Published online Jun 28, 2021. doi: 10.13105/wjma.v9.i3.317
Peer-review started: April 23, 2021
First decision: June 7, 2021
Revised: June 13, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: June 28, 2021
Processing time: 79 Days and 15.1 Hours
Listeria monocytogenes (L. monocytogenes) is one of the most important zoonotic bacteria that is transmitted to humans through infected animal products and is the cause of human listeriosis. Pregnant women and immunocompromised patients are more susceptible to the bacterium than healthy people. Recent studies have reported extensive evidence on the role of L. monocytogenes infection and the risk of spontaneous abortion.
To evaluate the possible connection with L. monocytogenes in the risk of spontaneous abortion in pregnancy.
We conducted a systematic literature review using several databases to search the relevant case-control studies on the association between L. monocytogenes infection and spontaneous abortion. Finally, the impact of infection with L. monocytogenes and risk of spontaneous abortion was assessed via odds ratio at corresponding 95% confidence intervals.
In the present study, we evaluated the data of 4059 pregnant women who had a spontaneous abortion, and interestingly their colonization rate of L. monocytogenes was about 20.5%.
Therefore, based on statistical analysis, we found that there is a significant relationship between the infection with L. monocytogenes and spontaneous abortion.
Core Tip: Listeria monocytogenes is one of the most common bacterial infections among developing countries. Acquisition of this bacterium during pregnancy is dangerous for the health of neonates. Our results suggested that Listeria monocytogenes can significantly increase the risk of spontaneous abortion during pregnancy.
- Citation: Yousefi A, Karbalaei M, Keikha M. Extraintestinal infection of Listeria monocytogenes and susceptibility to spontaneous abortion during pregnancy: A systematic review and meta-analysis. World J Meta-Anal 2021; 9(3): 317-326
- URL: https://www.wjgnet.com/2308-3840/full/v9/i3/317.htm
- DOI: https://dx.doi.org/10.13105/wjma.v9.i3.317
Spontaneous abortion is one of the biggest health challenges that has increased in recent decades, especially due to in vitro fertilization methods, such as in vitro fertilization, gamete intrafallopian transfer, and intracytoplasmic sperm injection[1]. About 1% of women suffer from recurrent miscarriage, so this phenomenon can be consi
L. monocytogenes is a gram-positive, non-spore forming, facultative anaerobe, and motile bacterium that naturally resides in sources such as water, soil, vegetables, dairy products, and processed food[7]. These bacteria enter the human body through the fecal-oral route and can withstand harsh environmental conditions such as acidic pH, high salt concentration, and low temperature[8]. This bacterium can cross the intestinal barrier and enter various tissues through hematogenous dissemination, particularly the placenta-fetal unit and the cerebrospinal fluid[9,10]. Studies show that pregnant women are about 17 times more likely to be infected with L. monocytogenes than the general population[11]. Numerous studies have been performed on the effects of infection of L. monocytogenes and abortion in humans and animals[12]. The aim of this study was to investigate the effects of extraintestinal infection with this pathogen and susceptibility to spontaneous abortion. We estimated the frequency of L. monocytogenes colonization in the women who had a spontaneous miscarriage.
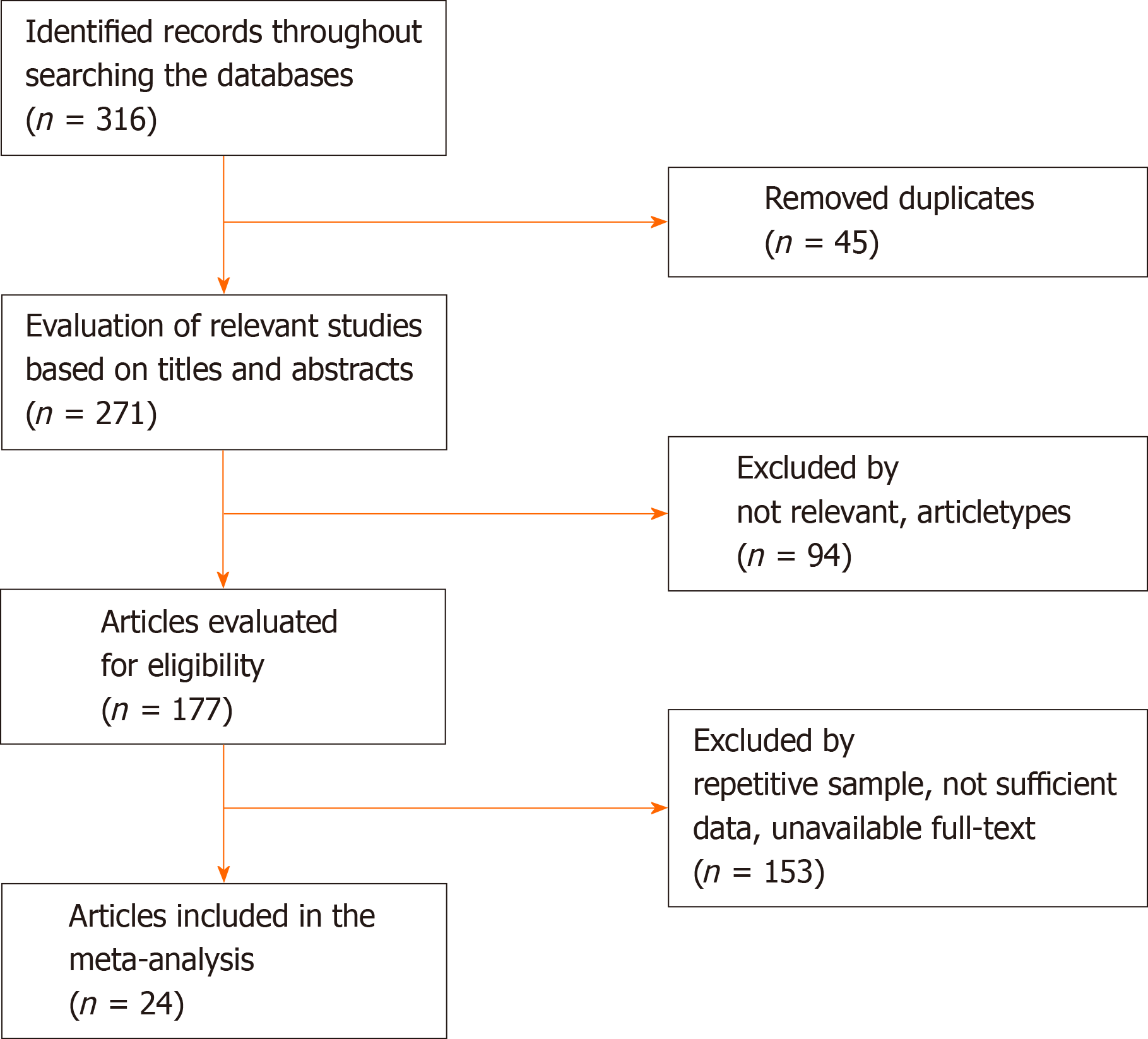
Initially, a systematic search of global databases such as PubMed, Scopus, Google Scholar, and SID was conducted to collect all articles on the effects of L. monocytogenes infection on spontaneous abortion, regardless of publication date or language restrictions. Search terms were selected based on Medical Subject Headings browser including “Listeria monocytogenes,” “Spontaneous abortion,” “Pregnant women,” and “Pregnancy.” Articles published in Persian, Arabic, and English were the only articles evaluated. After reviewing the titles and abstracts of eligible original articles (case-control studies, cross-sectional, and longitudinal studies), we selected those studies that were related to the effect of L. monocytogenes infection on spontaneous abortion. The process of searching and evaluating articles was done by two authors independently, and the discrepancies were examined by the third author. Duplicate articles, insufficient and vague information, case reports, and review articles were excluded from the study (Figure 1).
The required information such as first author, publication year, country, number of cases with at least one spontaneous abortion, number of L. monocytogenes strains, frequency of hlyA gene, frequency of serotypes, diagnostic methods, and the reference number were extracted from the eligible studies and are presented in Table 1. An important evaluation checklist of the Joanna Briggs Institute was used to evaluate the quality of the studies in the present meta-analysis.
| Ref. | City | Number of patients | Number of positive culture for L. monocytogenes | Number of hylA positive strains | Frequency of serotypes | Diagnostic method | ||
| Case | Control | Case | Control | |||||
| Aljicević et al[13], 2005 | Bosnia and Herzegovina | 30 | 30 | 18 | 8 | ND | ND | Serology |
| Kaur et al[14], 2007 | India | 61 | ND | 4 | ND | 3 | ND | Convectional |
| Saeedi et al[15], 2009 | Iran | 118 | 99 | 9 | 3 | ND | ND | IgG |
| Jamshidi et al[16], 2009 | Iran | 250 | 200 | 89 | 35 | ND | ND | IgG |
| Tahery et al[17], 2009 | Iran | 102 | 102 | 82 | 20 | ND | ND | IFA |
| Nazeri[18], 2011 | Iran | 512 | ND | 6 | ND | ND | ND | PCR |
| Lotfollahi et al[19], 2011 | Iran | 100 | ND | 9 | ND | ND | ND | Convectional |
| Jahangirisiskht et al[20], 2012 | Iran | 190 | 120 | 9 | 2 | 11/107 | ND | Convectional |
| Jahangirsisakht et al[21], 2013 | Iran | 64 | ND | 7 | ND | 10.28 | ND | PCR |
| Shindang et al[22], 2013 | Nigeria | 200 | ND | 14 | ND | ND | 4b (71%); 1/2a (28) | Convectional |
| Eslami et al[23], 2014 | Iran | 96 | ND | 16 | ND | 16 | ND | PCR |
| Shoukat et al[24], 2014 | India | 141 | ND | 4 | ND | 4 | ND | Convectional |
| Haghroosta et al[25], 2015 | Iran | 120 | 60 | 25 | 4 | ND | ND | IFA |
| Pourkaveh et al[26], 2016 | Iran | 317 | ND | 54 | ND | 11 | PCR | |
| Bobade et al[27], 2016 | Nagpur | 113 | ND | 11 | ND | 11 | 4b (81%); 1/2b (18%) | PCR |
| Tajedini et al[28], 2017 | Iran | 58 | ND | 21 | ND | ND | ND | IFA |
| Pour et al[29], 2018 | Iran | 123 | ND | 28 | ND | 28 | 1/2a (50%); 4b (35%) | PCR |
| Heidari et al[30], 2018 | Iran | 100 | ND | 4 | ND | 4 | ND | Convectional |
| Al-dorri[31], 2018 | Iraq | 94 | ND | 11 | ND | 11 | ND | PCR |
| Ohadi et al[32], 2019 | Iran | 96 | ND | 16 | ND | 16 | 1/2b (31%); 1/2a (25%); 4b (12.5%) | PCR |
| Mozaffari et al[33], 2019 | Iran | 130 | 100 | 35 | 11 | ND | ND | IFA |
| Zahirnia et al[34], 2019 | Iran | 124 | 76 | 31 | 28 | 3 | ND | PCR |
| Fall et al[35], 2020 | Senegal | 43 | ND | 2 | ND | ND | ND | PCR |
| Al-Mayahi and Jaber[36], 2020 | Iraq | 90 | ND | 15 | ND | 15 | ND | PCR |
The comprehensive meta-analysis software ver 2.0 (Biostat, Englewood, NJ, United States) was used for data pooling as well as statistical analysis of the extracted data. The frequency of L. monocytogenes infection in women with spontaneous abortion was measured as the incidence rate (event rate) with 95% confidence intervals (CIs) using cross-sectional and case-control studies. In addition, the frequency of the hlyA gene as well as common serotypes isolated from women with spontaneous abortion were also examined as the incidence rate. The effect of infection with this bacterium on the susceptibility to spontaneous abortion was assessed according to odds ratio with the 95%CIs using eligible case-control studies. Finally, the frequency of resistance of L. monocytogenes strains to different antibiotics was calculated and reported as the incidence rate and heterogeneity between studies was determined using I2 index and Cochran’s Q-test. In cases with high heterogeneity (I2 > 25% and Cochran’s Q-test P > 0.05), the analysis was done using the random-effects model. The publication bias was also determined using Egger’s P value, Begg’s P value, and asymmetry of funnel plots.
Of the 732 initial studies collected, 24 studies were eligible and entered in our meta-analysis (Figure 1)[13-36]. Of these, 17 studies were conducted in Iran, 2 studies in India, 2 studies in Iraq, 1 study in Bosnia and Herzegovina, 1 study in Nigeria, 1 study in Nagpur, and 1 study in Senegal. Overall, in selected studies L. monocytogenes (strains were isolated from urine, blood, placenta, vaginal swabs, and cervix swabs). In general, microbiological methods such as cold-enrichment, culture on PALCAM agar, culture on blood agar, as well as Gram-staining, oxidase, catalase, carbohydrates fermentation, methyl red/Voges-Proskauer, and motility at 25 °C were used to isolate and identify L. monocytogenes strains. In addition, the authors had used polymerase chain reaction technique (for genes such as hlyA, InlA, InlB, actA, iap, plcA, and PrfA) and serological tests on blood samples in their studies (Table 1).
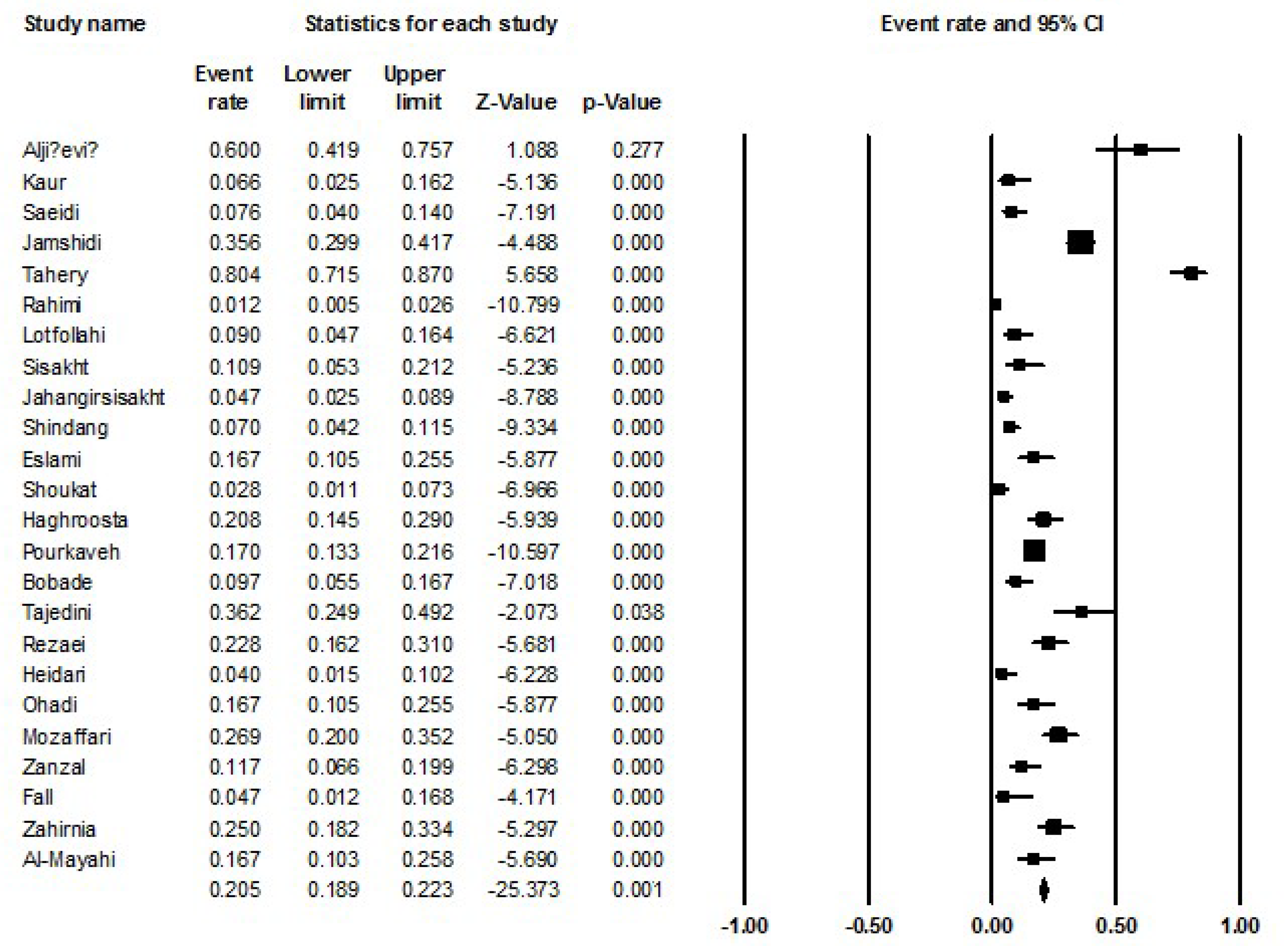
The prevalence of L. monocytogenes infection in women undergoing spontaneous abortion was approximately 20.5% (18.9-22.3 with 95%CIs; P = 0.001; I2: 93.85; Q-value: 374.16; P = 0.001; Egger’s P = 0.05; Begg’s P = 0.01) was estimated (Figure 2).
The frequency of the hlyA gene in L. monocytogenes strains isolated from patients was estimated to be about 28.0% (21.0%-36.3% with 95%CI; P= 0.001; I2: 87.34; Q-value: 94.84; P = 0.001; Begg’s P = 0.03; Egger’s P = 0.001). Also, abundance of serotypes 1/2a, 12/b, and 4b were measured to be about 38.7% (26.8%-52.2% with 95%CIs), 26.5% (13.1%-46.3% with 95%CIs), and 49.5% (32.8%-59.5% with 95%CIs), respectively.
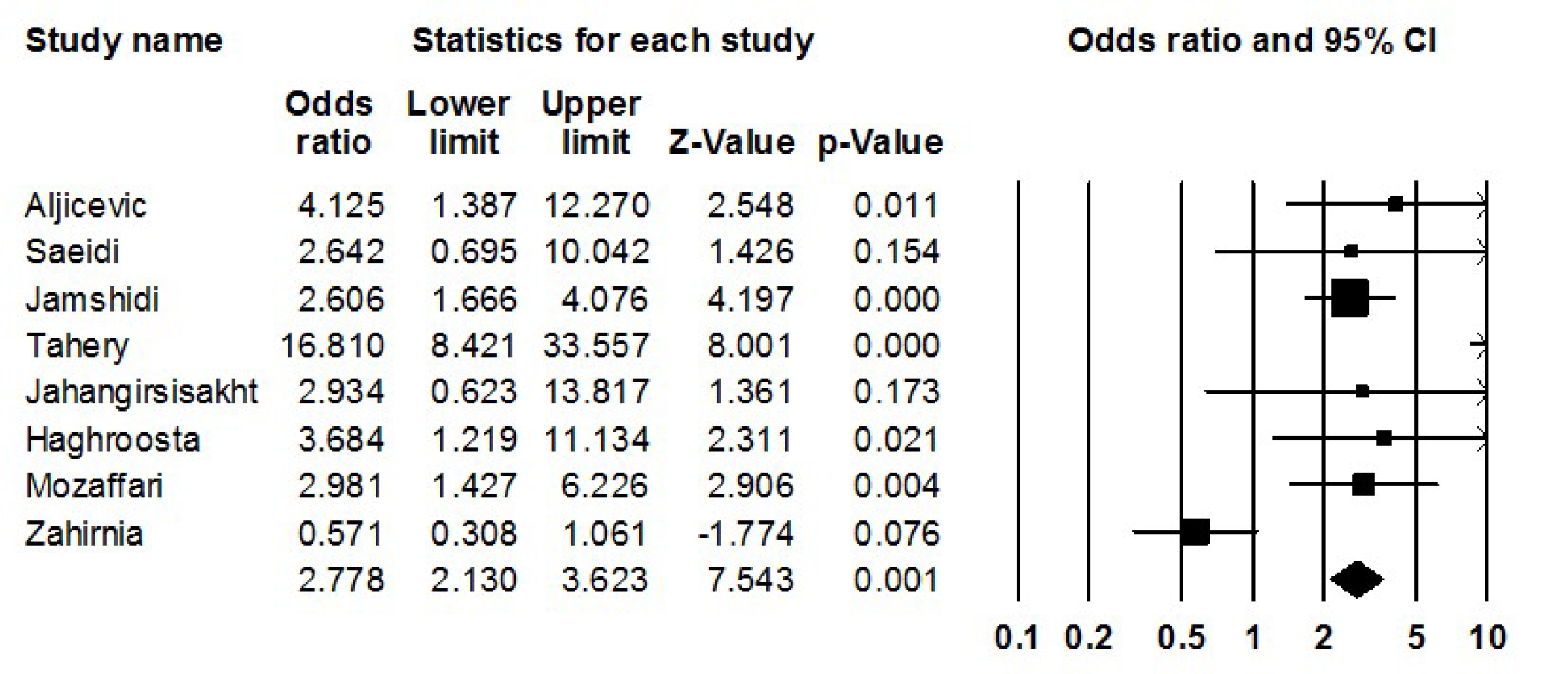
Furthermore, it was revealed that the infection with L. monocytogenes significantly increases the risk of spontaneous abortion in pregnant women (odds ratio: 2.778; 2.130-3.623 with 95%CIs; P = 0.001; I2: 76.30; Q-Value: 21.01; P = 0.001; Egger’s P = 0.38; Begg’s P = 0.51) (Figure 3).
The current analysis showed that L. monocytogenes strains isolated from spontaneous abortion cases were resistant to various antibiotics. The resistance to different antibiotics was as follows: ampicillin 31.8% (25.5-38.9 with 95%CIs; I2: 92.96; Q-Value: 56.84; P = 0.001), penicillin G 56.8% (51.4-62.0 with 95%CIs; I2: 94.64; Q-Value: 74.67; P = 0.001), cotrimoxazole 33.0% (24.5-42.8 with 95%CIs; I2: 0.00; Q-Value: 0.00; P = 1.00), cephalothin 50.0% (40.3-59.7 with 95%CIs; I2: 0.00; Q-Value: 0.00; P = 1.00), tetracycline 29.2% (23.2-36.1 with 95%CIs; I2: 91.75; Q-Value: 36.37; P = 0.001), erythromycin 15.0% (9.5-22.8 with 95%CIs; I2: 91.96; Q-Value: 37.31 ; P = 0.001), cefotaxime 77.0% (70.7-82.3 with 95%CIs; I2: 0.00; Q-Value: 0.00; P = 1.00), chloramphenicol 11.0% (7.4-16.1 with 95 % CIs; I2: 0.00; Q-Value: 0.00; P = 1.00), trimethoprim 38.3% (30.5-46.8 with 95%CIs; I2: 95.47; Q-Value: 66.26; P = 0.01), ciprofloxacin 11.1% (6.8-17.7 with 95%CIs; I2: 82.1; Q-Value: 11.18; P = 0.04), gentamycin 28.0% (20.0-37.6 with 95%CIs; I2: 0.00; Q-Value: 0.00; P = 1.00), and streptomycin 11.0% (7.4-16.1 with 95%CIs; I2: 0.00; Q-Value: 000; P = 1.00). No resistance has been reported to the three antibiotics amikacin, meropenem, and norfloxacin.
Listeriosis is one of the most well-known foodborne diseases that is transmitted to humans through the consumption of contaminated animal products. Several outbreaks of listeriosis have been reported worldwide, with children being the main victims[37]. According to studies, the prevalence of listeriosis per 100000 people in children, the elderly, and pregnant women is estimated at 3.4, 10.0, and 12.0, respectively. Pregnant women can pass listeriosis to their baby in the womb or at birth[38]. Latent listeriosis in pregnant women can have fatal effects on the fetus such as granulomatosis infantiseptica, spontaneous abortion, stillbirth, premature birth, and meningitis[39]. According to the Centers for Disease Control and Prevention, about 14% of human listeriosis occurs in pregnant women. Therefore, the diagnosis of listeriosis during pregnancy is essential, especially in developing areas (Listeria spp. are more likely to be contaminating food in these areas)[40].
Based on the current analysis, it was shown that the rate of colonization with L. monocytogenes in women who had spontaneous abortions was about 20.5%, although in some studies, L. ivanovii and L. seeligeri were the cause of infection and abortion. For the first time, in the current meta-analysis, we evaluated the association of colonization with L. monocytogenes in pregnant women with spontaneous abortion, and most of the studies considered in the current analysis were related to Iran. Iran as a developing country has a high rate of L. monocytogenes infection, which in turn is related to working conditions, lifestyle, and geographical location. In another meta-analysis study from Iran, the rate of colonization with L. monocytogenes in humans, animals, and food products was estimated at 10%, 7%, and 4%, respectively[41].
Cell-mediated immunity is somewhat suppressed during pregnancy, while humoral immunity is as active as ever. Therefore, pregnancy increases the risk of infection with facultative intracellular bacteria such as L. monocytogenes. Meanwhile, the human placenta also provides a protective niche for the growth and proliferation of this bacterium, which in turn increases the risk of spontaneous abortion[42]. We found that infection with L. monocytogenes can significantly increase the risk of miscarriage during pregnancy (odds ratio: 2.7; 2.1-3.6 with 95%CI; P = 0.01).
So far, four lineages have been identified from L. monocytogenes strains, and it should be noted that lineages I (serotypes 1/2b and 4b) and II (1/2a) are responsible for most human infections. In this study, the frequencies of serotypes 1/2a, 1/2b, and 4b were reported as 38.7%, 26.5%, and 49.5%, respectively, which were similar to the distribution of serotypes in other human listeriosis infections[27,29]. In pathogenic strains, numerous virulence factors are encoded by pathogenicity island 1. The hlyA gene encodes hemolysin (listeriolysin-O), which is one of the most important virulence factors of L. monocytogenes[43]. According to our analysis, only 28% of strains isolated from women with spontaneous miscarriage had this gene, indicating the low diagnostic value of this gene for diagnosis of L. monocytogenes infection in pregnant women.
Based on the evidence, β-lactam antibiotics alone or in combination with aminoglycosides as well as cotrimoxazole are recommended treatment options for severe listeriosis infections. Although, nowadays, the resistance of Listeria spp. to β-lactams and aminoglycosides is increasing[44,45]. Some L. monocytogenes strains are also resistant to other antibiotics such as fluoroquinolones, macrolides, and tetracyclines, and the emergence and spread of the drug-resistant strains in pregnant women can be life-threatening for both mother and child[44]. Our results showed that the antibiotic resistance of L. monocytogenes strains isolated from the women with spontaneous abortion was high. The highest resistance was related to cefotaxime (77%), and the lowest was related to chloramphenicol and streptomycin (both 11%); all strains were sensitive to norfloxacin, meropenem, and amikacin. In a recent meta-analysis study by Khademi and Sahebkar[44], resistance of L. monocytogenes strains isolated from Iranian patients to penicillin, ampicillin, and gentamicin was reported to be 56.8%, 29.5%, and 32.4%, respectively, which was close to the results of our study[44]. Quentin et al[46] also identified a multi-resistant strain of L. monocytogenes in a septic abortion[46]. It is important to note that increasing drug resistance of L. monocytogenes strains to β-lactams and aminoglycosides in the coming years will lead to the ineffectiveness of these antibiotics in the treatment of human listeriosis.
Our meta-analysis has several limitations as following: (1) The population sample size was low; (2) Heterogeneity was significant; (3) There is a slight publication bias; and (4) We included only available articles in Persian, Arabic, and English. Overall, our results showed that there is a significant relationship between L. monocytogenes infection and the increased risk of miscarriage during pregnancy. The results of the present analysis also showed that drug resistance is increasing among L. monocytogenes strains, which needs to be re-evaluate. If necessary, treatment guidelines should be updated to reduce the incidence of human listeriosis.
Overall, regarding the importance of microorganisms such as Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, L. monocytogenes, herpes simplex viruses, adenoviruses, human polyomaviruses, and cytomegalovirus in the induction of spontaneous abortion, it is important that all aspects of these pathogens, such as diagnosis, treatment, control, and vaccination, be considered by all researchers around the world.
Spontaneous abortion is one of the most important concerning issues in pregnant women, and it has been suggested that Listeria monocytogenes (L. monocytogenes) infection can play a key role in pathogenesis of this disease.
We conducted the present study to estimate the risk of infection with L. monocytogenes in the development of spontaneous abortion during pregnancy.
The aim of this study was evaluation of the probable connection between infection with L. monocytogenes and risk of spontaneous abortion in pregnancy.
We conducted a systematic literature review using several databases to search the relevant case-control studies on the association between L. monocytogenes infection and spontaneous abortion. Finally, the impact of infection with L. monocytogenes and risk of spontaneous abortion was assessed via odds ratio at corresponding 95% confidence intervals.
The frequency of L. monocytogenes infection was significantly increased in pregnant women with spontaneous abortion in comparison with healthy subjects. There is significant association between infection with L. monocytogenes and development of spontaneous abortion in pregnant women (odds ratio: 2.778; 2.130-3.623 with 95% confidence interval).
Our results suggested the infection with L. monocytogenes is a marker for prediction of the risk of development of spontaneous abortion during pregnancy.
Regarding the importance of L. monocytogenes in the initiation and development of spontaneous abortion, it is important that all aspects of this pathogen, such as diagnosis, treatment, control, and vaccination, be considered by all researchers around the world.
| 1. | Wang JX, Norman RJ, Wilcox AJ. Incidence of spontaneous abortion among pregnancies produced by assisted reproductive technology. Hum Reprod. 2004;19:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | JS, Buxbaum RE, Mankuta D. Spontaneous first trimester miscarriage rates per woman among parous women with 1 or more pregnancies of 24 weeks or more. BMC Pregnancy Childbirth. 2017;17: 437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Alberman E. Spontaneous abortions: epidemiology. Spontaneous Abortion: Springer; 1992: 9-20. |
| 4. | Moradinazar M, Najafi F, Nazar ZM, Hamzeh B, Pasdar Y, Shakiba E. Lifetime Prevalence of Abortion and Risk Factors in Women: Evidence from a Cohort Study. J Pregnancy. 2020;2020:4871494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Otgonjargala B, Becker K, Batbaatar G, Tsogtsaikhan S, Enkhtsetseg J, Enkhjargal A, Pfeffer K, Adams O, Battogtokh C, Henrich B. Effect of Mycoplasma hominis and cytomegalovirus infection on pregnancy outcome: A prospective study of 200 Mongolian women and their newborns. PLoS One. 2017;12:e0173283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update. 2016;22:116-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 281] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 7. | Buchanan RL, Gorris LG, Hayman MM, Jackson TC, Whiting RC. A review of Listeria monocytogenes: an update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food control. 2017;75:1-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 562] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 8. | Letchumanan V, Wong PC, Goh BH, Long CM, Lee LH. A review on the characteristics, taxanomy and prevalence of Listeria monocytogenes. Microbe Mol Biol. 2018;1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Barikbin P, Sallmon H, Hüseman D, Sarioglu N, Weichert A, von Weizsäcker K, Bührer C, Koehne P. Clinical, Laboratory, and Placental Findings in Perinatal Listeriosis. Fetal Pediatr Pathol. 2016;35:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Lamont RF, Sobel J, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E, Kim SK, Uldbjerg N, Romero R. Listeriosis in human pregnancy: a systematic review. J Perinat Med. 2011;39:227-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Mylonakis E, Paliou M, Hohmann EL, Calderwood SB, Wing EJ. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore). 2002;81:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 197] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Stephen S, Indrani R, Achyutha Rao K, Padma Rao A. Listeriosis in human abortions—including a brief review of literature. J Obstet Gynecol India. 1978;28:497-501. |
| 13. | Aljicević M, Beslagić E, Zvizdić S, Hamzić S, Mahmutović S. Listeria monocytogenes as the possible cause of the spontanous abortion in female of the fertile age. Bosn J Basic Med Sci. 2005;5:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Kaur S, Malik SV, Vaidya VM, Barbuddhe SB. Listeria monocytogenes in spontaneous abortions in humans and its detection by multiplex PCR. J Appl Microbiol. 2007;103:1889-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Saeedi M, Bakhshandeh Nosrat S, Moradi A, Hedayat Mofidi S, Behnampoor N. Comparative Study of Cytomegalovirus, Listeria monocytogen and Toxoplasma gondii infections in successful and non-successful pregnancy in Gorgan. Med Lab J. 2009;3. |
| 16. | Jamshidi M, Jahromi AS, Davoodian P, Amirian M, Zangeneh M, Jadcareh F. Seropositivity for Listeria monocytogenes in women with spontaneous abortion: a case-control study in Iran. Taiwan J Obstet Gynecol. 2009;48:46-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Tahery Y, Kafilzadeh F, Momtaz YA. Listeria monocytogenesis and abortion: A case study of pregnant women in Iran. Afr J Microbiol Res. 2009;3:826-832. |
| 18. | Nazeri M. Evaluation of indirect immunofluorescence assay for diagnosis of Listeria monocytogenes in abortion. Adv Environ Biolo. 2011;5:1487-1490. |
| 19. | Lotfollahi L, Nowrouzi J, Irajian G, Masjedian F, Kazemi B, Falahat LEA. Prevalence and antimicrobial resistance profiles of Listeria monocytogenes in spontaneous abortions in humans. Afr J Microbiol Res. 2011;5:1990-1993. |
| 20. | Jahangirisiskht A, Kargar M, Mirzaee A, Aramesh S, Akbartabar M, Mohamadkhani N, Rezaee Z. Comparison the standard culture method and polymerase chain reaction in diagnosis of Listeria monocytogenes in pregnant women. Armaghane Danesh. 2012;17:156-163. |
| 21. | Jahangirsisakht A, Kargar M, Mirzaee A, Akbartabar M, Aramesh S, Mohamadkhani N. Assessing Listeria monocytogenes hly A genome in pregnant women with spontaneous abortion using PCR method in Yasuj, south west of Iran. Afr J Microbiol Res. 2013;7:4257-4260. |
| 22. | Shindang J, Shindang CO, Ekwempu AI. Incidence of Listeria monocytogenes and other bacteria in spontaneous abortion cases in Jos. Niger J Biotechnol. 2013;25:1-7. |
| 23. | Eslami G, Goudarzi H, Ohadi E, Taherpour A, Pourkaveh B, Taheri S. Identification of Listeria monocytogenes virulence factors in women with abortion by polymerase chain reaction. Arch Clin Infect Dis. 2014;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Shoukat S, Malik S, Rawool D, Kumar A, Kumar S, Shrivastava S, Barbuddhe SB, Das DP, Das S. A study on detection of pathogenic Listeria monocytogenes in ovine’s of Kashmir region having abortion or history of abortion. Proc Nati Acad Sci India. 2014;84:311-316. |
| 25. | Haghroosta A, Shakh AF, Shooshtari MM. Investigation on the seroprevalence of Listeria monocytogenes in women with spontaneous abortion. Comp Clin Path. 2015;24:153-156. |
| 26. | Pourkaveh B, Ahmadi M, Eslami G, Gachkar L. Factors contributes to spontaneous abortion caused by Listeria monocytogenes, in Tehran, Iran, 2015. Cell Mol Biol (Noisy-le-grand). 2016;62:3-10. [PubMed] |
| 27. | Bobade S, Warke S, Kalorey D. Virulence gene profiling and serotyping of Listeria monocytogenes from infertility cases of women. Int J Health Sci Res. 2016;6:440-449. |
| 28. | Tajedini E, Talebi S, Vandyousefi J. Evaluation and Comparison of ELISA and Indirect Immunofluorescence Methods for the Detection of Anti-Listeria Antibodies Among Women with Spontaneous Abortion Referred to Kamali Hospital in Karaj, Iran. Ira J Med Microbiol. 2017;11:98-106. |
| 29. | Pour NK, Rezaei M, Vandyousefi J, Zamin FR, Irajian G. Determination of dominant serovars of Listeria monocytogenes strains isolated from spontaneous human abortion in Tehran/IRAN. Eur J Bio Scis. 2018;12:377-383. |
| 30. | Heidari S, Soltan Dallal MM. Prevalence of Listeria monocytogenes isolated from pregnant women with and without history of abortion and detection of hemolysin (hlyA) gene in clinical samples. Sci J Kur Uni Med Sci. 2018;23:96-107. |
| 31. | Al-dorri AZRa. Study of bacteria Listeria monocytogenes in spontaneous aborted women in Salah Al-deen province. Tik J Pur Sci. 2018;21:12-17. |
| 32. | Ohadi E, Goudarzi H, Kalani BS, Taherpour A, Shivaee A, Eslami G. Serotyping of Listeria monocytogenes Isolates from Women with Spontaneous Abortion Using Polymerase Chain Reaction Method. J Med Bacteriol. 2019;8:8-17. |
| 33. | Mozaffari M, Mozafari M, Mohebbi M. Clinical Significance and Seroprevalence of L. monocytogenes in Pregnant Women with Spontaneous Abortion: Personalized Medicine to improve Outcome (Diagnosis and monitoring). City. 2019;72:51. |
| 34. | Zahirnia Z, Mansouri S, Saffari F. Pregnancy-related listeriosis: frequency and genotypic characteristics of L. monocytogenes from human specimens in Kerman, Iran. Wien Med Wochenschr. 2019;169:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Fall NS, Sarr M, Diagne N, Bassène H, Sokhna C, Lagier JC, Raoult D. Listeria monocytogenes detected in vaginal self-samples of 2 women after spontaneous miscarriage, Senegal, West Africa. Eur J Clin Microbiol Infect Dis. 2020;39:393-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Al-Mayahi FSA, Jaber SM. Multiple drug resistance of Listeria monocytogenes isolated from aborted women by using serological and molecular techniques in Diwaniyah city/Iraq. Iran J Microbiol. 2020;12:305-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Martinez-Rios V, Dalgaard P. Prevalence of Listeria monocytogenes in European cheeses: A systematic review and meta-analysis. Food Control. 2018;84:205-214. |
| 38. | Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology: Elsevier Health Sciences. 2015. |
| 39. | Craig AM, Dotters-Katz S, Kuller JA, Thompson JL. Listeriosis in Pregnancy: A Review. Obstet Gynecol Surv. 2019;74:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Overturf GD. Indications for the immunological evaluation of patients with meningitis. Clin Infect Dis. 2003;36:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Ranjbar R, Halaji M. Epidemiology of Listeria monocytogenes prevalence in foods, animals and human origin from Iran: a systematic review and meta-analysis. BMC Public Health. 2018;18:1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Parida SK, Domann E, Rohde M, Müller S, Darji A, Hain T, Wehland J, Chakraborty T. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol Microbiol. 1998;28:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Johnson J, Jinneman K, Stelma G, Smith BG, Lye D, Messer J, Ulaszek J, Evsen L, Gendel S, Bennett RW, Swaminathan B, Pruckler J, Steigerwalt A, Kathariou S, Yildirim S, Volokhov D, Rasooly A, Chizhikov V, Wiedmann M, Fortes E, Duvall RE, Hitchins AD. Natural atypical Listeria innocua strains with Listeria monocytogenes pathogenicity island 1 genes. Appl Environ Microbiol. 2004;70:4256-4266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Khademi F, Sahebkar A. A systematic review and meta-analysis on the prevalence of antibiotic-resistant Listeria species in food, animal and human specimens in Iran. J Food Sci Technol. 2019;56:5167-5183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Lambotte O, Fihman V, Poyart C, Buzyn A, Berche P, Soumelis V. Listeria monocytogenes skin infection with cerebritis and haemophagocytosis syndrome in a bone marrow transplant recipient. J Infect. 2005;50:356-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Quentin C, Thibaut MC, Horovitz J, Bebear C. Multiresistant strain of Listeria monocytogenes in septic abortion. Lancet. 1990;336:375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang LL S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH