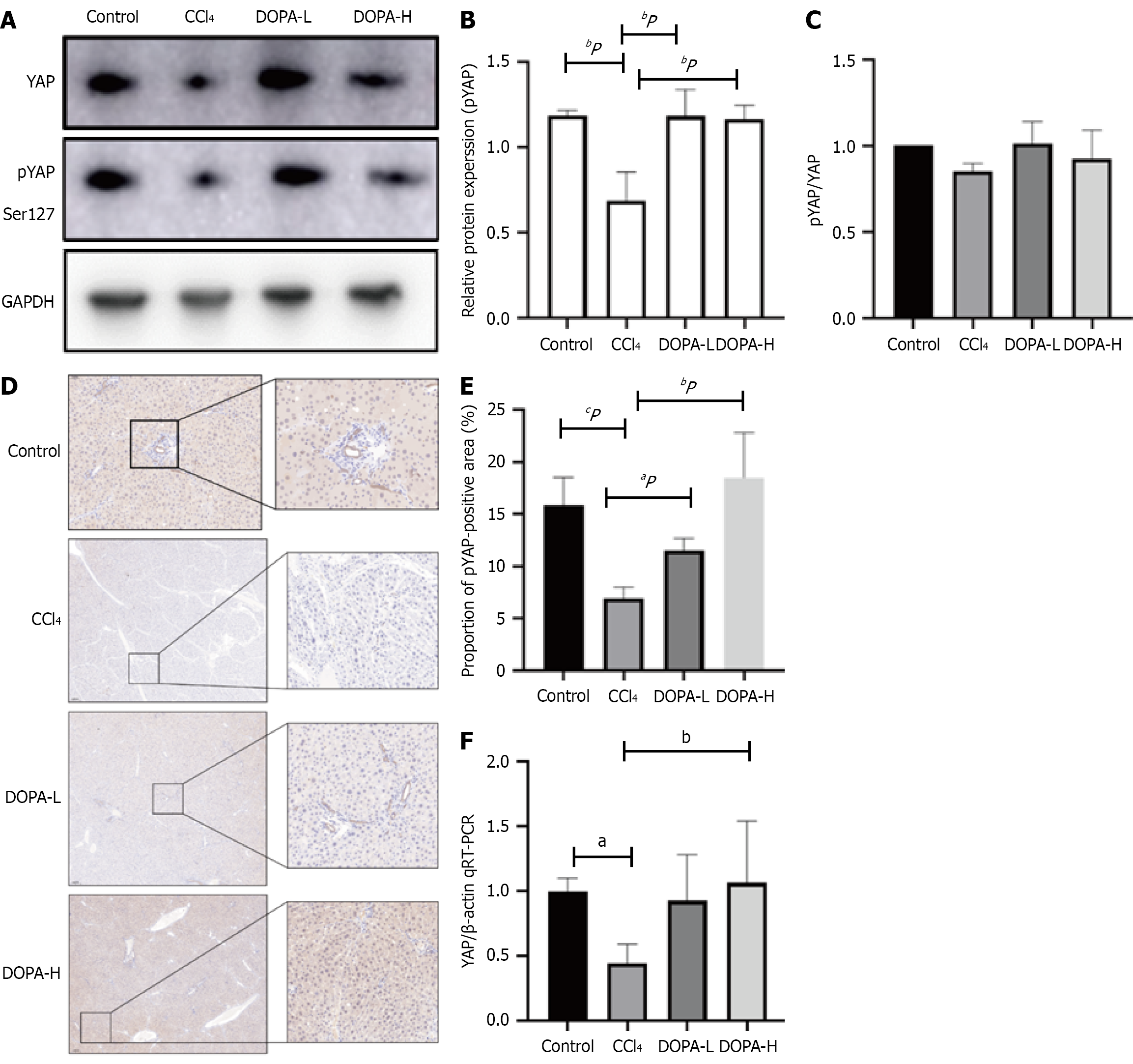
Notably, Western blotting revealed that, compared with the blank control group, YAP expression was significantly decreased following levodopa treatment (P = 0.0143 and P = 0.0013; Figure 4A-C) but showed no significant difference between the DOPA-L and DOPA-H groups (P = 0.0996). IHC results also confirmed that the phosphorylation level of YAP significantly increased in both DOPA-L and DOPA-H groups (P = 0.0432 and P < 0.0001; Figure 4D-F).
Finally, RNA levels were assessed. Reverse transcription-qPCR showed that the YAP levels in the model group were significantly lower in the CCl4 group than in the blank control group and the levodopa treatment groups (P = 0.0405 and P = 0.009). These results suggest that levodopa inhibits YAP/TAZ in the Hippo pathway, thereby reducing liver fibrosis.
Recent studies have reported that dopamine receptor expression is associated with liver fibrosis[21-23]. Dopamine regulates physiological processes such as metabolism and hormone secretion through two subfamilies of GPCRs: D1-like receptors (DRD1 and DRD5) and D2-like receptors (DRD2, DRD3, and DRD4). DRD1 has been confirmed to be associated with YAP/TAZ in the Hippo pathway and is coupled to stimulatory GCPRs.
It is worth noting that studies have found that DRD1 is preferentially expressed in fibroblasts relative to endothelial and epithelial cells in lung and liver tissues, and DRD1 agonists can alter lung fibroblasts from a profibrotic to a fibrotic regression phenotype[15]. However, the mechanism by which DRD1 regulates liver fibrosis remains unclear.
Our present study for the first time used levodopa, the precursor of dopamine, which is also a drug for the treatment of Parkinson’s disease, to attenuate CCl4-induced liver fibrosis. Our study demonstrated that levodopa reduced collagen deposition in rat liver tissue and significantly decreased α-SMA expression; however, no significant difference in collagen deposition was observed between DOPA-L and DOPA-H groups, which may be explained by the fact that the effect of levodopa tends to be saturated after reaching a certain dose. In other words, once a certain dose is reached, increasing the dose may not significantly enhance efficacy, but may lead to side effects. Therefore, the therapeutic effect of levodopa may be non-dose-dependent. We will further investigate these aspects in future studies to better understand the underlying mechanisms and refine the experimental design. Additionally, levodopa is an amino acid that is converted into dopamine by the enzyme dopa decarboxylase (DDC) in the body. Dopamine binds to DRD1 on the cell surface with the help of transporters, initiating downstream signaling pathway. This can alter the factors related to the dopamine pathway in vivo, such as DRD1[24,25]. In the present study, levodopa upregulated DRD1 in liver tissue through DDC without increasing the level of DRD1.
The Hippo signaling pathway regulates several aspects of liver function. Specific knockout of YAP/TAZ in hepatocytes has been shown to reduce inflammation and myofibroblast proliferation in mice with liver fibrosis[26]. To verify whether levodopa can attenuate liver fibrosis by inhibiting YAP in the Hippo signaling pathway, we extracted proteins from the liver tissues of rats treated with levodopa, and the phosphorylation level of YAP was measured. Western blotting showed that, compared with the blank control group, the level of YAP phosphorylation in the liver of the levodopa treatment groups increased, and immunofluorescence assays also revealed a significant increase in YAP phosphorylation. These findings indicated that pYAP was reduced in rats with liver fibrosis, which was consistent with the previous studies[27]. Moreover, the levels of YAP and pYAP were correlated, suggesting that levodopa may influence pYAP by affecting YAP level to play a role in treating liver fibrosis.
We also found that the indicators of liver fibrosis showed no significant changes in SD rats treated with different doses of levodopa, which may be attributed to the minimal differences in levodopa dosages. Nevertheless, we observed that levodopa holds potential as a therapeutic agent for liver fibrosis. Based on these findings, low-dose levodopa may be employed in the treatment regimen, potentially combined with dopamine receptor agonists to optimize efficacy while minimizing required dose. For individuals with liver fibrosis, levodopa dosage may be tailored, along with close liver function monitoring.
The present study had certain limitations. First, while the results indicated that levadopa alleviated CCl4-induced liver fibrosis in rats, its role in other animal models (e.g., bile duct ligation-induced liver fibrosis models) warrant further investigations. Second, neither cell nor gene experiments were performed in this animal study. Third, although it has been reported that the half-life of levodopa in the brain and blood is short (1.5 to 2 hours)[28], the pharmacokinetics of levodopa in the liver remains unclear.
Levodopa has shown potential as a long-term treatment for liver fibrosis, primarily through its antioxidant properties, modulation of HSC activation, and promotion of hepatocyte repair. While most research has focused on animal models, further studies are needed to confirm its efficacy in humans. Monotherapy in liver fibrosis often yields suboptimal outcomes, suggesting that levodopa may be more effective when combined with other therapeutic agents. Combinations with antioxidants, anti-inflammatory agents, immunomodulators, and other drugs may produce synergistic effects, enhancing its anti-fibrotic properties. In future clinical studies, levodopa could emerge as a promising therapeutic option for liver fibrosis, especially when used in combination with other treatments to improve patient outcomes.
In summary, levodopa may exert a potentially protective role against liver fibrosis (Figure 5). This is the first study to demonstrate that levodopa can ameliorate CCl4-induced liver fibrosis by inhibiting the Hippo/YAP signaling pathways, thereby serving as a new drug for the prevention and treatment of liver fibrosis. However, the action of mechanism and clinical safety of levodopa remain to be explored.
| 1. | Zhang YL, Li ZJ, Gou HZ, Song XJ, Zhang L. The gut microbiota-bile acid axis: A potential therapeutic target for liver fibrosis. Front Cell Infect Microbiol. 2022;12:945368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 2. | Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 1389] [Article Influence: 277.8] [Reference Citation Analysis (0)] |
| 3. | Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 988] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 4. | Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75 Suppl 1:S49-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 267] [Article Influence: 53.4] [Reference Citation Analysis (1)] |
| 5. | Cai J, Hu M, Chen Z, Ling Z. The roles and mechanisms of hypoxia in liver fibrosis. J Transl Med. 2021;19:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Bangru S, Arif W, Seimetz J, Bhate A, Chen J, Rashan EH, Carstens RP, Anakk S, Kalsotra A. Alternative splicing rewires Hippo signaling pathway in hepatocytes to promote liver regeneration. Nat Struct Mol Biol. 2018;25:928-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell. 2024;187:1563-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Alsamman S, Christenson SA, Yu A, Ayad NME, Mooring MS, Segal JM, Hu JK, Schaub JR, Ho SS, Rao V, Marlow MM, Turner SM, Sedki M, Pantano L, Ghoshal S, Ferreira DDS, Ma HY, Duwaerts CC, Espanol-Suner R, Wei L, Newcomb B, Mileva I, Canals D, Hannun YA, Chung RT, Mattis AN, Fuchs BC, Tager AM, Yimlamai D, Weaver VM, Mullen AC, Sheppard D, Chen JY. Targeting acid ceramidase inhibits YAP/TAZ signaling to reduce fibrosis in mice. Sci Transl Med. 2020;12:eaay8798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Zhao S, Xu K, Jiang R, Li DY, Guo XX, Zhou P, Tang JF, Li LS, Zeng D, Hu L, Ran JH, Li J, Chen DL. Evodiamine inhibits proliferation and promotes apoptosis of hepatocellular carcinoma cells via the Hippo-Yes-Associated Protein signaling pathway. Life Sci. 2020;251:117424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Bou Saleh M, Louvet A, Ntandja-Wandji LC, Boleslawski E, Gnemmi V, Lassailly G, Truant S, Maggiotto F, Ningarhari M, Artru F, Anglo E, Sancho-Bru P, Corlu A, Argemi J, Dubois-Chevalier J, Dharancy S, Eeckhoute J, Bataller R, Mathurin P, Dubuquoy L. Loss of hepatocyte identity following aberrant YAP activation: A key mechanism in alcoholic hepatitis. J Hepatol. 2021;75:912-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Pepe-Mooney BJ, Dill MT, Alemany A, Ordovas-Montanes J, Matsushita Y, Rao A, Sen A, Miyazaki M, Anakk S, Dawson PA, Ono N, Shalek AK, van Oudenaarden A, Camargo FD. Single-Cell Analysis of the Liver Epithelium Reveals Dynamic Heterogeneity and an Essential Role for YAP in Homeostasis and Regeneration. Cell Stem Cell. 2019;25:23-38.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 12. | Saranza G, Lang AE. Levodopa challenge test: indications, protocol, and guide. J Neurol. 2021;268:3135-3143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 13. | Cilia R, Cereda E, Akpalu A, Sarfo FS, Cham M, Laryea R, Obese V, Oppon K, Del Sorbo F, Bonvegna S, Zecchinelli AL, Pezzoli G. Natural history of motor symptoms in Parkinson's disease and the long-duration response to levodopa. Brain. 2020;143:2490-2501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 14. | Azkona G, Sagarduy A, Aristieta A, Vazquez N, Zubillaga V, Ruíz-Ortega JA, Pérez-Navarro E, Ugedo L, Sánchez-Pernaute R. Buspirone anti-dyskinetic effect is correlated with temporal normalization of dysregulated striatal DRD1 signalling in L-DOPA-treated rats. Neuropharmacology. 2014;79:726-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yan Y, Pan J, Chen Y, Xing W, Li Q, Wang D, Zhou X, Xie J, Miao C, Yuan Y, Zeng W, Chen D. Increased dopamine and its receptor dopamine receptor D1 promote tumor growth in human hepatocellular carcinoma. Cancer Commun (Lond). 2020;40:694-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Sang L, Wang XM, Xu DY, Sang LX, Han Y, Jiang LY. Morin enhances hepatic Nrf2 expression in a liver fibrosis rat model. World J Gastroenterol. 2017;23:8334-8344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 17. | Yu J, Wang Y, Qian H, Zhao Y, Liu B, Fu C. Polyprenols from Taxus chinensis var. mairei prevent the development of CCl₄-induced liver fibrosis in rats. J Ethnopharmacol. 2012;142:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Tsironis C, Marselos M, Evangelou A, Konitsiotis S. The course of dyskinesia induction by different treatment schedules of levodopa in Parkinsonian rats: is continuous dopaminergic stimulation necessary? Mov Disord. 2008;23:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Cui G, Yang X, Wang X, Zhang Z, Yue X, Shi H, Shen X. Ranitidine reduced levodopa-induced dyskinesia in a rat model of Parkinson's disease. Neuropsychiatr Dis Treat. 2014;10:39-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Gu Y, Ding C, Yu T, Liu B, Tang W, Wang Z, Tang X, Liang G, Peng J, Zhang X, Li Z. SIRT7 promotes Hippo/YAP activation and cancer cell proliferation in hepatocellular carcinoma via suppressing MST1. Cancer Sci. 2024;115:1209-1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Qing J, Ren Y, Zhang Y, Yan M, Zhang H, Wu D, Ma Y, Chen Y, Huang X, Wu Q, Mazhar M, Wang L, Liu J, Ding BS, Cao Z. Dopamine receptor D2 antagonism normalizes profibrotic macrophage-endothelial crosstalk in non-alcoholic steatohepatitis. J Hepatol. 2022;76:394-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | Zhao B, Li S, Guo Z, Chen Z, Zhang X, Xu C, Chen J, Wei C. Dopamine receptor D2 inhibition alleviates diabetic hepatic stellate cells fibrosis by regulating the TGF-β1/Smads and NFκB pathways. Clin Exp Pharmacol Physiol. 2021;48:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Yue S, Wang T, Yang Y, Fan Y, Zhou L, Li M, Fu F. Lipopolysaccharide/D-galactosamine-induced acute liver injury could be attenuated by dopamine receptor agonist rotigotine via regulating NF-κB signaling pathway. Int Immunopharmacol. 2021;96:107798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Xiao P, Yan W, Gou L, Zhong YN, Kong L, Wu C, Wen X, Yuan Y, Cao S, Qu C, Yang X, Yang CC, Xia A, Hu Z, Zhang Q, He YH, Zhang DL, Zhang C, Hou GH, Liu H, Zhu L, Fu P, Yang S, Rosenbaum DM, Sun JP, Du Y, Zhang L, Yu X, Shao Z. Ligand recognition and allosteric regulation of DRD1-Gs signaling complexes. Cell. 2021;184:943-956.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 25. | Xue Z, Zhang Y, Zhao R, Liu X, Grützmann K, Klink B, Zhang X, Wang S, Zhao W, Sun Y, Han M, Wang X, Hu Y, Liu X, Yang N, Qiu C, Li W, Huang B, Li X, Bjerkvig R, Wang J, Zhou W. The dopamine receptor D1 inhibitor, SKF83566, suppresses GBM stemness and invasion through the DRD1-c-Myc-UHRF1 interactions. J Exp Clin Cancer Res. 2024;43:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Mooring M, Fowl BH, Lum SZC, Liu Y, Yao K, Softic S, Kirchner R, Bernstein A, Singhi AD, Jay DG, Kahn CR, Camargo FD, Yimlamai D. Hepatocyte Stress Increases Expression of Yes-Associated Protein and Transcriptional Coactivator With PDZ-Binding Motif in Hepatocytes to Promote Parenchymal Inflammation and Fibrosis. Hepatology. 2020;71:1813-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 27. | Shan S, Liu Z, Liu Z, Zhang C, Song F. MitoQ alleviates carbon tetrachloride-induced liver fibrosis in mice through regulating JNK/YAP pathway. Toxicol Res (Camb). 2022;11:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Contin M, Martinelli P. Pharmacokinetics of levodopa. J Neurol. 2010;257:S253-S261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/