Published online Jun 28, 2021. doi: 10.13105/wjma.v9.i3.309
Peer-review started: April 23, 2021
First decision: May 19, 2021
Revised: May 24, 2021
Accepted: June 17, 2021
Article in press: June 17, 2021
Published online: June 28, 2021
Processing time: 79 Days and 15.5 Hours
Psoriasis is one of the most common chronic systemic diseases, mainly appearing as lesions on the skin and joints, and is associated with a high mortality due to a lack of standard treatment. The exact mechanism of this disease is not fully understood, but the inflammatory response and dysregulation of the immune system are the most important molecular processes that trigger this disease. The skin microflora is one of the main factors involved in inducing, maturing, and dysregulating the immune system, which may underly the development of psoriasis.
To determine the impact of Streptococcus pyogenes (S. pyogenes) infection and susceptibility to psoriasis using available case-control studies.
In this study, we conducted a comprehensive literature search using PubMed, Scopus, Web of science, and Google scholar databases to obtain all available relevant studies on the association between S. pyogenes and psoriasis. We pooled the data using Comprehensive Meta-analysis software to investigate the role of S. pyogenes infection in the development of psoriasis. The probable connection between S. pyogenes and susceptibility to psoriasis was assessed using the odds ratio (OR) with corresponding 95% confidence intervals (CIs).
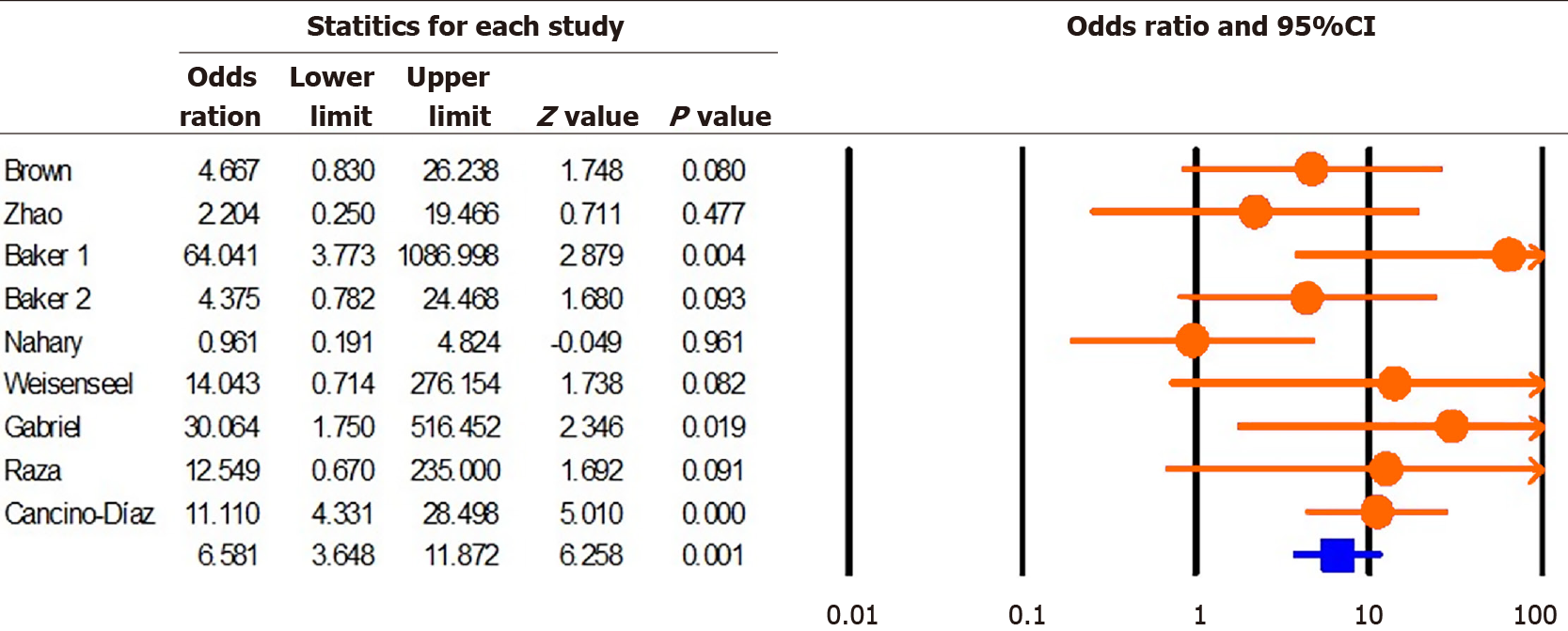
Data from 781 cases were evaluated in this study. Our results showed that the rate of infection with S. pyogenes in psoriatic patients and healthy individuals was 33.4% (95%CI: 27.8-39.6) and 16.2% (95%CI: 9.7-25.9), respectively. S. pyogenes infection significantly increased the risk of psoriasis (OR: 6.58; 95%CI: 3.64-11.87; P = 0.001).
S. pyogenes infection can significantly increase the risk of psoriasis. Thus, infection with S. pyogenes is a risk factor for the initiation and development of psoriatic events.
Core Tip: Currently, the causes of autoimmune diseases are being seriously considered. According to various studies, both genetic and epigenetic events are reasonable hypotheses for these diseases. Psoriasis is one of the most common autoimmune diseases, and studies conducted in recent decades have shown that infectious diseases caused by Streptococcus pyogenes (S. pyogenes) (e.g., pharyngitis) may be associated with psoriasis. Based on these studies, autoantibodies produced against streptococcal M12 protein potentially react with human keratin. Statistical analyses in this study showed that infection with S. pyogenes increased the risk of psoriasis. Thus, long-term treatment as well as strategies to prevent streptococcal infections will be effective in reducing the risk of psoriasis.
- Citation: Yousefi A, Karbalaei M, Keikha M. Impact of Streptococcus pyogenes infection in susceptibility to psoriasis: A systematic review and meta-analysis. World J Meta-Anal 2021; 9(3): 309-316
- URL: https://www.wjgnet.com/2308-3840/full/v9/i3/309.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i3.309
Psoriasis is one of the most prevalent multisystem chronic cutaneous inflammatory diseases, usually characterized by lesions including red, dry, itchy, and scaly plaques on the elbows, knees, scalp, and lower back[1]. This disease occurs at all ages and in all parts of the world[1,2]. Based on recent studies, the prevalence of psoriasis is estimated to be 0%-2.1% in children and 0.91%-8.5% in adults[2]. According to the World Health Organization, the prevalence of psoriasis in different countries is reportedly 0.09%-11.43%, and 100 million people worldwide suffer from this disease annually[3]. This disease affects the quality of life of patients, as well as their physical activities, and emotional and social health. Unfortunately, there are no specific and effective treatments for this disease[2-4].
The underlying cause of psoriasis is not yet fully recognized. However, several factors such as smoking, stress, alcohol consumption, genetics, hormones, and infectious agents can affect the susceptibility of people to psoriasis[5]. Meanwhile, the role of infectious agents in the development of psoriasis is prominent, as the microbiome can dysregulate the immune response by stimulating the inflammatory process, which triggers the development of autoimmune diseases, particularly psoriasis[1-3]. According to the literature, infectious agents such as Staphylococcus aureus, Streptococcus pyogenes (S. pyogenes), Propionibacterium acnes, Helicobacter pylori, and Candida spp. can induce psoriasis[5,6]. In general, Streptococci are a predominant microbiome of the human skin and mucous membrane in the healthy population that can cause numerous opportunistic infections[7]. This group of bacteria contains a variety of enzymes, toxins, and superantigens that can stimulate the immune system and cause psoriasis[8].
The possible interaction between previous infection with S. pyogenesis and the development of psoriasis remain unknown. In addition, the potential association between S. pyogenes and psoriasis has been contradictory in some previously published studies. Hence, for the first time, we conducted this meta-analysis to discuss the molecular aspects of S. pyogenes infection in the development of various psoriatic events using available case-control documents.
We conducted a systematic search using global databases such as PubMed (available at: https://pubmed.ncbi.nlm.nih.gov/), Scopus (Available at: https://www.scopus.com/search/form.uri?display=basic#basic), Web of Science online databases (available at: https://mjl.clarivate.com/search-results), and Google scholar (available at: https://scholar.google.com/) to collect all of the studies related to the possible association between S. pyogenes infection and psoriasis published up to October 2020 without time limits. In this regard, two authors independently searched related articles using the keywords "Streptococcus pyogenes," "Streptococcus group A," "S. pyogenes” and “psoriasis” according to medical subject heading terms.
The initial documents collected met the criteria for selecting eligible documents. Inclusion criteria were: cross-sectional, case-control, and longitudinal studies on the association between S. pyogenes and psoriasis; studies with available full-text; studies on the evaluation of infection frequency in the case and control groups; studies on the assessment of S. pyogenes infection using reliable methods; and studies published in the English language. However, studies including review articles, letters and case-reports; Congress articles; duplicate studies; full-text articles with unclear methods and results; and full-text with repetitive samples were excluded. Disagreements between two authors were resolved by a third author.
The Newcastle-Ottawa Scale checklist was used to evaluate the quality of eligible articles. Subsequently, the required information such as: the first author, publication year, location of studies, number of patients as well as healthy controls, number of S. pyogenes strains in the case/control groups, diagnostic method, and relevant reference number were carefully reviewed by two independent authors. The characteristics of eligible studies are listed in the Table 1.
| Ref. | Location | Total cases | Number of streptococcal strains | Diagnostic method | NOS score | ||
| Case | Control | Case | Control | ||||
| Baker et al[10], 1991 | Iceland | 78 | 27 | 42 | 0 | Antistreptolysin-O | 8 |
| Baker et al[11], 1993 | Iceland | 9 | 18 | 5 | 4 | Skin TCL | 8 |
| Villeda-Gabriel et al[12], 1998 | Mexico | 68 | 56 | 14 | 0 | Immunofluorescence | 6 |
| Brown et al[13], 2000 | United Kingdom | 13 | 12 | 10 | 5 | Skin TCL | 7 |
| Cancino-Díaz et al[14], 2004 | Mexico | 218 | OR: 11.11; 4.33-28.49 | Hsp60 | 5 | ||
| Weisenseel and Prinz[15], 2005 | Germany | 19 | 9 | 8 | 0 | Antistreptolysin-0 | 6 |
| Zhao et al[16], 2005 | China | 98 | 42 | 5 | 1 | Conventional | 7 |
| Raza et al[17], 2007 | Pakistan | 40 | 40 | 5 | 0 | Conventional | 8 |
| Nahary et al[18], 2008 | United Kingdom | 24 | 10 | 7 | 3 | Anti-beta-hemolytic streptococci | 7 |
The meta-analysis was conducted using Comprehensive Meta-Analysis software version 2.2 (Biostat, Englewood, NJ, United States). First, we assessed the frequency of S. pyogenes infection in both patients with psoriasis (case) and healthy individuals groups with 95%CIs. We used the OR with 95%CIs to evaluate the effect of S. pyogenes infection on developing psoriasis. Heterogeneity between studies was determined using the statistical inconsistency and Cochrane Q-test (P < 0.05). The random-effects model, based on the Dersimonian and Laird method, was used when I2> 25% and Cochrane P > 0.05; otherwise, we used the fixed-effects model. In addition, the presence of publication bias was measured using Begg’s and Egger’s tests[9].
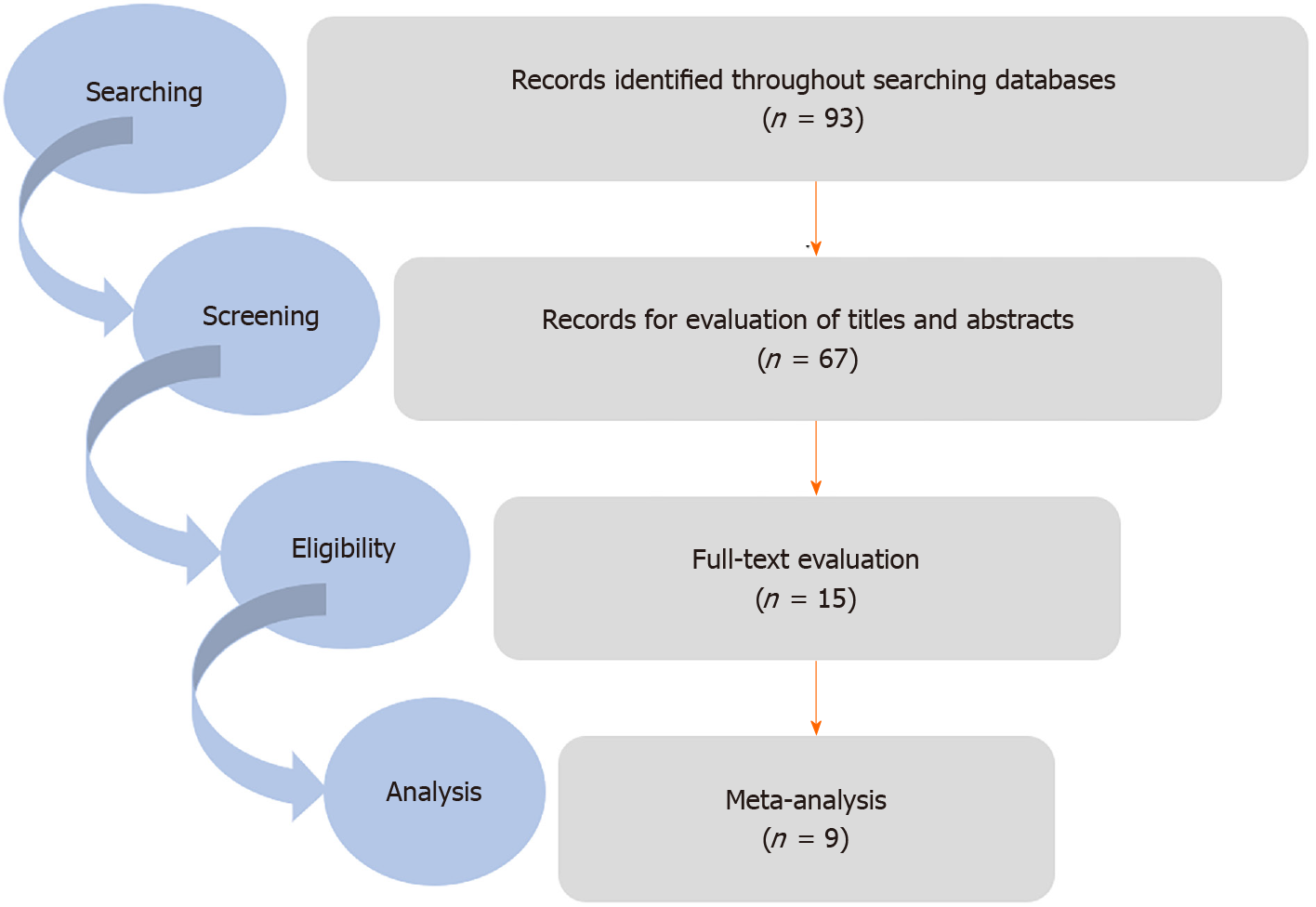
In the initial search, 93 articles were selected. In the next step, duplicate studies were deleted, and finally nine articles were recognized as eligible studies and entered used for quantitative analyses (Figure 1).
In this study, the data were evaluated from 781 cases, including 458 psoriatic patients and 323 healthy individuals. Of the studies included, the number of studies was as follows: two in the United Kingdom, two in Iceland, two in Mexico, one in Pakistan, one in Germany, and one in China[10-18]. These studies were conducted during 1991-2008. In these studies, psoriasis was diagnosed using clinical manifestations or pathology examinations, and infection with S. pyogenes was diagnosed using tests such as skin CD4+ T cell-producing interferon gamma (IFN-γ), conventional microbiology, antistreptolysin-O (ASO) tetier, anti-beta-hemolytic Streptococci, immuno
According to the results of this study, the rate of infection with S. pyogenes in psoriatic patients and healthy individuals was 33.4% (95%CI: 27.8-39.6; P = 0.001; I2 = 88.36; Q = 60.18; P = 0.01; Egger's P = 0.33 and Begg’s P = 0.45) and 16.2% (95%CI: 9.7-25.9; P = 0.001; I2 = 69.52; Q = 22.97; P = 0.01; Egger’s P = 0.04; Begg’s = 0.02), respectively. Moreover, we found that infection with S. pyogenes significantly increased the risk of developing psoriasis (OR: 6.58; 95%CI: 3.64-11.87; P = 0.001; I2 = 33.32; Q = 11.99; P = 0.15; Egger's P = 0.38; Begg's P = 0.23) (Figure 2).
Although the exact etiology of psoriasis is not clear, similar to acute rheumatic fever, the onset of clinical manifestations of only type I (but not type II) of psoriasis are associated with pharyngitis caused by S. pyogenes[19,20]. However, factors such as hyperproliferation of keratinocytes and dysregulation of the immune system are among the most important aspects that play a key role in the immunopathogenesis of psoriasis. The interaction among the microbiome, keratinocytes, T cell lymphocytes, neutrophils, monocytes, and dendritic cells is highly significant in the development of psoriasis[21,22]. Skin microflora, particularly Streptococci, contain several enzymes, toxins, and superantigens that can induce proinflammatory responses, including the production of tumor necrosis factor alpha (TNF-α), IFN-γ, and interleukin 8 (IL-8), as well as production of chemokine receptors, e.g., E-selectin, P-selectin, and CD4 + T cells[23,24]. In addition, recent studies have demonstrated that HLA-Cw*06 effectively presents Streptococci antigens to CD8+ cytotoxic T lymphocytes, leading to a balance distribution between T helper type 1 (Th1) and Th2[23-25]. During chronic inflammation, T-cell lymphocytes are recruited to cutaneous lymph nodes in response to IL-8 and chemokine receptors, and hyperproliferation of keratinocytes also occurs due to high levels of TNF-α and IFN-γ[25,26]. In addition, molecular mimicry between Streptococci antigens and auto-antigens of normal skin can also induce the production of antibodies that cross-react with some normal skin auto-antigens. In a study by Muto et al[27], it was shown that the serum titers of antibodies against the protein M12 (similar to sub-units of human keratin) of S. pyogenes were higher in patients with psoriasis than in control subjects[27-29]. The presence of Streptococci in the chronic psoriasis plaques indicates the role of these bacteria in increasing the risk of psoriasis[30].
For the first time, Shelley et al[31] found that intradermal injection of heat-inactivated group A streptococcus (GAS) in the finger of a 39-year-old woman with psoriasis exacerbated psoriasis skin lesions in her finger. Gross et al[32] showed that psoriatic patients have a specific cellular immune response against GAS antigen. In their studies, Baker et al[10] found that skin T cells in the psoriasis patients are activated and react with M proteins of S. pyogenes. In this study, we found that the infection with S. pyogenes significantly increased the risk of psoriasis (OR: 6.58; 95%CI: 3.64-11.87). According to the literature, the rate of infection with S. pyogenes is high in psoriatic patients. For instance, El-Rachkidy et al[33] demonstrated that a large population of psoriatic patients had high immunoglobulin G titers (> 500) against S. pyogenes. According to studies by Tervaert and Esseveld[34], McFadden et al[35], and Lilja et al[36], the rate of S. pyogenes infection in the psoriatic patients is reportedly 88%-97%. Kim et al[37] showed that the rate of psoriasis in children is significantly associated with ASO serum levels. It seems that streptococci can survive as a facultative intracellular pathogen within the epithelial cells of the tonsils, and as a stable source, continuously inject their antigens into the bloodstream and increase the risk of psoriasis[36,38,39]. Based on an in vitro study by Ruiz-Romeu et al[40] it was demonstrated that S. pyogenes could increase the activity of T cell lymphocytes by inducing Th17 responses in patients with guttate psoriasis. In general, according to previous studies as well as our study, on the one hand, infection with S. pyogenes increases the risk of psoriasis, and on the other hand, long-term treatment and prevention of its infection reduce the risk of psoriasis.
This study had several limitations including: low sample size, the use of English articles only, publication bias, presence of significant heterogeneity, and lack of subgroup analysis or sensitivity analyses to reduce heterogeneity. However, we showed in this study that the rate of S. pyogenes infection in patients with psoriasis was about twice as high as that in healthy individuals. We also showed that infection with S. pyogenes could significantly increase the risk of psoriasis.
S. pyogenes can stimulate the skin through its enzymes, toxins, superantigens, and T-cell lymphocytes and increases the risk of psoriasis by dysregulating the immune response. Hence, S. pyogenes can be considered a risk factor for psoriasis, and prevention from streptococcal infection as well as effective treatment of S. pyogenes infections are among the best strategies to reduce the risk of psoriasis.
Psoriasis is a multifactorial autoimmune disease, and it has been suggested that bacterial infection can contribute to the initiation or development of this disease.
We performed this study to discover the association between infection with Streptococcus pyogenes (S. pyogenes) as potential factors and risk of develop to psoriasis.
The objective of this study was to determine the impact of S. pyogenes infection and susceptibility to psoriasis using available case-control studies.
We used a computer-assisted comprehensive literature search to obtain relevant case-control regarding to the possible connection between S. pyogenes infection and psoriasis. Finally, the impact of infection with S. pyogenes and susceptibility to psoriasis was measured using odds ratio (OR) with 95% confidence intervals (CIs).
The rate of infection with S. pyogenes in psoriatic patients vs healthy individuals was 33.4% and 16.2%, respectively. Furthermore, there is a significant association between S. pyogenes infection and development of psoriasis (OR: 6.58; 95%CI: 3.64-11.87; P = 0.001)
Infection with S. pyogenes is a risk factor for susceptibility to psoriasis.
Further long-term cohort studies are needed to investigate the relationship between S. pyogenes infection and psoriasis. Also, studies are needed to evaluate the clinical efficacy of the treatment of S. pyogenes infection in decreasing the number of psoriatic events.
We thank our colleagues from Mashhad University of Medical Sciences and Jiroft University of Medical Sciences.
| 1. | Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1315] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 2. | Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1887] [Cited by in RCA: 1773] [Article Influence: 136.4] [Reference Citation Analysis (0)] |
| 3. | Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM; Global Psoriasis Atlas. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 741] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 4. | Pietrzak A, Grywalska E, Socha M, Roliński J, Franciszkiewicz-Pietrzak K, Rudnicka L, Rudzki M, Krasowska D. Prevalence and Possible Role of Candida Species in Patients with Psoriasis: A Systematic Review and Meta-Analysis. Mediators Inflamm. 2018;2018:9602362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Dhana A, Yen H, Cho E. All-cause and cause-specific mortality in psoriasis: A systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1332-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Totté JE, van der Feltz WT, Bode LG, van Belkum A, van Zuuren EJ, Pasmans SG. A systematic review and meta-analysis on Staphylococcus aureus carriage in psoriasis, acne and rosacea. Eur J Clin Microbiol Infect Dis. 2016;35:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | DeMuri GP, Wald ER. The Group A Streptococcal Carrier State Reviewed: Still an Enigma. J Pediatric Infect Dis Soc. 2014;3:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Lewis DJ, Chan WH, Hinojosa T, Hsu S, Feldman SR. Mechanisms of microbial pathogenesis and the role of the skin microbiome in psoriasis: A review. Clin Dermatol. 2019;37:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Youssefi M, Tafaghodi M, Farsiani H, Ghazvini K, Keikha M. Helicobacter pylori infection and autoimmune diseases; Is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? J Microbiol Immunol Infect. 2021;54:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 10. | Baker BS, Powles AV, Malkani AK, Lewis H, Valdimarsson H, Fry L. Altered cell-mediated immunity to group A haemolytic streptococcal antigens in chronic plaque psoriasis. Br J Dermatol. 1991;125:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Baker BS, Bokth S, Powles A, Garioch JJ, Lewis H, Valdimarsson H, Fry L. Group A streptococcal antigen-specific T lymphocytes in guttate psoriatic lesions. Br J Dermatol. 1993;128:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Villeda-Gabriel G, Santamaría-Cogollos LC, Pérez-Lorenzo R, Reyes-Maldonado E, Saúl A, Jurado-Santacruz F, Jiménez-Zamudio L, García-Latorre E. Recognition of Streptococcus pyogenes and skin autoantigens in guttate psoriasis. Arch Med Res. 1998;29:143-148. [PubMed] |
| 13. | Brown DW, Baker BS, Ovigne JM, Hardman C, Powles AV, Fry L. Skin CD4+ T cells produce interferon-gamma in vitro in response to streptococcal antigens in chronic plaque psoriasis. J Invest Dermatol. 2000;114:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Cancino-Díaz ME, Ruiz-González V, Ramírez-Reséndiz L, Ortiz B, Domínguez-López ML, Paredes-Cabrera GC, León-Dorantes G, Blancas-González F, Jiménez-Zamudio L, García-Latorre E. IgG class antibodies from psoriasis patients recognize the 60-KDa heat-shock protein of Streptococcus pyogenes. Int J Dermatol. 2004;43:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Weisenseel P, Prinz JC. Incidental detection of S. pyogenes-DNA in psoriatic skin by PCR. Arch Dermatol Res. 2005;296:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Zhao G, Feng X, Na A, Yongqiang J, Cai Q, Kong J, Ma H. Acute guttate psoriasis patients have positive streptococcus hemolyticus throat cultures and elevated antistreptococcal M6 protein titers. J Dermatol. 2005;32:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Raza N, Usman M, Hameed A. Chronic plaque psoriasis: streptococcus pyogenes throat carriage rate and therapeutic response to oral antibiotics in comparison with oral methotrexate. J Coll Physicians Surg Pak. 2007;17:717-720. [PubMed] |
| 18. | Nahary L, Tamarkin A, Kayam N, Sela S, Fry L, Baker B, Powles A, Rogers S, Benhar I. An investigation of antistreptococcal antibody responses in guttate psoriasis. Arch Dermatol Res. 2008;300:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Weisenseel P, Laumbacher B, Besgen P, Ludolph-Hauser D, Herzinger T, Roecken M, Wank R, Prinz JC. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Allen HB, Miller B, Durkin J, Joshi SG. Psoriasis: a sequela of streptococcal infection similar to acute rheumatic fever. Clin Microbiol. 2016;5:2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Eberle FC, Brück J, Holstein J, Hirahara K, Ghoreschi K. Recent advances in understanding psoriasis. F1000Res. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol. 2012;9:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 276] [Article Influence: 19.7] [Reference Citation Analysis (1)] |
| 23. | El Ferezli J, Jenbazian L, Rubeiz N, Kibbi AG, Zaynoun S, Abdelnoor AM. Streptococcus sp. and Staphylococcus aureus isolates from patients with psoriasis possess genes that code for toxins (superantigens): clinical and therapeutic implications. Immunopharmacol Immunotoxicol. 2008;30:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Maciejewska-Radomska A, Szczerkowska-Dobosz A, Rębała K, Wysocka J, Roszkiewicz J, Szczerkowska Z, Placek W. Frequency of streptococcal upper respiratory tract infections and HLA-Cw*06 allele in 70 patients with guttate psoriasis from northern Poland. Postepy Dermatol Alergol. 2015;32:455-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Muto M, Fujikura Y, Hamamoto Y, Ichimiya M, Ohmura A, Sasazuki T, Fukumoto T, Asagami C. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37 Suppl 1:S54-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Prinz JC. Disease mimicry--a pathogenetic concept for T cell-mediated autoimmune disorders triggered by molecular mimicry? Autoimmun Rev. 2004;3:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Ovigne JM, Baker BS, Davison SC, Powles AV, Fry L. Epidermal CD8+ T cells reactive with group A streptococcal antigens in chronic plaque psoriasis. Exp Dermatol. 2002;11:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Shelley WB, Wood MG, Beerman H. Pustular psoriasis elicited by streptococcal antigen and localized to the sweat pore. J Invest Dermatol. 1975;65:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Gross WL, Packhäuser U, Hahn G, Westphal E, Christophers E, Schlaak M. Lymphocyte activation by streptococcal antigens in psoriasis. Br J Dermatol. 1977;97:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | El-Rachkidy RG, Hales JM, Freestone PP, Young HS, Griffiths CE, Camp RD. Increased blood levels of IgG reactive with secreted Streptococcus pyogenes proteins in chronic plaque psoriasis. J Invest Dermatol. 2007;127:1337-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Tervaert WC, Esseveld H. A study of the incidence of haemolytic streptococci in the throat in patients with psoriasis vulgaris, with reference to their role in the pathogenesis of this disease. Dermatologica. 1970;140:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | McFadden JP, Baker BS, Powles AV, Fry L. Psoriasis and streptococci: the natural selection of psoriasis revisited. Br J Dermatol. 2009;160:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Lilja M, Silvola J, Räisänen S, Stenfors LE. Where are the receptors for Streptococcus pyogenes located on the tonsillar surface epithelium? Int J Pediatr Otorhinolaryngol. 1999;50:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Kim SK, Kang HY, Kim YC, Lee ES. Clinical comparison of psoriasis in Korean adults and children: correlation with serum anti-streptolysin O titers. Arch Dermatol Res. 2010;302:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Fry L, Powles AV, Corcoran S, Rogers S, Ward J, Unsworth DJ. HLA Cw*06 is not essential for streptococcal-induced psoriasis. Br J Dermatol. 2006;154:850-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, Olafsson JH, Petersen H, Sigurdsson MI, Gudjonsson JE, Johnston A, Valdimarsson H. HLA-Cw6 homozygosity in plaque psoriasis is associated with streptococcal throat infections and pronounced improvement after tonsillectomy: A prospective case series. J Am Acad Dermatol. 2016;75:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Ruiz-Romeu E, Ferran M, Sagristà M, Gómez J, Giménez-Arnau A, Herszenyi K, Hóllo P, Celada A, Pujol R, Santamaria-Babí LF. Streptococcus pyogenes-induced cutaneous lymphocyte antigen-positive T cell-dependent epidermal cell activation triggers TH17 responses in patients with guttate psoriasis. J Allergy Clin Immunol 2016; 138: 491-499. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Muthu S, Sun Q S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li X