©The Author(s) 2025.
World J Gastroenterol. Nov 7, 2025; 31(41): 111174
Published online Nov 7, 2025. doi: 10.3748/wjg.v31.i41.111174
Published online Nov 7, 2025. doi: 10.3748/wjg.v31.i41.111174
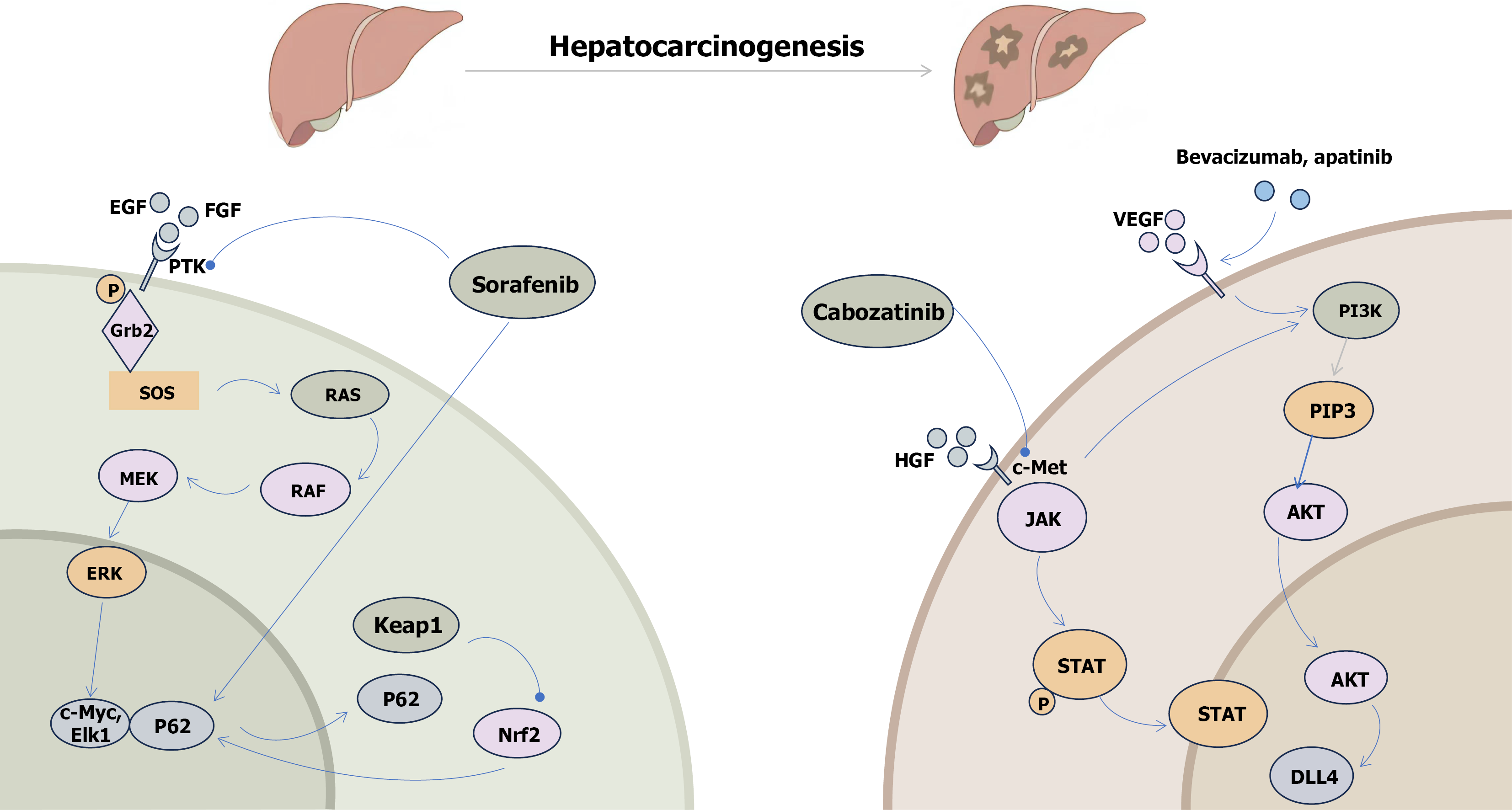
Figure 1 The hepatocarcinogenesis-related signaling pathways and their drugs of action are critical for understanding liver cancer biology and developing targeted therapies.
Sorafenib inhibits the expression of c-Myc and ETS transcription factor 1 through multiple rapidly accelerated fibrosarcoma/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling pathways, including the Janus kinase/signal transducer and activator of the transcription pathway, while promoting the expression of pro-death markers like P62. Cabozantinib exerts its anti-liver cancer effects primarily through the phosphatidylinositol 3-kinase/phosphatidylinositol-(3,4)-P2/protein kinase B pathway. Additionally, bevacizumab and apatinib target liver cancer by inhibiting key downstream signaling molecules in the phosphatidylinositol 3-kinase/phosphatidylinositol-(3,4)-P2/protein kinase B pathway, thereby modulating the cellular response to these anti-tumor therapies. These findings underscore the importance of understanding the molecular mechanisms of drug action in the development of more effective liver cancer treatments. EGF: Epidermal growth factor; FGF: Fibroblast growth factor; PTK: Protein tyrosine kinases; RAF: Rapidly accelerated fibrosarcoma; MEK: Mitogen-activated protein kinase kinase; ERK: Extracellular signal-regulated kinase; Elk1: ETS transcription factor 1; Keap1: Kelch-like ECH-associated protein 1; Nrf2: Nuclear factor erythroid 2-related factor 2; VEGF: Vascular endothelial growth factor; PI3K: Phosphatidylinositol 3-kinase; PIP3: Phosphatidylinositol-(3,4,5)-P3; HGF: Hepatocyte growth factor; AKT: Protein kinase B; STAT: Signal transducer and activator of the transcription; DLL4: Delta-like ligand 4.
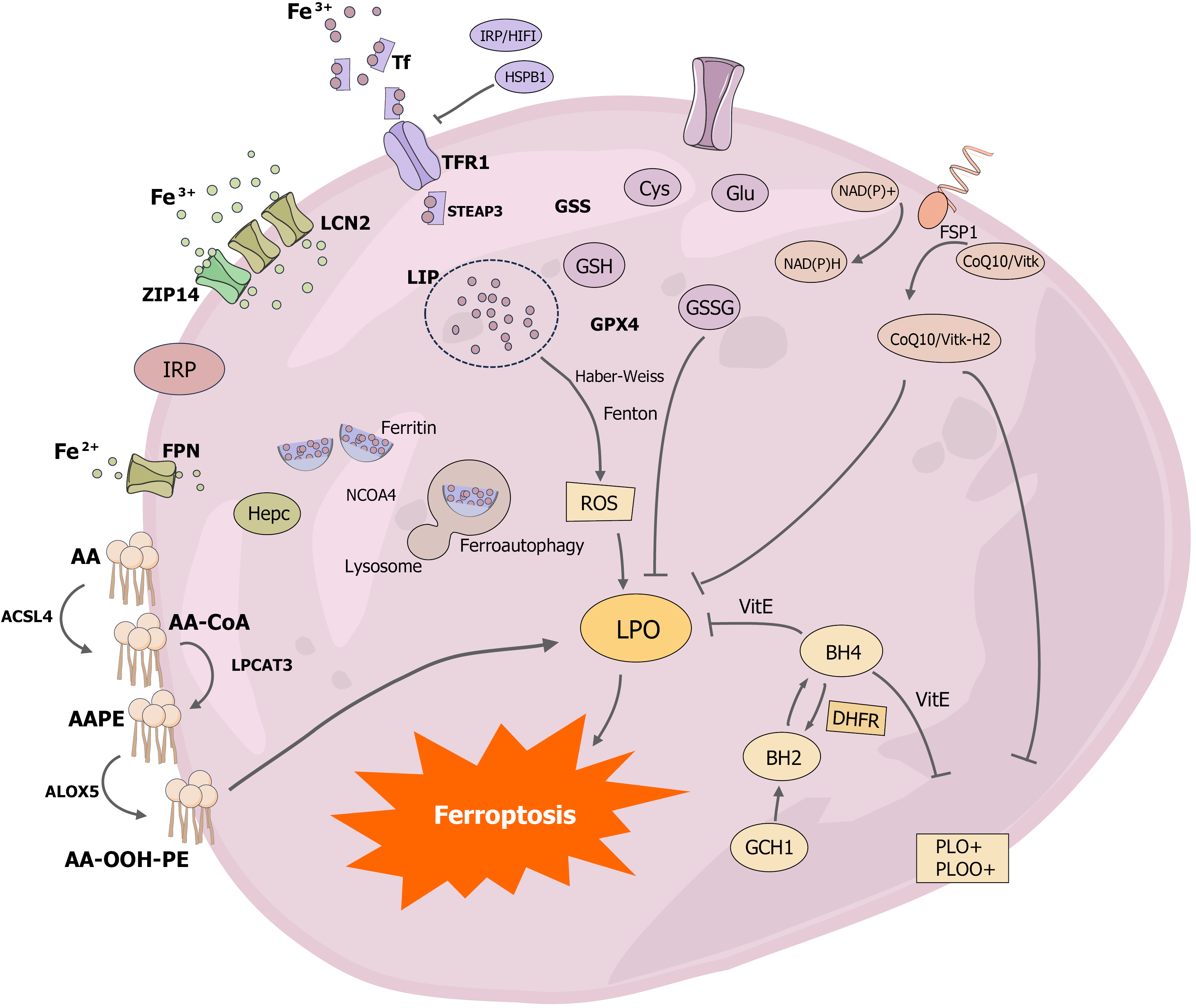
Figure 2 Diagram of the mechanism of development of ferroptosis.
The key molecular mechanisms of ferroptosis have been systematically investigated, with a focus on understanding the pathways and mechanisms of iron-related processes. Ferritin, the primary storage protein for iron in the body, plays a central role in iron metabolism. Iron is primarily acquired in the bloodstream as Fe3+, which enters cells through the transferrin receptor 1 (TFR1) on the cell surface. The expression of TFR1 is regulated by iron regulatory protein and hypoxia-inducible factor-1, while heat shock protein B1 inhibits TFR1 expression. Fe3+ is reduced to Fe2+ by the lipid carrier protein 2, which allows free iron to enter the cytoplasmic iron-unstable pools. Additionally, ZRT/IRT-like proteins such as ZRT/IRT-like protein 14 can also mediate iron entry into the cell. Ferritin, the sole channel protein for Fe2+ export, is primarily regulated by hemosiderin, and ferritin autophagy is a critical mechanism for maintaining intracellular free Fe2+ levels. When autophagy receptor nuclear coactivator 4 is present, ferritin binds to autophagosomes, increasing intracellular free Fe2+ levels. Free iron in the cytoplasmic iron-unstable pools generates reactive oxygen species through the Fenton reaction and Haber-Weiss reaction. Enzymatic pathways, such as long-chain family 4 catalyzing arachidonoyl-CoA formation, lysophosphatidylcholine acyltransferase 3 controlling the esterification of arachidonoyl-CoA to arachidonoyl-phosphatidylethanolamine, and arachidonic acid lipoxygenases oxidizing arachidonoyl-phosphatidylethanolamine to AA-OOH-PE, contribute to the generation of reactive oxygen species. The primary antioxidant pathways in ferroptosis include the cystine/glutamate antiporter/glutathione peroxidase 4/glutathione axis, the ferroptosis suppressor protein 1-CoQ10-NAD(P)H axis, and the GTP cyclohydrolase 1-dihydrofolate reductase-BH4 axis. Notably, the ferroptosis suppressor protein 1-CoQ10-NAD(P)H axis and the GTP cyclohydrolase 1-dihydrofolate reductase-BH4 axis function to inhibit fatty acid synthesis, thereby contributing to the oxidative stress response in ferroptosis. Tf: Transferrin; IRP: Iron regulatory protein; HIF1: Hypoxia-inducible factor 1; HSPB1: Heat shock protein B1; TFR1: Transferrin receptor 1; LCN2: Lipid carrier protein 2; GSS: Glutathione synthetase; ZIP14: ZRT/IRT-like protein 14; GSH: Glutathione; GSSG: Glutathione disulfide; FSP1: Ferroptosis suppressor protein 1; GPX4: Glutathione peroxidase 4; FPN: Ferroportin; ROS: Reactive oxygen species; AA: Arachidonic acid; AA-CoA: Arachidonoyl-CoA; AA-PE: Arachidonoyl-phosphatidylethanolamine; ACSL4: Acyl coenzyme A synthetase long-chain family 4; LPCAT3: Lyso-phosphatidylcholine acyltransferase-3; ALOX5: Arachidonic acid lipoxygenases 5; LPO: Lipid peroxidation; DHFR: Dihydrofolate reductase; GCH1: GTP cyclohydrolase 1.
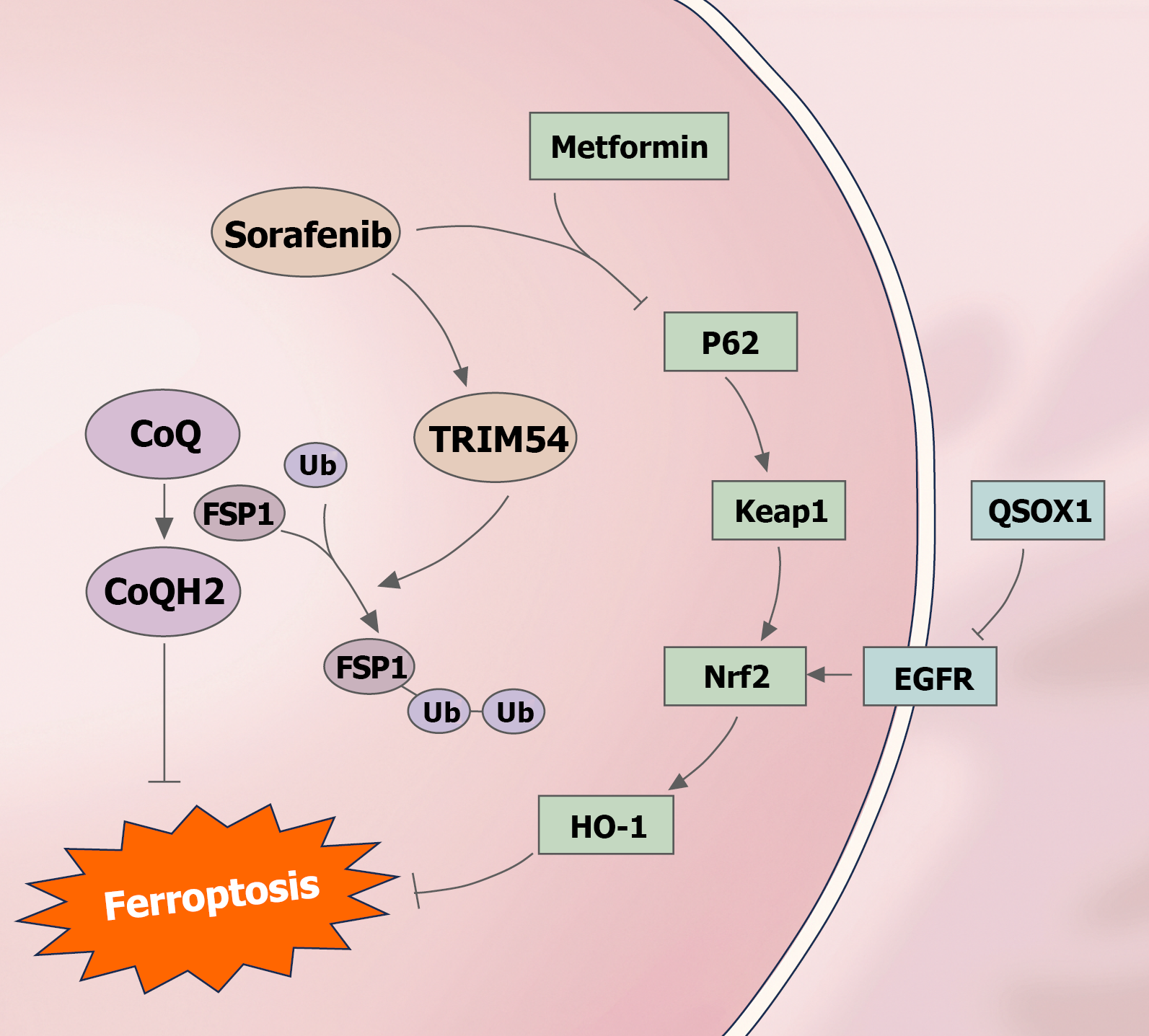
Figure 3 Effects of sorafenib, metformin and quiescin sulfhydryl oxidase 1 on ferroptosis.
Sorafenib enhances the ubiquitination of ferroptosis suppressor protein 1 through TRIM54, leading to the induction of ferroptosis by decreasing CoQH2. Furthermore, quiescin sulfhydryl oxidase 1 induces ferroptosis by inhibiting epidermal growth factor receptor, which subsequently inhibits nuclear factor erythroid 2-related factor 2. Metformin induces ferroptosis by inhibiting the P62/Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2/heme oxygenase 1 pathway. FSP1: Ferroptosis suppressor protein 1; Keap1: Kelch-like ECH-associated protein 1; Nrf2: Nuclear factor erythroid 2-related factor 2; EGFR: Epidermal growth factor receptor; HO-1: Heme oxygenase 1.
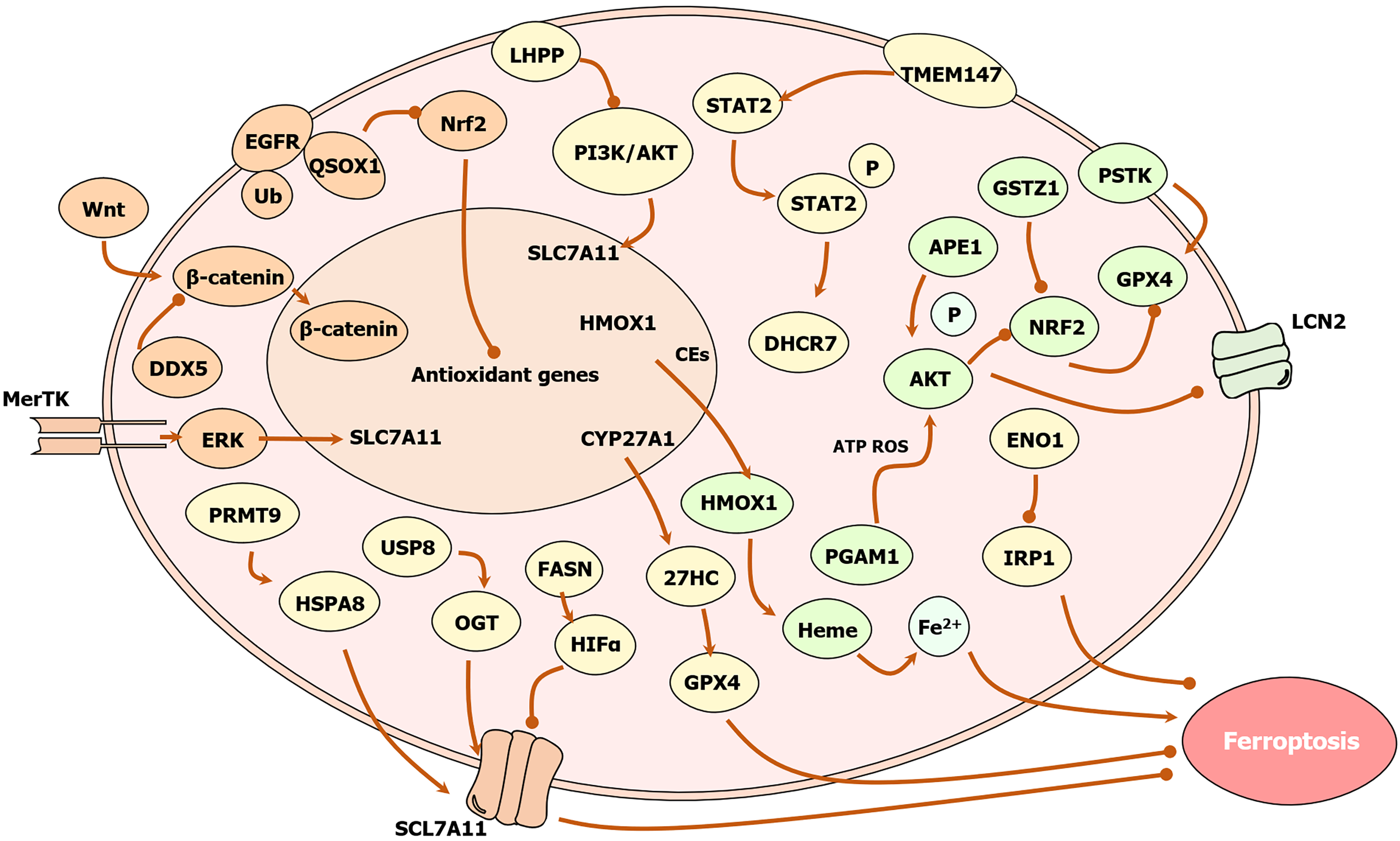
Figure 4 Effect of related enzymes on iron death in hepatocellular carcinoma cells.
Phosphoglycerate mutase 1 inhibits ferroptosis through an energy stress/reactive oxygen species (ROS)-dependent protein kinase B (AKT)/Lipid carrier protein 2 pathway; nicotinamide methyltransferase inhibits ferroptosis via the multifunctional microneedle array/ROS axis; lysine-specific demethylase 4 inhibits ferroptosis via the murine double minute 2/P53/SLC7A11/glutathione peroxidase 4 (GPX4) axis; 7-dehydrocholesterol reductase inhibits ferroptosis through the TMEM147/signal transducer and activator of transcription 2/7-dehydrocholesterol reductase/27HC axis; ubiquitin-specific peptidase 8 (USP8) inhibits ferroptosis through the USP8/O-GlcNAc transferase/SLC7A11 signaling pathway and the Wnt/β-catenin/GPX4 axis; protein arginine methyltransferase 9 inhibits ferroptosis through the hepatitis B virus X protein/protein arginine methyltransferase 9/heat shock protein A8/CD44 axis; α-enolase 1 inhibits ferroptosis through the α-enolase 1-iron regulatory protein 1-Mfrn1 pathway; apurinic/apyrimidinic endonuclease 1 inhibits ferroptosis through the AKT/glycogen synthase kinase-3β/nuclear factor erythroid 2-related factor 2 (Nrf2)/SLC7A11/GPX4 axis; phospho-serine acyl-tRNA kinase inhibits ferroptosis through the thioredoxin reductase/thioredoxin/glutathione/GPX4 pathway; inhibits ferroptosis; dual-specific phosphatase 4 inhibits ferroptosis via the YTHDC1/FTL/ferritin heavy chain 1/Lipid ROS pathway; MER proto-oncogene tyrosine kinase inhibits ferroptosis via the extracellular signal-regulated kinase/SP1/SLC7A11 pathway; ubiquitin C-terminal hydrolase L3 inhibits ferroptosis via the Ub-proteasome/Wnt/β-catenin/GPX4 pathway; aldehyde reductase 1C3 inhibits ferroptosis via the Hippo/YAP/TAZ/SLC7A11 pathway to inhibit ferroptosis; the deubiquitinating enzyme EIF3H inhibits ferroptosis via the O-GlcNAc transferase/Lipid ROS/glutathione pathway; the USP24 promotes ferroptosis via the K48/Beclin1/ferroportin pathway promotes ferroptosis; lysine phosphatase promotes ferroptosis via the lysine phosphatase/phosphatidylinositol-3-kinase/AKT pathway; quiescin sulfhydryl oxidase 1 promotes ferroptosis via the epidermal growth factor receptor/Nrf2/GPX4 pathway; RNA helicase DDX5 promotes ferroptosis via the Wnt/β-catenin/GPX4 signaling pathway to promote ferroptosis; glutathione S-transferase zeta 1 promotes ferroptosis via the Kelch-like ECH-associated protein 1/Nrf2/GPX4 pathway; fatty acid synthase promotes ferroptosis via the fatty acid synthase/hypoxia-inducible factor-1α/SLC7A11 pathway; heme oxygenase 1 promotes ferroptosis through the Nrf2/heme oxygenase 1/GPX4 axis; flavin monooxygenase 3 promotes ferroptosis through the flavin monooxygenase 3/trimethylamine N-oxide/signal transducer and activator of transcription 3/SLC7A11/GPX4 axis. LHPP: Lysine phosphatase; EGFR: Epidermal growth factor receptor; QSOX1: Quiescin sulfhydryl oxidase 1; Nrf2: Nuclear factor erythroid 2-related factor 2; PI3K: Phosphoinositol-3-kinase; AKT: Protein kinase B; STAT2: Signal transducer and activator of transcription 2; GSTZ1: Glutathione S-transferase zeta 1; PSTK: Phospho-serine acyl-tRNA kinase; APE1: Apurinic/apyrimidinic endonuclease 1; MerTK: MER proto-oncogene tyrosine kinase; HMOX1: Heme oxygenase 1; CEs: Cholesterol esters; DHCR7: 7-dehydrocholesterol reductase; GPX4: Glutathione peroxidase 4; LCN2: Lipid carrier protein 2; ERK: Extracellular signal-regulated kinase; ROS: Reactive oxygen species; PRMT9: Protein arginine methyltransferase 9; USP8: Ubiquitin-specific peptidase 8; ENO1: Α-enolase 1; HSPA8: Heat shock protein A8; OGT: O-GlcNAc transferase; FASN: Fatty acid synthase; HIFα: Hypoxia-inducible factor-α; PGAM1: Phosphoglycerate mutase-1; IRP1: Iron regulatory protein 1.
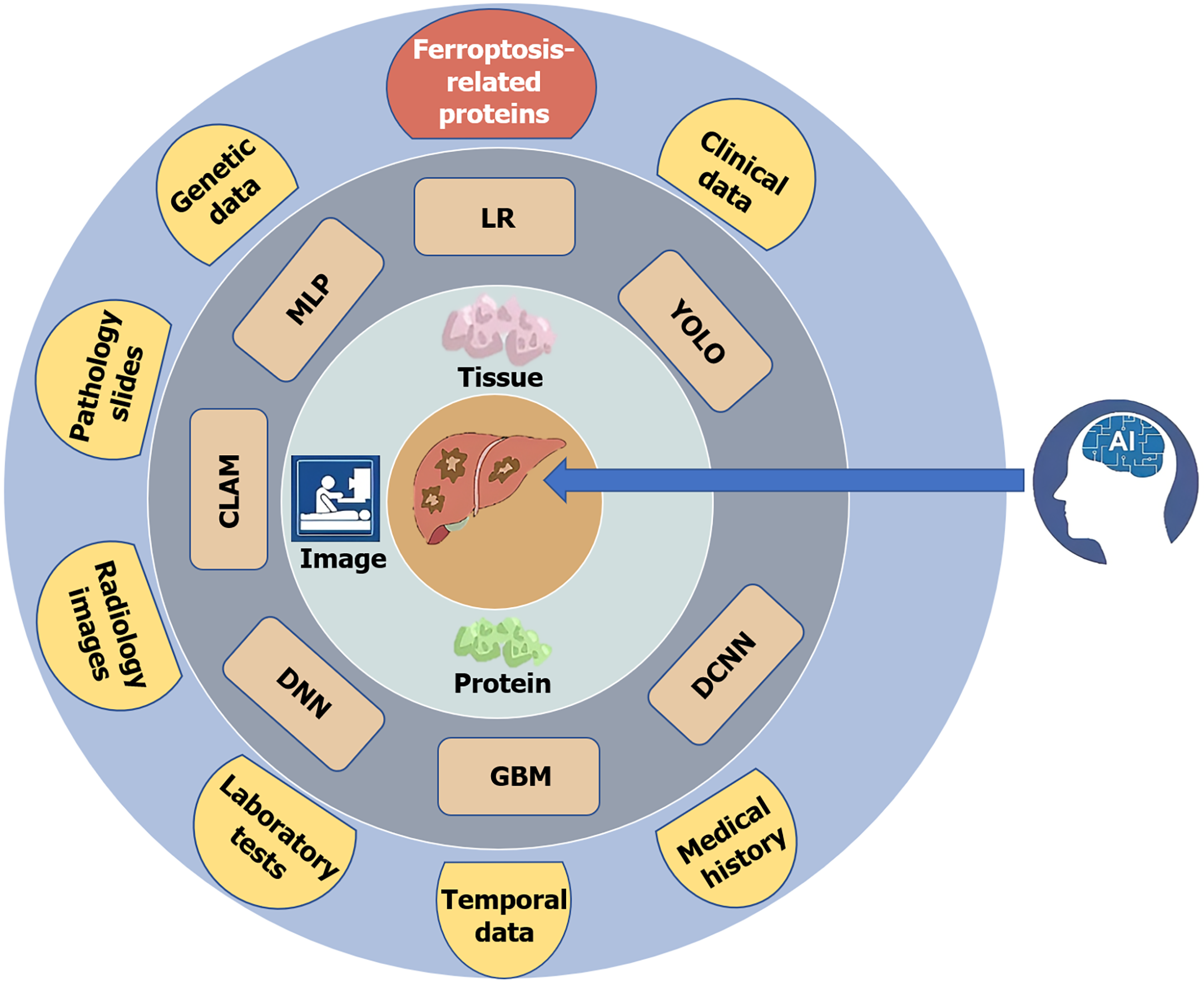
Figure 5 Liver cancer prediction models and associated databases are primarily constructed based on three directions: Images, tissue pathology grading, and protein markers.
Commonly used algorithms include deep neural network, gradient boosting machine, deep convolutional neural network, you only look once, logistic regression, multilayer perceptron, and compressed linear approximation model. During model training, data are mainly drawn from eight sources: Genetic data, pathology slides, radiology images, laboratory tests, temporal data, medical history, clinical data, and ferroptosis-related proteins. LR: Logistic regression; YOLO: You only look once; DCNN: Deep convolutional neural network; GBM: Gradient boosting machine; DNN: Deep neural network; CLAM: Compressed linear approximation model; MLP: Multilayer perceptron; AI: Artificial intelligence.
- Citation: Han JF, Jia ZY, Fan X, Zhao XY, Cheng LY, Xia YX, Ji XR, Zang WQ. Mechanisms of ferroptosis in primary hepatocellular carcinoma and progress of artificial intelligence-based predictive modeling in hepatocellular carcinoma. World J Gastroenterol 2025; 31(41): 111174
- URL: https://www.wjgnet.com/1007-9327/full/v31/i41/111174.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i41.111174