Copyright
©The Author(s) 2026.
World J Gastroenterol. Feb 28, 2026; 32(8): 114268
Published online Feb 28, 2026. doi: 10.3748/wjg.v32.i8.114268
Published online Feb 28, 2026. doi: 10.3748/wjg.v32.i8.114268
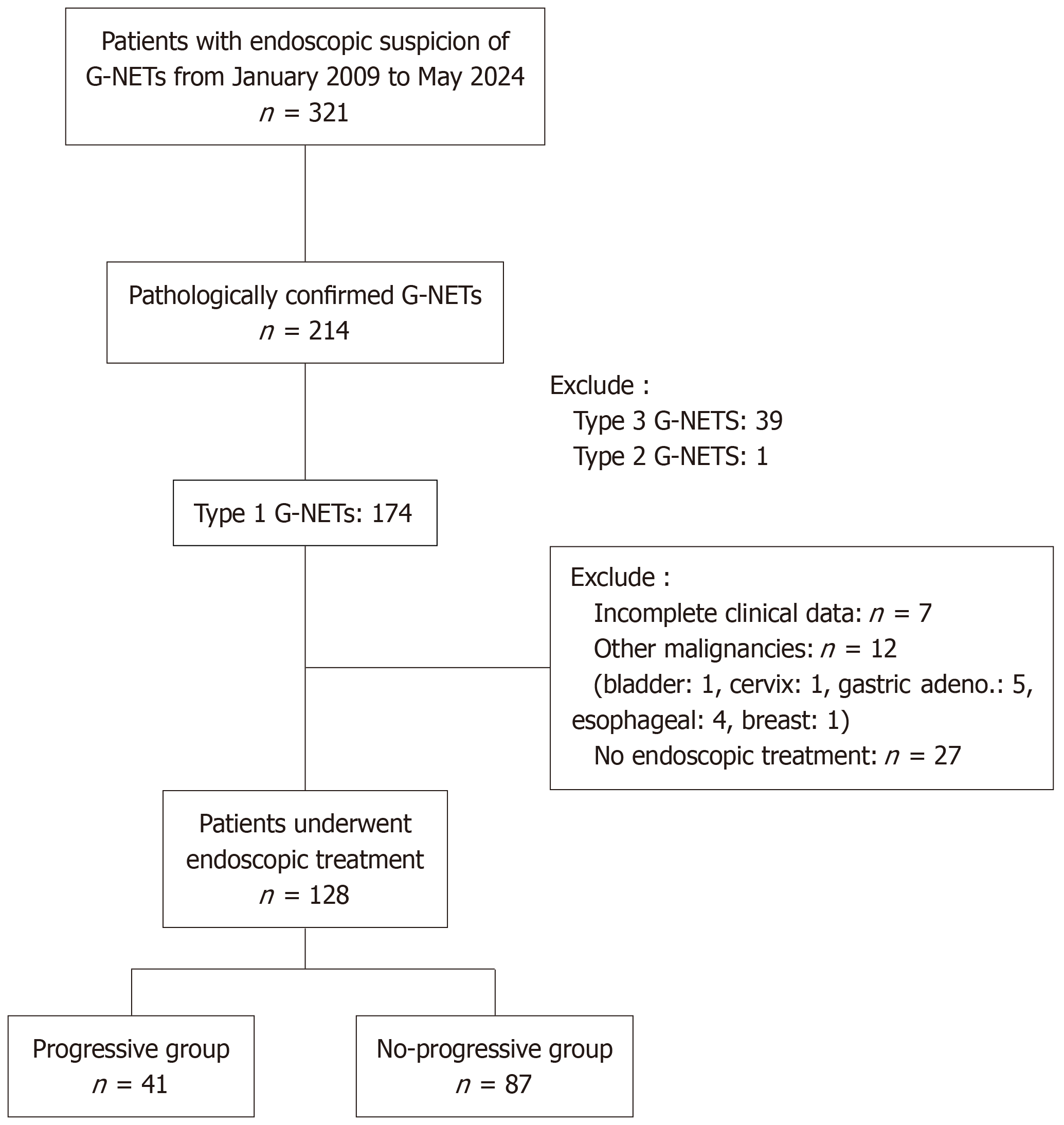
Figure 1 Patient selection flowchart.
G-NETs: Gastric neuroendocrine tumors.
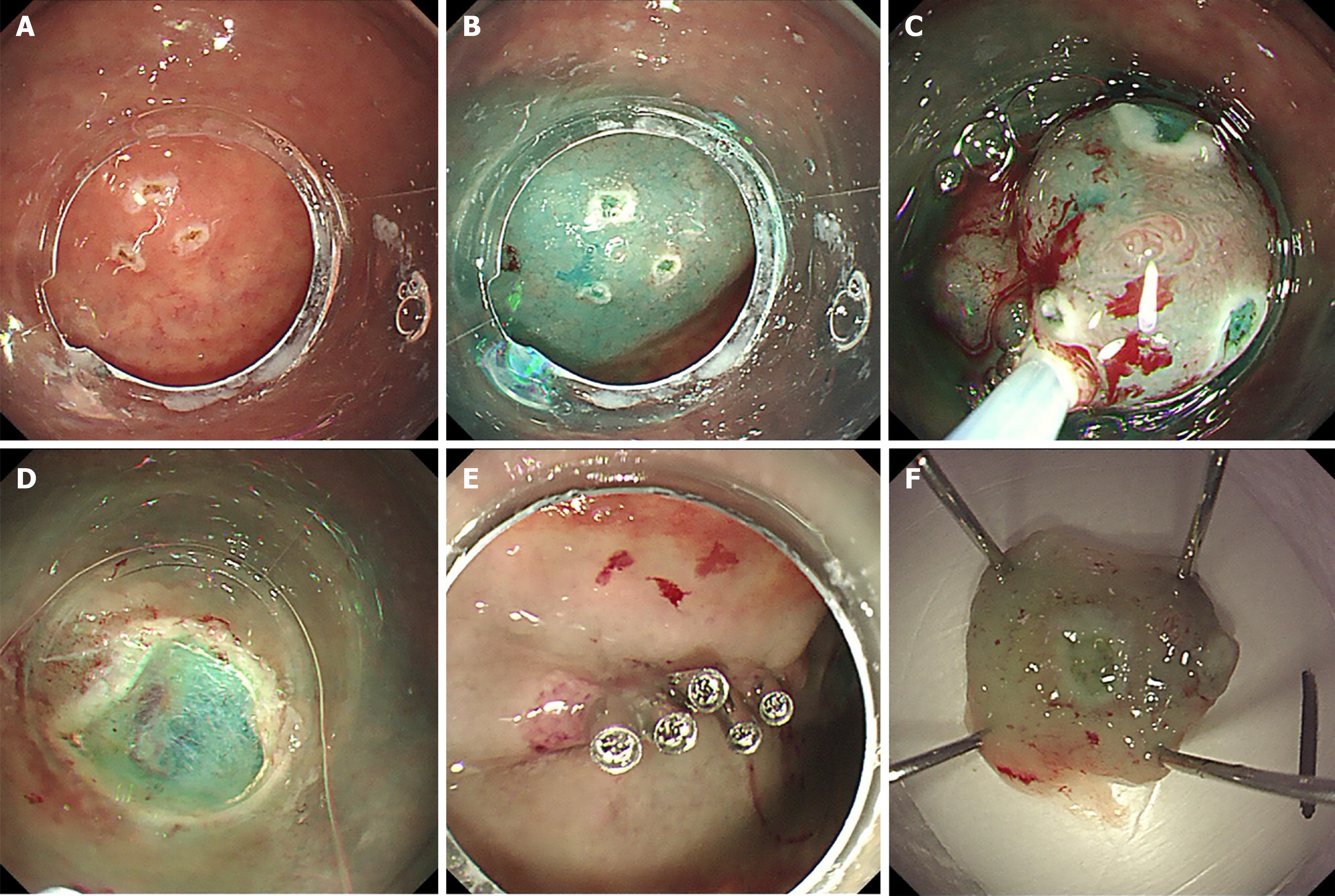
Figure 2 Endoscopic mucosal resection.
A: Circumferential marking of the lesion; B: Submucosal injection to create a fluid cushion; C: Resection using a snare; D and E: Targeted coagulation for hemostasis; F: Resected specimen.
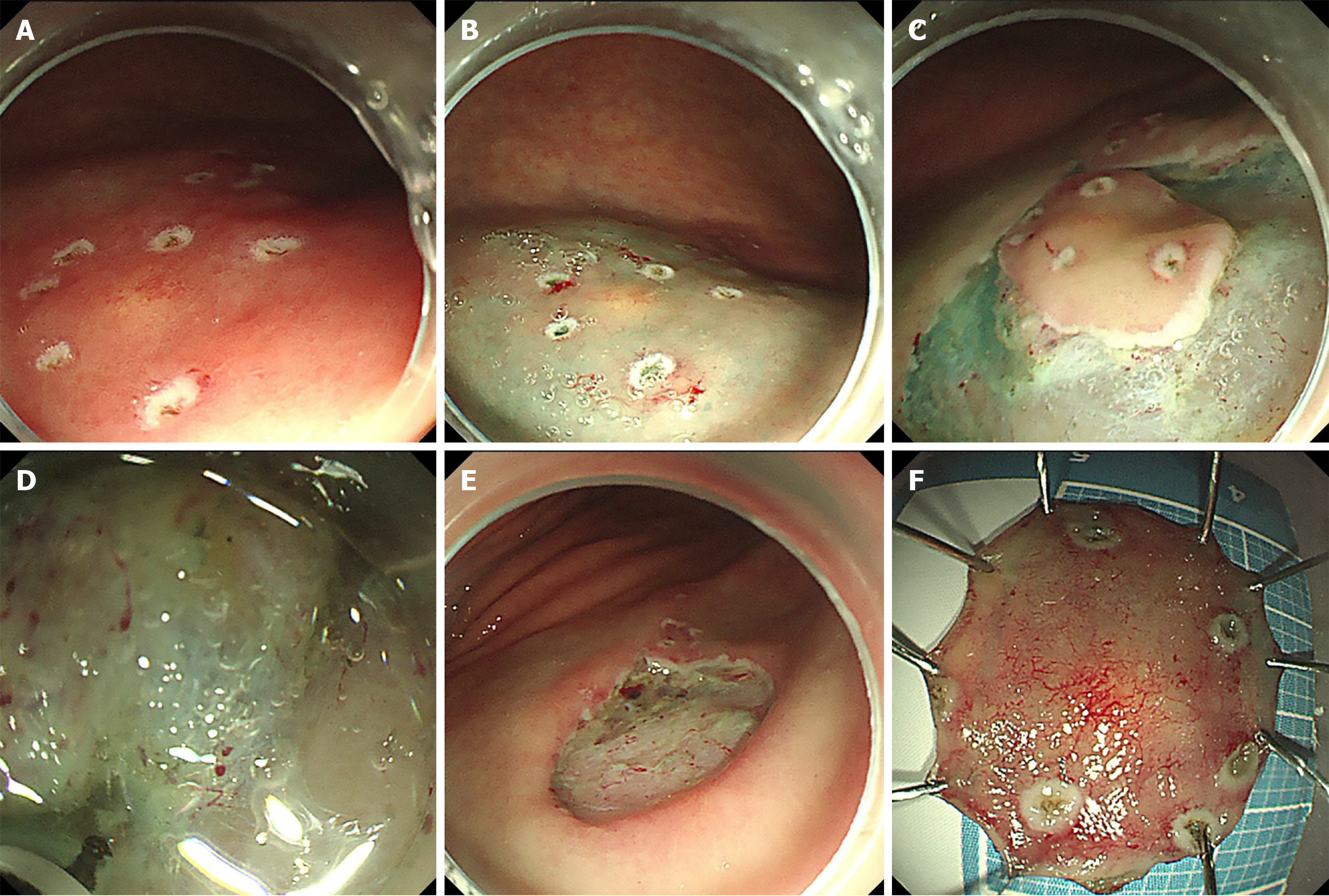
Figure 3 Endoscopic submucosal dissection.
A: Circumferential marking; B: Submucosal injection for mucosal elevation; C: Mucosal pre-cutting; D: Submucosal dissection for en bloc excision; E: Hemostasis and wound closure; F: Resected specimen.
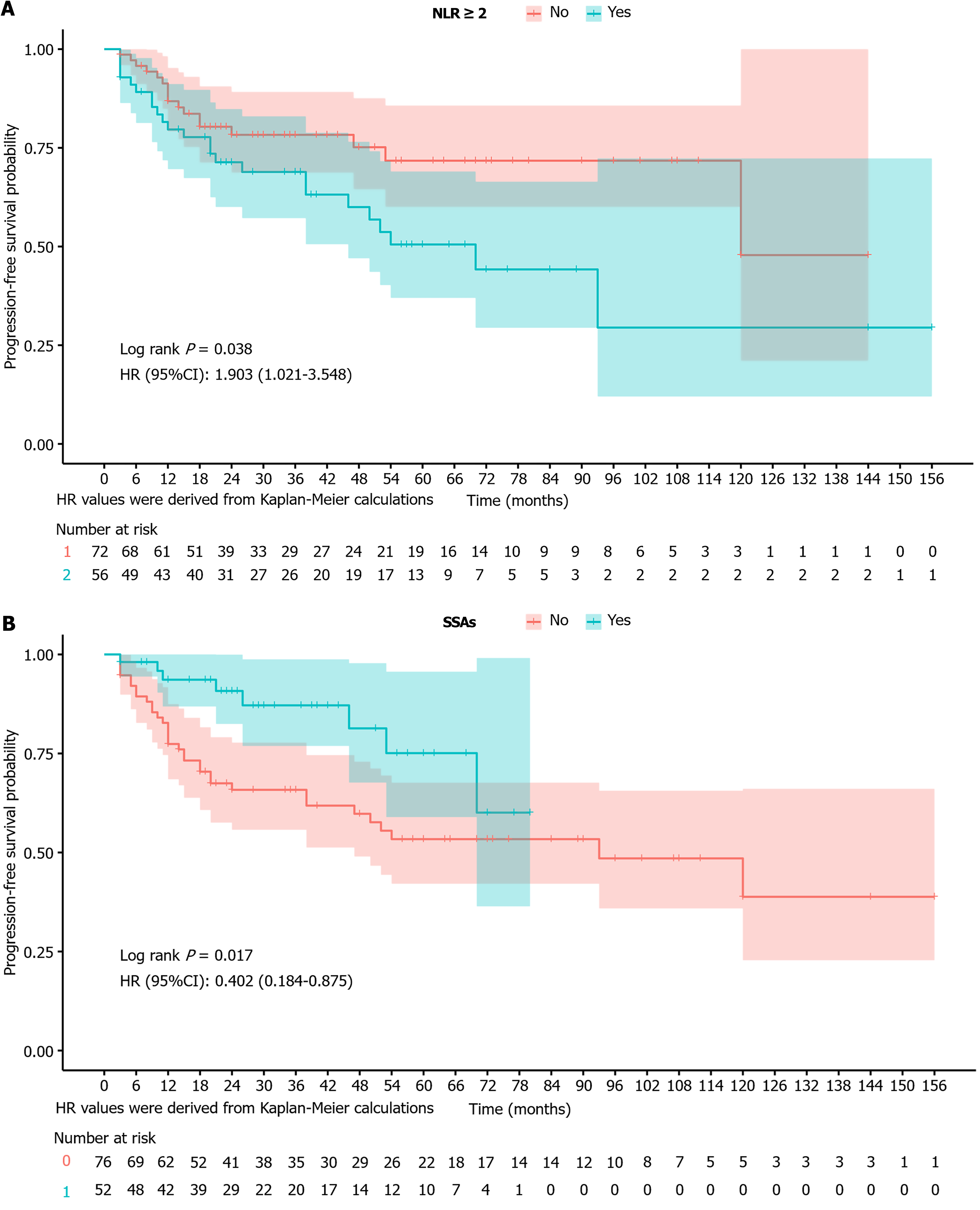
Figure 4 Progression-free survival curve.
A: Progression-free survival curve based on the neutrophil-to-lymphocyte ratio; B: Progression-free survival curve based on somatostatin analog use. Hazards ratio values were derived from Kaplan-Meier calculations. HR: Hazards ratio; CI: Confidence interval; NLR: Neutrophil-to-lymphocyte ratio; SSA: Somatostatin analog.
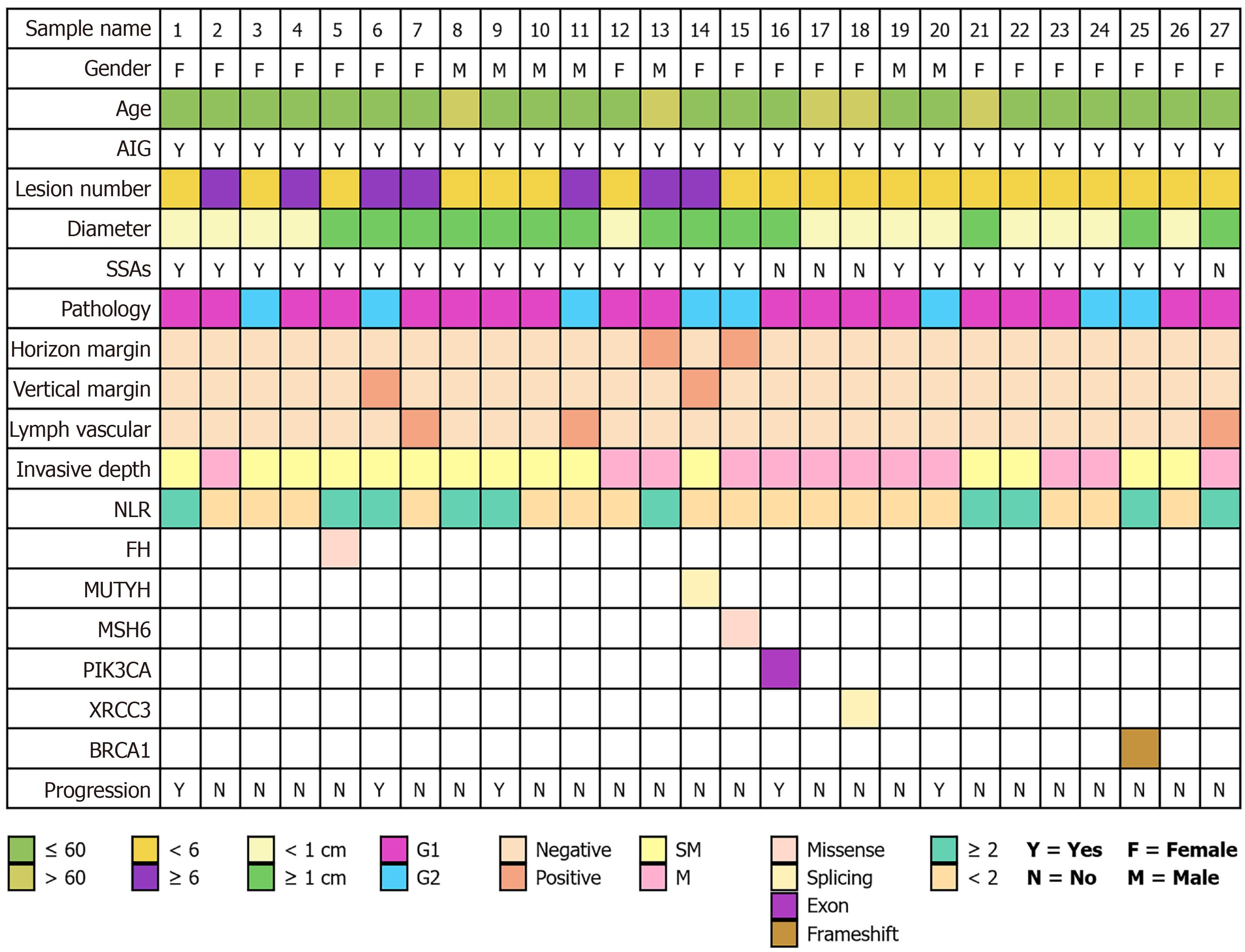
Figure 5 Baseline information and gene sequencing mutation conditions of 27 patients with type I gastric neuroendocrine tumors.
AIG: Autoimmune gastritis; SSAs: Somatostatin analogs; NLR: Neutrophil-to-lymphocyte ratio; FH: Fumarate hydratase; MUTYH: MutY DNA glycosylase; MSH6: MutS homolog 6; PIK3CA: Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; XRCC3: X-ray repair cross-complementing protein 3; BRCA1: Breast cancer 1.
- Citation: Yang ZL, Wang HK, Liu Y, Dou LZ, Zhang YM, Ng HI, He S, Chi YB, Wang GQ. Progression after endoscopic treatment for type I gastric neuroendocrine tumors: A single-center retrospective study. World J Gastroenterol 2026; 32(8): 114268
- URL: https://www.wjgnet.com/1007-9327/full/v32/i8/114268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i8.114268