Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8836
Peer-review started: January 3, 2015
First decision: January 22, 2015
Revised: February 14, 2015
Accepted: March 27, 2015
Article in press: March 27, 2015
Published online: August 7, 2015
Processing time: 217 Days and 17.9 Hours
AIM: To investigate the expression and oncogenic role of nemo-like kinase (NLK) in colorectal cancer.
METHODS: Expression of NLK protein was assessed by immunohistochemistry in tissue specimens from 56 cases of normal colorectal mucosa, 51 cases of colorectal adenoma, and 712 cases of colorectal cancer. In addition, NLK expression was knocked down using a lentivirus carrying NLK small hairpin RNA in colorectal cancer cells. Cell viability methylthiazoletetrazolium assays, colony formation assays, flow cytometry cell cycle assays, Transwell migration assays, and gene expression assays were performed to explore its role on proliferation and migration of colorectal cancer.
RESULTS: Expression of NLK protein progressively increased in tissues from the normal mucosa through adenoma to various stages of colorectal cancer. Overexpression of NLK protein was associated with advanced tumor-lymph node-metastasis stages, poor differentiation, lymph node and distant metastases, and a higher recurrence rate of colorectal cancer (P < 0.05). Multivariate analyses showed that NLK expression was an independent prognostic factor to predict overall survival (hazard ratio 2.57, 95% confidence interval: 1.66-3.98; P < 0.001) and disease-free survival (hazard ratio 1.96, 95% confidence interval: 1.40-2.74: P < 0.001) of colorectal cancer patients. Furthermore, knockdown of NLK expression in colorectal cancer cell lines reduced cell viability, colony formation, and migration, and arrested tumor cells at the G0/G1 phase of the cell cycle. At the gene level, knockdown of NLK expression inhibited matrix metalloproteinase-2 expression in colorectal cancer cells.
CONCLUSION: NLK overexpression is an independent prognostic factor in colorectal cancer and knockdown of NLK expression inhibits colorectal cancer progression and metastasis.
Core tip: Altered expression of nemo-like kinase (NLK) protein is associated with cancer development. This study systematically evaluated NLK expression in different stages of colorectal cancer (CRC) for association with CRC prognosis. NLK expression progressively increased from normal tissues through adenoma, stage I, II, and III, to stage IV CRC. However, knockdown of NLK expression significantly inhibited CRC cell growth, migration, cell cycle progression, and matrix metalloproteinase-2 expression. These data demonstrate that NLK overexpression is an independent CRC prognostic indicator and that knockdown of NLK expression inhibits CRC cell progression and metastasis.
- Citation: Zhang W, He J, Du Y, Gao XH, Liu Y, Liu QZ, Chang WJ, Cao GW, Fu CG. Upregulation of nemo-like kinase is an independent prognostic factor in colorectal cancer. World J Gastroenterol 2015; 21(29): 8836-8847
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8836.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8836
Colorectal cancer (CRC) remains a significant worldwide health problem and was responsible for more than 1.2 million new cancer cases and over 600000 cancer-related deaths in 2008 worldwide[1]. Currently, the tumor-lymph node-metastasis (TNM) staging system is utilized to stage the disease, guide treatment selections, and predict the prognosis for CRC patients[2,3]. However, utilization of this staging system is not always possible, such as in CRC detected by colonoscopy or malignant colorectal polyps resected under endoscopy[4]. In this regard, biologic markers could help us to precisely predict the prognosis, treatment responses, or even treatment selections (such as targeted therapy)[5-7]; in addition, it is easy to assess a small amount of tumor tissues[4]. Such an approach has been used to predict the long-term survival of CRC patients[8]. To date, due to the advancement of surgical techniques and the integration of chemoradiotherapy and targeted therapy, the prognosis of CRC patients has been significantly improved[9,10]. However, in advanced CRC patients and those with local recurrence or distant metastasis, the prognosis is still very poor[11,12]. Therefore, the molecular mechanisms and key regulators of CRC progression and metastasis need to be investigated to provide biomarkers to predict the risk of developing CRC local recurrence and distant metastasis[13], which could in turn enable us to optimally select treatment strategies and eventually improve patient prognosis.
Nemo-like kinase (NLK), an evolutionarily conserved serine/threonine protein kinase[14], regulates many transcription factors and signaling pathways that are important for determining cell fate[15,16]. NLK is an important regulator of several signal transduction pathways including Wnt and Notch signaling pathways, both of which play critical roles in tumorigenesis. NLK is able to regulate the Wnt/β-catenin signaling pathway by phosphorylation of lymphoid enhancer-binding factor 1 in neural progenitor cells[15]. In addition, NLK, as a negative regulator of the Notch signaling pathway, is able to inhibit formation of the transcriptionally active ternary complex of the notch intracellular domain, CSL, and mastermind[16]. Altered NLK expression also is associated with the development and progression of several human cancers[17-20]. Upregulation of NLK protein occurs in hepatocellular carcinoma[17], whereas NLK expression is downregulated in prostate cancer cells[18]. In other human cancers, knockdown of NLK expression was able to reduce tumor cell viability[19,20]. Furthermore, NLK gene variations are associated with ovarian cancer risk[21]. Because NLK is a member of the mitogen-activated protein kinase family, which functions to promote cell proliferation, in this study, we first evaluated the NLK expression level via immunohistochemistry in normal mucosa, adenoma, and CRC tissue specimens from 712 cases. We also included 16 familial adenomatous polyposis (FAP) patients and 21 metastatic CRC patients. Next, we knocked down NLK expression using a lentivirus carrying NLK small hairpin RNA (shRNA) to assess the effects on CRC cells, including cell viability, cell cycle distribution, colony formation, migration, and gene expression.
The study population has been described previously[22]. Specifically, there were five groups of patients enrolled in this study. Group A consisted of 56 patients with normal rectal mucosa. Samples were obtained from patients with severe mixed hemorrhoids who underwent the procedure for prolapse and hemorrhoids. All patients had morphologically normal colorectal mucosa that were free of neoplastic or inflammatory diseases and confirmed by preoperative colonoscopy[22]. Group B included 51 patients with colorectal adenomatous polyps. Group C included 742 patients with sporadic histologically confirmed CRC, including 53 stage I, 312 stage II, 322 stage III, and 55 stage IV patients. Group D consisted of 16 FAP patients with concomitant CRC, each with a set of three matched specimens (normal mucosa, adenoma, and carcinoma). Group E consisted of 21 patients with metastatic CRC and concurrently resected metastatic carcinoma. Each of these metastatic patients also had a set of three matched specimens (normal mucosa and primary and metastatic tumors). Of these 21 metastatic carcinomas, 19 had liver metastases and 2 had greater omental metastases. All patients underwent surgical treatment in the Department of Colorectal Surgery, Changhai Hospital, The Second Military Medical University (Shanghai, China) between December 1999 and December 2009. All diagnoses were confirmed histopathologically by two independent pathologists. This study was approved by the Ethics Committee of Changhai Hospital, and written informed consent was obtained from all patients.
Tissue specimens were processed and embedded in paraffin, and paraffin blocks from each lesion were used to construct the tissue microarrays (TMAs) as described previously[22]. A total of six TMA blocks covering all the tissue specimens were constructed and used for immunostaining of NLK expression.
Immunohistochemistry was performed as described previously[22]. Briefly, the deparaffinized sections were incubated with 0.3% hydrogen peroxide and then with 20% goat serum to block nonspecific binding. Next, the TMA sections were incubated with a monoclonal anti-NLK antibody (ab69933; Abcam, Cambridge, United Kingdom) at a dilution of 1:100 for 2 h in a humidified chamber at room temperature. After that, the sections were further incubated with a secondary antibody and underwent a color reaction.
All immunostained TMA sections were reviewed and scored by two investigators (Gao XH and He J) who were blinded to the clinical information. The concordance rate was high (> 94%) and any disagreements were resolved by consensus. The level of NLK expression was scored using the criteria available on the ATLAS web site[23] and as described previously[22]. In particular, immunostaining of NLK protein was scored using the multiplication of the intensity and percentage of staining with a scale from 0 to 12. Staining of tumor cells with final staining scores of 0, 1-4, 5-8, and 9-12 was assigned as negative (-), slightly positive (+), moderately positive (++), and strongly positive (+++), respectively[22]. The scores were further categorized into lower expression (- to +) and higher expression (++ to +++) groups for analyses.
An embryonic kidney HEK293T cell line and CRC SW480, SW620, RKO, DLD-1, HCT116, and HT-29 cell lines were obtained from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Science (Shanghai, China) and maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified incubator with 5% CO2.
According to a previous protocol[20], we selected the NLK sequence (5′-GATAGACCTATTGGATATG-3′) according to GenBank data (NM_016231) to knock down NLK expression, and the negative control sequence used was 5′-TTCTCCGAACGTGTCACGT-3′. The double-strand oligonucleotides were synthesized and then cloned into the pFH-L vector (Shanghai Preii, Shanghai, China). Next, the lentivirus was generated, packaged, and used to infect cells according to the manufacturer’s instructions. In brief, 293T cells were cotransfected with shRNA-expressing plasmids and the two helper plasmids pCMV∆R8.92 and pVSVG-I (Shanghai Preii) using Lipofectamine 2000 (Invitrogen of Thermo Fisher Scientific, Waltham, MA, United States). After 48 h, culture medium containing the packaged lentivirus was harvested and concentrated. To infect cells, HT-29 cells (5 × 104/well) were infected with lentivirus carrying NLK (Lv-shNLK) or negative control (Lv-shCon) shRNA with a multiplicity of infection of 50 using 2 μL of Polybrene (at a stock of 4 μg/μL) in 1 mL of virus/media at a final concentration of 8 μg/mL; 24 h later, the culture medium was replaced with a regular medium. The NLK knockdown efficiency was validated by quantitative real-time reverse transcription (qRT)-PCR and Western blotting 5 d after lentivirus infection. After confirming the knockdown efficiency, cells were seeded into 96-well plates for the methylthiazoletetrazolium (MTT) cell proliferation assay and 6-well plates for the colony formation assay and cell cycle analysis.
Total cellular RNA was isolated using Trizol reagent (Invitrogen) and reversely transcribed into cDNA using M-MLV-RTase (Promega, Madison, WI, United States) according to the manufacturers’ instructions. cDNA samples were then used for qPCR amplification of NLK using the SYBR-Green Master PCR Mix (Applied Biosystems of Thermo Fisher Scientific) in triplicate. qPCR amplification and data collection were performed on the TP800 qPCR System (Takara Bio Inc., Otsu, Shiga, Japan). All data were normalized to an endogenous control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The relative value of NLK mRNA expression compared to the control was expressed as 2-(Ct-Cc) (Ct and Cc were the mean threshold cycle differences after normalizing to GAPDH). The primers used for qPCR were as follows: GAPDH, 5′-TGACTTCAACAGCGACACCCA-3′ and 5′-GGAGTGTTGGAGAAGTCATATTAC-3′; and NLK, 5′-ATCATCAGCACTCGCATCATC-3′ and 5′-GACCAGACAACACCAAAGGC-3′.
Total cellular protein was extracted using a lysis buffer containing 100 mmol/L Tris, 4% sodium dodecyl sulfate (SDS), 10% glycerol, 200 mmol/L NaCl, and 2 mmol/L EDTA, and then quantified. For Western blot, cell lysates were separated in 12% SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA, United States). The membranes were then blocked in a 5% skim milk solution, followed by incubation in milk containing mouse anti-NLK monoclonal antibody (Abcam). Western blotting was developed using a horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Dallas, TX, United States) and was detected by an enhanced chemiluminescence reagent (Santa Cruz Biotechnology). GAPDH was used as an internal control for Western blotting analysis.
Cells infected with Lv-shNLK or Lv-shCon were seeded in 96-well plates at a density of 2000 cells per well. At the indicated time points, 20 μL of MTT solution (5 mg/mL) was added to each well. The plates were further incubated for 4 h at 37 °C, and 150 μL of dimethyl sulfoxide was added into each well to dissolve the crystals. After incubation for 10 min at room temperature, the absorbance was recorded at 490 nm.
CRC HT-29 cells were infected with Lv-shNLK or Lv-shCon. Five days later, the cells were collected and reseeded at 300 cells per well in 6-well plates in triplicate. The cells were incubated at 37 °C in 5% CO2, and the growth medium was renewed every 3 d. After 14 d of culture, the plates were stained with Giemsa, and the numbers of colonies were counted and recorded.
HT-29 cells infected with Lv-shNLK or Lv-shCon were inoculated in a 6-cm dish and cultured for 40 h. At the end of the experiments, 1 × 106 cells from each well were harvested and fixed in 70% ethanol for 1 h. After washing three times with ice-cold PBS, the cells were treated with 50 μL/mL propidium iodide solution (Sigma-Aldrich, St. Louis, MO, United States) and 100 μL/mL RNase in PBS for 15 min at room temperature in the dark and analyzed by flow cytometry (BD FACS Calibur; BD Biosciences, San Jose, CA, United States) according to the manufacturers’ instructions.
The Transwell migration assay was conducted as described previously[24]. Briefly, 2 × 104 parental and Lv-shCon- or Lv-shNLK-infected HT-29 cells were suspended in 200 μL of DMEM without FBS and placed into the upper chamber of the Transwells. The lower chamber was filled with 500 μL of DMEM containing 10% FBS. The cells were allowed to grow and migrate through these polycarbonate membranes with 8.0-μm-sized pores (Corning Inc., Corning, NY, United States) at 37 °C with 5% CO2 for 24 h. Cells remaining on the upper surface of the filter were removed, and those that had migrated to the lower compartment were fixed with methanol, stained with crystal violet, and counted visually in five random fields under a light microscope. In addition, the migrated cells were dissociated, lysed, and quantified using a spectrophotometer at 570 nm. All of the experiments were performed in triplicate and repeated three times.
Associations between NLK expression and clinicopathologic variables were assessed by nonparametric (Mann-Whitney U or Kruskal-Wallis) tests. NLK expression in the paired tissue specimens, such as FAP and metastatic CRC, was compared using a paired nonparametric test (Wilcoxon test). Disease-free survival (DFS) was defined as the period from the date of surgery to the date of confirmed tumor relapse for relapsed patients or to the date of the last follow-up for non-recurrent patients. The Kaplan-Meier method was used to estimate the overall survival (OS) and DFS, and analyzed using the log-rank test. Cox proportional hazards models were used to estimate the survival distributions and hazard ratios. Data on the in vitro experiments were compared using the Student’s t test. All statistical analyses were two-sided and conducted using SPSS software version 18.0 (SPSS Inc., Chicago, IL, United States). A P < 0.05 was considered as statistically significant.
TMAs containing CRC tissue specimens from 742 cases from our previous study[22] were used. During cutting, tissue samples from 30 cases were lost, leading to 712 CRC samples for the current study.
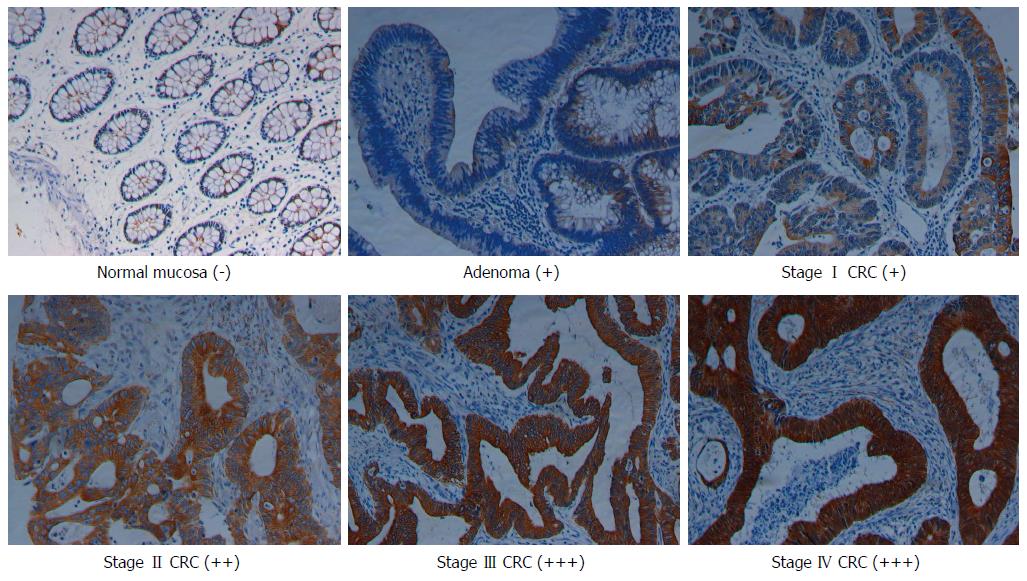
Immunostaining data showed that NLK was primarily expressed in the cytoplasm of epithelial cells and was significantly upregulated from the normal mucosa through adenoma to CRC (Figure 1). There were significant differences in NLK expression among these six groups of tissue specimens (P < 0.001; Table 1). Furthermore, overexpression of NLK protein in the tumor tissues was verified using Western blot, and overexpression of NLK mRNA was verified using qRT-PCR with ten cases of CRC and paired normal mucosae (Supplemental materials). The level of NLK mRNA in the tumor tissues was significantly higher than in the normal tissues (P < 0.05).
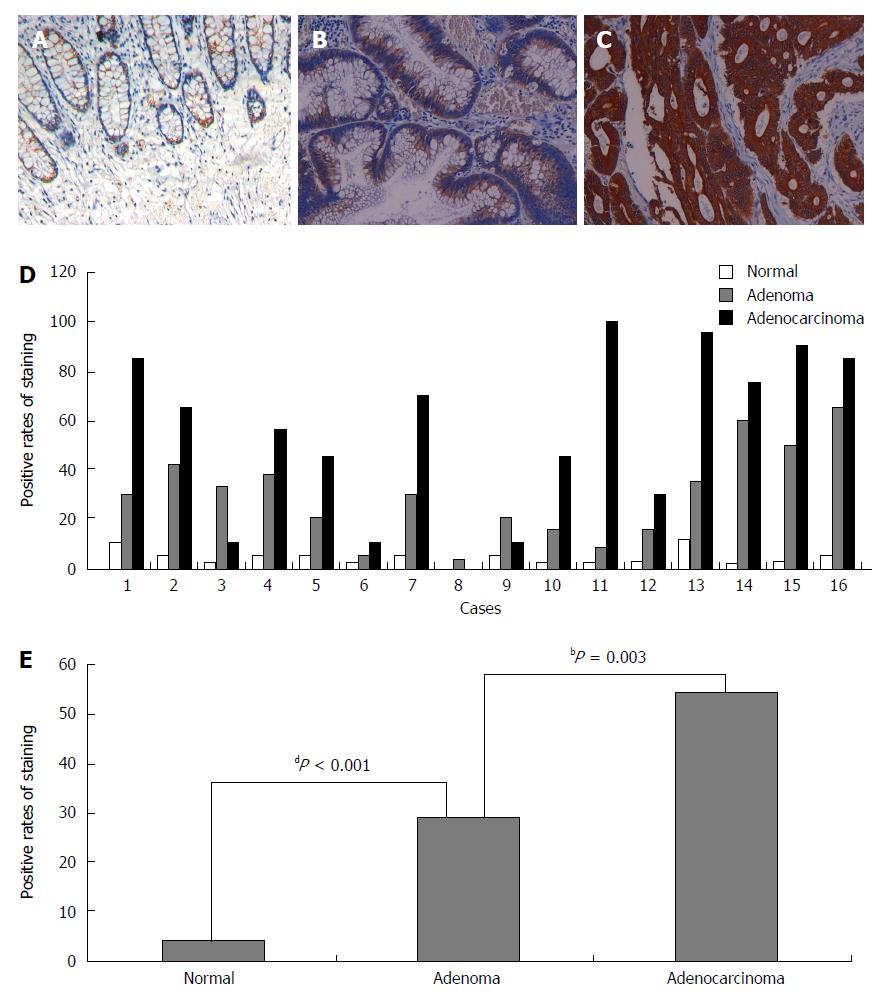
Next, we analyzed NLK expression in mucosa, adenoma, and adenocarcinoma tissues from 16 FAP patients. These data showed that NLK expression was significantly increased between normal mucosa and adenoma tissues (P < 0.001), as well as between adenoma and adenocarcinoma tissues (P = 0.003; Figure 2).
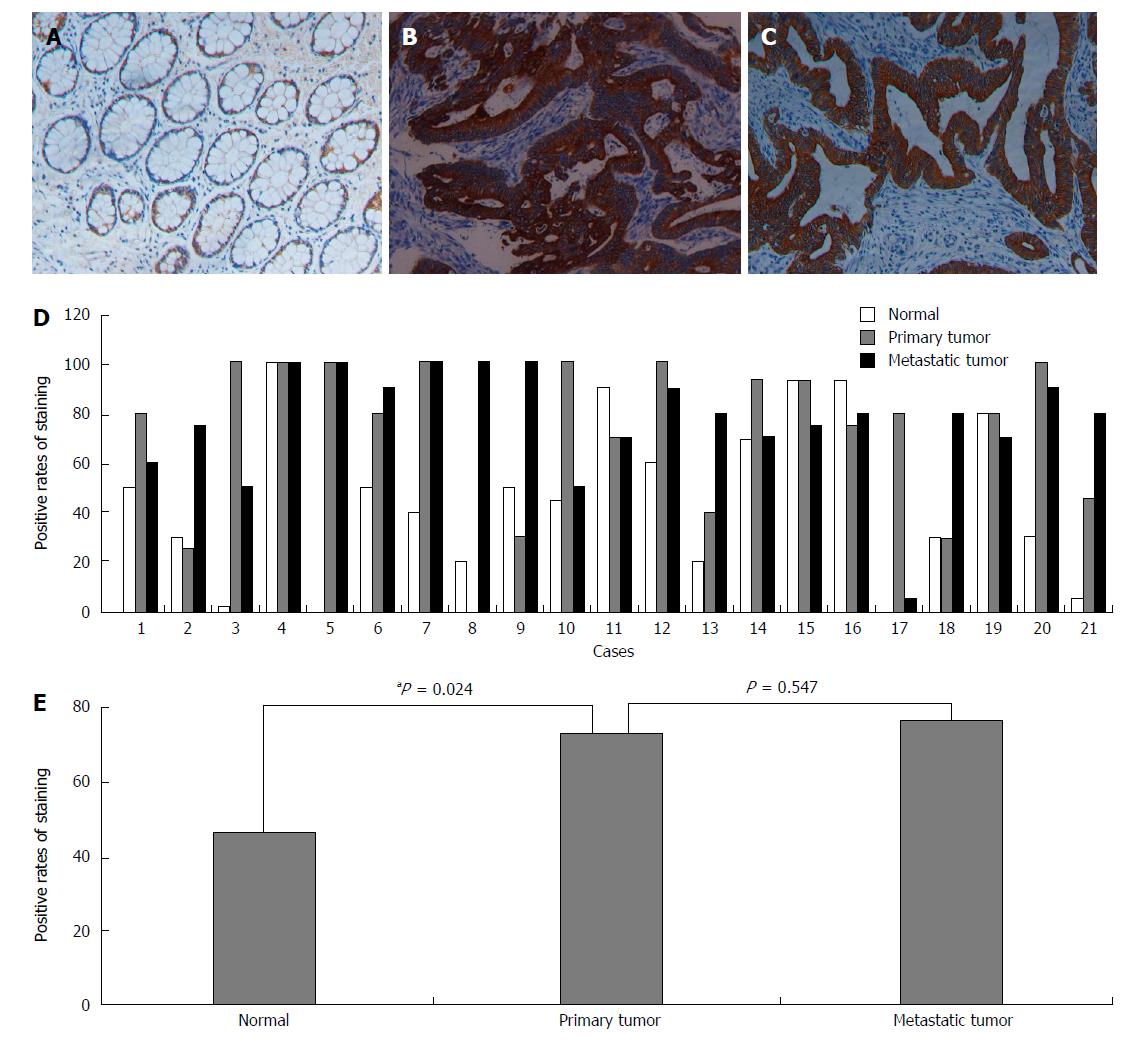
We also analyzed the NLK expression in normal mucosa as well as primary and metastatic CRC tissues from 21 patients. We found that the NLK expression in primary CRC was significantly higher than in the normal mucosa (P = 0.024), but there was no significant difference between metastatic and primary tumors (Figure 3).
The clinicopathologic variables are shown in Table 2. In brief, overexpression of NLK protein was significantly associated with tumor lymph node metastases, distant metastasis, advanced TNM stages, poorer tumor differentiation, and higher recurrence rate (all P < 0.05). Moreover, NLK expression was significantly higher in rectal cancer than in colon cancer (P < 0.001). There was no significant association between NLK expression and other clinicopathologic factors.
| Parameter | NLK immunostaining | P value | |||
| - | + | ++ | +++ | ||
| Sex | 0.1651 | ||||
| Male | 29 | 112 | 210 | 55 | |
| Female | 31 | 90 | 147 | 38 | |
| Age (yr) | 0.4081 | ||||
| < 60 | 33 | 129 | 211 | 65 | |
| ≥ 60 | 27 | 73 | 146 | 28 | |
| Tumor location | < 0.0011 | ||||
| Colon cancer | 48 | 110 | 145 | 36 | |
| Rectal cancer | 12 | 92 | 212 | 57 | |
| Invasion depth | 0.9801 | ||||
| T1-T2 | 6 | 27 | 42 | 12 | |
| T3-T4 | 54 | 175 | 315 | 81 | |
| Lymph node metastasis | 0.0272 | ||||
| N0 | 35 | 122 | 180 | 43 | |
| N1 | 21 | 54 | 120 | 36 | |
| N2 | 4 | 26 | 57 | 14 | |
| Distant metastasis | < 0.0011 | ||||
| M0 | 59 | 195 | 340 | 69 | |
| M1 | 1 | 7 | 17 | 24 | |
| TNM stage | < 0.0012 | ||||
| I | 4 | 22 | 21 | 6 | |
| II | 31 | 97 | 154 | 30 | |
| III | 24 | 76 | 165 | 33 | |
| IV | 1 | 7 | 17 | 24 | |
| Tumor differentiation | 0.0191 | ||||
| Well, moderate | 42 | 182 | 326 | 82 | |
| Poor, mucinous | 18 | 20 | 31 | 11 | |
| Serum CEA (ng/mL) | 0.5701 | ||||
| < 5 | 39 | 123 | 220 | 63 | |
| ≥ 5 | 21 | 79 | 137 | 30 | |
| Serum CA19-9 (U/mL) | 0.2641 | ||||
| < 37 | 51 | 178 | 294 | 79 | |
| ≥ 37 | 9 | 24 | 63 | 14 | |
| Recurrence | < 0.0011 | ||||
| No | 56 | 176 | 257 | 68 | |
| Yes | 4 | 26 | 80 | 25 | |
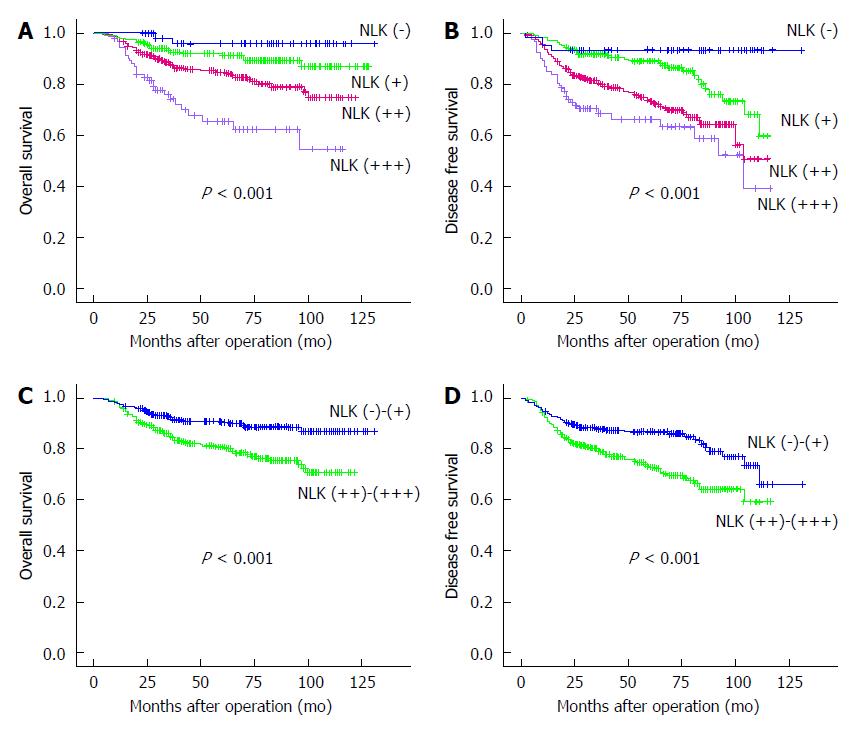
Patients with NLK-overexpressing tumors had a worse OS and DFS compared to those without or with weakly NLK-expressing tumors (all P < 0.001; Figure 4). Univariate analyses identified advanced TNM stages, colon cancer, elevated serum carcinoembryonic antigen (CEA), elevated serum cancer antigen (CA)19-9, overexpression of NLK protein, and postoperative radiochemotherapy as associated with a shorter OS (all P < 0.01), whereas advanced TNM stages, elevated serum CEA, elevated serum CA19-9, higher expression of NLK, and postoperative radiochemotherapy were associated with a shorter DFS (all P < 0.001) (Table 3). Multivariate analysis showed that advanced TNM stages, poor tumor differentiation, rectal cancer, elevated serum CEA, and higher expression of NLK were independent predictive factors for a shorter OS (all P < 0.05), whereas advanced TNM stage, elevated serum CA19-9, and overexpression of NLK protein were independent predictive factors for a shorter DFS (all P < 0.01).
| Variables | Overall survival | Disease-free survival | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Univariate analyses | ||||
| Sex (male vs female) | 0.84 (0.58-1.23) | 0.376 | 0.86 (0.63-1.18) | 0.342 |
| Age (≥ 60 yr vs < 60 yr) | 1.24 (0.85-1.80) | 0.259 | 0.95 (0.69-1.30) | 0.734 |
| TNM stage (IV/III vs II/I) | 3.11 (2.06-4.70) | < 0.001 | 2.37 (1.72-3.27) | < 0.001 |
| Differentiation (poor/mucinous adenocarcinoma vs well/moderate) | 1.54 (0.93-2.55) | 0.094 | 1.33 (0.85-2.08) | 0.219 |
| Position (rectal vs colon) | 0.56 (0.38-0.83) | 0.003 | 0.92 (0.66-1.23) | 0.506 |
| CEA (≥ 5 ng/mL vs < 5 ng/mL) | 1.97 (1.36-2.85) | < 0.001 | 1.63 (1.19-2.21) | 0.002 |
| CA19-9 (≥ 37 U/mL vs < 37 U/mL) | 2.27 (1.50-3.42) | < 0.001 | 1.89 (1.31-2.72) | 0.001 |
| NLK (++/+++ vs -/+) | 2.19 (1.44-3.35) | < 0.001 | 2.71 (1.86-3.94) | < 0.001 |
| Postoperative radiochemotherapy (yes vs no) | 2.42 (1.30-4.51) | 0.005 | 2.69 (1.60-4.50) | < 0.001 |
| Multivariate analyses | ||||
| TNM stage (IV/III vs II/I) | 3.02 (1.99-4.59) | < 0.001 | 2.34 (1.70-3.22) | < 0.001 |
| Differentiation (poor/mucinous adenocarcinoma vs well/moderate) | 1.78 (1.06-2.98) | 0.029 | 1.45 (0.94-2.24) | 0.091 |
| Position (rectal vs colon) | 0.49 (0.33-0.72) | < 0.001 | 0.80 (0.59-1.08) | 0.143 |
| CEA (≥ 5 ng/mL vs < 5 ng/mL) | 1.56 (1.04-2.35) | 0.032 | 1.31 (0.95-1.81) | 0.099 |
| CA19-9 (≥ 37 U/mL vs < 37 U/mL) | 1.54 (0.99-2.41) | 0.058 | 1.76 (1.24-2.49) | 0.002 |
| NLK (++/+++ vs -/+) | 2.57 (1.66-3.98) | < 0.001 | 1.96 (1.40-2.74) | < 0.001 |
| Postoperative radiochemotherapy (yes vs no) | 1.04 (0.50-2.16) | 0.921 | 1.64 (0.93-2.92) | 0.090 |
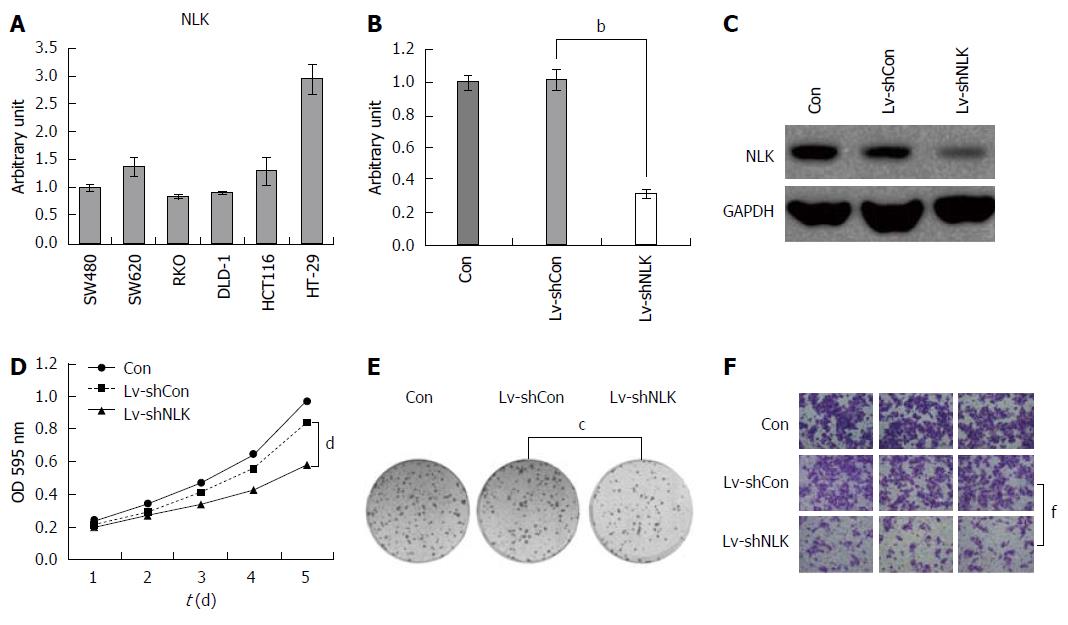
To explore the role of NLK in CRC, we first detected NLK expression in six different colorectal cell lines using qRT-PCR (Figure 5A). These data showed that NLK was expressed in all cell lines, with the highest NLK level in HT-29 cells. Thus, we selected HT-29 cells for our knockdown experiments. Specifically, NLK expression was downregulated by Lv-shNLK compared to Lv-shCon. At the highest infection efficiency, green fluorescent protein was expressed in > 90% of infected HT-29 cells. Five days after infection, the levels of NLK mRNA and protein were reduced significantly in HT-29 cells infected with Lv-shNLK compared to Lv-shCon (P < 0.05; Figure 5B and C).
Next, we assessed the effects of NLK knockdown on regulation of tumor cell viability and colony formation. We found that knockdown of NLK expression reduced the numbers of HT-29 cells compared to the control group (P < 0.05; Figure 5D). The colony formation assay also showed that NLK knockdown in HT-29 cells resulted in fewer colonies compared to the control group (P < 0.05; Figure 5E). The Transwell tumor cell migration assay showed that a significantly lower proportion of HT-29 cells with NLK knockdown was able to migrate into the bottom chambers compared to the control group (P < 0.01; Figure 5F).
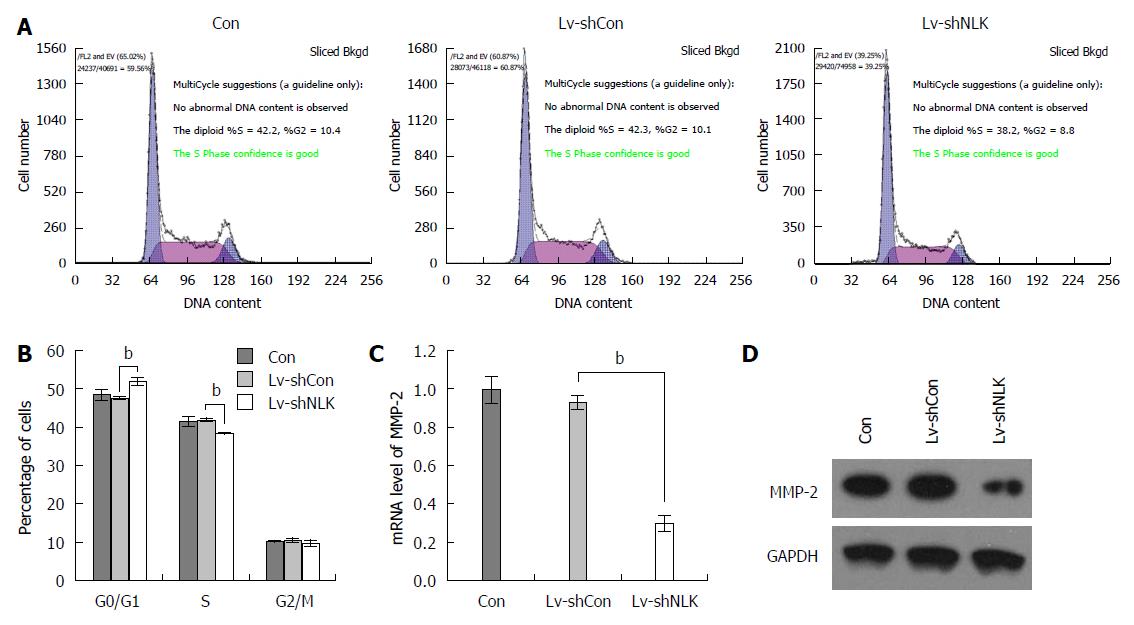
A flow cytometric cell cycle assay was performed using HT-29 cells 5 d after infection. These data showed that after HT-29 cells were infected with Lv-shNLK, the number of G0/G1 phase cells was increased significantly (P < 0.01), whereas the number of S phase cells was reduced significantly (P < 0.01), indicating that Lv-shNLK infection arrested HT-29 cells at the G0/G1 phase of the cell cycle (Figure 6A and B). NLK knockdown also reduced the levels of matrix metalloproteinase-2 mRNA and protein (both P < 0.01; Figure 6C and D).
To date, numerous studies have reported an association between NLK expression and cancer development[25]. In the current study, NLK expression was analyzed in five groups of colorectal tissue specimens and then in vitro experiments were performed to assess the potential role of NLK in CRC development and progression. The results show that NLK protein is progressively overexpressed from the normal mucosa through adenoma to various stages of CRC, and that NLK overexpression is associated with advanced tumor TNM stages, poor differentiation, lymph node and distant metastases, and a higher recurrence rate of CRC. NLK expression was also one of the independent prognostic factors to predict OS and DFS of CRC patients. These results are consistent with the those reported by Chen et al[26] who showed that patients with NLK-positive tumors had higher rates of recurrence and mortality than patients with NLK-negative tumors, and that NLK expression was an independent factor of OS and DFS in CRC patients[26]. However, Han et al[27] found different results based on expression of NLK mRNA in 92 CRC cases. This inconsistency may be explained by the difference between expression of NLK protein and mRNA. In addition, the in vitro data from the current study also support the ex vivo results; for example, knockdown of NLK expression in vitro reduced CRC cell viability, colony formation, and migration, and arrested tumor cells at the G0/G1 phase of the cell cycle. Knockdown of NLK expression also inhibited matrix metalloproteinase-2 expression in CRC cells. Taken together, results from the current study support the notion that NLK overexpression plays an important role in CRC development and progression.
TMA technology[28] allows for analysis of an entire cohort of tissue samples in one batch of experiments, which eliminates the staining bias of multiple day experiments[29]. However, it may produce sample selection bias. Our data demonstrate that NLK protein is overexpressed in CRC, but not in normal mucosae, with intermediate expression in adenoma tissues. Overexpression of NLK protein was associated with poorer clinicopathologic parameters, such as an advanced TNM stage, poor differentiation, lymph node and distant metastases, and a higher recurrence rate in CRC, thus contributing to poor OS and DFS of these CRC patients. These data are consistent with the overexpression of NLK in other organ sites of cancer[7]. Indeed, NLK is a member of the mitogen-activated protein kinase family that functions to promote cell proliferation; thus, overexpression of NLK could lead to cell proliferation and malignant transformation. Furthermore, the multivariate analysis in this study confirmed that NLK expression is one of the independent prognostic factors of the OS and DFS for CRC patients.
One unique feature of this study is that overexpression of NLK was confirmed in the paired tissues of normal mucosa, adenoma, and adenocarcinoma from 16 FAP patients, as well as in the paired tissues of normal mucosa and primary and metastatic CRC tissues from 21 patients. In general, hereditary heterogeneity produces variable gene expression in different tissues; however, evaluation of tissues from the same patient at different developing stages of CRC can minimize such heterogeneity. The data presented here further support the notion that NLK overexpression by cancer cells plays an oncogenic role in transformation of normal mucosa through adenoma to adenocarcinoma. NLK expression was not significantly different between primary and secondary tumor tissues, but the overall data show that NLK overexpression is associated with CRC lymph node and distant metastases, suggesting that NLK overexpression could induce tumor metastasis.
To assess the effects of NLK knockdown on tumor cell phenotypes in vitro, this study utilized shRNA-carrying lentiviruses, which effectively silence gene expression in vitro and in vivo[20]. The data show that knockdown of NLK expression significantly reduces HT-29 cell viability, colony formation, and migration capacity. These in vitro data further support the in vivo data, suggesting that NLK may play an oncogenic role in CRC. Therefore, NLK might serve as a potential therapeutic target for the future control of CRC. However, a previous study by Yasuda et al[30] showed that overexpression of NLK is associated with a decrease in tumor cell growth and induced apoptosis of the human colon cancer cell line DLD-1. Their data suggest that NLK might function as a tumor suppressor in colon cancer, which is contrary to the current findings. The reason for this discrepancy is unknown; but in their study, induction of wild-type NLK induced growth suppression of DLD-1 cells, whereas the kinase-negative mutant did not have such an effect, indicating that NLK may not function as a tumor suppressor in DLD-1 cells. The data of the current study also confirmed this finding, further suggesting that NLK does not play any role in DLD-1 tumorigenesis or maintenance of tumor phenotypes. In this case, NLK overexpression or an NLK kinase-negative mutation may either have no effect on cell behavior or, in their case, NLK overexpression had tumor suppressive effects on CRC DLD-1 cells. CRC is a heterogeneous disease and different CRC cell lines have specific subgroup characteristics. Furthermore, the ex vivo data presented here further support our current hypothesis. In addition, NLK has been shown to exhibit dual and opposite effects in Wnt/β-catenin signaling in different in vivo situations[25]. A previous study showed that NLK might be involved as an oncogene in the tumorigenesis and progression of CRC[31]. Thus, further studies are needed to clarify this discrepancy and the overall role of NLK in CRC.
The current study has several limitations that should be addressed. For example, this study was retrospective, the patients were not randomly selected, and loss of follow-up could have introduced possible biases. Furthermore, the patients included in this study did not receive neoadjuvant chemoradiotherapy, as the treatment would affect gene expression. Thus, the results obtained from this population may not generalize to the clinical setting. In addition, the TMA technology, like any technology, has certain limitations[32]. We chose this method for its reduced time, cost, and tissue samples needed[29]. Lastly, NLK knockdown was only performed in HT-29 cells, and more cell lines are needed to confirm the finding.
Nemo-like kinase (NLK) is an important regulator of several signal transduction pathways, including the Wnt and Notch signaling pathways, both of which play a critical role in tumorigenesis. Dysregulation of NLK expression was closely associated with progression of different human cancers.
Altered NLK expression is associated with cancer development and progression; for example, upregulated NLK protein occurs in hepatocellular carcinoma, whereas NLK expression is downregulated in prostate cancer cells. In other human cancers, knockdown of NLK expression reduces tumor cell viability.
In this study, the authors systematically evaluated NLK expression in different developing stages of colorectal cancer (CRC) and investigated the prognostic value of NLK expression. We observed that NLK expression gradually increased in colorectal samples from normal tissues, adenoma, stage I, stage II, and stage III, to stage IV CRC. Knockdown of NLK in CRC cells significantly inhibited cell growth, migration, cell cycle progression, and matrix metalloproteinase-2 expression. The data of this study suggest that NLK overexpression is an independent prognostic factor in CRC, and that knockdown of NLK expression inhibits CRC progression and metastasis.
The current study demonstrated that NLK overexpression plays an important role in CRC development and progression. This finding may help to understand the mechanism of CRC metastasis and to promote the prevention, diagnosis, and management for CRC patients.
Tissue microarray technology has the advantage that the entire cohort of studied tissue samples in one batch of experiments. It has multiple advantages such as conserving reagents, saving time, and decreasing the amount of required tissue. However, the main limitation is sample selection bias, as the selected specimen may not be representative of the entire tissue.
This is an interesting paper assessing the role of NLK in CRC. The authors analyze a large series of clinical cases using TMA and correlate NLK expression with clinical parameters, and added some in vitro functional data of NLK knockdown to confirm the ex vivo data on the biomarker analysis. Their data indicated that NLK is a relevant molecule predicting CRC aggressiveness and prognosis.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25606] [Article Influence: 1707.1] [Reference Citation Analysis (11)] |
| 2. | Ou J, Peng Y, Deng J, Miao H, Zhou J, Zha L, Zhou R, Yu L, Shi H, Liang H. Endothelial cell-derived fibronectin extra domain A promotes colorectal cancer metastasis via inducing epithelial-mesenchymal transition. Carcinogenesis. 2014;35:1661-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Lönnroth C, Andersson M, Asting AG, Nordgren S, Lundholm K. Preoperative low dose NSAID treatment influences the genes for stemness, growth, invasion and metastasis in colorectal cancer. Int J Oncol. 2014;45:2208-2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Gao XH, Yu ZQ, Zhang C, Bai CG, Zheng JM, Fu CG. DNA topoisomerase II alpha: a favorable prognostic factor in colorectal caner. Int J Colorectal Dis. 2012;27:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Glockzin G, Gerken M, Lang SA, Klinkhammer-Schalke M, Piso P, Schlitt HJ. Oxaliplatin-based versus irinotecan-based hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal metastasis from appendiceal and colorectal cancer: a retrospective analysis. BMC Cancer. 2014;14:807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Nakanishi M, Kuriu Y, Murayama Y, Konishi H, Komatsu S, Shiozaki A, Ikoma H, Kubota T, Ichikawa D, Fujiwara H. Efficacy of perioperative chemotherapy in patients with colorectal cancer undergoing hepatectomy for resectable synchronous liver metastasis. Hepatogastroenterology. 2014;61:1582-1587. [PubMed] |
| 7. | Ji K, Ye L, Ruge F, Hargest R, Mason MD, Jiang WG. Implication of metastasis suppressor gene, Kiss-1 and its receptor Kiss-1R in colorectal cancer. BMC Cancer. 2014;14:723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ahmed IA, Kelly SB, Anderson JJ, Angus B, Challen C, Lunec J. The predictive value of p53 and p33(ING1b) in patients with Dukes’C colorectal cancer. Colorectal Dis. 2008;10:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Ishihara S, Hayama T, Yamada H, Nozawa K, Matsuda K, Miyata H, Yoneyama S, Tanaka T, Tanaka J, Kiyomatsu T. Prognostic impact of primary tumor resection and lymph node dissection in stage IV colorectal cancer with unresectable metastasis: a propensity score analysis in a multicenter retrospective study. Ann Surg Oncol. 2014;21:2949-2955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Lou X, Kang B, Zhang J, Hao C, Tian X, Li W, Xu N, Lu Y, Liu S. MFAP3L activation promotes colorectal cancer cell invasion and metastasis. Biochim Biophys Acta. 2014;1842:1423-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Duan B, Shen C, Wu B, Luo J, Zhao G. Treatment and multivariate analysis of colorectal cancer with liver metastasis. Hepatogastroenterology. 2014;61:1568-1573. [PubMed] |
| 12. | Ji D, Chen Z, Li M, Zhan T, Yao Y, Zhang Z, Xi J, Yan L, Gu J. MicroRNA-181a promotes tumor growth and liver metastasis in colorectal cancer by targeting the tumor suppressor WIF-1. Mol Cancer. 2014;13:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Zhou Y, Rideout WM, Bressel A, Yalavarthi S, Zi T, Potz D, Farlow S, Brodeur J, Monti A, Reddipalli S. Spontaneous genomic alterations in a chimeric model of colorectal cancer enable metastasis and guide effective combinatorial therapy. PLoS One. 2014;9:e105886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Katoh M. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 2007;3:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Ota S, Ishitani S, Shimizu N, Matsumoto K, Itoh M, Ishitani T. NLK positively regulates Wnt/β-catenin signalling by phosphorylating LEF1 in neural progenitor cells. EMBO J. 2012;31:1904-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042-4045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 611] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 17. | Jung KH, Kim JK, Noh JH, Eun JW, Bae HJ, Xie HJ, Ahn YM, Park WS, Lee JY, Nam SW. Targeted disruption of Nemo-like kinase inhibits tumor cell growth by simultaneous suppression of cyclin D1 and CDK2 in human hepatocellular carcinoma. J Cell Biochem. 2010;110:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Emami KH, Brown LG, Pitts TE, Sun X, Vessella RL, Corey E. Nemo-like kinase induces apoptosis and inhibits androgen receptor signaling in prostate cancer cells. Prostate. 2009;69:1481-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Mendes-Pereira AM, Lord CJ, Ashworth A. NLK is a novel therapeutic target for PTEN deficient tumour cells. PLoS One. 2012;7:e47249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Tan Z, Li M, Wu W, Zhang L, Ding Q, Wu X, Mu J, Liu Y. NLK is a key regulator of proliferation and migration in gallbladder carcinoma cells. Mol Cell Biochem. 2012;369:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Stevens KN, Kelemen LE, Wang X, Fridley BL, Vierkant RA, Fredericksen Z, Armasu SM, Tsai YY, Berchuck A, Narod SA. Common variation in Nemo-like kinase is associated with risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Gao XH, Liu QZ, Chang W, Xu XD, Du Y, Han Y, Liu Y, Yu ZQ, Zuo ZG, Xing JJ. Expression of ZNF148 in different developing stages of colorectal cancer and its prognostic value: a large Chinese study based on tissue microarray. Cancer. 2013;119:2212-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Knut and Alice Wallenberg Foundation. Assays and Annotation. The Human Protein Atlas. Accessed 2012, April 16. Available from: http://www.proteinatlas.org/about/assays1annotation. |
| 24. | Ding Y, Huang Y, Song N, Gao X, Yuan S, Wang X, Cai H, Fu Y, Luo Y. NFAT1 mediates placental growth factor-induced myelomonocytic cell recruitment via the induction of TNF-alpha. J Immunol. 2010;184:2593-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Ishitani T, Ishitani S. Nemo-like kinase, a multifaceted cell signaling regulator. Cell Signal. 2013;25:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Chen J, Han Y, Zhao X, Yang M, Liu B, Xi X, Xu X, Liang T, Xia L. Nemo-like kinase expression predicts poor survival in colorectal cancer. Mol Med Rep. 2015;11:1181-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Han Y, Kuang Y, Xue X, Guo X, Li P, Wang X, Guo X, Yuan B, Zhi Q, Zhao H. NLK, a novel target of miR-199a-3p, functions as a tumor suppressor in colorectal cancer. Biomed Pharmacother. 2014;68:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2991] [Cited by in RCA: 2996] [Article Influence: 107.0] [Reference Citation Analysis (1)] |
| 29. | Khouja MH, Baekelandt M, Sarab A, Nesland JM, Holm R. Limitations of tissue microarrays compared with whole tissue sections in survival analysis. Oncol Lett. 2010;1:827-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Yasuda J, Tsuchiya A, Yamada T, Sakamoto M, Sekiya T, Hirohashi S. Nemo-like kinase induces apoptosis in DLD-1 human colon cancer cells. Biochem Biophys Res Commun. 2003;308:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Dieter SM, Ball CR, Hoffmann CM, Nowrouzi A, Herbst F, Zavidij O, Abel U, Arens A, Weichert W, Brand K. Distinct types of tumor-initiating cells form human colon cancer tumors and metastases. Cell Stem Cell. 2011;9:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 32. | Baisse B, Bouzourene H, Saraga EP, Bosman FT, Benhattar J. Intratumor genetic heterogeneity in advanced human colorectal adenocarcinoma. Int J Cancer. 2001;93:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Lakatos PL, Linnebacher M, Xia HXX S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Wang CH