Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8848
Peer-review started: February 12, 2015
First decision: March 26, 2015
Revised: April 12, 2015
Accepted: June 9, 2015
Article in press: June 10, 2015
Published online: August 7, 2015
Processing time: 178 Days and 4.1 Hours
AIM: To optimize the perfusates used for hypothermic machine perfusion (HMP).
METHODS: Sprague-Dawley rats were assigned randomly to three groups (n = 12 per group) that received either saline, University of Wisconsin cold-storage solution (UW) or histidine-tryptophan-ketoglutarate solution (HTK) as the perfusate. Each group was divided into two subgroups: static cold storage (SCS) and HMP (n = 6 per subgroup). The liver graft was retrieved according to the method described by Kamada. For the SCS group, the graft was directly placed into cold perfusate (0-4 °C) for 6 h after liver isolation while the portal vein of the graft was connected to the perfusion machine for the HMP group. Then the perfusates were collected at different time points for analysis of aspartate aminotransferase (AST), alanine transaminase (ALT) and lactate dehydrogenase (LDH) levels. Liver tissues were obtained for evaluation of histology, dry/wet weight (D/W) ratio, and malondialdehyde (MDA) and adenosine-triphosphate (ATP) levels. The portal vein pressure and velocity were monitored in real time in all HMP subgroups.
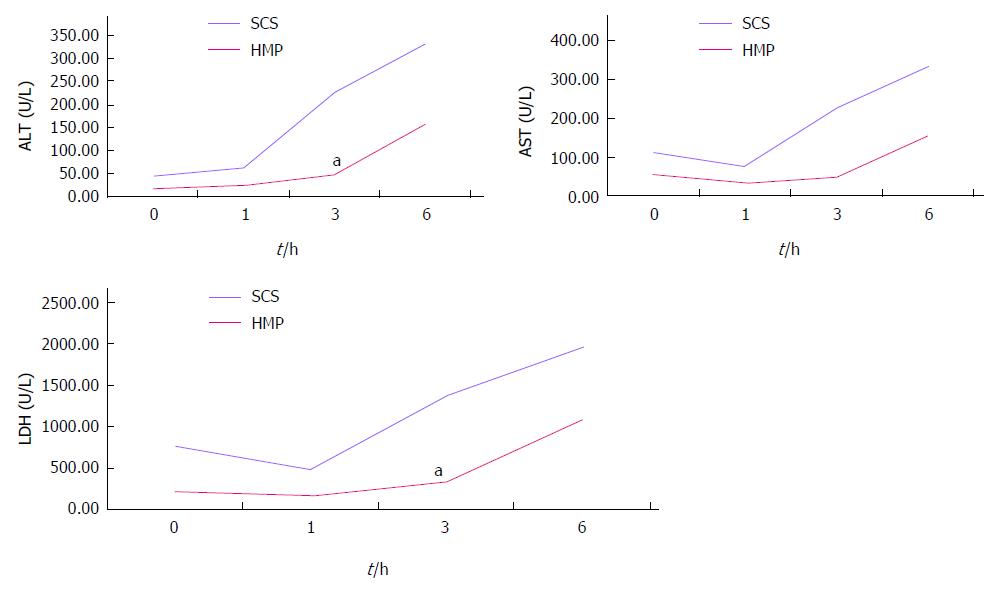
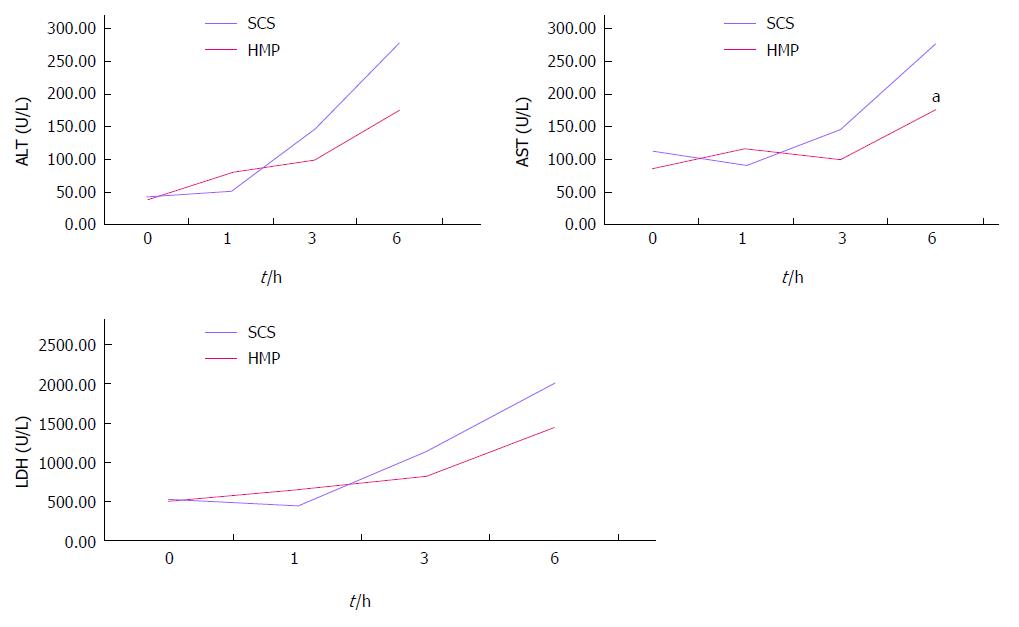
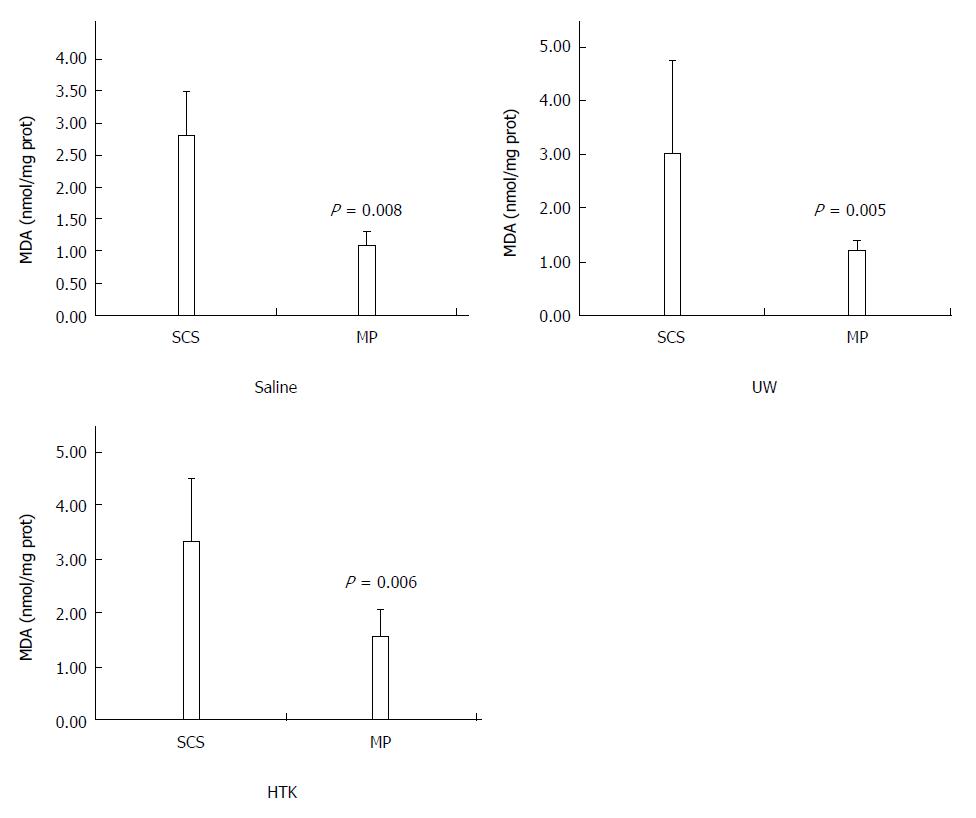
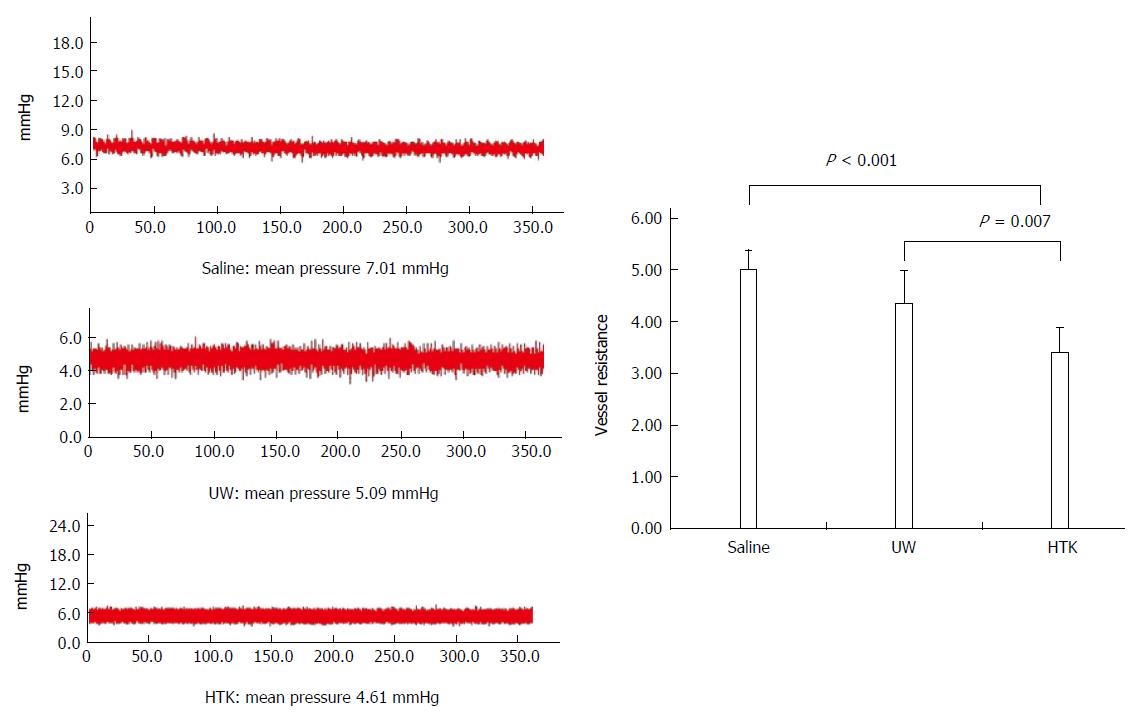
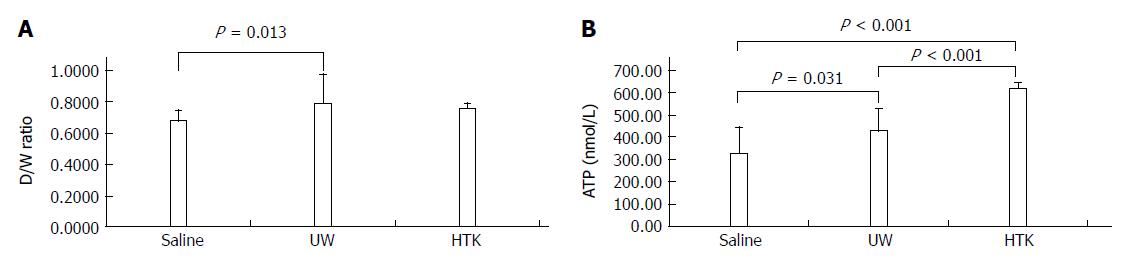
RESULTS: Comparison of HMP and SCS: Regardless of the perfusate, HMP improved the architecture of donor graft in reducing the congestion around sinusoids and central vein and maintaining sinusoid lining in morphology; HMP improved liver function in terms of ALT, AST and LDH, especially during the 3-6 h period (SCS vs HMP using saline: ALT3, 225.00 ± 105.62 vs 49.50 ± 18.50, P = 0.047; LDH3, 1362.17 ± 563.30 vs 325.75 ± 147.43, P = 0.041; UW: LDH6, 2880.14 ± 948.46 vs 2135.00 ± 174.27, P = 0.049; HTK, AST6, 307.50 ± 52.95 vs 185.20 ± 20.46, P = 0.041); HMP decreased MDA level (saline, 2.79 ± 0.30 vs 1.09 ± 0.09, P = 0.008; UW, 3.01 ± 0.77 vs 1.23 ± 0.68, P = 0.005; HTK, 3.30 ± 0.52 vs 1.56 ± 0.22, P = 0.006). Comparison among HMP subgroups: HTK showed less portal vein resistance than UW and saline (vs saline, 3.41 ± 0.49 vs 5.00 ± 0.38, P < 0.001; vs UW, 3.41 ± 0.49 vs 4.52 ± 0.63, P = 0.007); UW reduced edema most efficiently (vs saline, 0.68 ± 0.02 vs 0.79 ± 0.05, P = 0.013), while HTK maintained ATP levels best (vs saline, 622.60 ± 29.11 vs 327.43 ± 44.66, P < 0.001; vs UW, 622.60 ± 29.11 vs 301.80 ± 37.68, P < 0.001).
CONCLUSION: HMP is superior to SCS in maintaining both architecture and function of liver grafts. Further, HTK was found to be the optimal perfusate for HMP.
Core tip: Although static cold storage (SCS) is the gold standard for liver transplantation, hypothermic machine perfusion (HMP) is currently challenging the limitations of SCS. However, there is no consensus on the basic setting for HMP, including the ideal perfusate. Here we compared the most common preservation solutions [University of Wisconsin cold-storage solution and histidine-tryptophan-ketoglutarate solution (HTK), saline as control] and found that HMP is superior to SCS regardless of different solutions and HTK seems to be the optimal perfusate for HMP.
- Citation: Jia JJ, Zhang J, Li JH, Chen XD, Jiang L, Zhou YF, He N, Xie HY, Zhou L, Zheng SS. Influence of perfusate on liver viability during hypothermic machine perfusion. World J Gastroenterol 2015; 21(29): 8848-8857
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8848.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8848
Static cold storage (SCS) is currently the gold standard method for preservation of the donor graft for liver transplantation (LT), with University of Wisconsin cold-storage solution (UW) and histidine-tryptophan-ketoglutarate solution (HTK) being the most commonly used preservation solutions[1]. The principal aim of SCS is to minimize preservation-related injury by rapidly cooling the graft core temperature to reduce metabolism[2]. Hypothermic machine perfusion (HMP) was first proposed by Belzer in the early 1960s, but the complex nature of this technique, the cumbersome equipment required, and lack of clarity regarding the mechanisms have limited its wide usage in clinical settings. However, in the era of the donor organ shortage, HMP is of increasing interest in transplantation centers because it allows better monitoring of the graft, improves washout of injurious waste products, and allows pharmaceutical intervention, leading to decreased ischemia reperfusion (I/R) injury which could be indirectly measured by malondialdehyde (MDA), and extended preservation, even in cases of resuscitated marginal organs or those obtained from cardiac death donors (DCD)[3,4]. Mounting evidence shows that HMP challenges the limitations of SCS in terms of reduced preservation-related injury and improved LT outcome[5-7]. However, there is no consensus on the basic conditions of HMP, including the optimal perfusate, pressure and flow velocity, double or single vessel perfusion (retrograde or antegrade, pulsatile or not), and with or without oxygen. In this study, we aimed to identify the optimal perfusate by comparison of the influence of different perfusates on liver viability during HMP.
Adult male Sprague-Dawley rats (weight, 250-300 g) were used as the experimental animals. The rats were housed in a temperature-controlled environment (25-30 °C) and provided with a standard diet with water ad libitum. Rats were assigned randomly to three groups (n = 12 per group) that received either saline, UW or HTK solutions as the perfusate. Each group was then divided into two subgroups: SCS and HMP (n = 6 per subgroup). All procedures used in this study were approved by the Ethics Committee for the Use of Experimental Animals of Zhejiang University (China) and were carried out in accordance with the ARRIVE (Animal Research: Reporting in vivo Experiments) guidelines (http://www.nc3rs.org/ARRIVE).
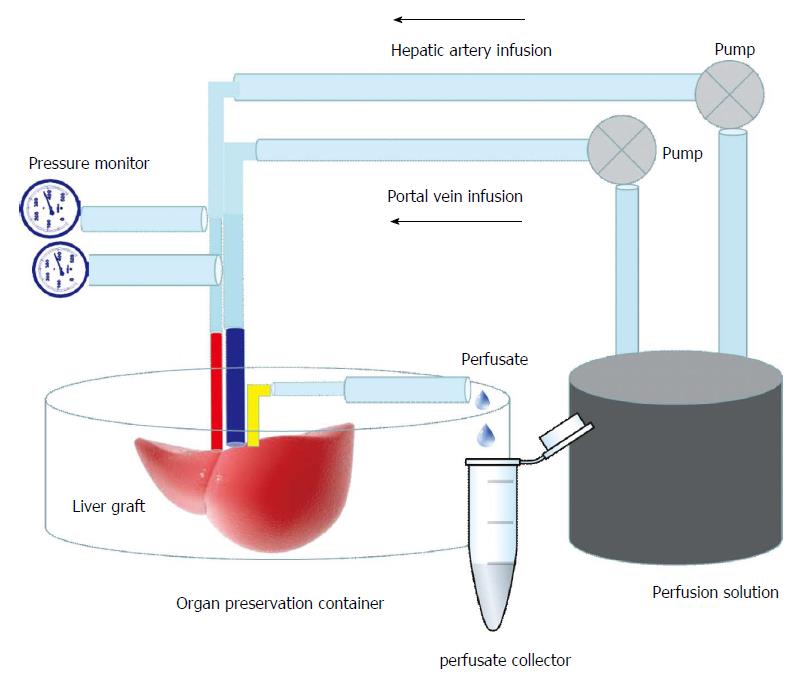
The donor animal was anesthetized by intraperitoneal injection of 4% chloral hydrate (Shanghai No. 1 Biochemical and Pharmaceutical Company, China) and the liver graft was retrieved according to the method described by Kamada[8]. After the donor liver was isolated, the graft was perfused through the portal vein with cooled saline containing 25 U/mL heparin. In the SCS group, the graft was then placed into cold perfusate (0-4 °C) for 6 h. In the HMP group, the portal vein of the graft was connected to the perfusion machine (Figure 1), which consisted of a BT200-2J low flow peristaltic pump (Xi’an Yima Opto-electrical Company, China), a four-channel physiological recorder (BL-420S, Biomart, China), a low-temperature thermostat (DC-1015, Shanghai Bilon Instrument Company, China) and a computer for 6 h. The perfusion machine settings were as follows: temperature, 4 °C; portal vein velocity, 2 rpm (1.4 mL/min non-pulsatile delivery); perfusate volume, 60 mL. The pressure of the portal vein was monitored in real time and recorded using the computer.
At 0, 1, 3, and 6 h during the perfusion process, 2 mL perfusate samples were collected from each group for the analysis of liver function. At 6 h, liver tissues were obtained and fixed in 10% neutral formalin for later histological evaluation. The left lateral lobe was collected for determination of the dry/wet weight (D/W) ratio and other liver tissues were stored at -80 °C for further analysis.
Excised liver specimens were fixed in 10% neutral buffered formalin for 48 h before being paraffin-embedded and sectioned (thickness, 4 μm) according to standard procedures. The sections were deparaffinized and hydrated gradually, and examined by hematoxylin and eosin staining. Morphological assessment was performed by an experienced liver pathologist in a blinded fashion. The levels of aspartate aminotransferase (AST), alanine transaminase (ALT) and lactate dehydrogenase (LDH) were analyzed with a Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan).
Malondialdehyde (MDA) is considered to represent an indirect measurement of oxidative damage induced by reactive oxygen species (ROS)[9]. Here MDA was measured using MDA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) to evaluate the lipid peroxidation in liver tissue according to the manufacturer’s protocols. All assays were performed with a Beckman Coulter DU-800 Spectrophotometer (USA). After 6 h of preservation, the left lateral lobe of the liver was weighed [wet weight (W), g] immediately after removal. The lobe was then dried overnight at 60 °C and reweighed [dry weight (D), g].
Liver tissue adenosine triphosphate (ATP) levels represent a good indicator of mitochondrial respiratory function and were measured using ATP kit (Beyotime, JiangSu, China) according to manufacturer’s instructions. Portal VR (mmHg・min/mL) was calculated as portal vein pressure (mmHg)/velocity (mL/min).
All experimental results are shown as mean ± SD. Statistical differences were calculated using one-way analysis of variance (ANOVA) followed by the Least Squares Difference (LSD) or Dunnett’s test for multiple comparisons. P < 0.05 was considered to indicate statistical significance.
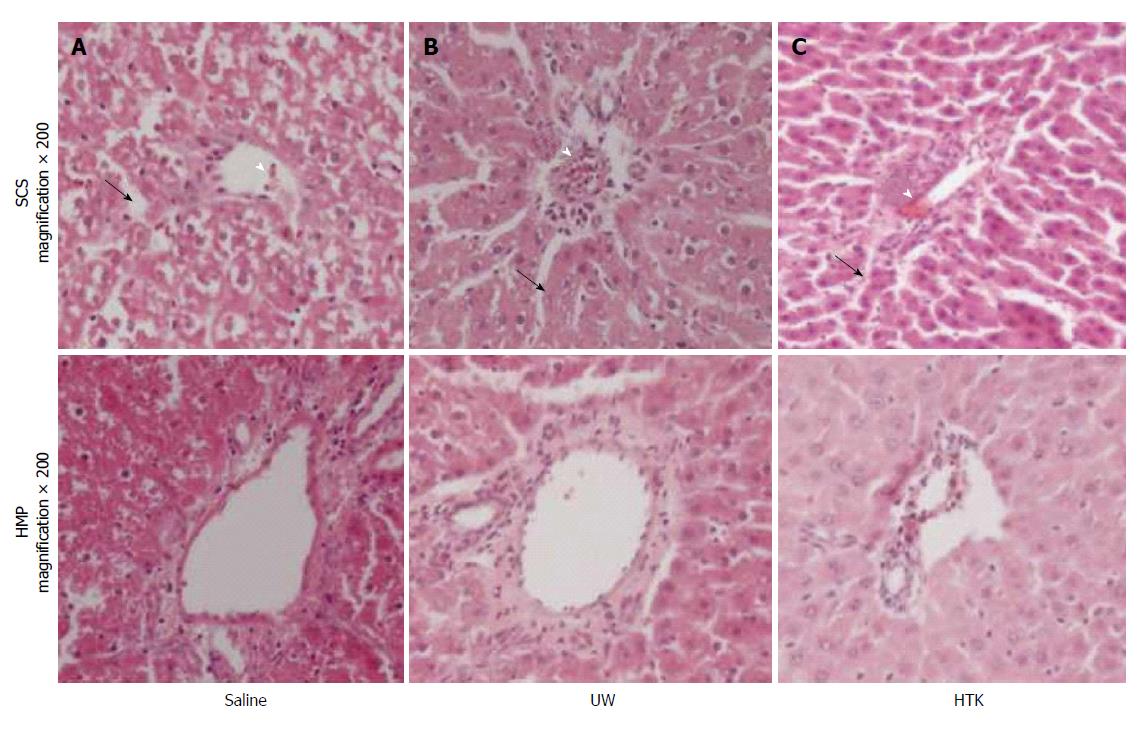
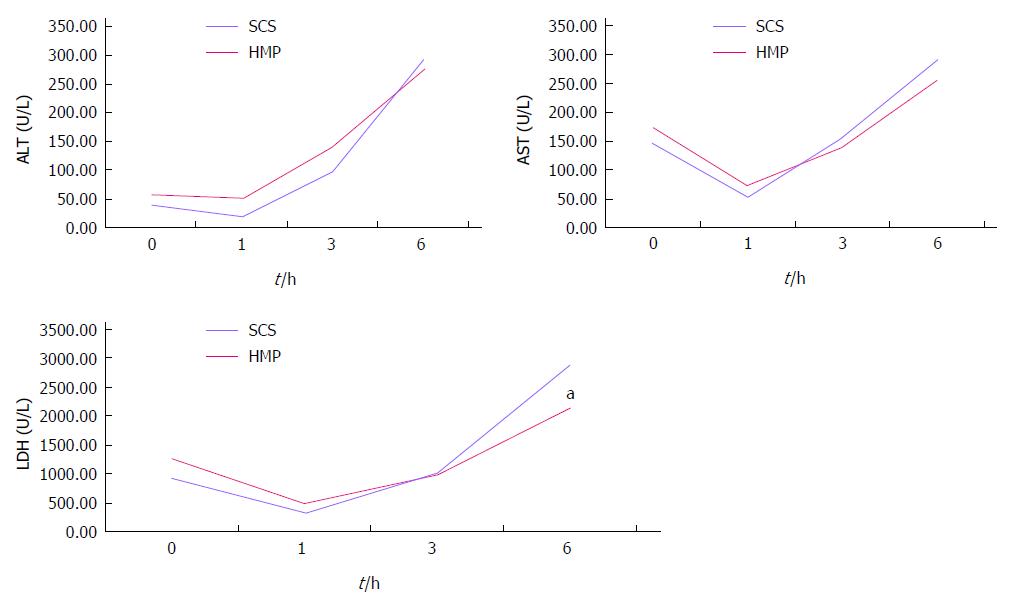
HMP improves the architecture and function of donor grafts compared to SCS regardless of the perfusate: Using saline as the perfusate, compared to SCS, HMP reduced the congestion around sinusoids and central vein, maintained sinusoid lining in morphology (Figure 2A) and decreased ALT, AST and LDH release, especially during the 3-6 h period (Figure 3). Similar results were found using UW (Figure 2B, Figure 4) and HTK (Figure 2C, Figure 5) as the perfusate.
HMP decreases MDA levels regardless of the perfusate: The MDA levels determined as an indirect measurement of oxidative damage induced by ROS in the HMP and SCS livers are shown in Figure 6. HMP induced significantly lower MDA level compared to SCS regardless of the perfusate (saline, 2.79 ± 0.30 vs 1.09 ± 0.09, P = 0.008; UW, 3.01 ± 0.77 vs 1.23 ± 0.68, P = 0.005; HTK, 3.30 ± 0.52 vs 1.56 ± 0.22, P = 0.006).
HTK shows less portal VR than UW and saline: Portal VR among the HMP subgroups is shown in Figure 7. The VR level gradually decreased among the saline, UW and HTK groups, with HTK showing the lowest VR (vs saline, 3.41 ± 0.49 vs 5.00 ± 0.38, P < 0.001; vs UW, 3.41 ± 0.49 vs 4.52 ± 0.63, P = 0.007)
UW reduces edema most efficiently, while HTK maintains ATP levels best: The D/W ratios and ATP levels among the HMP subgroups are shown in Figure 8. The D/W ratio gradually decreased between the subgroups perfused with UW, HTK and saline, indicating that UW was associated with the least edema, followed by HTK and then saline, which was associated with the most edema (Figure 8A, UW vs saline, 0.68 ± 0.02 vs 0.79 ± 0.05, P = 0.013). The ATP levels gradually increased between the subgroups perfused with saline, UW and HTK, demonstrating that HTK and UW maintained ATP level more effectively than saline, with HTK providing the best effect (Figure 8B, saline vs HTK, 327.43 ± 44.66 vs 622.60 ± 29.11, P < 0.001; saline vs UW, 327.43 ± 44.66 vs 301.80 ± 37.68, P = 0.031; UW vs HTK, 301.80 ± 37.68 vs 622.60 ± 29.11, P < 0.001)
Currently, more than 5500 cases of LT are performed each year in Europe and in the United States[10,11] with 5-year patient survival rates exceeding 70% in most centers. The success of LT has subsequently resulted in a substantial worldwide organ shortage. To reduce the gap between the patients’ need and the availability of donors, the use of “extended criteria donor” (ECD) grafts, which were previously considered unsuitable for transplantation, has regained interest. Consequently, there is a critical need for improved methods of organ preservation. Currently, SCS and HMP are the two major methods used for liver preservation. SCS is widely accepted because of its simplicity and for economic reasons; however, recent ex vivo and in vivo transplantation studies suggest that HMP provides improved preservation compared to SCS, especially for ECD grafts[12,13]. Nevertheless, the optimal conditions for HMP remain controversial due to a lack of consensus regarding the conditions in terms of perfusates, oxygenation, perfusion route (dual vs portal), and reperfusion pressure. Optimizing techniques would help to reduce preservation injury and increase the donor pool.
In this study, we established a stable, simplified HMP system and compared the performance of three perfusate solutions using this system. Two of the perfusates (UW and HTK) are commonly used in the clinical LT setting. Our results showed that HMP is superior to SCS in terms of AST, ALT, LDH release and MDA levels, regardless of the perfusate used, which is in accordance with previous reports[14,15]. Among the three perfusates evaluated, UW and HTK are superior to saline in HMP in terms of D/W ratio, VR and ATP levels. However, UW and HTK are associated with their own individual advantages and disadvantages. For instance, UW reduced edema more efficiently, but was associated with higher VR and ATP consumption than HTK, which may be due to its high viscosity and the hydroxyethyl starch (HES) component. Although UW is the most commonly used perfusate, its high viscosity may result in initial poor perfusion and incomplete distribution of UW between the intravascular space and the liver parenchyma in the donor grafts[16]. Furthermore, HES in UW causes hyperaggregating effects on erythrocytes, which hampers a complete washout from the liver and loss of ATP[17]. Compared with UW, HTK solution contains lower sodium and potassium concentrations, no HES and a much lower viscosity (approximately one-third that of UW), thus facilitating its release into the circulation[18]. Experimentally, studies have recently reported HTK is as good as or even better than UW for short periods of perfusion[19,20]. The low viscosity and absence of HES in HTK partially contribute to the relatively low VR and ATP consumption associated with its use as a perfusate[21].
Historically, a number of perfusates have been developed such as UW, HTK, Celsior, Cardiosol, or Custodiol[22]. Belzer et al[23], who proposed the concept of HMP, developed the first perfusate, UW, which remains the gold standard today. However, the intrinsic shortage of intracellular electrolytes and the HES component imposes marked changes in the ionic environment and produces high viscosity[2], resulting in deterioration of cellular components, with loss of ATP energy stores and alteration in the biochemical function and cellular architecture, which is even more deleterious in ECD[17]. Subsequently, other perfusates such as modified UW alone[24] and later with diluted, heparinized and oxygenated blood[25,26], Polysol[27], HTK or Custodiol-N solution[24] sometimes enriched with Kidney Perfusion Solution-1 and Aqix RS-I[28], were introduced to achieve better preservation. However, these perfusates have not resulted in significant improvements in the preservation of donor organs, with the exception of diluted blood. This perfusate has been shown to be safe and effective as an oxygen carrier in an animal model[29] and the first clinical HMP trial by Oxford University has shown an excellent outcome.
In this study, particular attention has been paid to the energy balance of the liver in its preservation. Recovered levels of ATP appear to be a good indicator of mitochondrial respiratory function. Improvement of mitochondrial condition, reflected here by augmentation of tissue ATP content during HMP, may result in improved tolerance to I/R injury[30]. Moreover, many clinical studies have shown that the preimplantation ATP content correlates with liver failure[31] and post-transplantation graft function[32].
There are some limitations of our study that should be noted. First, the time period for liver preservation (SCS or HMP) was only 6 h, while the benefits of UW associated with the ischemic period may extend to longer than 6 h. The selection of 6 h in our experimental design was mainly based on our clinical experience with 1500 LT cases, in which the optimized cold ischemia time was less than 6-8 h.
In conclusion, innovative and improved perfusates are an important approach to the future development of HMP techniques for LT. We established a stable HMP system for use in optimizing HMP conditions and demonstrated that HMP is superior to SCS in terms of both the architecture and function of the liver. Among the perfusates evaluated in our study, HTK was identified as the optimized machine perfusion solution (MPS). However, some optimized MPS as Polysol and diluted blood might have set a new standard for liver organ preservation solutions.
Although static cold-storage (SCS) is the gold standard for liver transplantation, hypothermic machine perfusion (HMP) is currently challenging the limitations of SCS. However, there is no consensus on the basic conditions used for HMP, including the optimal perfusates.
Machine perfusion is an alternative preservation method for liver graft. Recent ex vivo and in vivo transplantation studies suggest that HMP provides improved preservation compared to SCS, especially for “extended criteria donor” grafts.
Plenty of articles compare UW and HTK in static cold storage, but we made first try to compare them in HMP setting and found HTK is superior to UW in terms of ATP maintaining and portal vein resistance.
Although countless studies show that HMP is superior to SCS in kidney transplantation, SCS is still gold standard in liver transplantation. In the future stable HMP system will be taken into practice and the optimized perfusate will reach an agreement.
HMP preserves the organ by a constant perfusion through its blood vessels with a machine perfusion solution.
The aim of this study was clear and relevant. It was relatively hard to evaluate the results because formation of UW and HTK was not explained. Comparison of UW and HTK would provide basis for the future experiments. Different reagents between UW and HTK would be a clue to the difference of storage performance. Investigation of the difference might pave the way to a better storage solution.
| 1. | Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus Med Hemother. 2011;38:125-142. [PubMed] [DOI] [Full Text] |
| 2. | Eghtesad B, Aucejo F, Fung JJ. Preservation solutions in liver transplantation: what are the options? Liver Transpl. 2006;12:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Monbaliu D, Brassil J. Machine perfusion of the liver: past, present and future. Curr Opin Organ Transplant. 2010;15:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Graham JA, Guarrera JV. “Resuscitation” of marginal liver allografts for transplantation with machine perfusion technology. J Hepatol. 2014;61:418-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF, Lee HT, Brown RS. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 6. | Henry SD, Nachber E, Tulipan J, Stone J, Bae C, Reznik L, Kato T, Samstein B, Emond JC, Guarrera JV. Hypothermic machine preservation reduces molecular markers of ischemia/reperfusion injury in human liver transplantation. Am J Transplant. 2012;12:2477-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | op den Dries S, Karimian N, Sutton ME, Westerkamp AC, Nijsten MW, Gouw AS, Wiersema-Buist J, Lisman T, Leuvenink HG, Porte RJ. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64-69. [PubMed] |
| 9. | Nardo B, Caraceni P, Pasini P, Domenicali M, Catena F, Cavallari G, Santoni B, Maiolini E, Grattagliano I, Vendemiale G. Increased generation of reactive oxygen species in isolated rat fatty liver during postischemic reoxygenation. Transplantation. 2001;71:1816-1820. [PubMed] |
| 10. | Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, Yersiz H, Holt C, Harlander-Locke M, Hong JC, Rana AR. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg. 2013;258:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 662] [Article Influence: 47.3] [Reference Citation Analysis (5)] |
| 12. | Schlegel A, Rougemont Od, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 13. | Fondevila C, Hessheimer AJ, Maathuis MH, Muñoz J, Taurá P, Calatayud D, Leuvenink H, Rimola A, García-Valdecasas JC, Ploeg RJ. Hypothermic oxygenated machine perfusion in porcine donation after circulatory determination of death liver transplant. Transplantation. 2012;94:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Shigeta T, Matsuno N, Huai-Che H, Obara H, Mizunuma H, Hirano T, Uemoto S, Enosawa S. A basic consideration for porcine liver preservation using a novel continuous machine perfusion device. Transplant Proc. 2012;44:942-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Monbaliu D, Liu Q, Libbrecht L, De Vos R, Vekemans K, Debbaut C, Detry O, Roskams T, van Pelt J, Pirenne J. Preserving the morphology and evaluating the quality of liver grafts by hypothermic machine perfusion: a proof-of-concept study using discarded human livers. Liver Transpl. 2012;18:1495-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Yamauchi JI, Richter S, Vollmar B, Menger MD, Minor T. Warm preflush with streptokinase improves microvascular procurement and tissue integrity in liver graft retrieval from non-heart-beating donors. Transplantation. 2000;69:1780-1784. [PubMed] |
| 17. | van der Plaats A, ‘t Hart NA, Morariu AM, Verkerke GJ, Leuvenink HG, Ploeg RJ, Rakhorst G. Effect of University of Wisconsin organ-preservation solution on haemorheology. Transpl Int. 2004;17:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Fridell JA, Mangus RS, Tector AJ. Clinical experience with histidine-tryptophan-ketoglutarate solution in abdominal organ preservation: a review of recent literature. Clin Transplant. 2009;23:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Lynch RJ, Kubus J, Chenault RH, Pelletier SJ, Campbell DA, Englesbe MJ. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin preservation in renal transplantation. Am J Transplant. 2008;8:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Cullen J, Lansing K, Bozorgzadeh A. University of Wisconsin versus histidine-tryptophan-ketoglutarate for tissue preservation in live-donor liver transplantation. Exp Clin Transplant. 2006;4:451-457. [PubMed] |
| 21. | Olschewski P, Hunold G, Eipel C, Neumann U, Schöning W, Schmitz V, Vollmar B, Neuhaus P, Puhl G. Improved microcirculation by low-viscosity histidine- tryptophan-ketoglutarate graft flush and subsequent cold storage in University of Wisconsin solution: results of an orthotopic rat liver transplantation model. Transpl Int. 2008;21:1175-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Baicu SC, Taylor MJ. Acid-base buffering in organ preservation solutions as a function of temperature: new parameters for comparing buffer capacity and efficiency. Cryobiology. 2002;45:33-48. [PubMed] |
| 23. | Belzer FO, Hoffman RM, Stratta RJ, D’Alessandro A, Pirsch J, Kalayoglu M, Sollinger HW. Combined cold storage-perfusion preservation of the kidney with a new synthetic perfusate. Transplant Proc. 1989;21:1240-1241. [PubMed] |
| 24. | Stegemann J, Hirner A, Rauen U, Minor T. Use of a new modified HTK solution for machine preservation of marginal liver grafts. J Surg Res. 2010;160:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Brettschneider L, Bell PR, Martin AJ, Tarr JS, Taylor PD, Starzl TE. Conservation of the liver. Transplant Proc. 1969;1:132-137. [PubMed] |
| 26. | Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392-415. [PubMed] |
| 27. | Bessems M, Doorschodt BM, Dinant S, de Graaf W, van Gulik TM. Machine perfusion preservation of the pig liver using a new preservation solution, polysol. Transplant Proc. 2006;38:1238-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Vekemans K, Liu Q, Heedfeld V, Van de Vel K, Wylin T, Pirenne J, Monbaliu D. Hypothermic Liver Machine Perfusion With EKPS-1 Solution vs Aqix RS-I Solution: In Vivo Feasibility Study in a Pig Transplantation Model. Transplant Proc. 2009;41:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Uygun K, Tolboom H, Izamis ML, Uygun B, Sharma N, Yagi H, Soto-Gutierrez A, Hertl M, Berthiaume F, Yarmush ML. Diluted blood reperfusion as a model for transplantation of ischemic rat livers: alanine aminotransferase is a direct indicator of viability. Transplant Proc. 2010;42:2463-2467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Ferrigno A, Rizzo V, Boncompagni E, Bianchi A, Gringeri E, Neri D, Richelmi P, Freitas I, Cillo U, Vairetti M. Machine perfusion at 20°C reduces preservation damage to livers from non-heart beating donors. Cryobiology. 2011;62:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Lanir A, Jenkins RL, Caldwell C, Lee RG, Khettry U, Clouse ME. Hepatic transplantation survival: correlation with adenine nucleotide level in donor liver. Hepatology. 1988;8:471-475. [PubMed] |
| 32. | Bruinsma BG, Berendsen TA, Izamis ML, Yarmush ML, Uygun K. Determination and extension of the limits to static cold storage using subnormothermic machine perfusion. Int J Artif Organs. 2013;36:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Tomizawa M S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM