Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11369
Peer-review started: May 26, 2021
First decision: June 15, 2021
Revised: June 15, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: December 26, 2021
Processing time: 211 Days and 4.3 Hours
Anorectal melanoma is a tumour that is difficult to identify due to its rarity and variability of presentation. Insufficient data published in the literature do not allow for diagnostic and treatment guidelines to be established. Anorectal melanoma has the worst prognosis among mucosal melanomas and is frequently misdiagnosed by standard identification methods.
A 66-year-old woman presented with intermittent anal bleeding, pain, and tenesmus in the past month, with no associated weight loss. Colonoscopy revealed a cauliflower-like tumour with a diameter of 1.5 cm, with exulcerated areas and an adherent clot but without obstruction. Biopsy results identified an inflammatory rectal polyp with nonspecific chronic rectitis. Tumour markers CA 19-9 and CEA were within the normal range. After 6 mo, due to the persistence of symptoms, a pelvic magnetic resonance imaging scan was performed. A lesion measuring 2.8 cm × 2.7 cm × 2.1 cm was identified at the anorectal junction, along with two adjacent lymphadenopathies. No distant metastases were detected. Immunohistochemistry was performed on the second set of biopsies, and a diagnosis of anorectal melanoma was established. Surgical treatment by abdominoperineal resection was performed. Evolution was marked by the appearance of lung metastases at 1 mo postoperatively, detected on a positron emission tomography-computer tomography scan, and perineal recurrence after 5 mo. After molecular testing, the patient was included in an immunotherapy trial.
This case highlights the difficulty of establishing a definitive early diagnosis of anorectal melanoma, the importance of performing histological analysis on a well-represented biopsy specimen, and the poor prognosis, even with radical surgery.
Core Tip: Anorectal melanoma is a rare type of melanoma with the worst prognosis among mucous melanomas. The location and nonspecific symptoms of anorectal melanoma make it difficult to detect. Even histological examination can be misleading in the absence of obvious pigmentation and suspicion raised by the clinician to induce immunohistochemical examination. In this context, the diagnosis is late, in advanced stages, and the rarity of cases has not allowed the development of treatment guidelines.
- Citation: Apostu RC, Stefanescu E, Scurtu RR, Kacso G, Drasovean R. Difficulties in diagnosing anorectal melanoma: A case report and review of the literature. World J Clin Cases 2021; 9(36): 11369-11381
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11369.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11369
Anorectal melanoma is a rare disease accounting for 0.5%-4% of all anorectal malignancies[1]. After the skin and retina, the anorectum is the third most common site for malignant melanoma and the second most frequent mucosal melanoma site[2-5]. Although the prevalence is 1.6-2.3 times higher in women, survival is longer in women than in men (15.7% vs 10.6%)[4,6].
The characteristic onset of anorectal melanoma is nonspecific regarding symptoms, hence the diagnosis is late at advanced stages upon its identification[4,6].
Treatment is mainly surgical, but even with radical resection, the prognosis is extremely poor even compared to other mucosal melanomas, with a median survival rate of 20%, compared with 5%-25% for vaginal melanoma, 24%-77% for vulvar melanoma, and 12%-30% for head and neck melanoma[6]. The median survival in patients with recurrent or metastatic disease is less than 10 mo[1,7].
We present the case of a 66-year-old female patient whose final diagnosis of anal melanoma was immunohistochemically established 6 mo after the onset of symptoms.
A 66-year-old woman presented with intermittent anal bleeding, two to three times per day, and tenesmus every 2 d.
Symptoms were present in the last month, and no weight loss was registered.
The patient had a medical history of hepatitis C and hypertension, with a surgical history of cholecystectomy.
The patient had a family history of colon and liver cancer.
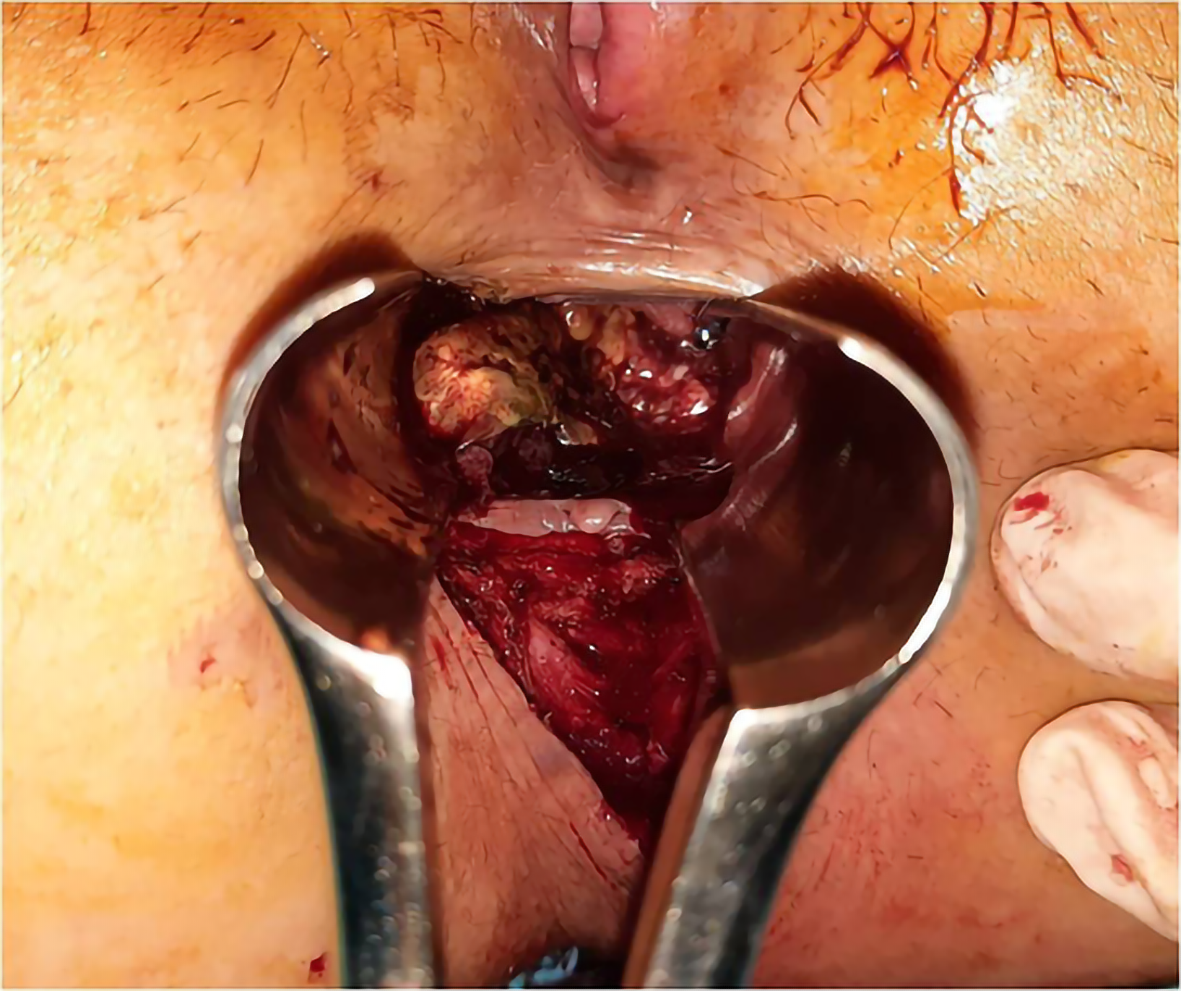
On digital rectal examination, a protrusive, sessile tumour was noted, located near the anal verge on the posterolateral anorectal wall (Figure 1). The tumour was 1.5 cm in diameter, and no other pathological changes of the rest of the anorectal wall were noted.
Serum tumour markers: A malignant origin of the tumour was suspected, and tumour markers CA 19-9 and CEA were evaluated, with both being within the normal range. Gastroenterological monitoring was initiated.
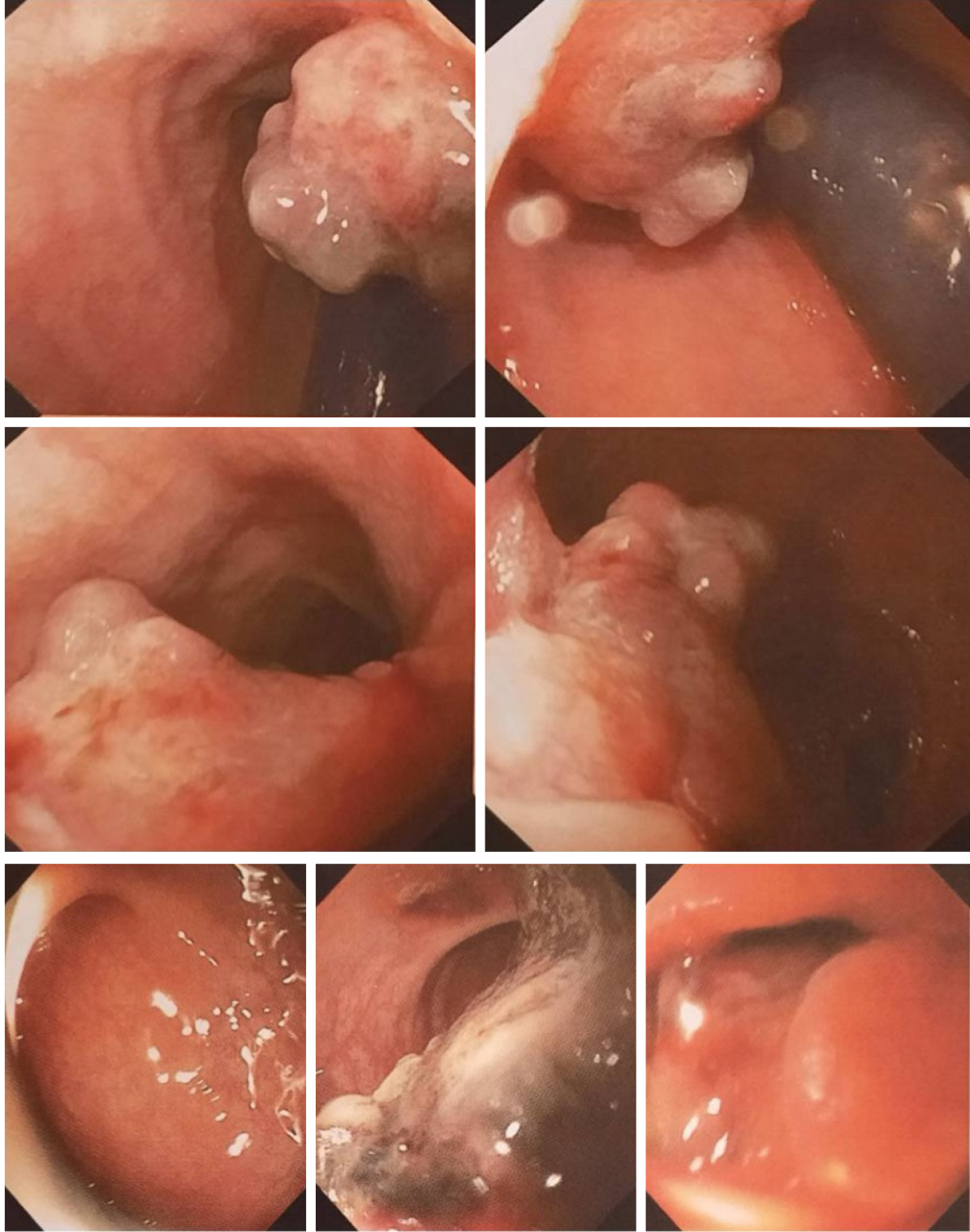
Lower digestive endoscopy: Colonoscopy showed a cauliflower-like tumour, with a 1-cm-wide base, exulcerated areas, and an adherent clot but no luminal narrowing (Figure 2). A biopsy was taken, and the result identified an exulcerated inflammatory rectal polyp with nonspecific chronic rectitis.
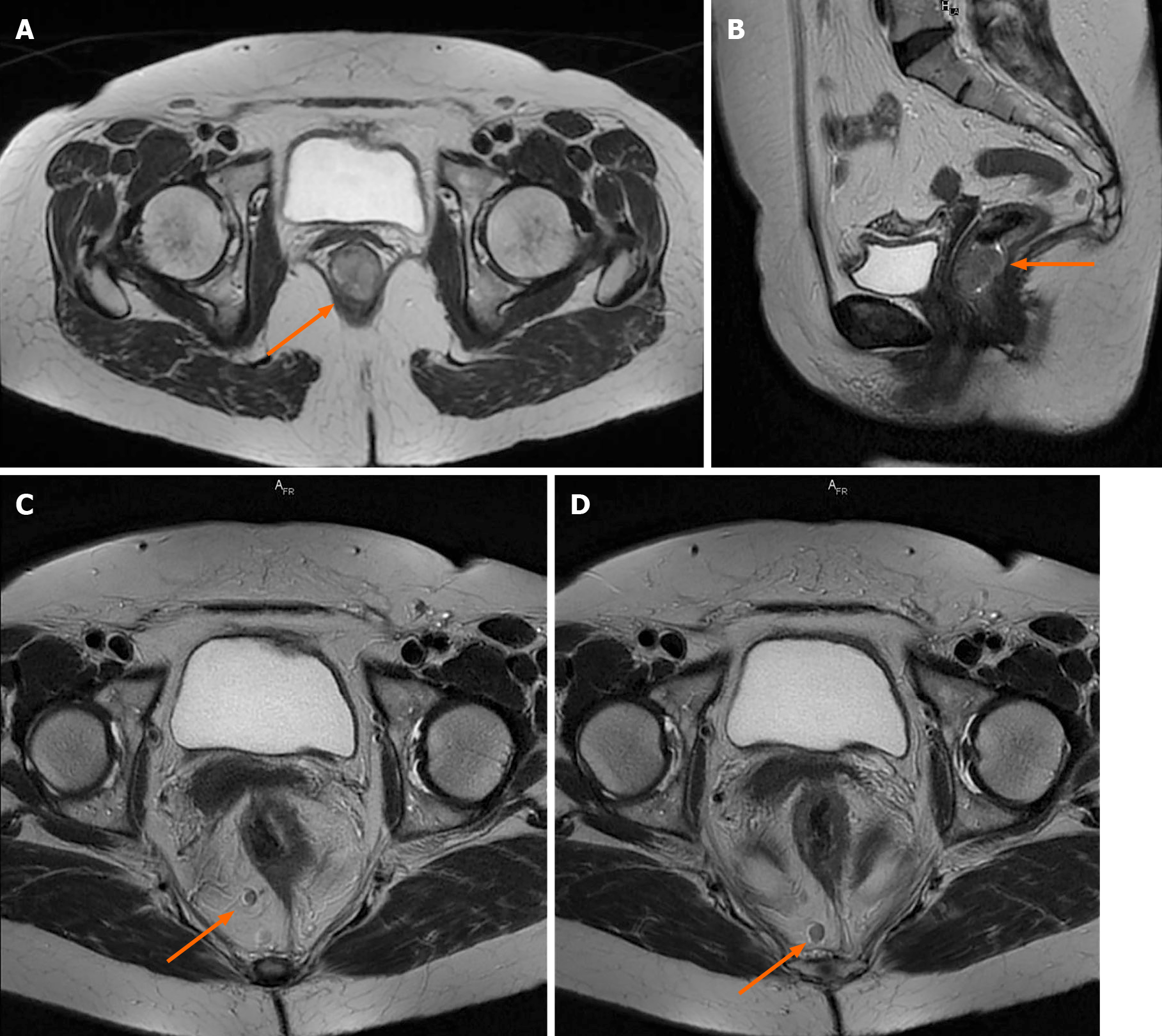
After 6 mo, due to the persistence of symptoms, an oncological consultation was obtained, and pelvic magnetic resonance imaging (MRI) with intravenous contrast was indicated. The examination found a circumferential process at the anorectal junction, with measured dimensions of 2.8 cm × 2.7 cm × 2.1 cm on axial and sagittal T2 images, that did not exceed or infiltrate the rectal tunica muscularis; homogeneous contrast was captured, and no areas of necrosis were observed. The lesion presented restricted diffusion on diffusion-weighted imaging (DWI) with an apparent diffusion coefficient map characteristic of malignant tumours. Target lymphadenopathies were identified in primary stations, without mesorectal fascia retraction or pseudofibrosis (Figure 3). These target lymphadenopathies also presented restricted diffusion on DWI images, with a specificity of secondary malignant lesions.
The colonoscopy was repeated, and the biopsy results identified malignant tumour proliferation, with discoid cells having a diffuse distribution. The cells were intensely positive for SOX10 but negative for CD5, CD10, CD20, MUM1, BCL6, c-Kit, chromogranin, CKAE1/AE3, and Ki67. The suspicion of an anal canal melanoma was raised.
A CT scan of the chest, abdomen, and pelvis was performed, showing no distant metastasis, and according to the anal and rectal cancer staging system, the tumour was staged as IIIA (T2N1M0), while the mucosal melanoma staging system placed the tumour in stage II.
The patient has a surgical indication as curative in intent upfront therapy. Extensive genetic tumour testing (NGS or equivalent) is desirable. If not possible, at least BRAF-V600E and c-kit IHC is mandatory. If unresectable, PD-L1 IHC is added. Adjuvant therapy will be considered according to the final pathological report. If generous Ro radical surgery and no pTNM upstaging are performed, RT might be considered after wide excision but not after amputation. If R1 resection is performed, a 2nd surgery would be preferable to postoperative radiotherapy; if not possible or refused, a combination of external beam and brachytherapy boost might be considered.
As there is no systemic therapy standard of care indication for locally advanced nonmetastatic anorectal mucosal melanoma, inclusion in a clinical trial (if available) could be considered. If cumulative negative prognostic factors are identified in the pathological report (pT3-4/extensive lymph node involvement, L1, V1, Pn1, R1), targeted therapy options according to molecular genetic testing will be discussed with the patient, emphasizing the lack of level 1-2 evidence in this scenario and sometimes the lack of drug reimbursement in this setting by the National Health Insurance System.
Postoperative regular follow-up will be carried out, including 3-mo cross-sectional thoraco-abdomino-pelvic ± brain imaging (MRI or CT scan or both).
The patient shows an indication for surgical treatment with abdominoperineal resection.
The final preoperative diagnosis of the presented case was anorectal melanoma stage IIIA (cT2N1M0).
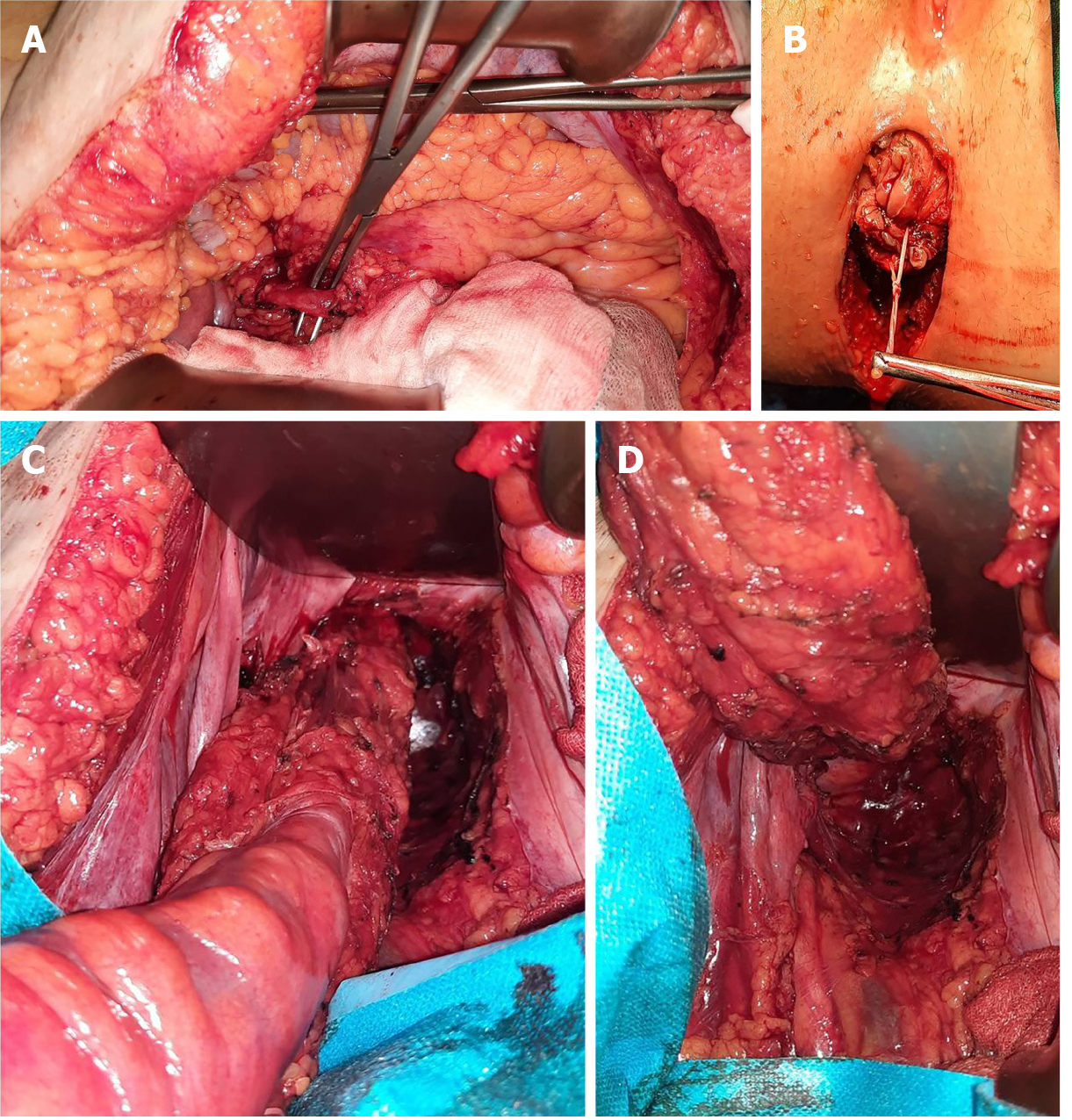
Abdominoperineal resection was planned for the patient. Operative findings showed no liver or peritoneal metastasis. The inferior mesenteric artery was isolated and sectioned. Total mesocolic and mesorectal excision was performed (Figure 4). The tumour was identified to involve the anorectal junction (Figure 5). Postoperative recovery was uneventful, and the patient was discharged home.
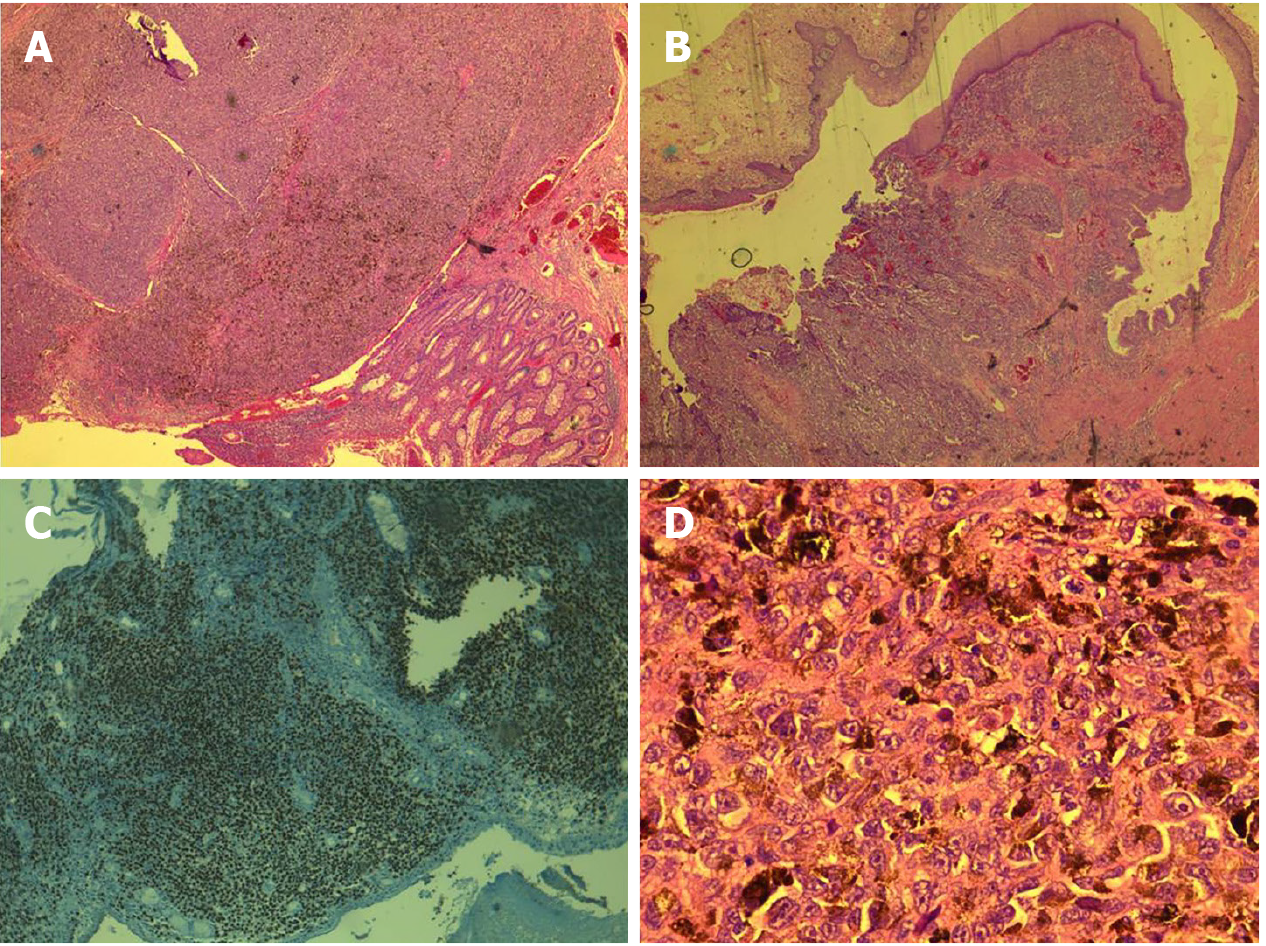
The histopathology of the resected specimen showed a polypoid tumour of 2.6 cm × 2.4 cm × 1.6 cm that was brown in colour and located at the level of the rectum and anal canal; the tumour had an ulcerated surface and infiltrated up to the submucosa, - 5 mm, with a maximum thickness of 9 mm. Venous but not perineural invasion was noted, and the distal resection edge (anal verge) was 1.2 cm from the tumour. No lymph node metastases were identified. Epithelioid cells, with focal fusiform aspects, marked nuclear pleomorphism, and atypical mitosis > 15/mm², were characteristic. Some cells contained melanocyte pigment (Figure 6). These features were consistent with those of anorectal malignant melanoma, and a final pathological classification of early stage II (pT1N0V1M0) was made.
After 1 mo, a positron emission tomography-computer tomography (PET-CT) scan was performed, which showed no local recurrence but revealed pulmonary metastasis. Molecular testing was performed, and the patient was included in an immunotherapy trial. After 5 mo, the patient was still under treatment, and perineal recurrence was identified. The tumour was 5 cm in diameter and located under the perineal scar. Given the presence of distant metastases, resection was not performed.
Mucosal melanoma is a rare pathology comprising 1% of all melanomas, fewer than 25% of which are anorectal and 0.05% are colorectal malignancies[1,8]. Amelanotic melanoma accounts for 20%-30% of anorectal cases[6,9]. Patients are diagnosed at an average age of 55 years, with an interval ranging from 29 to 91 years[1,4,5]. To date, no known risk factors have been associated with anorectal melanoma[1,8].
The pathogenesis of the disease is based on several theories. In the anorectal region, melanocytes are located in the epithelial lining of the dentate line[10]. These melanocytes have antioxidant activity and modulate the regional immune response[5]. The vast majority of tumours therefore develop at the dentate line and are located in the anal canal[10] or within 6 cm of the anal rim[5,6]. Lymphatic spread involves the mesenteric and inguinal lymph nodes, while the most common distant metastasis site is the brain, followed by the liver and lung[4].
Commonly, patients present with nonspecific complaints, such as rectal bleeding, anal pain (14%-27%)[2,4,6], a palpable anal mass (12%-16%), obstipation (6%), diarrhoea (4%), pruritus, tenesmus, and changes in bowel habits[1,4,6]. Haematochezia is the most frequent symptom (54%-78%)[3,4,6,10]. The median duration of symptoms before diagnosis is 4-5 mo[11], with up to 8%-16% of patients being diagnosed after a haemorrhoidectomy[4]. In our study, the diagnosis was established 6 mo after the onset of haematochezia. Kaya et al[10] described a median period of 11 mo until the final diagnosis.
A definite diagnosis is usually established by biopsy and immunohistochemical staining. The lesions are characterized by histological examination[6], with several histological variants of anorectal melanomas being described, including epithelioid, spindle-cell, lymphoma-like, or pleomorphic[5]. The main characteristics are epithelioid cells, high pleomorphism in the nucleus, and melanin granules[6]. Histological misdiagnosis can still be frequent, as 80% of lesions lack pigmentation and 20%-30% are histologically amelanotic[2,11]. In these cases, non-Hodgkin lymphoma, adenocarcinoma, or sarcoma should be considered, and an immunohistochemistry panel must be performed[5,6,9]. Additionally, the histological distinction between amelanotic melanoma and anaplastic squamous cell carcinoma can be difficult[3].
The immunohistochemical stains required for the diagnosis are HMB-45 (human melanoma black 45), S-100 (soluble 100%), melan A (melanoma-associated protein A), or vimentin. Anti-S-100 protein staining is highly sensitive for melanocytic differentiation[2,10,12]. These markers are intensely positive in 78%-100% of anorectal melanomas[12], and negative cases for HMB-45 are also recorded[13]. Anorectal melanoma is negative for pan-cytokeratin. Up to 10% of anorectal melanomas can express a keratin or epithelial marker, making possible a misdiagnosis of poorly differentiated rectal carcinoma[5]. In some cases, positivity for CD30 and CD68 can be identified, while negativity for AE1/AE3 (anti-cytokeratin monoclonal antibodies), CD17, or desmin can also occur. Antibodies against Mart-1 (melanoma-associated antigen recognized by T cells-1) are also used[6].
In this study, histopathological examination of the biopsy specimen revealed a morphological and immunohistochemical profile, which raises the issue of differential diagnosis of undifferentiated neuroendocrine carcinoma, melanoma, and CD5 and CD20-negative lymphoproliferations. The discoid cells isolated from the tumour were negative for CD20, CD5, CD10, BCL6, and MUM1 (multiple myeloma 1). A recommendation to complete the immunohistochemical profile with CKAE1/AE3, LCA (leukocyte common antigen), SOX10, chromogranin, and c-Kit stains was issued. The second set revealed negative cellular expression of c-Kit, chromogranin, and CLAE1/AE3 and intense and diffuse staining for SOX10. These results allowed the establishment of a definitive diagnosis. The evaluation of the entire specimen, performed after surgery, identified melanocyte pigment in the epithelioid cells and tumour venous thrombi. All these aspects were conclusive for the diagnosis.
To obtain a prompt diagnosis, the determination of various serum tumour markers has been proposed. High LDH values have been found in the serum of patients with advanced melanoma. S-100B is a protein with a role in cell cycle progression with serum correspondence, while MIA (melanoma inhibitor activity protein) is strongly expressed by malignant melanocytes. Other markers being studied are neuroenolase, with a high predictive value in melanoma, tumour-associated antigen 90 immune complex, and YKL-40 in advanced metastatic melanoma, a marker that correlates with survival, poor performance status, and the site of metastasis[6,14].
As an investigative tool, pelvic MRI scans are crucial for the diagnosis and the determination of the extent of disease because classic paramagnetic MRI characteristics have been described. The melanocytic component of anorectal melanoma is thought to shorten T1 relaxation time and increase T2 relaxation time, and the tumour is hyperintense on T1W and hypointense on T2W images. As not all anorectal melanomas will display these specifications and many studies have demonstrated mixed-intensity image signals, a CT scan is recommended for differential diagnosis[12]. A correlation analysis of the MRI findings with the clinical aspects concluded that a polypoid mass in the anorectum without obstruction, with T1 hyperintensity, T2 mixed signal intensity, hyperenhancement, adjacent infiltration, and lymphadenopathies, can be considered characteristic[15]. In addition to MRI and CT scans, PET-CT and MRI of the brain can be considered preoperatively if radical resection is planned[16]. Additionally, PET-CT is superior for the evaluation of patients with locoregional and systemic involvement[5].
There is no pathologic staging system specific to anorectal melanoma[3]. The disease is staged based on clinical and pathological findings. A frequent staging system used is based only on the pattern of the spread, with stage I for local disease, stage II for local disease with regional lymph nodes, and stage III for distant metastasis[6]. Another clinical staging system used also considers the thickness of the lesion and the presence of ulceration[17]. Other systems classify mucosal melanomas at three levels: Level 1 for local or in situ, level 2 for regional invasion of the lamina propria, and level 3 for disseminated, with each level having corresponding survival time[18].
A site-specific staging method is missing from the 8th edition of the American Joint Committee on Cancer (AJCC) staging system[7,16], so three systems are currently used: Clinical system, rectal TNM staging system, and anal TNM staging system. Although the differences are not significant regarding prognosis, it has been concluded that the accuracy of prognosis in patients diagnosed with stage III anorectal melanoma would be improved using the rectal TNM staging system, with additional information about the number of lymph node metastases[3].
There are no consensus guidelines for treating anorectal melanoma[8]. The main method of treatment is surgery combined with adjuvant therapy: Chemotherapy, immunotherapy, targeted therapy, or antiangiogenetic therapy can be applied[6].
Surgery is the most effective treatment but is not associated with improvement in overall survival[5]. Resection with curative or palliative intent can be performed by EMR (endoscopic mucosal resection) or ESD (endoscopic submucosal dissection) as wide local excision or abdominoperineal resection[16]. Curative intent is associated with stage I and some stage II patients[7]. The aim of surgical treatment is to obtain R0 resection margins with local excision. Repeated local excisions can be performed if sphincter function is not compromised, and abdominoperineal resection is no longer routinely recommended as a standard of care[16].
Although 60% of patients are diagnosed with metastatic lesions, endoscopic submucosal dissection has been proposed as a diagnostic and therapeutic tool for early-stage anorectal melanoma, with local recurrence registered after 6 mo[9]. Better results are described with tumours confined to the mucosa, with no recurrence or distant metastases found at up to 2 years of follow-up[19]. An advantage of endoscopic submucosal resection is the provision of a specimen with adequate pathological findings compared to biopsy specimens[9]. This method determines a prompt diagnosis without delaying the concrete identification of the tumour, as described in this study. Here, the biopsy results showed nonspecific chronic rectitis, and tumoural markers were within normal values.
Endoscopic mucosal resection has also been applied, with a 5-year disease-free survival when combined with adjuvant interferon therapy[20]. This method has also been applied for cases where tumour invasion was confined to the submucosal layer, with a long-term survival up to 9 years, despite repeated local relapses, all of which were resolved in the same manner[21].
There is no consensus on which approach is favourable, wide local excision or abdominoperineal resection, as recurrence is not associated with the initial surgical procedure, and no difference in survival was reported between the two procedures[3,6,22,23]. Local excision with > 10 mm cutting edge is preferred because abdominoperineal resection has a worse prognosis postoperatively[2,7,16]. Tumours < 1 mm in thickness can undergo local resection with a 1 cm margin, while a lesion thickness up to 4 mm must be treated with 2 cm margin resection[5]. Local excision has the advantage of a quicker recovery and sphincter preservation and is a reasonable palliative treatment[6,8]. Local excision is also curative for stage 0 and ensures an appropriate quality of life[4]. One of the disadvantages of the local excision is the local recurrence rate, which is as high as 65%[8]. Abdominoperineal resection has the advantage of a better local disease and lymphatic spread control[7,8,24]. Abdominoperineal resection is also associated with higher R0 resection rates than local excision. Despite this, no differences in 5-year overall survival between the local excision and abdominoperineal resection (21% vs 17%) were reported[25], while others have obtained a 13% increase in survival in cases with R0 resections[26]. Laparoscopic APR can reduce the morbidity associated with an open procedure and control local disease and should be considered in these cases, with a recurrence-free interval of at least one year being recorded[5,27]. Laparoscopic APR should also be used when there is obstruction or the need for salvage surgery[4].
Better survival can be described in patients with wide local excision because the procedure is more applicable in earlier stages than abdominoperineal resection[10], although other studies have shown no differences in stage-specific survival between the procedures[28].
In patients with large primary tumours or distant metastases, palliative surgery, such as local segmental resection or diverting colostomy, is suggested[6]. If melanoma is at an advanced stage, the surgical procedure should be selected based on the quality of life. In these cases, wide local excision and adjuvant radiotherapy or systemic therapy should be performed[4].
The surgical method has been demonstrated to affect the local recurrence rate but not the survival rate[9,24]. Recurrence is more frequent in rectal involvement than in anal involvement alone[5]. Most recurrences occur systemically. Locoregional failure occurs at the inguinal lymph nodes more than the pelvic lymph nodes[4,10,13], with significant differences reported (47% vs 7%)[29]. As no surgical procedure involves these lymph nodes, no advantage in controlling locoregional recurrences can be obtained[4,10,13]. The absence of an advantage in resecting these nodes can be explained by the complex lymphatic drainage of the anal canal, with more than 10 lymphatic routes being documented[10]. The use of prophylactic inguinal lymph node dissection is not supported[7], as it does not improve survival but increases the risk of complications. However, elective dissection should be considered in clinically palpable disease[5]. Mesenteric lymphadenectomy is indicated in the presence of metastatic regional nodal disease[16].
Local failures can be managed by salvage surgery. The focus should be on minimizing morbidity and maximizing quality of life[24]. Repeated local excisions have been described as a preferred treatment in these cases[30].
Sentinel lymph node mapping could be used in mucous melanoma to prevent understaging and plan the extent of surgery[4]. A biopsy is recommended to direct adjuvant treatment or allow entry into a clinical trial. The completion of the nodal dissection can be performed in accordance with a multidisciplinary consensus[16].
Adjuvant therapy has a controversial role in treating anorectal melanoma[3]. Even though resistance to radiotherapy has been stated, some efficacy was demonstrated with adjuvant treatment after wide local excision, with a reduction in the locoregional recurrence rate from 50% to 17% compared to the surgical procedure alone[31]. Additionally, the prognosis of patients with mucosal involvement is thought to be much better with this association[10]. Despite that, the routine use of radiotherapy after curative resection is not recommended in anorectal melanoma. R1 resection should be followed by radiotherapy[16]. Regional lymph nodes should not be included in the radiation field routinely because of the risk of complications, such as lymphedema and proctitis[16,31]. Additionally, a radical dose equivalent should be administered[16]. Neoadjuvant radiotherapy has a minimal effect, while adjuvant treatment cannot control distant relapse[4].
Chemotherapy treatment possibilities are limited, and the response is poor[1,2]. The efficiency of chemotherapy is similar to that of chemoimmunotherapies[5]. Dacarbazine has a 20% partial response in 4 to 6 mo, alone or associated with high-dose interferon or interleukin-2[2,4,6]. Immunotherapy with α-interferon in patients with nodal involvement has demonstrated significant disease-free survival and overall survival in patients with mucous melanoma[32]. The regression of the tumour and metastatic lesions was demonstrated using temozolomide, an oral alternative to dacarbazine, along with cisplatin and doxorubicin[4]. A randomized trial demonstrated a benefit of adjuvant chemotherapy in mucosal melanoma compared to resection alone (20.8 mo vs 5.4 mo)[33].
Mutation detection tests provide an opportunity for targeted therapy[1]. Molecular testing is recommended at the time of the first diagnosis to allow entry into clinical trials[16]. Up to 25%-35.5% of mucosal melanomas exhibit amplification of the Kit gene[6,9,12]. Only 3%-10% contain activating mutations of the BRAF gene, whereas mutations in the NRAS gene are rarely described to be related to mucosal melanoma[5,34]. Kit-mutated melanomas are commonly located at acral (23%) and mucosal sites (15.6%)[1,34]. Randomized trials have reported positive results in treatment with tyrosine kinase inhibitors, with a response rate of 23%-54%[9]. Good response or even remission for up to 15 mo has been described in studies with sunitinib treatment[6]. However, not all c-Kit mutations are successfully targeted[16]. In patients with BRAF mutations, dabrafenib has been effective, with a 50%-70% response rate[6].
Triple-negative melanomas (no Braf, c-Kit, or RAS mutation) can benefit from novel anti-CTLA-4 (cytotoxic T-lymphocyte associated antigen) and anti-PD-L1 (programmed cell death ligand 1) immunotherapy[34]. Combined treatment is suggested for use in selected patients, with care regarding toxicity[16].
Ipilimumab has demonstrated long-term survival success in up to 20% of treated patients and was the first anti-CTLA-4 agent approved for treating advanced melanoma[6], followed by nivolumab and pembrolizumab (anti-PD-1 antibodies)[5].
Vemurafenib and ipilimumab have been evaluated for treating patients with BRAF mutations, while in the absence of this mutation, ipilimumab is used alone or along with chemotherapy as dacarbazine[6]. IL-2 with ipilimumab proved efficient in patients with unresectable disease[5].
There are no randomized clinical trials using anti-PD-1 inhibitors for mucosal melanoma, but the efficacy has been demonstrated in a series of studies[9]. Hamid et al[35] reported a response rate to pembrolizumab of 19% with a median overall survival of 11.3 mo. Some retrospective studies reported a response rate of 23% for anti-PD-L1 inhibitors[36], while other studies have reported a near-complete response in advanced mucosal melanoma using anti-PD1 treatment[6]. With nivolumab, a significant reduction in liver and bone metastasis has been registered, and no recurrences were registered 17 mo after treatment[5]. The use of a single-agent anti-PD-1 inhibitor is recommended for stage III or IV unresectable disease[16].
In anorectal melanoma, the depth and size of the tumour are considered as prognostic factors. A thickness less than 2 mm is a determining factor for long-term survival[4,5]. Additionally, a tumour size < 2 cm is associated with a greater overall survival[5]. Nodal status at the diagnosis and duration of the initial symptom are also predictive factors[4,5]. Regarding the pattern of spread, bilateral node involvement is a worse prognostic factor[10]. There were no differences in prognosis related to tumour location. A lesion located proximal to the dentate line has a more advanced stage at diagnosis, while a distal tumour is associated with a higher lymph node recurrence rate[4,5].
The poor overall prognosis is related to delayed diagnosis and biological differences in melanocytes of this anatomical area compared to other sites[5]. Compared to other mucosal locations, anorectal melanoma has the highest vertical tumour thickness, a more advanced nodal status, and a higher percentage of metastatic disease at diagnosis, up to 67%[3,4,12,37]. This group is also associated with most local relapses and the poorest prognosis[37].
The median survival of anorectal melanoma is 24 mo, ranging from 19 to 26.4 mo[2,4,10], while survival in patients with recurrent or metastatic disease is less than 10 months[1,7]. The 5-year survival rate ranges between 3% and 22%[1,4,7,8], with a 37%-50% survival rate for tumours confined locally and 17%-0% for patients with regional or distant metastasis[4,9,16]. Amelanotic melanoma has a poor prognosis due to its difficult detection and invasive nature[3,6]. Long-term survival was demonstrated in patients with abdominoperineal resection and extended lymph node dissection[6].
A rare association is described in the literature of anorectal melanoma and synchronous primary colorectal adenocarcinoma, with only six reported cases, all with relatively late presentation, distant metastasis, and treatment with abdominoperineal resection[38].
Anorectal melanoma is a rare, complex tumour whose biological heterogeneity makes it difficult to diagnose and treat. Given its high aggressiveness, it is important to perform rapid and adequate treatment in a timely manner. Even in cases such as the one we presented above, a T1 tumour may have a poor prognosis with rapid metastatic evolution, which should underline the importance of a rapid therapeutic decision. In the absence of guidelines, a multidisciplinary treatment plan must be initiated, considering tumour aggressiveness and the patient’s quality of life.
| 1. | Kobakova I, Stoyanov G, Popov H, Spasova-Nyagulova S, Stefanova N, Stoev L, Yanulova N. Anorectal Melanoma - a Histopathological Case Report and a Review of the Literature. Folia Med (Plovdiv). 2018;60:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Nafees R, Khan H, Ahmed S, Ahmed Samo K, Siraj Memon A. Primary Rectal Amelanotic Malignant Melanoma: A Rare Case Report. Cureus. 2020;12:e8115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Chae WY, Lee JL, Cho DH, Yu CS, Roh J, Kim JC. Preliminary Suggestion about Staging of Anorectal Malignant Melanoma May Be Used to Predict Prognosis. Cancer Res Treat. 2016;48:240-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Nam S, Kim CW, Baek SJ, Hur H, Min BS, Baik SH, Kim NK. The clinical features and optimal treatment of anorectal malignant melanoma. Ann Surg Treat Res. 2014;87:113-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Paolino G, Didona D, Macri G, Calvieri S, Mercuri SF. Anorectal melanoma. In: Scott JF, Gerstenblith MR. Noncutaneous melanoma. Brisbane (AU): Codon Publications, 2018: 83-98. |
| 6. | Malaguarnera G, Madeddu R, Catania VE, Bertino G, Morelli L, Perrotta RE, Drago F, Malaguarnera M, Latteri S. Anorectal mucosal melanoma. Oncotarget. 2018;9:8785-8800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Guo J, Qin S, Liang J, Lin T, Si L, Chen X, Chi Z, Cui C, Du N, Fan Y, Gu K, Li F, Li J, Li Y, Liang H, Liu J, Lu M, Lu A, Nan K, Niu X, Pan H, Ren G, Ren X, Shu Y, Song X, Tao M, Wang B, Wei W, Wu D, Wu L, Wu A, Xu X, Zhang J, Zhang X, Zhang Y, Zhu H; written; Chinese Society of Clinical Oncology (CSCO) Melanoma Panel. Chinese Guidelines on the Diagnosis and Treatment of Melanoma (2015 Edition). Ann Transl Med. 2015;3:322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 8. | Yeung HM, Gupta B, Kamat B. A Rare Case of Primary Anorectal Melanoma and a Review of the Current Landscape of Therapy. J Community Hosp Intern Med Perspect. 2020;10:371-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Manabe S, Boku Y, Takeda M, Usui F, Hirata I, Takahashi S. Endoscopic submucosal dissection as excisional biopsy for anorectal malignant melanoma: A case report. World J Clin Cases. 2019;7:1652-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Kaya S, Kement M, Altuntas YE, Altin O, Seker A, Mazmanoglu S, Kaptanoglu L, Bildik N, Kucuk HF. Anal melanoma: Outcomes of current surgical approaches. Niger J Clin Pract. 2018;21:1622-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Juanmartiñena Fernández JF, Fernández-Urien I, Córdoba A. Primary anorectal malignant melanoma: an uncommon anorectal pathology. Rev Esp Enferm Dig. 2016;108:604-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 12. | Bohan S, Ramli Hamid MT, Poh KS, Chow TK, Chan WY. Primary anorectal malignant melanoma: A clinical, radiology and pathology correlation. Malays J Pathol. 2020;42:461-467. [PubMed] |
| 13. | Dai JJ, Qu CS, Wang W, Wang YB, Mao XW, Li QS, Chen JF. Primary anorectal malignant melanoma: a case report. Int J Clin Exp Pathol. 2020;13:272-276. [PubMed] |
| 14. | Kluger HM, Hoyt K, Bacchiocchi A, Mayer T, Kirsch J, Kluger Y, Sznol M, Ariyan S, Molinaro A, Halaban R. Plasma markers for identifying patients with metastatic melanoma. Clin Cancer Res. 2011;17:2417-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Park HJ, Kim HJ, Park SH, Lee JS, Kim AY, Kim SW, Hong SM. JOURNAL CLUB: Primary Anorectal Melanoma: MRI Findings and Clinicopathologic Correlations. AJR Am J Roentgenol. 2018;211:W98-W108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Smith HG, Bagwan I, Board RE, Capper S, Coupland SE, Glen J, Lalondrelle S, Mayberry A, Muneer A, Nugent K, Pathiraja P, Payne M, Peach H, Smith J, Westwell S, Wilson E, Rodwell S, Gore M, Turnbull N, Smith MJF. Ano-uro-genital mucosal melanoma UK national guidelines. Eur J Cancer. 2020;135:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA, Haydu LE, Eggermont AMM, Flaherty KT, Balch CM, Thompson JF; for members of the American Joint Committee on Cancer Melanoma Expert Panel and the International Melanoma Database and Discovery Platform. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1703] [Article Influence: 189.2] [Reference Citation Analysis (0)] |
| 18. | Mikkelsen LH, Larsen AC, von Buchwald C, Drzewiecki KT, Prause JU, Heegaard S. Mucosal malignant melanoma - a clinical, oncological, pathological and genetic survey. APMIS. 2016;124:475-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Lian J, Xu A, Chu Y, Chen T, Xu M. Early primary anorectal malignant melanoma treated with endoscopic submucosal dissection: a case report. Int J Colorectal Dis. 2020;35:959-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Park JH, Lee JR, Yoon HS, Jung TY, Lee EJ, Lim JG, Ko SY, Wang JH, Lee JD, Kim HY. Primary anorectal malignant melanoma treated with endoscopic mucosal resection. Intest Res. 2015;13:170-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Tanaka S, Ohta T, Fujimoto T, Makino Y, Murakami I. Endoscopic mucosal resection of primary anorectal malignant melanoma: a case report. Acta Med Okayama. 2008;62:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Kiran RP, Rottoli M, Pokala N, Fazio VW. Long-term outcomes after local excision and radical surgery for anal melanoma: data from a population database. Dis Colon Rectum. 2010;53:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Fields AC, Goldberg J, Senturk J, Saadat LV, Jolissaint J, Shabat G, Irani J, Bleday R, Melnitchouk N. Contemporary Surgical Management and Outcomes for Anal Melanoma: A National Cancer Database Analysis. Ann Surg Oncol. 2018;25:3883-3888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Matsuda A, Miyashita M, Matsumoto S, Takahashi G, Matsutani T, Yamada T, Kishi T, Uchida E. Abdominoperineal resection provides better local control but equivalent overall survival to local excision of anorectal malignant melanoma: a systematic review. Ann Surg. 2015;261:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Ford MM, Kauffmann RM, Geiger TM, Hopkins MB, Muldoon RL, Hawkins AT. Resection for anal melanoma: Is there an optimal approach? Surgery. 2018;164:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Nilsson PJ, Ragnarsson-Olding BK. Importance of clear resection margins in anorectal malignant melanoma. Br J Surg. 2010;97:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Ramalingam G, Gan EY, Kutt-Sing W. Laparoscopic abdominoperineal resection for anorectal melanoma: a case report and review of the literature. Surg Laparosc Endosc Percutan Tech. 2009;19:e149-e151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Droesch JT, Flum DR, Mann GN. Wide local excision or abdominoperineal resection as the initial treatment for anorectal melanoma? Am J Surg. 2005;189:446-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Ross M, Pezzi C, Pezzi T, Meurer D, Hickey R, Balch C. Patterns of failure in anorectal melanoma. A guide to surgical therapy. Arch Surg. 1990;125:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 93] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Li Z, Šandera P, Beer M, Weber M. A rare case of recurrent primary anorectal melanoma emphasizing the importance of postoperative follow-ups. BMC Surg. 2020;20:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Kelly P, Zagars GK, Cormier JN, Ross MI, Guadagnolo BA. Sphincter-sparing local excision and hypofractionated radiation therapy for anorectal melanoma: a 20-year experience. Cancer. 2011;117:4747-4755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1410] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 33. | Lian B, Si L, Cui C, Chi Z, Sheng X, Mao L, Li S, Kong Y, Tang B, Guo J. Phase II randomized trial comparing high-dose IFN-α2b with temozolomide plus cisplatin as systemic adjuvant therapy for resected mucosal melanoma. Clin Cancer Res. 2013;19:4488-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Ponti G, Manfredini M, Greco S, Pellacani G, Depenni R, Tomasi A, Maccaferri M, Cascinu S. BRAF, NRAS and C-KIT Advanced Melanoma: Clinico-pathological Features, Targeted-Therapy Strategies and Survival. Anticancer Res. 2017;37:7043-7048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Hamid O, Robert C, Ribas A, Hodi FS, Walpole E, Daud A, Arance AS, Brown E, Hoeller C, Mortier L, Schachter J, Long J, Ebbinghaus S, Ibrahim N, Butler M. Antitumour activity of pembrolizumab in advanced mucosal melanoma: a post-hoc analysis of KEYNOTE-001, 002, 006. Br J Cancer. 2018;119:670-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 36. | Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, Rapisuwon S, Eroglu Z, Sullivan RJ, Luke JJ, Gangadhar TC, Salama AK, Clark V, Burias C, Puzanov I, Atkins MB, Algazi AP, Ribas A, Wolchok JD, Postow MA. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;122:3354-3362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 37. | Heppt MV, Roesch A, Weide B, Gutzmer R, Meier F, Loquai C, Kähler KC, Gesierich A, Meissner M, von Bubnoff D, Göppner D, Schlaak M, Pföhler C, Utikal J, Heinzerling L, Cosgarea I, Engel J, Eckel R, Martens A, Mirlach L, Satzger I, Schubert-Fritschle G, Tietze JK, Berking C. Prognostic factors and treatment outcomes in 444 patients with mucosal melanoma. Eur J Cancer. 2017;81:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 38. | Lim A, Grant B, Avramovic J, Ho YH, Wallace C. Synchronous primary anorectal melanoma and sigmoid adenocarcinoma: a case report. Int Surg. 2015;100:814-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naswhan AJ S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ