Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10222
Peer-review started: July 7, 2021
First decision: August 18, 2021
Revised: August 18, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: November 26, 2021
Processing time: 138 Days and 6.3 Hours
The clinical role of ground glass opacity (GGO) on computed tomography (CT) in stage I pulmonary adenocarcinoma patients currently remains unclear.
To explore the prognostic value of GGO on CT in lung adenocarcinoma patients who were pathologically diagnosed with tumor-node-metastasis stage I.
A comprehensive and systematic search was conducted through the PubMed, EMBASE and Web of Science databases up to April 3, 2021. The hazard ratio (HR) and corresponding 95% confidence interval (CI) were combined to assess the association between the presence of GGO and prognosis, representing overall survival and disease-free survival. Subgroup analysis based on the ratio of GGO was also conducted. STATA 12.0 software was used for statistical analysis.
A total of 12 studies involving 4467 patients were included. The pooled results indicated that the GGO predicted favorable overall survival (HR = 0.44, 95%CI: 0.34-0.59, P < 0.001) and disease-free survival (HR = 0.35, 95%CI: 0.18-0.70, P = 0.003). Subgroup analysis based on the ratio of GGO further demonstrated that the proportion of GGO was a good prognostic indicator in pathological stage I pulmonary adenocarcinoma patients, and patients with a higher ratio of GGO showed better prognosis than patients with a lower GGO ratio did.
This meta-analysis manifested that the presence of GGO on CT predicted favorable prognosis in tumor-node-metastasis stage I lung adenocarcinoma. Patients with a higher GGO ratio were more likely to have a better prognosis than patients with a lower GGO ratio.
Core Tip: Our manuscript demonstrated that the ,ground glass opacity (GGO) predicted favorable overall survival (P < 0.001) and disease-free survival (P = 0.003). Subgroup analysis based on the ratio of GGO further demonstrated that the proportion of GGO was a good prognostic indicator in pathological stage I pulmonary adenocarcinoma patients and patients with a higher ratio of GGO showed better prognosis than patients with a lower GGO ratio did. This meta-analysis manifested that the presence of GGO on computed tomography predicted favorable prognosis in tumor-node-metastasis stage I lung adenocarcinoma. Patients with a higher GGO ratio were more likely to have a better prognosis than patients with a lower GGO ratio.
- Citation: Pan XL, Liao ZL, Yao H, Yan WJ, Wen DY, Wang Y, Li ZL. Prognostic value of ground glass opacity on computed tomography in pathological stage I pulmonary adenocarcinoma: A meta-analysis. World J Clin Cases 2021; 9(33): 10222-10232
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10222.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10222
Due to great advances in technology and the gradual popularity of high-resolution computed tomography (HRCT), many more cases of cancer can be screened and diagnosed at very early stages than previously possible[1,2]. Meanwhile, the proportion of different pathologic subtypes of lung cancer have changed significantly, and adenocarcinoma occupies a considerable proportion among non-small cell lung cancer[3,4]. With the increasing incidence of lung adenocarcinoma in recent years, a novel term, ground glass opacity (GGO), has been reported and received widespread attention. GGO refers to the increase in local density in the pulmonary nodules and blurred shadow that does not cover the blood vessels and bronchi in the lungs.
According to previous research, the presence of GGO in lung adenocarcinoma usually indicates the indolent nature of the lesions, and pure GGO nodules are related to pathologically preinvasive lesions[5-8]. In other words, the proportion of GGO reflects the malignant degree of pulmonary adenocarcinoma to a certain extent. Compared with pure GGO and subsolid lesions with a mixture of solid portion and GGO portion, lung adenocarcinomas representing as pure solid lesions are typically related with more aggressive behaviors and worse prognosis[9-13]. Therefore, the presence or absence of GGO and the specific ratio should be considered for the diagnosis and formulation of treatment.
Miao et al[14] conducted a meta-analysis by including 13 studies and demonstrated that the GGO ratio was significantly associated with overall survival (OS) [hazard ratio (HR) = 0.8, 95% confidence interval (CI): 0.78-0.93, P = 0.009], and the GGO area measured on HRCT showed a good prognostic value in small lung adenocarcinoma[14]. However, most of the studies included in their meta-analysis did not focus on stage I lung adenocarcinoma patients, and the clinical guiding significance of GGO in early stage lung adenocarcinoma is more important.
Thus, the aim of this meta-analysis was to explore the prognostic value of the presence of GGO on computed tomography (CT) in pathologic stage I lung adenocarcinoma patients, with the expectation that our findings will help with the clinical management and treatment of this group of patients.
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020) checklist.
The PubMed, EMBASE and Web of Science electronic databases were searched until April 3, 2020. The following key words were used: Adenocarcinoma, lung, pulmonary and GGO. A combination of medical subject heading terms and free words was applied. In detail, the specific search strategy was as follows: Adenocarcinoma AND (lung OR pulmonary) AND (ground glass opacity OR GGO). In addition, the references cited in included studies were also reviewed for availability.
The inclusion criteria were as follows: (1) Patients were pathologically diagnosed with lung adenocarcinoma and tumor-node-metastasis (TNM) stage I; (2) Patients received the radical operation; (3) Patients were divided into different groups according to the ratio of GGO on C,T and the prognosis was compared between groups; (4) The endpoints in the studies included the OS and disease-free survival (DFS); and (5) The HR with 95%CI were reported, if not, the Kaplan-Meier survival curves were provided to calculate them.
The exclusion criteria were as follows: (1) The HR with 95%CI were not reported, and the survival curves were also not obtained; (2) Reviews, case reports, meeting abstracts, animal trials and editorials; and (3) Duplicated or severely overlapped data.
The following information was extracted from included studies: The author, publication year, country, sample size, ratio of GGO, TNM stage (IA or IB), endpoints and HR with corresponding 95%CI.
The quality of included studies were assessed according to the Newcastle Ottawa Scale (NOS), and studies with a NOS of 6 or higher were regarded as high-quality studies[15].
The literature retrieval, selection, data extraction and quality assessment were performed by two investigators independently (Xue-Lin Pan and Zi-Ling Liao), and any disagreement was resolved by team discussion.
All statistical analysis were conducted by STATA 12.0 software (College Station, TX, United States). The HR with 95%CI were combined to evaluate the association between the presence of GGO and prognosis. When the HR with corresponding 95%CI were not provided directly, they were calculated from the Kaplan-Meier survival curves using the method reported by Tierney et al[16]. The heterogeneity was evaluated by Cochran’s Q test and Higgins I2 statistic; P < 0.10 and/or I2> 50% was defined as significant heterogeneity among studies, and the random-effects model was applied for the pooled effect estimates, otherwise the fixed-effects model was used[17]. Subgroup analyses stratified by the ratio of GGO (0% vs > 0%) were conducted. Sensitivity analysis for OS and DFS were performed by removing individual study from the meta-analysis each time.
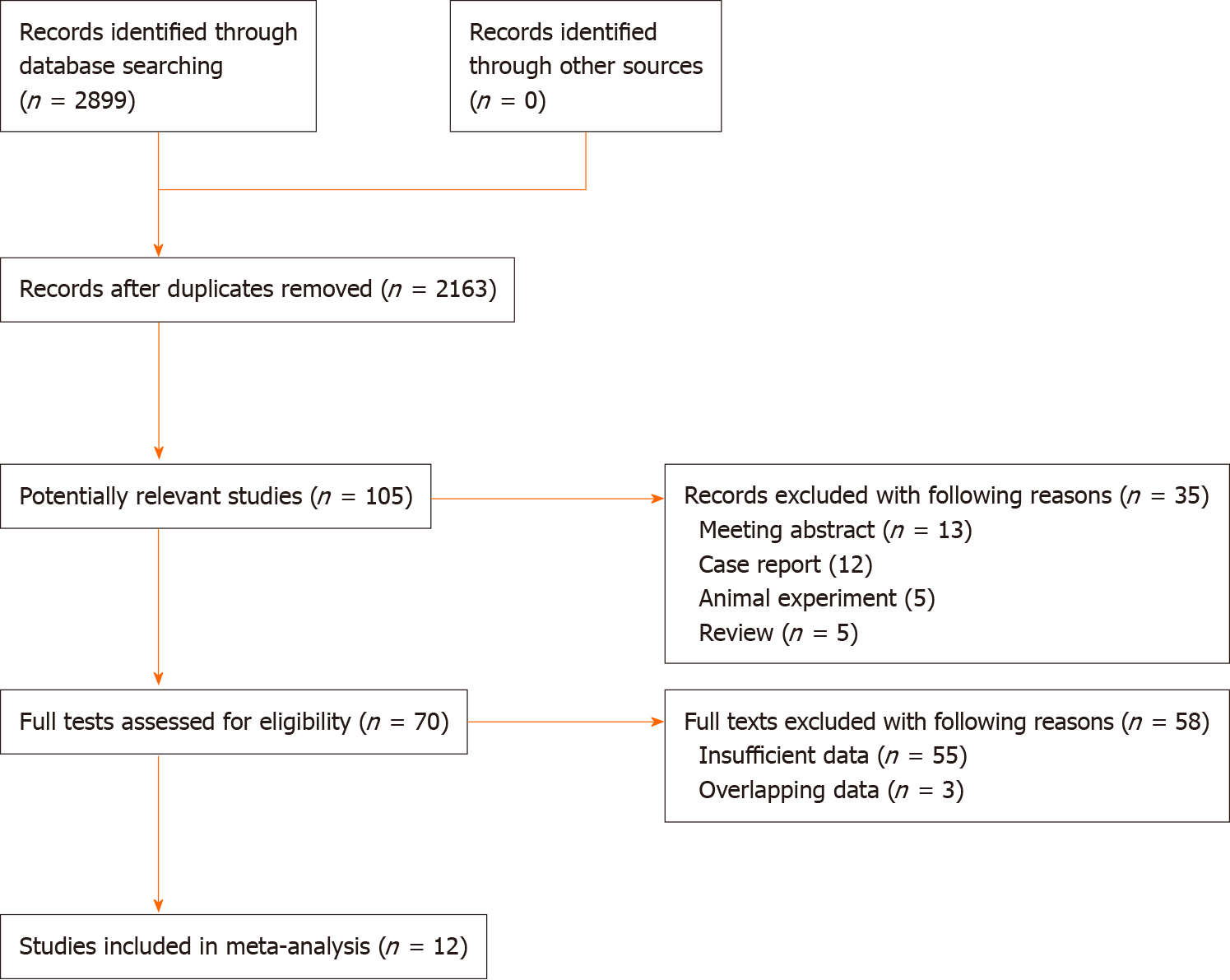
Initially, 2899 records were yielded from the three databases. After removing 736 duplicated records, 105 publications were found to be potentially related with the topic of this meta-analysis. Then 35 records were excluded due to the following reasons: Conference abstracts (n = 13), case reports (n = 12), animal trials (n = 5) and reviews (n = 5). Seventy full tests were reviewed for eligibility, and 58 publications were excluded because of insufficient data (n = 55) and overlapping data (n = 3). Finally, a total of 12 studies were included in this meta-analysis for further analysis[18-29] (Figure 1).
Among the included 12 studies, a total of 4467 patients were enrolled, with a range of sample size from 79 to 809. Most of included studies were from Asian countries, including Japan, China and Korea. Meanwhile, seven of the 12 studies divided patients into two groups according to the presence or absence of GGO in pulmonary nodules. All included studies were high-quality researches with a NOS of 6 or higher. Detailed information is presented in Table 1.
| Ref. | Country | Sample size | GGO ratio | TNM | NOS | Endpoint |
| Takamochi et al[18], 2004 | Japan | 189 | 0.8 | IA + IB | 7 | OS |
| Nakayama et al[19], 2010 | Japan | 201 | 0.5 | IA | 6 | DFS |
| Yanagawa et al[20], 2014 | Japan | 145 | 0.37 | IA + IB | 7 | OS, DFS |
| Nakamura et al[21], 2015 | Japan | 113 | 0.5 | IB | 7 | OS, DFS |
| Wang et al[22], 2016 | United States | 79 | 0 | IA + IB | 6 | OS |
| Zhong et al[23], 2018 | Japan | 354 | 0.5 | IA | 7 | DFS |
| Miyoshi et al[25], 2019 | Japan | 809 | 0 | IA | 7 | OS |
| Kinoshita et al[24], 2019 | Japan | 274 | 0 | IA + IB | 8 | DFS |
| Zhong et al[28], 2021 | China | 620 | 0 | IA + IB | 6 | OS, DFS |
| Han et al[26], 2020 | Korea | 544 | 0.5 | IA | 8 | DFS |
| Phillips et al[27], 2020 | United States | 357 | 0 | IA | 7 | OS, DFS |
| Shigefuku et al[29], 2021 | Japan | 782 | 0 | IA + IB | 7 | OS |
Eight studies explored the association between the presence of GGO on CT and OS of stage I lung adenocarcinoma patients[18,20-23,27-29]. The pooled results indicated that GGO was significantly related with better OS (HR = 0.44, 95%CI: 0.34-0.59, P < 0.001; I2= 24.3%, P = 0.236) (Figure 2). Subgroup analysis based on the ratio of GGO demonstrated that the presence of GGO was an independent predictor for OS, and patients with a higher ratio of GGO had better OS than patients with a lower ratio of GGO did (Figure 3 and Table 2).
| No. studies | HR | 95%CI | P value | I2 (%) | P value | |
| Overall survival | 8 [18,20-23,27-29] | 0.44 | 0.34-0.59 | < 0.001 | 24.3 | 0.236 |
| > 01 | 3 [18,20,21] | 0.14 | 0.05-0.36 | < 0.001 | 0.0 | 0.943 |
| 01 | 5 [22,23,27-29] | 0.49 | 0.37-0.66 | < 0.001 | 0.0 | 0.583 |
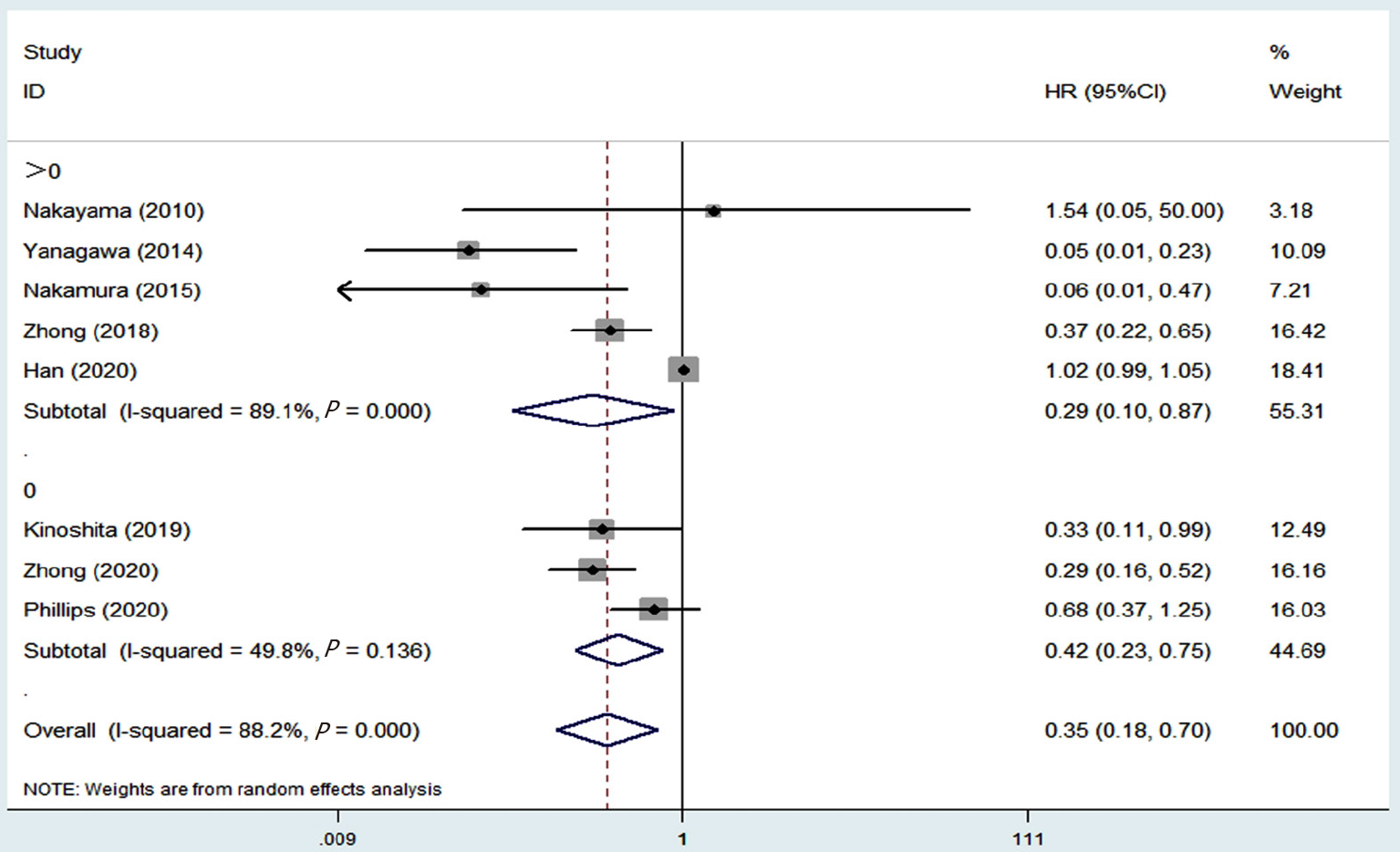
| Disease-free survival | 8 [19-21,23,24,26-28] | 0.35 | 0.18-0.70 | 0.003 | 88.2 | < 0.001 |
| > 01 | 5 [19-21,23,26] | 0.29 | 0.10-0.87 | 0.027 | 89.1 | < 0.001 |
| 01 | 3 [24,27,28] | 0.42 | 0.23-0.75 | 0.004 | 49.8 | 0.136 |
Eight studies investigated the relationship between the presence of GGO on CT and DFS[19-21,23,24,26-28]. The pooled results demonstrated that the presence of GGO was significantly related with improved DFS (HR = 0.35, 95%CI: 0.18-0.70, P = 0.003; I2 = 88.2%, P < 0.001) (Figure 4). Subgroup analysis stratified by the proportion of GGO in nodules also manifested that the presence of GGO was a significant predictive indicator for DFS, and patients with a higher ratio of GGO were more likely to experience a better DFS (Figure 5 and Table 2).
The results of sensitivity analysis for the OS (Figure 6A) and DFS (Figure 6B) indicated that the pooled results of this meta-analysis were stable and reliable.
The current meta-analysis demonstrated that the presence of GGO on CT was a predictive indicator for improved OS and DFS of pathologic stage I lung adenocarcinoma patients. In addition, the proportion of GGO played an essential role in predicting the survival of this group of patients.
Although we conducted subgroup analysis based on the GGO ratio in pulmonary nodules and manifested that patients with a higher ratio of GGO on CT would experience better prognosis than patients with a lower ratio of GGO did, we still deemed that it was necessary to explore the association between the proportion of GGO and survival risk. Half of the included studies simply divided patients into the GGO group (presence of GGO) and non-GGO group (absence of GGO) and only identified the prognostic value of presence of GGO on CT in stage I lung adenocarcinoma patients[22,24,25,27-29]. The other studies divided patients into the higher GGO ratio group and lower GGO ratio group, and most of them defined the 50% as the threshold ratio[18,21,23,26]. However, after combining the four studies comparing the DFS between the GGO dominant group and solid dominant group, no significant difference in the DFS was observed (HR = 0.47, 95%CI: 0.17-1.27, P = 0.136; I2= 85.4%, P < 0.001) (Supplementary Figure 1)[19,21,23,26], which indicated that 50% may not be a reliable critical value in distinguishing the prognosis of patients with different ratios of GGO on CT. Besides, Takamochi et al[18] identified 80% as the threshold value and found that patients with a GGO ratio > 80% had improved OS than patients with a GGO ratio < 80% did (HR = 0.158, 95%CI: 0.045-0.554, P = 0.004)[18]. However, they did report the source of this threshold. Yanagawa et al[20] identified the optimal cutoff value of GGO proportion on CT according to the receiver operating characteristic analysis, and 37% was defined as the optimal threshold value[20]. Notably, in their study, GGO ratio < 37% was verified to be a strong predictive indicator for poor OS (HR = 9.60, 95%CI: 1.17-78.91, P = 0.036) and DFS (HR = 18.45, 95%CI: 4.34-78.49, P < 0.001)[20]. Thus, it is believed that a reliable statistical method is vital in dividing patients into different groups according to the ratio of GGO when exploring the prognostic value of GGO ratio on CT in future relevant studies.
Besides, the study by Shigefuku et al[29] reported the association between the presence of GGO on CT and cancer-specific survival (CSS) of stage I lung adenocarcinoma patients and manifested that GGO was also a significant predictive indicator for improved CSS (HR = 0.509, 95%CI: 0.260-0.997, P = 0.049)[29]. Actually, we deemed that CSS was more valuable than OS in pathologic TNM stage I pulmonary adenocarcinoma. For lung adenocarcinoma patients without other malignancies, the 5-year OS rate exceeds 80%[3]. Thus, defining the CSS, as well as the DFS, as the endpoint might help with exploring the impact of pulmonary nodules and its components on the prognosis.
Although we demonstrated that the presence of GGO on CT predicted favorable prognosis in TNM stage I lung adenocarcinoma and patients with a higher GGO ratio had an improved prognosis than patients with a lower GGO ratio did, there are still many fields worthy of in-depth investigating about the GGO ratio in stage I lung adenocarcinoma patients. First, as mentioned above, the optimal cutoff value of GGO proportion in distinguishing survival risk of patients with different ratios of GGO on CT remains unclear. Second, a combination of GGO proportion and other imaging features such as the spiculation sign and lobulation sign should be better in predicting prognosis of lung adenocarcinoma patients. Third, the association of GGO ratio on CT with the therapeutic effect of targeted therapy or chemoradiotherapy is unclear, although most of patients with stage I lung adenocarcinoma do not received these adjuvant therapies. However, multiple primary lung adenocarcinomas are receiving increasing attention in recent years, and these adjuvant therapies might be applied in multiple primary pulmonary adenocarcinoma patients undergoing diagnostic pulmonary resection.
There are several limitations in this meta-analysis. First, all included studies are retrospective, which may cause some bias. Second, most of patients are from Asian countries, and there might be some regional heterogeneity. Third, due to the lack of detailed information about the age, sex and pathological subtype of adenocarcinoma, we failed to conduct subgroup analysis based on these parameters.
We demonstrated that the presence of GGO on CT predicted favorable prognosis in TNM stage I lung adenocarcinoma by combining 12 relevant studies involving 4467 patients. Patients with a higher GGO ratio were more likely to have a better prognosis than patients with a lower GGO ratio. However, more prospective studies with high quality are still needed to verify our findings.
The presence of ground glass opacity (GGO) in lung adenocarcinoma usually indicates the indolent nature of lesions, and the proportion of GGO reflects the malignant degree of pulmonary adenocarcinoma to a certain extent
The prognostic role of GGO on computed tomography (CT) in stage I pulmonary adenocarcinoma patients remains unclear now.
To identify the prognostic value of GGO on CT in lung adenocarcinoma patients who were pathologically diagnosed with tumor-node-metastasis stage I.
Several databases were searched for relevant studies. The hazard ratio and corresponding 95% confidence interval were combined to assess the association between the presence of GGO and prognosis, representing as the overall survival and disease-free survival. Subgroup analysis based on the ratio of GGO was also conducted.
GGO predicted favorable overall survival (P < 0.001) and disease-free survival (P = 0.003). Subgroup analysis based on the ratio of GGO further demonstrated that the proportion of GGO was a good prognostic indicator in pathological stage I pulmonary adenocarcinoma patients, and patients with a higher ratio of GGO showed better prognosis than patients with a lower GGO ratio did.
The presence of GGO on CT predicted favorable prognosis in tumor-node-metastasis stage I lung adenocarcinoma.
Patients with a higher GGO ratio were more likely to have a better prognosis than patients with a lower GGO ratio.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saueressig MG S-Editor: Fan JR L-Editor: Filipodia P-Editor: Liu JH
| 1. | Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70:443-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 789] [Article Influence: 131.5] [Reference Citation Analysis (1)] |
| 2. | Sun K, You A, Wang B, Song N, Wan Z, Wu F, Zhao W, Zhou F, Li W. Clinical T1aN0M0 lung cancer: differences in clinicopathological patterns and oncological outcomes based on the findings on high-resolution computed tomography. Eur Radiol. 2021;31:7353-7362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1979] [Article Influence: 395.8] [Reference Citation Analysis (3)] |
| 4. | Wang Y, Li S, Hu X, Wang Y, Wu Y, Li P, Che G. The prognostic value of serum albumin-globulin ratio in early-stage non-small cell lung cancer: a retrospective study. Cancer Manag Res. 2019;11:3545-3554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Hattori A, Matsunaga T, Hayashi T, Takamochi K, Oh S, Suzuki K. Prognostic Impact of the Findings on Thin-Section Computed Tomography in Patients with Subcentimeter Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Importance of Ground Glass Opacity Component in Clinical Stage IA Radiologic Invasive Lung Cancer. Ann Thorac Surg. 2017;104:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Lee KH, Goo JM, Park SJ, Wi JY, Chung DH, Go H, Park HS, Park CM, Lee SM. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol. 2014;9:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Ye T, Deng L, Xiang J, Zhang Y, Hu H, Sun Y, Li Y, Shen L, Wang S, Xie L, Chen H. Predictors of Pathologic Tumor Invasion and Prognosis for Ground Glass Opacity Featured Lung Adenocarcinoma. Ann Thorac Surg. 2018;106:1682-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Liu K, Li K, Wu T, Liang M, Zhong Y, Yu X, Li X, Xie C, Zhang L, Liu X. Improving the accuracy of prognosis for clinical stage I solid lung adenocarcinoma by radiomics models covering tumor per se and peritumoral changes on CT. Eur Radiol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Su H, Dai C, She Y, Ren Y, Zhang L, Xie H, Xie D, Jiang G, Chen C. Which T descriptor is more predictive of recurrence after sublobar resection: whole tumour size vs solid component size? Eur J Cardiothorac Surg. 2018;54:1028-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Dennie C, Bayanati H, Souza CA, Peterson R, Shamji FM. Role of the Thoracic Radiologist in the Evaluation and Management of Solid and Subsolid Lung Nodules. Thorac Surg Clin. 2021;31:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Wang B, Hamal P, Sun K, Bhuva MS, Yang Y, Ai Z, Sun X. Clinical Value and Pathologic Basis of Cystic Airspace Within Subsolid Nodules Confirmed as Lung Adenocarcinomas by Surgery. Clin Lung Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Lai J, Li Q, Fu F, Zhang Y, Li Y, Liu Q, Chen H. Subsolid Lung Adenocarcinomas: Radiological, Clinical and Pathological Features and Outcomes. Semin Thorac Cardiovasc Surg. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Miao XH, Yao YW, Yuan DM, Lv YL, Zhan P, Lv TF, Liu HB, Song Y. Prognostic value of the ratio of ground glass opacity on computed tomography in small lung adenocarcinoma: A meta-analysis. J Thorac Dis. 2012;4:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 5:80-84. |
| 16. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 5082] [Article Influence: 267.5] [Reference Citation Analysis (1)] |
| 17. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48390] [Article Influence: 2103.9] [Reference Citation Analysis (4)] |
| 18. | Takamochi K, Yoshida J, Nishimura M, Yokose T, Sasaki S, Nishiwaki Y, Suzuki K, Nagai K. Prognosis and histologic features of small pulmonary adenocarcinoma based on serum carcinoembryonic antigen level and computed tomographic findings. Eur J Cardiothorac Surg. 2004;25:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Nakayama H, Okumura S, Daisaki H, Kato Y, Uehara H, Adachi S, Yoshimura M, Okada M. Value of integrated positron emission tomography revised using a phantom study to evaluate malignancy grade of lung adenocarcinoma: a multicenter study. Cancer. 2010;116:3170-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Yanagawa M, Tanaka Y, Leung AN, Morii E, Kusumoto M, Watanabe S, Watanabe H, Inoue M, Okumura M, Gyobu T, Ueda K, Honda O, Sumikawa H, Johkoh T, Tomiyama N. Prognostic importance of volumetric measurements in stage I lung adenocarcinoma. Radiology. 2014;272:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Nakamura S, Fukui T, Kawaguchi K, Fukumoto K, Hirakawa A, Yokoi K. Does ground glass opacity-dominant feature have a prognostic significance even in clinical T2aN0M0 lung adenocarcinoma? Lung Cancer. 2015;89:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Wang H, Schabath MB, Liu Y, Stringfield O, Balagurunathan Y, Heine JJ, Eschrich SA, Ye Z, Gillies RJ. Association Between Computed Tomographic Features and Kirsten Rat Sarcoma Viral Oncogene Mutations in Patients With Stage I Lung Adenocarcinoma and Their Prognostic Value. Clin Lung Cancer. 2016;17:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Zhong C, Sakurai H, Wei S, Fang W, Asamura H. Sublobar resections for small-sized stage Ia lung adenocarcinoma: a Sino-Japanese multicenter study. J Thorac Dis. 2018;10:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Kinoshita F, Toyokawa G, Matsubara T, Kozuma Y, Haratake N, Takamori S, Akamine T, Hirai F, Takenaka T, Tagawa T, Maehara Y. Prognosis of Early-stage Part-solid and Pure-solid Lung Adenocarcinomas. Anticancer Res. 2019;39:2665-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Miyoshi T, Aokage K, Katsumata S, Tane K, Ishii G, Tsuboi M. Ground-Glass Opacity Is a Strong Prognosticator for Pathologic Stage IA Lung Adenocarcinoma. Ann Thorac Surg. 2019;108:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Han SJ, Jeon JH, Jung W, Seong YW, Cho S, Kim K, Jheon S. Do ground-glass opacity-dominant features have prognostic significance in node-negative adenocarcinomas with invasive components of similar sizes? Eur J Cardiothorac Surg. 2020;57:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Phillips WW, Gill RR, Mazzola E, Armitage JR, de Forcrand C, Colson YL, Gibney BC. Impact of Nodule Density in Women With Sublobar Resection for Stage IA Adenocarcinoma. Ann Thorac Surg. 2021;112:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Zhong Y, Xu Y, Deng J, Wang T, Sun X, Chen D, Wu C, Hou L, Xie H, She Y, Xie D, Chen C. Prognostic impact of tumour spread through air space in radiological subsolid and pure solid lung adenocarcinoma. Eur J Cardiothorac Surg. 2021;59:624-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Shigefuku S, Shimada Y, Hagiwara M, Kakihana M, Kajiwara N, Ohira T, Ikeda N. Prognostic Significance of Ground-Glass Opacity Components in 5-Year Survivors With Resected Lung Adenocarcinoma. Ann Surg Oncol. 2021;28:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |