©The Author(s) 2025.
World J Gastroenterol. Nov 28, 2025; 31(44): 112833
Published online Nov 28, 2025. doi: 10.3748/wjg.v31.i44.112833
Published online Nov 28, 2025. doi: 10.3748/wjg.v31.i44.112833
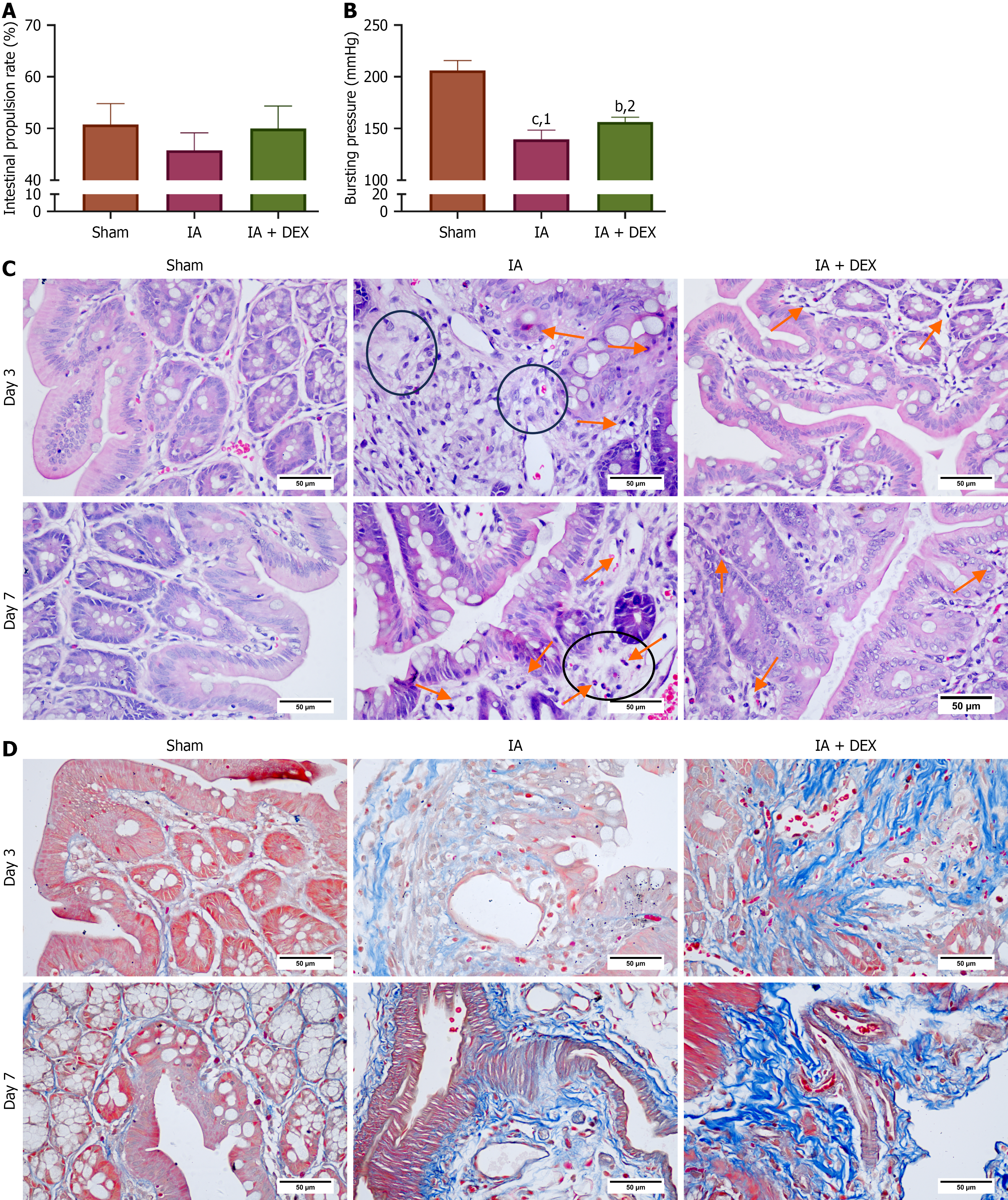
Figure 1 Dexmedetomidine promoted postoperative intestinal recovery.
A: Intestinal propulsion rate on postoperative day 6 (n = 6 per group). No significant differences were observed among groups; B: Anastomotic bursting pressure on postoperative day 7 (n = 6 per group); C: Representative hematoxylin-eosin-stained sections of the colon. Intestinal anastomosis (IA) group: Inflammatory cell infiltration (arrows) and edema (circles) in epithelium/crypt regions; Disorganized epithelium. IA + dexmedetomidine (DEX) group: Reduced inflammation and epithelial thickness; D: Representative Masson’s trichrome-stained sections demonstrating collagen deposition (IA + DEX > IA). Original magnification: 400 ×; scale bar: 50 μm. bP < 0.01. cP < 0.001. 1P compared with the Sham group. 2P compared with the intestinal anastomosis group. DEX: Dexmedetomidine; IA: Intestinal anastomosis; Sham group: Underwent abdominal only opening and closure; IA + DEX group: Dexmedetomidine was infused in the intestinal anastomosis model.
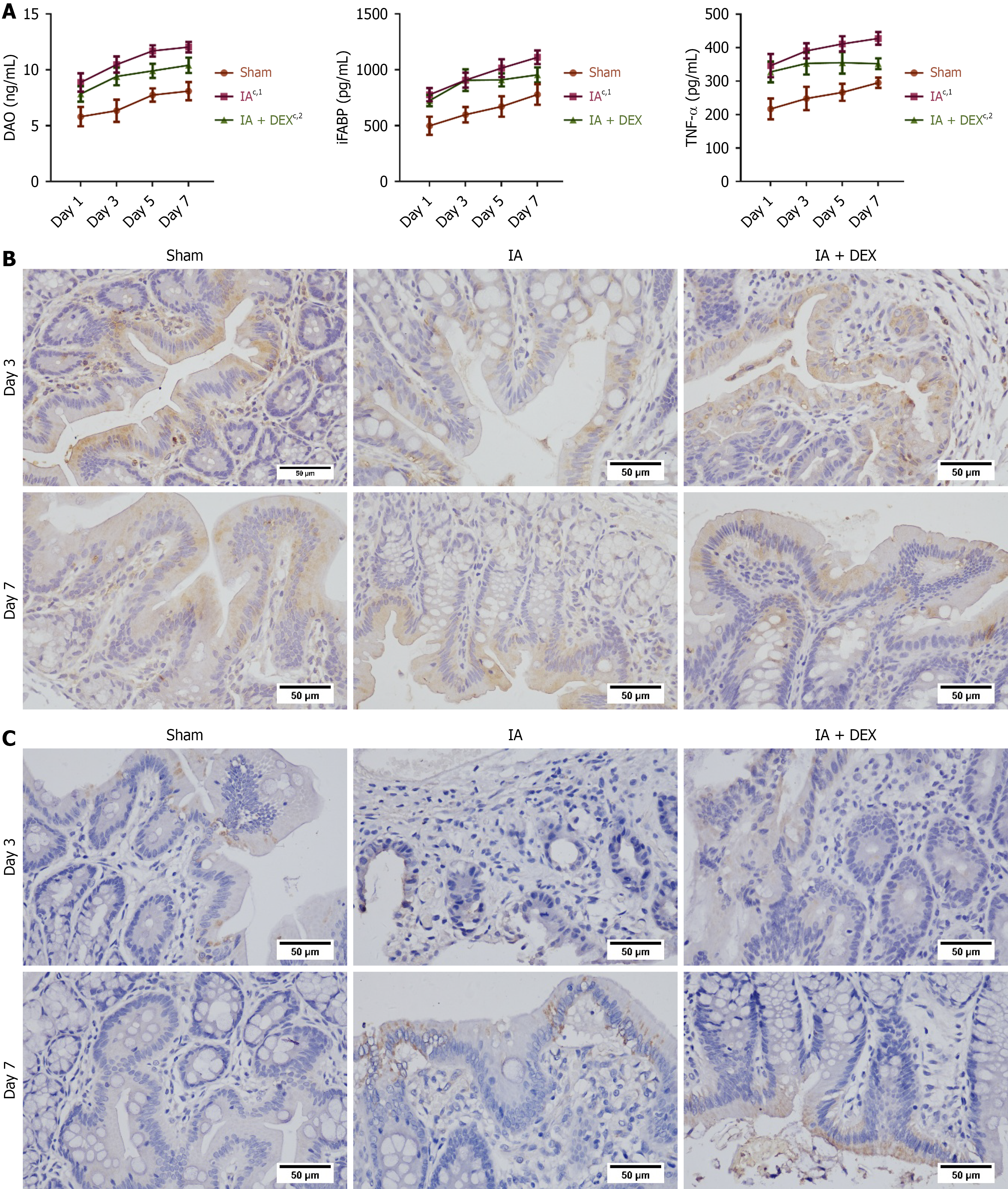
Figure 2 Dexmedetomidine improved intestinal barrier function and modulated inflammatory responses following intestinal anastomosis surgery.
A: Serum levels of barrier markers (diamine oxidase; intestinal fatty acid-binding protein) and tumor necrosis factor-alpha measured by enzyme-linked immunosorbent assay at indicated time points after surgery (n = 6 per group); B: Immunohistochemical analysis of claudin-1 expression in intestinal tissues on postoperative days 3 and 7 (n = 6 per group); C: Immunohistochemical analysis of zonula occludens-1 expression in intestinal tissues on postoperative days 3 and 7 (n = 6 per group). Original magnification: 400 ×; scale bar: 50 μm. cP < 0.001. 1P compared with the sham group. 2P compared with the intestinal anastomosis group. DAO: Diamine oxidase; iFABP: Intestinal fatty acid-binding protein; TNF-α: Tumor necrosis factor-alpha; DEX: Dexmedetomidine; IA: Intestinal anastomosis; Sham group: Underwent abdominal only opening and closure; IA + DEX group: Dexmedetomidine was infused in the intestinal anastomosis model.
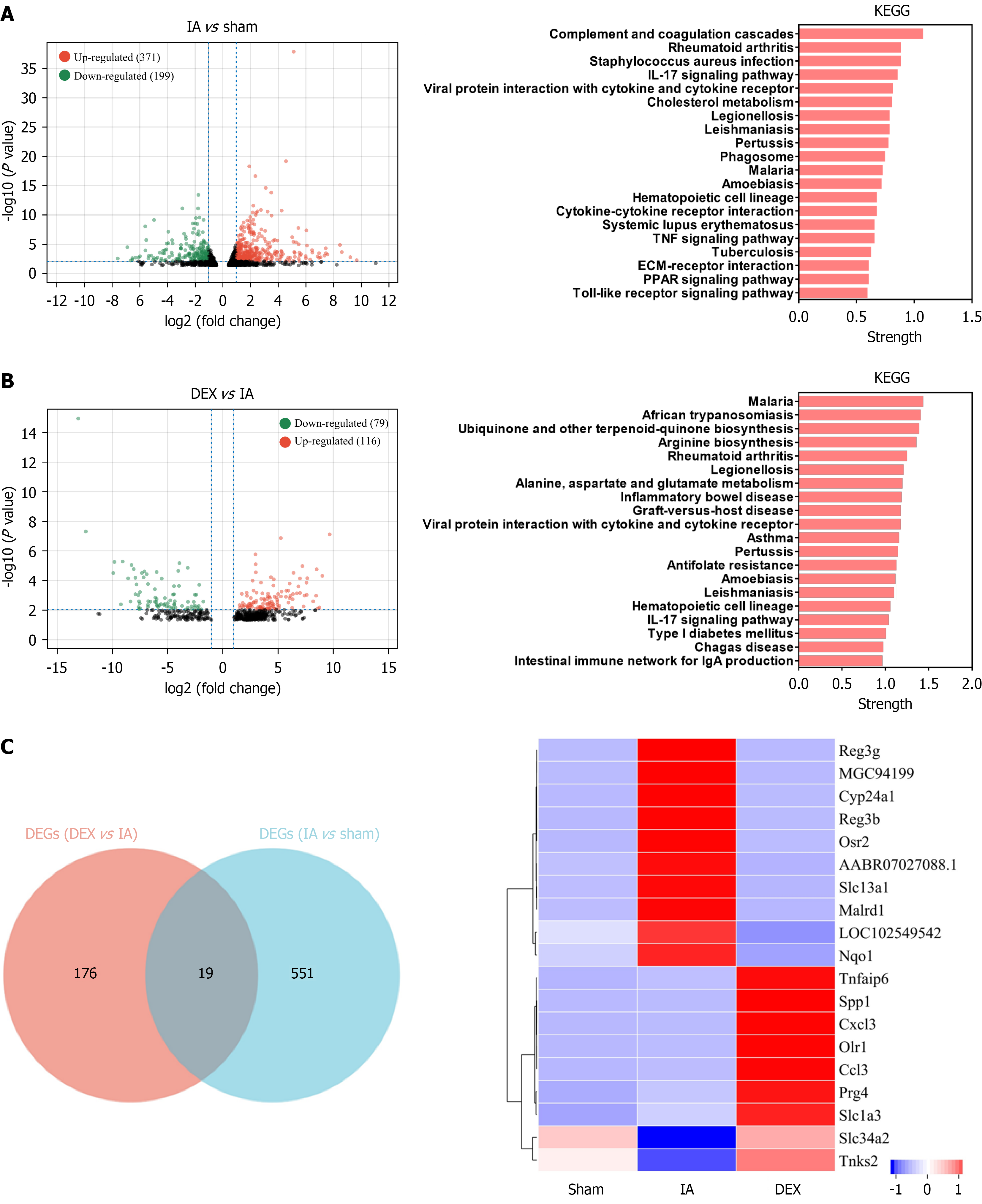
Figure 3 Exploring the potential molecular mechanisms underlying dexmedetomidine-mediated gut protection using transcriptome sequencing (RNA-sequencing).
A: A comparative analysis between the intestinal anastomosis (IA) and the sham groups (screening criteria: Fold change > 2 and P < 0.01) identified 570 differentially expressed genes (DEGs), with 371 upregulated and 199 downregulated genes. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of these 570 DEGs is shown in the right panel; B: Comparative analysis between the IA + dexmedetomidine (DEX) group and the IA group (screening criteria: Fold change > 2 and P < 0.01) identified 195 DEGs, including 116 upregulated and 79 downregulated DEGs. KEGG enrichment analysis of these 195 DEGs is presented in the right panel; C: The intersection of the two sets of DEGs revealed 19 overlapping genes. These genes, which both mediate IA progression and are modulated by DEX and represent potential therapeutic targets. A heatmap also illustrated the expression levels of these 19 genes across the three experimental groups. KEGG: Kyoto Encyclopedia of Genes and Genomes; TNF: Tumor necrosis factor; IL: Interleukin; ECM: Extracellular matrix; PPAR: Peroxisome proliferators-activated receptor; DEX: Dexmedetomidine; IA: Intestinal anastomosis; Sham group: Underwent abdominal only opening and closure.
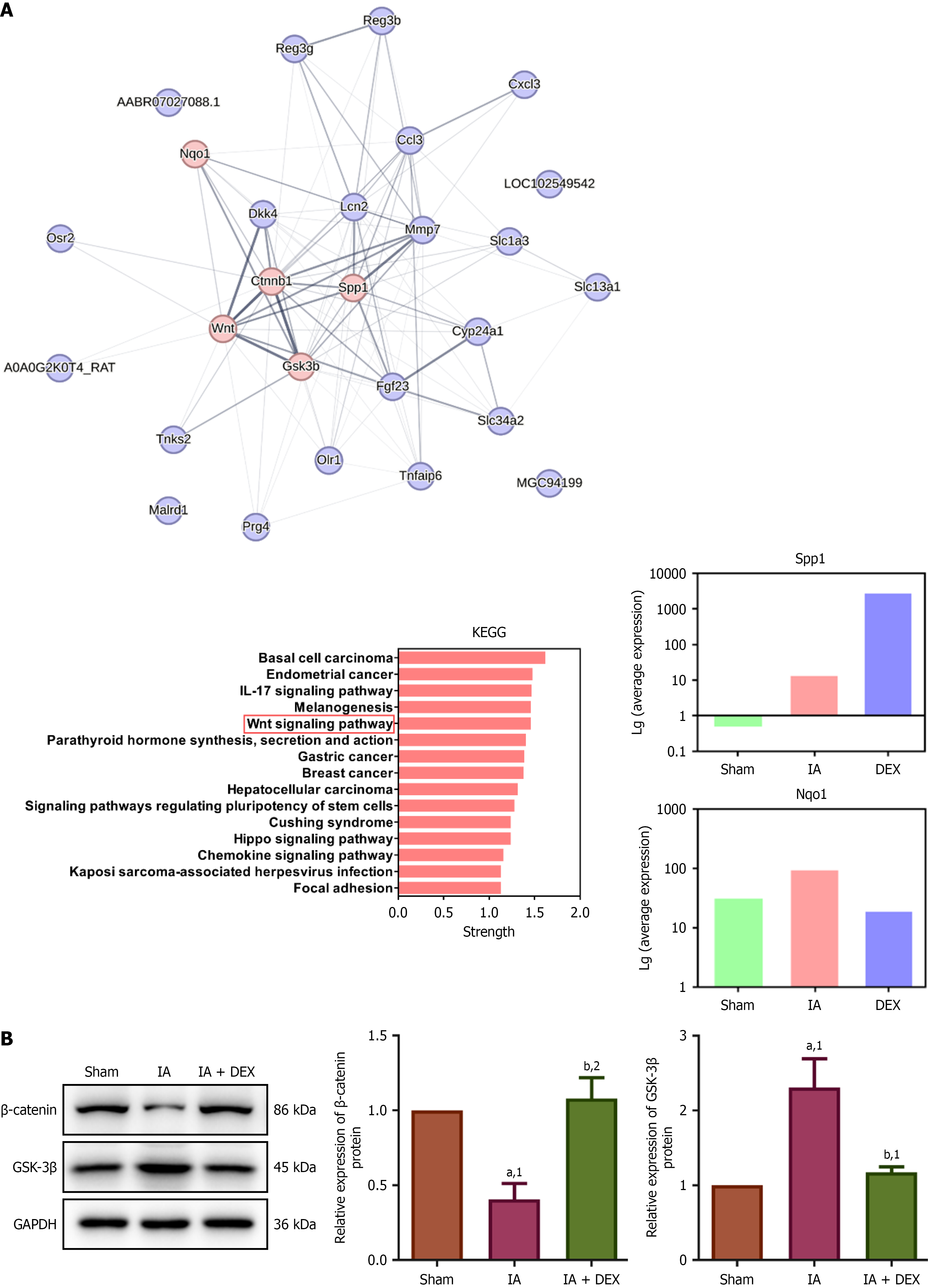
Figure 4 Dexmedetomidine upregulated Wnt signaling pathway activity in rats after intestinal anastomosis surgery.
A: Protein-protein interaction network and Kyoto Encyclopedia of Genes and Genomes enrichment analysis of 19 key genes, identifying significant involvement of the Wnt signaling pathway; B: Dexmedetomidine upregulated the Wnt signaling pathway after intestinal anastomosis surgery (n = 3 per group). aP < 0.05. bP < 0.01. 1P compared with the sham group. 2P compared with the intestinal anastomosis group. KEGG: Kyoto Encyclopedia of Genes and Genomes; IL: Interleukin; GSK-3β: Glycogen synthase kinase-3 beta; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; DEX: Dexmedetomidine; IA: Intestinal anastomosis; Sham group: Underwent abdominal only opening and closure; IA + DEX group: Dexmedetomidine was infused in the intestinal anastomosis model.
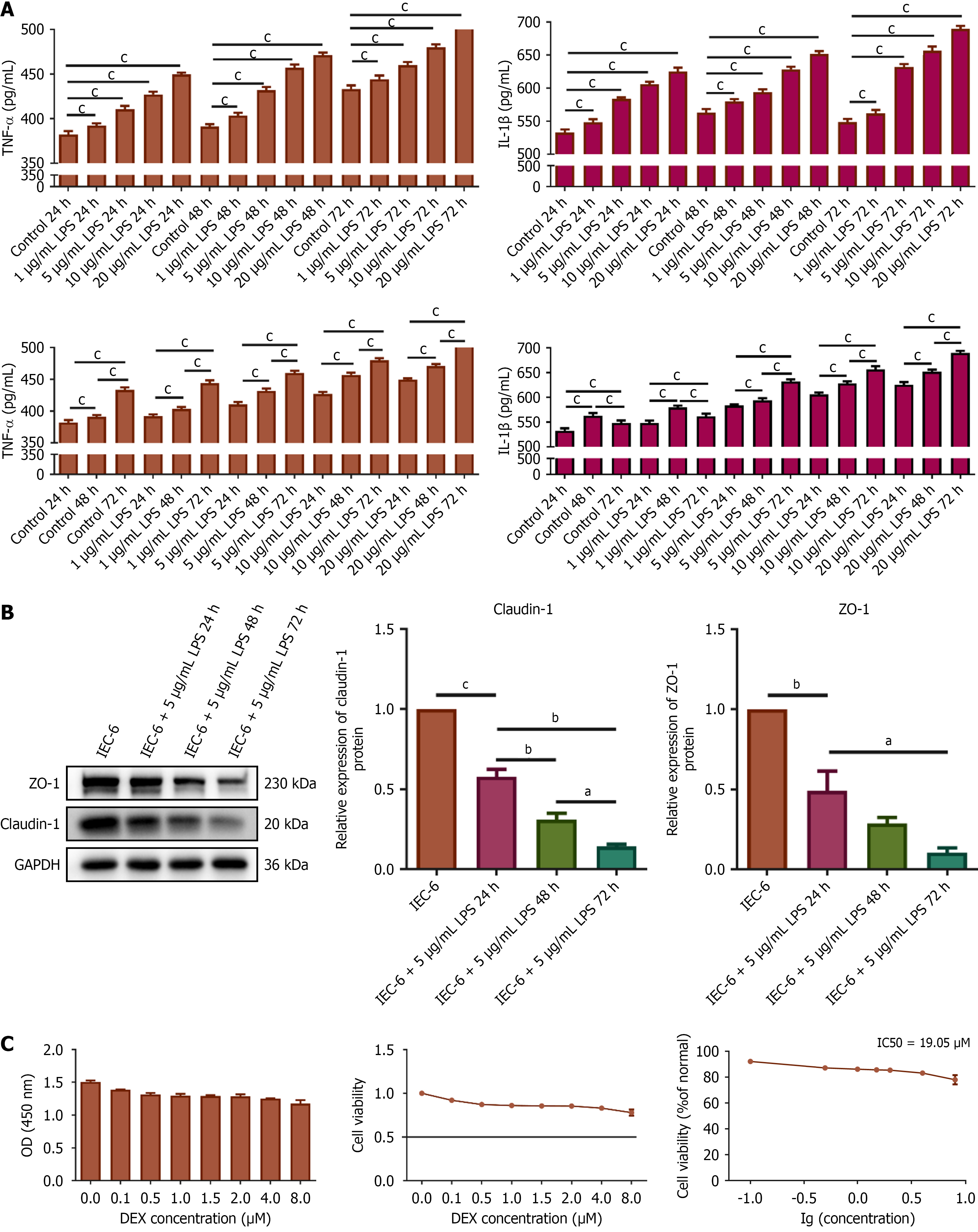
Figure 5 Establishing optimal in vitro experimental conditions.
A: Tumor necrosis factor-alpha and interleukin-1β levels in supernatants of IEC-6 cells treated with various concentrations and durations of lipopolysaccharide (LPS), measured by enzyme-linked immunosorbent assay; B: The protein expression of claudin-1 and zonula occludens-1 in IEC-6 cells treated with 5 μg/mL LPS for the indicated durations, assessed by Western blotting; C: IEC-6 cell viability assessed by cell counting kit-8 assay following treatment with different concentrations of dexmedetomidine. aP < 0.05. bP < 0.01. cP < 0.001. Dexmedetomidine concentrations selected for subsequent experiments: Dexmedetomidine-low (2.4 μM), Dexmedetomidine-high (4.8 μM). TNF: Tumor necrosis factor; IL: Interleukin; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; LPS: Lipopolysaccharide; ZO-1: Zonula occludens-1; OD: Optical density; DEX: Dexmedetomidine.
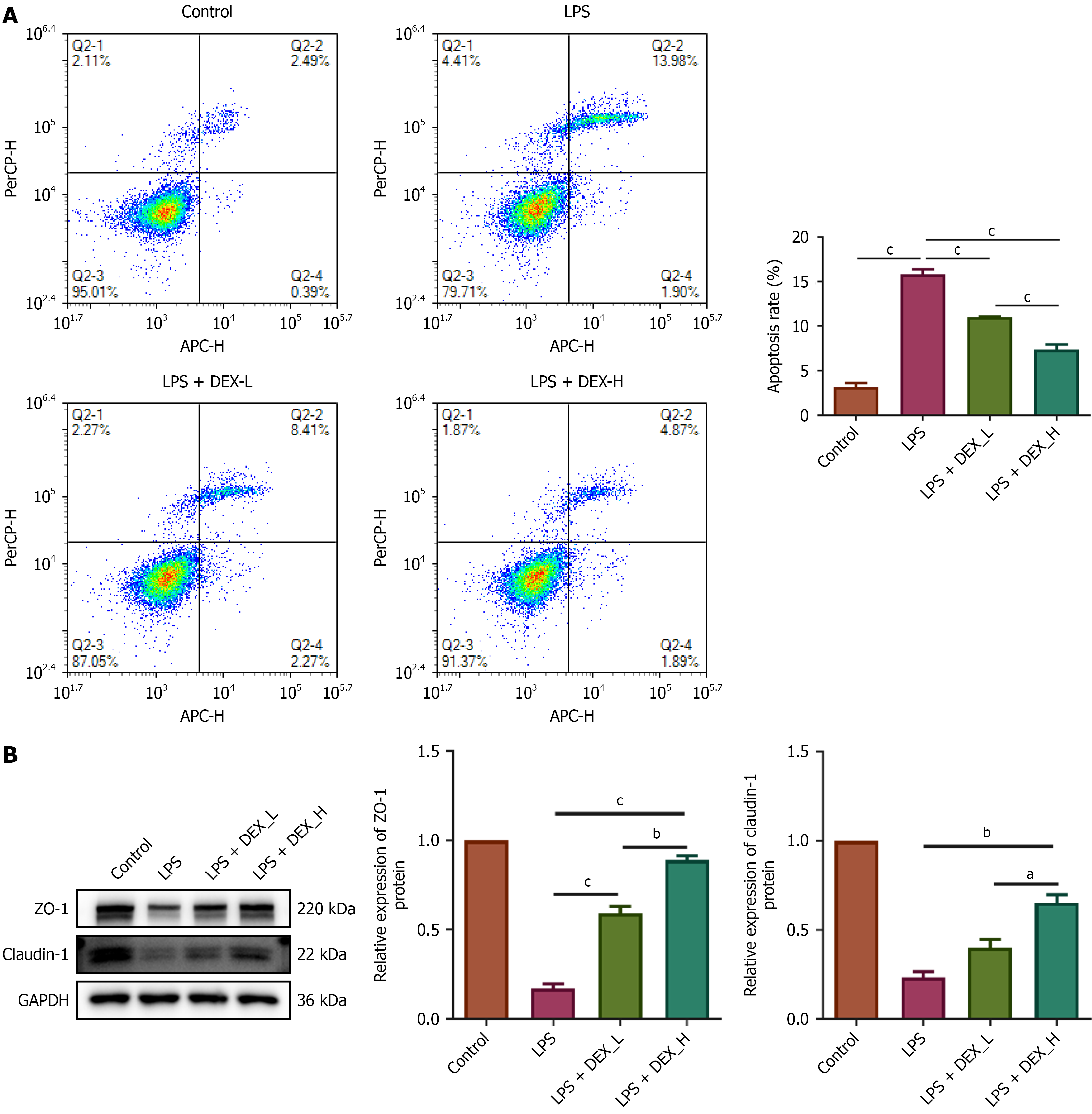
Figure 6 Dexmedetomidine attenuated lipopolysaccharide-induced apoptosis and upregulated the expression of tight junction proteins in IEC-6 cells.
A: Attenuated lipopolysaccharide-induced apoptosis; B: Upregulated the expression of tight junction proteins. aP < 0.01. bP < 0.001. cP < 0.0001. Dexmedetomidine (DEX)-low = 2.4 μM DEX; DEX-high = 4.8 μM DEX. Control group: IEC-6 cells maintained in standard culture medium; LPS group: IEC-6 cells treated with 5 μg/mL lipopolysaccharide for 72 hours; LPS + DEX-L group: IEC-6 cells pretreated with 2.4 μM dexmedetomidine for 24 hours followed by treatment with 5 μg/mL lipopolysaccharide for 72 hours; LPS + DEX-H group: IEC-6 cells pretreated with 4.8 μM dexmedetomidine for 24 hours followed by 5 μg/mL lipopolysaccharide for 72 hours; LPS: Lipopolysaccharide; DEX: Dexmedetomidine; L: Low; H: High; APC-H: Allophycocyanin-height; PerCP-H: Peridinin-chlorophyll-protein complex-height; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; ZO-1: Zonula occludens-1.
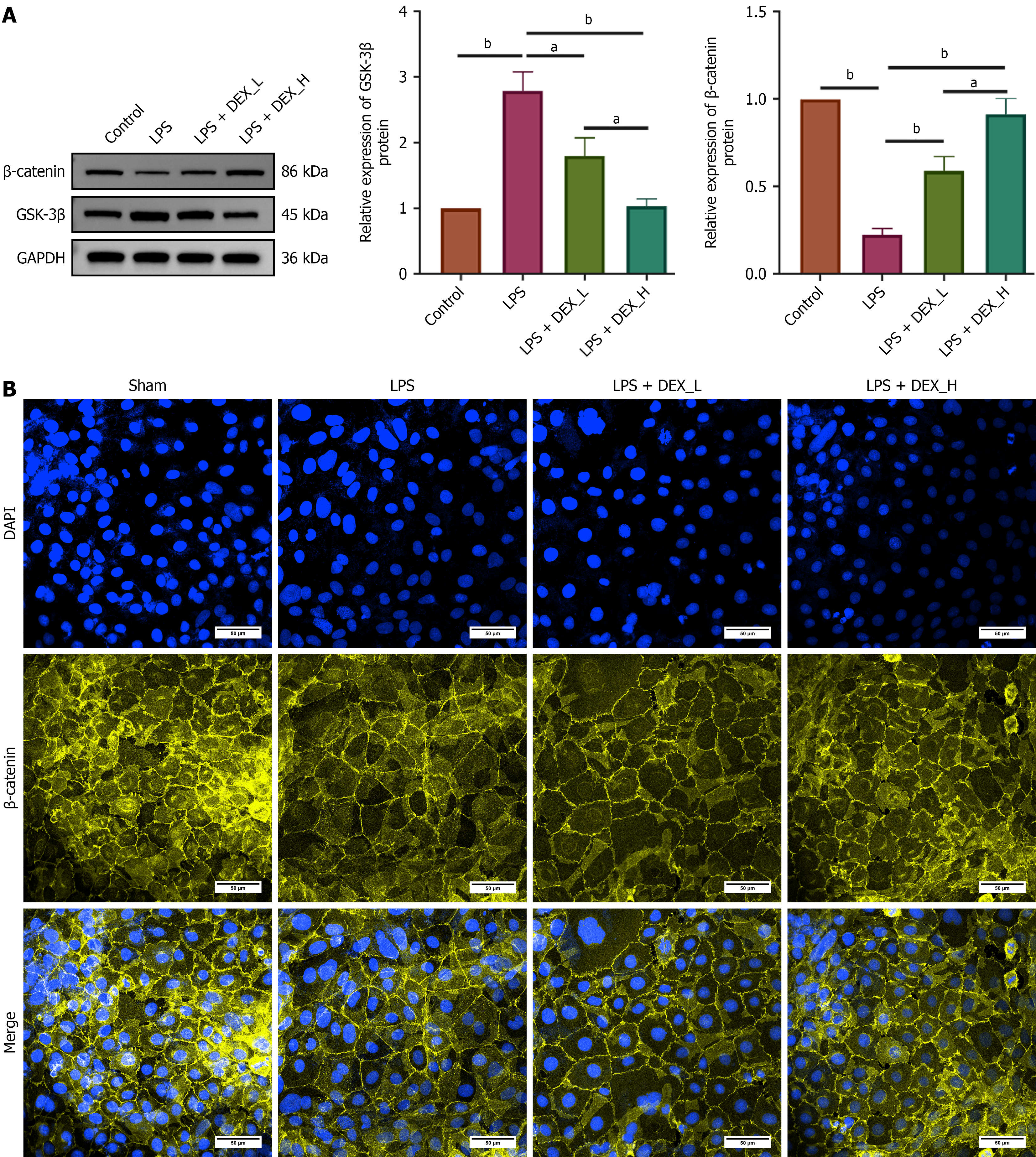
Figure 7 Effects of dexmedetomidine on the Wnt/β-catenin signaling pathway in IEC-6 cells.
A: Western blotting for the protein expression of β-catenin and glycogen synthase kinase-3 beta in IEC-6 cells treated with lipopolysaccharide and dexmedetomidine. Glyceraldehyde-3-phosphate dehydrogenase was used as the loading control; B: Immunofluorescence staining of β-catenin in IEC-6 cells (original magnification: 600 ×; scale bar: 50 μm). Data are presented as mean ± SD. aP < 0.01. bP < 0.001. 4’,6-diamidino-2-phenylindole (DAPI) (blue) was used to stain cell nuclei, while β-catenin (yellow) was used to visualize its subcellular localization. Merged images combine DAPI and β-catenin staining. Dexmedetomidine (DEX)-low = 2.4 μM DEX; DEX-high = 4.8 μM DEX. Control group: IEC-6 cells maintained in standard culture medium; LPS group: IEC-6 cells treated with 5 μg/mL lipopolysaccharide for 72 hours; LPS + DEX-L group: IEC-6 cells pretreated with 2.4 μM dexmedetomidine for 24 hours followed by treatment with 5 μg/mL lipopolysaccharide for 72 hours; LPS + DEX-H group: IEC-6 cells pretreated with 4.8 μM dexmedetomidine for 24 hours followed by 5 μg/mL lipopolysaccharide for 72 hours; LPS: Lipopolysaccharide; DEX: Dexmedetomidine; L: Low; H: High; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GSK-3β: Glycogen synthase kinase-3 beta.
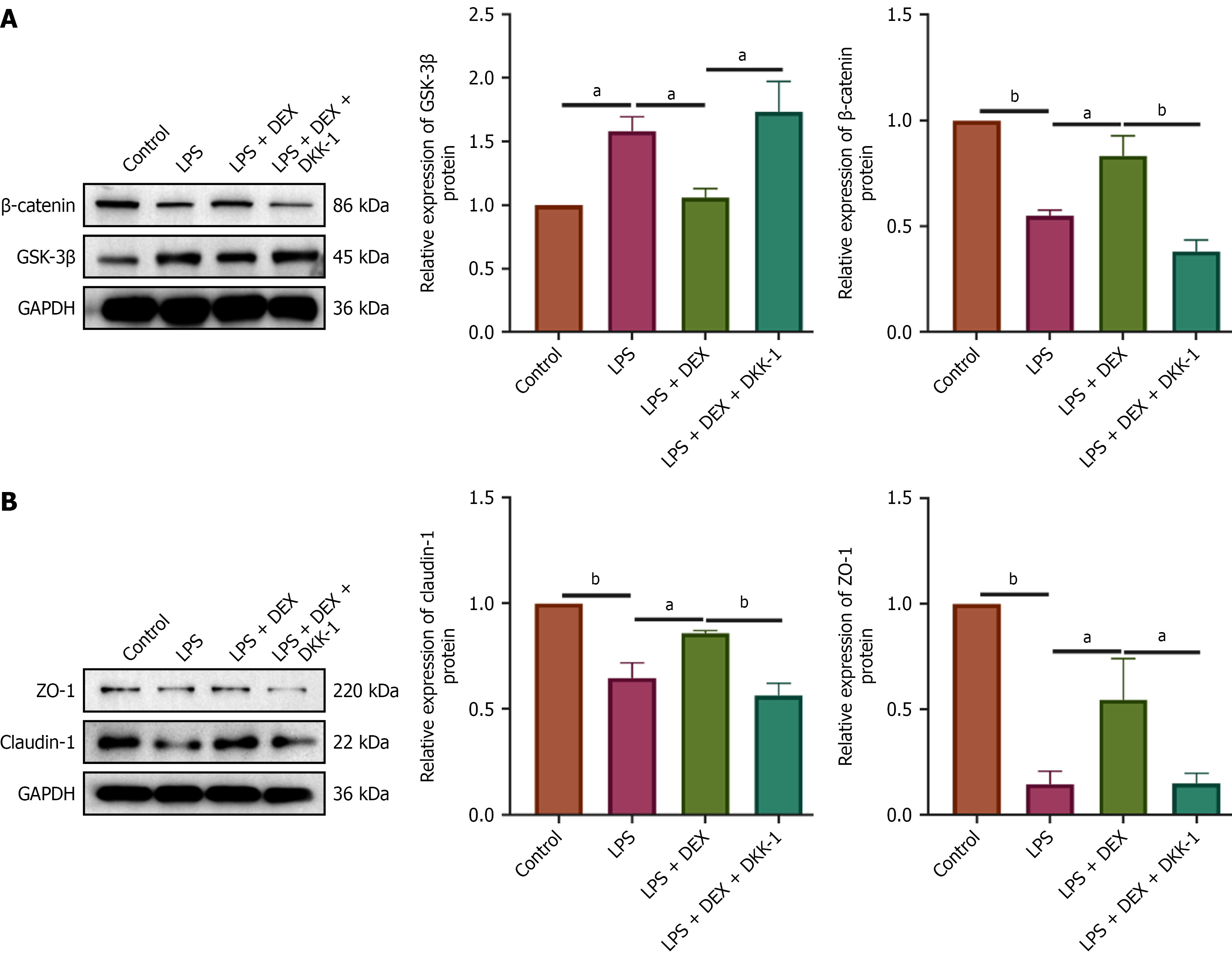
Figure 8 The Wnt/β-catenin signaling pathway inhibitor dickkopf-1 reverses the protective effects of dexmedetomidine on tight junctions in IEC-6 cells.
A: Western blot detection of β-catenin expression levels in each group of cells; B: Western blot detection of zonula occludens-1 (ZO-1) expression levels in each group of cells. Western blot results indicate that dickkopf-1 (DKK-1) effectively inhibits the Wnt signaling pathway, and that inhibiting Wnt signaling reverses dexmedetomidine’s upregulatory effect on claudin-1 and ZO-1 expression levels. aP < 0.01. bP < 0.001. Control group: IEC-6 cells maintained in standard culture medium; LPS group: IEC-6 cells treated with 5 μg/mL lipopolysaccharide for 72 hours; LPS + DEX group: IEC-6 cells pretreated with 4.8 μM dexmedetomidine for 24 hours followed by treatment with 5 μg/mL lipopolysaccharide for 72 hours; LPS + DEX + DKK-1 group: IEC-6 cells pretreated with 4.8 μM dexmedetomidine and 100 ng dickkopf-1 for 24 hours followed by 5 μg/mL lipopolysaccharide for 72 hours; LPS: Lipopolysaccharide; DEX: Dexmedetomidine; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GSK-3β: Glycogen synthase kinase-3 beta; DKK-1: Dickkopf-1; ZO-1: Zonula occludens-1.
- Citation: Chen Y, Li CT, Wang JB, Tang WL, Zhao Y, Chen Y, Liao LM, Zhang LC, Lin TH, Cao ZF. Dexmedetomidine enhances anastomotic healing partly via the Wnt/β-catenin pathway in a rat model of colon surgery. World J Gastroenterol 2025; 31(44): 112833
- URL: https://www.wjgnet.com/1007-9327/full/v31/i44/112833.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i44.112833