Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10126
Peer-review started: May 28, 2021
First decision: July 1, 2021
Revised: July 3, 2021
Accepted: October 11, 2021
Article in press: October 11, 2021
Published online: November 26, 2021
Processing time: 177 Days and 19.9 Hours
A gastric glomus tumor is relatively rare, and there is little knowledge on its endoscopic ultrasound findings.
To assess the accuracy of endoscopic ultrasonography (EUS) in the diagnosis of gastric glomus tumor and to discuss its value by reviewing the literature.
A retrospective analysis of the EUS characteristics of gastric glomus tumor (such as tumor location, shape, size, echogenicity, homogeneity, margins, layer of origin, and so on) was performed. The study included 12 cases of gastric glomus tumor confirmed by surgery and pathology (7 females and 5 males, age range 36-74 years, average age was 58.2 years).
All the lesions were located in the gastric antrum (12 cases), protruding into the cavity, with a diameter between 1 and 3.5 cm. Glomus tumor of the stomach manifested as a circumscribed and slightly hypoechoic mass in the fourth layer, with an internal heterogeneous echo mixed with hyperechogenic spots and a marginal more hypoechoic halo. Smooth muscle actin, h-caldesmon and vimentin were shown to be positive by immunohistochemistry.
Although glomus tumor of the stomach is relatively rare, a typical glomus tumor of the stomach has characteristic changes under EUS.
Core Tip: Gastric glomus tumor is a rare non-epithelial benign vascular tumor and is difficult to diagnose with upper gastrointestinal endoscopy. We summarized the characteristics of endoscopic ultrasonography (EUS), computed tomography (CT) and pathology of 12 cases of gastric glomus tumor confirmed by pathology. The EUS characteristics of a gastric glomus tumor include a hypoechoic lesion originating from the fourth layer with a peripheral acoustic halo. Clinically, the diagnosis can be confirmed by the EUS characteristics combined with CT imaging findings.
- Citation: Bai B, Mao CS, Li Z, Kuang SL. Endoscopic ultrasonography diagnosis of gastric glomus tumors. World J Clin Cases 2021; 9(33): 10126-10133
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10126.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10126
Glomus tumor is a rare non-epithelial benign vascular tumor occurring at the site of arteriovenous anastomoses. Tumor cells are derived from smooth muscle cells with vascular spheroid degeneration. Glomus tumors often occur under the nail bed at the end of the extremities, and can also occur in other parts, such as soft tissue, mediastinal lung, nose, pharynx, sacrococcygeal region etc. Glomus tumors of the gastrointestinal tract are relatively rare and more common in the stomach. Most gastric glomus tumors are benign and are occasionally found as subepithelial lesions[1,2]. Endoscopic ultrasound is currently more and more widely used in clinical practice, but there is little understanding of the endoscopic ultrasound manifestations of gastric glomus tumors, and their characteristics. This study summarized the endoscopic ultrasound manifestations and related pathological characteristics of 12 cases of gastric glomus tumors confirmed by surgery and pathology, combined with a review of relevant literature, to improve the diagnosis and understanding of the disease under endoscopic ultrasound.
This study was a retrospective observational study. We retrospectively collected data from medical records and endoscopic reports at Henan Provincial People’s Hospital (Zhengzhou China). Twelve cases of gastric glomus tumor confirmed by surgery and pathology in our hospital from January 2013 to October 2020 were enrolled, including 7 females and 5 males, aged 36-74 years, with an average age of 58.2 years. Six cases experienced epigastric pain and discomfort with acid reflux and heartburn, 1 case had nausea, 2 cases had a positive stool occult blood test and 3 cases were normal foll
Endoscopic ultrasound examination included the use of the EU-ME2 PP ultrasound host (Olympus Japan), MAJ-935 or MAJ-1720 ultrasound probe driver, UM-2R, UM-3R ultrasound microprobe, or the GF-UE160-AL5 radial array scanning endoscope. Ultrasound scanning was performed by the water injection method. The liquid and gas in the gastric cavity were first removed, and the target lesions of ultrasonic scanning were displayed and confirmed. Then, water was slowly injected at a low flow rate, and the lesion was completely immersed in degassed water following suction of gastric air. The micro-probe was taken out of the endoscopic channel for ultrasound scanning, and for larger lesions, a radial scanning ultrasound was used for ultrasound scanning. The following features were recorded: location, size, the presence of mucosal ulceration, shape, original layer, echogenicity, echo uniformity, the presence of marginal halos, cystic change, and calcification.
All specimens were fixed in 10% buffered formalin and routinely processed, followed by routine hematoxylin and eosin immunohistochemical staining. The immunohistochemical staining process was carried out according to the instructions. The pathology results were independently interpreted by 2 experienced pathologists. Under a light microscope, tumor cells were seen growing around blood vessels. Diagnosis was confirmed when immunohistochemistry showed positive smooth muscle actin (SMA), h-caldesmon and vimentin[3].
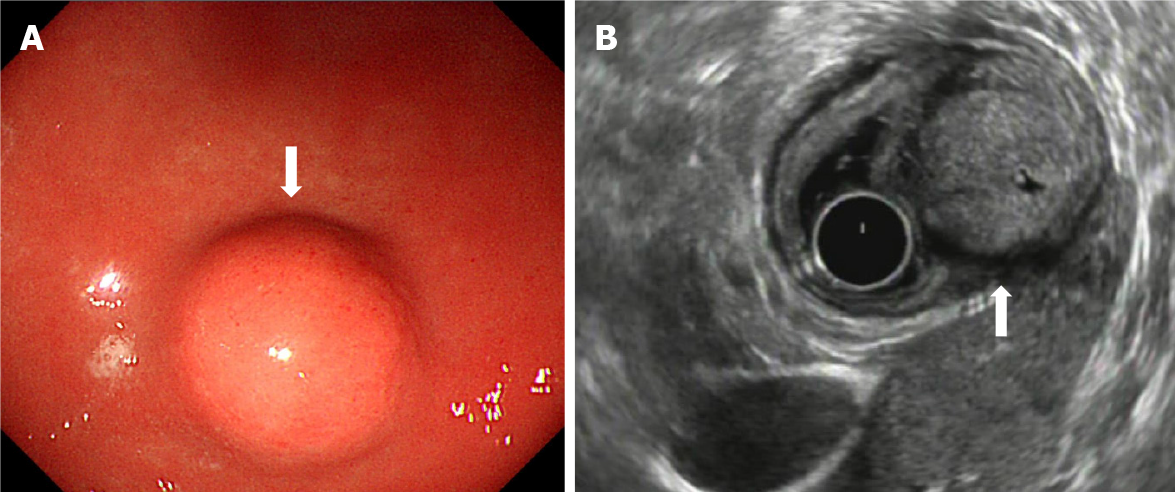
During gastroscopy, all lesions were located in the gastric antrum, and presented as a submucosal tumor that protruded into the lumen in a small semi-spherical shape (Figure 1A). Ten cases had smooth hemispherical bulges of the mucosa, the same color as the surrounding mucosa, and 2 cases had small concave ulcers on the mucosa surface, leading to hemorrhage. The lesions were located in the anterior wall of the gastric antrum in 3 cases and in the lesser curvature of the gastric antrum in 9 cases. Endoscopic ultrasonography (EUS) showed that the lesions were round, ranging in maximum diameter from 10 mm to 35 mm with a mean diameter of 20 mm. The echo was slightly hypoechoic, and was uniform, accompanied by small hyperechoic spots. There were small anechoic areas in the larger lesions, and halos with a lower echo. The outer boundary of the halo was clear, and the inner boundary was fuzzy. All lesions originated from the fourth layer, that is, the muscularis propria (Figure 1B). The clinical and endoscopic ultrasound features of the 12 cases of gastric glomus tumor are summarized in Table 1.
| Case | Gender | Age (yr) | Treatment method | Location | Symptom | Diameter (cm) | Endoscopic ultrasound features | Ulceration | Follow-up time (yr) | Recurrence |
| 1 | Female | 36 | ESD | Anterior wall of the gastric antrum | Normal | 1 | The fourth layer, round, hypoechoic, halos | N | 3 | N |
| 2 | Female | 47 | ESD | Lesser curvature of the gastric antrum | Normal | 1.2 | The fourth layer, round, hypoechoic, halos | N | 4 | N |
| 3 | Female | 56 | Operation | Lesser curvature of the gastric antrum | Positive OBT | 3.5 | The fourth layer, round, hypoechoic, halos, with anechoic areas | Y | 7 | N |
| 4 | Male | 63 | ESD | Anterior wall of the gastric antrum | Epigastric pain, heartburn | 1.5 | The fourth layer, round, hypoechoic, halos | N | 5 | N |
| 5 | Male | 65 | ESD | Lesser curvature of the gastric antrum | Epigastric pain, heartburn | 1.8 | The fourth layer, round, hypoechoic, halos | N | 2 | N |
| 6 | Male | 58 | ESD | Lesser curvature of the gastric antrum | Heartburn | 2.3 | The fourth layer, round, hypoechoic, halos, small hyperechoic spots | N | 4 | N |
| 7 | Female | 54 | ESD | Lesser curvature of the gastric antrum | Heartburn | 2.1 | The fourth layer, round, hypoechoic, halos | N | 6 | N |
| 8 | Male | 64 | ESD | Lesser curvature of the gastric antrum | Epigastric pain, heartburn | 2.2 | The fourth layer, round, hypoechoic, halos, small hyperechoic spots | N | 1 | N |
| 9 | Male | 70 | ESD | Anterior wall of the gastric antrum | Normal | 1.3 | The fourth layer, round, hypoechoic, halos | N | 5 | N |
| 10 | Female | 56 | ESD | Lesser curvature of the gastric antrum | Epigastric pain, heartburn | 1.7 | The fourth layer, round, hypoechoic, halos | N | 3 | N |
| 11 | Female | 55 | Operation | Lesser curvature of the gastric antrum | Positive OBT | 3.2 | The fourth layer, round, hypoechoic, halos, with anechoic areas | Y | 1 | N |
| 12 | Female | 74 | Operation | Lesser curvature of the gastric antrum | Nausea | 2.8 | The fourth layer, round, hypoechoic, halos, with anechoic areas | N | 0.5 | N |
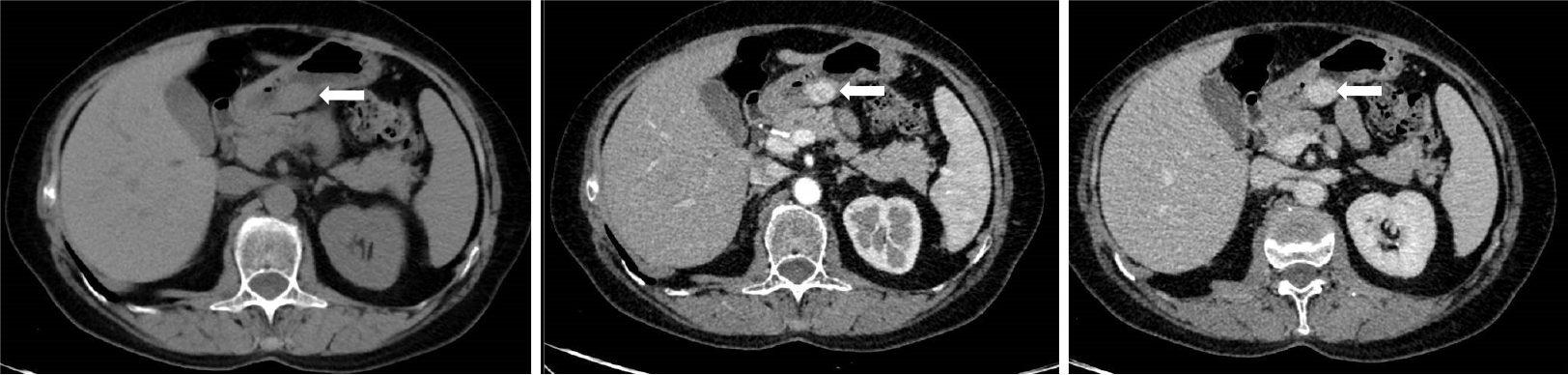
Enhanced CT examination was as follows: A Philips ingenuity 64 slice multi-slice spiral CT scanner was used, 1 mm thick, 5 mm reconstruction, whole abdomen plain scan and enhanced scan. The non-ionic contrast agent ioversol (100 mL; 320 mg I/mL) was injected with a Medrad Vistron CT high-pressure syringe at a rate of 3.0 mL/s via the elbow vein. The first phase (arterial phase) abdominal scan was started 30 s after injection, and the second phase (portal phase) abdominal scan was started 80 s after injection. CT showed well-defined subepithelial masses with homogeneous soft tissue densities with clear margins and perigastric adipose tissue. Dynamic contrast-enhanced CT showed strong peripheral nodular or homogeneous enhancement in the arterial and portal phases and prolonged enhancement in the delayed phase. The capsule of the tumor was slightly enhanced on CT scan, and no enlarged lymph nodes were observed (Figure 2).
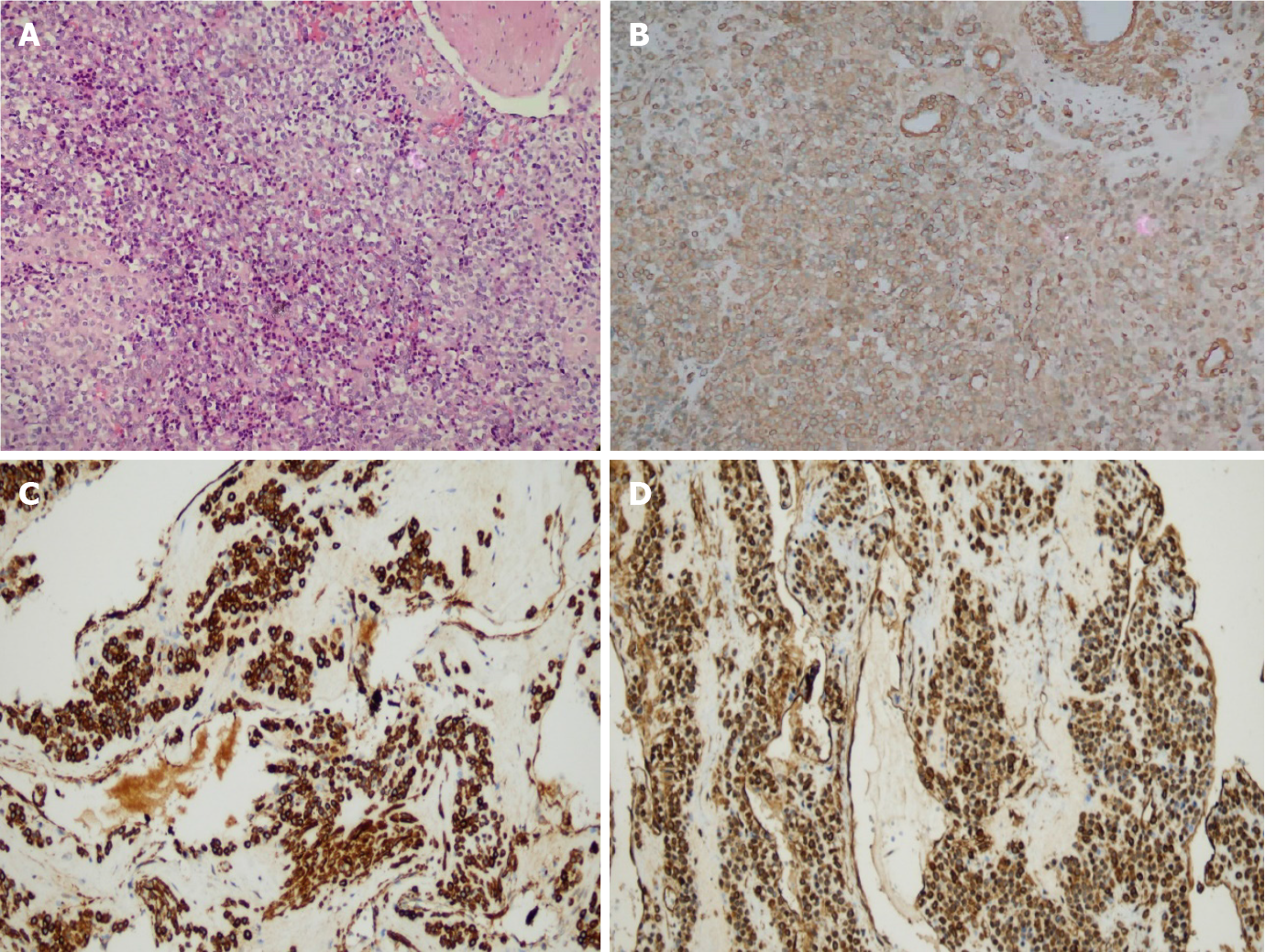
Gross examination showed the following: In 9 cases of ESD resection of tumor and 3 cases of partial gastric wall and tumor, the lesions were round, with clear boundaries, no obvious capsule, grayish-red, or grayish-white in color, and a tough texture. Histologically, the tumors were located in the gastric muscularis propria and composed of glomus cells surrounding capillaries. The tumor cells were small, uniform in size, arranged in nests, and round without nuclear pleomorphism, mitotic figures, or necrosis, and grew around blood vessels. The tumors had abundant blood supply; dilated blood vessels were visible in the surrounding muscularis along with clear or light red cytoplasm, and no atypia or mitosis (Figure 3A). Immunohistochemically, tumor cells showed diffuse immunostaining for SMA, h-caldesmon, and vimentin in 12 cases (igure 3B-D), and all tumors were negative for CD117, CD34, S-100, dog-1, desmin, and CD31.
A gastric glomus tumor is an organ-like tumor composed of glomus cells. The glomus is a network of capillaries connecting arteries and veins. The terminal organ-like tumor is composed of a small entering artery, anastomotic vessel/original collecting vein, endovascular reticular structure and a capsule. According to its contraction me
Miettinen et al[7] reported 32 cases of gastrointestinal glomus tumor. Among them, 31 cases originated from the stomach and 1 case from the cecum. Gastric glomus tumors account for 1% of gastric mesenchymal tumors. A gastric glomus tumor is usually located in the muscularis propria or submucosa of the stomach. Patients usually have no specific symptoms, abdominal discomfort, upper abdominal pain, and/or upper gastrointestinal bleeding are relatively common clinical manifestations. The lesion is more common in people over 60 years old, and more common in women than in men. The average age in our group was 58.2 years. Seven patients mainly had epigastric discomfort, acid reflux and heartburn. The lesion was found by gastroscopy examination in 3 patients, and by fecal occult blood in 2 patients. The data showed slightly more female patients than male patients, and the lesions were located in the gastric antrum, which was basically consistent with the literature reports.
All cases in this study were identified by endoscopy, and endoscopic ultrasound was then performed. The common endoscopic manifestations are subepithelial lesions, regular shape of the mucosa bulge, smooth surface mucosa, color is the same as the surrounding mucosa[8]. Endoscopic ultrasound of a gastric glomus tumor is characterized by a hypoechoic tumor, located in the third or fourth layer of the gastric wall, with an uneven internal echo and high echo spots. An acoustic halo at the edge can be seen[9]. We found that the lesions were round and hypoechoic, with a lower echoic halo around them, and some with small hyperechoic spots, which were consistent with previous reports. However, the echo was slightly hypoechoic, and all of them originated from the fourth layer, the muscularis propria. A slightly hypoechoic echo was observed compared with the echogenicity of gastrointestinal stromal tumors. The gastrointestinal stromal tumor is the most common interstitial tumor in the stomach. Gastrointestinal stromal tumors are characterized by subepithelial lesions, which is one of the most important features in the differential diagnosis of gastric glomus tumors. Typical endoscopic ultrasound features are clear boundary, low echogenicity and even relative echogenicity, which originate from the fourth layer, mostly at the fundus of the stomach. The echo intensity of a gastric glomus tumor is generally higher than that of a gastrointestinal stromal tumor. Of course, a more accurate method is to measure the gray value or relative gray value (compared with the intrinsic muscular layer beside the lesion). It is difficult to distinguish gastric glomus tumor or gastrointestinal stromal tumor under endoscopy, which is characterized by subepithelial lesions with a mucosal bulge. A gastric glomus tumor is usually isolated, located in the antrum or the lower part of the stomach. Gastrointestinal stromal tumor is single, occasionally multiple, mostly found in the stomach body or fundus.
A contrast-enhanced CT scan of gastric glomus tumors shows significant enhancement in the arterial, portal, and delayed phases, nodular or homogeneous enhancement may be seen around the mass in the arterial phase, and there is still significant enhancement in the portal and delayed phases, indicating that the lesion enhances substantially and simultaneously with the abdominal aorta at the same level[2,8,10]. Histopathologically, there are many thin-walled and tortuous vessels within the tumor tissue with an abundant blood supply. This internal structure of the tumor determines the pathologic basis for the apparent enhancement seen on the contrast-enhanced CT scan, which is significantly higher than that of other benign tumors.
Microscopically, a gastric glomus tumor is composed of a vascular lumen surrounded by small round cells of a single morphology with centrally placed nuclei and inconspicuous nucleoli. Pleomorphism, atypia, mitosis and necrosis are rare in gastric glomus tumors[8,11]. Immunohistochemically, the tumor is positive for SMA, vimentin, actin, collagen IV, and laminin, and negative for CD117, dog-1, S100, CD34, desmin and negative for neuroendocrine markers such as chromogranin, synaptophysin, CD56, and CD57[11]. Most gastrointestinal stromal tumors are strongly positive for CD117 and dog-1, and immunohistochemistry of gastric glomus tumors show negative CD117 and dog-1 as a means of differential diagnosis. The morphology and immunohistochemical features of the patients' lesions in our group were consistent with those in the literature.
Gastric glomus tumors are mostly benign and most do not require treatment, so their preoperative diagnosis is especially important. Endoscopic ultrasound combined with abdominal enhanced CT can diagnose gastric glomus tumors, but the final definitive diagnosis requires immunohistochemistry of the pathology. In this case group, 7 patients were diagnosed with gastric glomus tumors by endoscopic ultrasound, which shows that with the accumulation of endoscopic ultrasound experience, the endoscopic ultrasound characteristics of gastric glomus tumors can increase the understanding of the disease and make the diagnosis less difficult.
Although gastric glomus tumors are relatively rare, typical gastric glomus tumors have characteristic changes on endoscopic ultrasound. These characteristic changes include gastric antrum location, originate from the fourth layer, solitary, round and slightly hypoechoic lesions with halos at the margins are typical manifestations. The diagnosis of a gastric glomus tumor can be made clinically based on the endoscopic ultrasound features and contrast-enhanced CT findings; however, confirmatory diagnosis is always by pathological examination.
Gastric glomus tumors are relatively rare, and there is little knowledge regarding their endoscopic ultrasound findings.
Knowledge of the endoscopic characteristics of gastric glomus tumor is helpful in the diagnosis and differential diagnosis, and avoiding unnecessary surgery.
To summarize the endoscopic ultrasound characteristics of gastric glomus tumors confirmed by histopathology, and to verify the effectiveness of these characteristics in clinical cases.
The records of 12 consecutive patients undergoing endoscopic ultrasonography (EUS) examination, surgery and pathology analysis for gastric glomus tumors in one institution from 2013-2020 were reviewed. Analysis of the EUS characteristics of gastric glomus tumors (such as tumor location, shape, size, echogenicity, homogeneity, margins, layer of origin, and so on) was carried out.
Ten cases had smooth hemispherical bulges of the mucosa, the same color as the surrounding mucosa, and 2 cases had small concave ulcers on the mucosa surface, leading to hemorrhage. The lesions were located in the anterior wall of the gastric antrum in 3 cases and in the lesser curvature of the gastric antrum in 9 cases. EUS showed that the lesions were round, ranging in maximum diameter from 10 mm to 35 mm with a mean diameter of 20 mm. The echo was slightly hypoechoic, and uniform, accompanied by small hyperechoic spots in some. There were small anechoic areas in the larger lesions, and halos with a lower echo. The outer boundary of the halo was clear, and the inner boundary was fuzzy. All of the lesions originated from the muscularis propria.
Typical manifestations of gastric glomus tumors include gastric antrum location, originate from the fourth layer, solitary, round and slightly hypoechoic lesions with halos at the margins.
A multi-center study, with an expanded sample size and prospective verification is required.
| 1. | Casarotto A, Zarantonello FR, Piccirillo G, Criscenti P, Verza M, Zirillo M, Rebonato M. Gastric glomus tumor: a rare case of dyspepsia. Endoscopy. 2015;47 Suppl 1:E75-E76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Wu M, Zhou T, Cao D, Qu L, Cao X. Glomus tumor of the stomach: A case report. Medicine (Baltimore). 2018;97:e13132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Han Anjia YX, Wang J. Expert consensus on the selection of immunohistochemical indicators for pathological diagnosis of soft tissue tumors (2015). J Clin Exp Path. 31:5-8. [DOI] [Full Text] |
| 4. | Xu M, Jiang XM, He YL, Zhang YL, Xu MD, Yao LQ. Glomus tumor of the stomach: a case treated by endoscopic submucosal dissection. Clin Res Hepatol Gastroenterol. 2011;35:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 420] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Zhang QW, XY, Chi Z. A case of giant gastric glomus tumor and literature review. Xiandai Zhongliu Yixue. 2010;18:103-105. [DOI] [Full Text] |
| 7. | Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Rossi UG, Rutigliani M, Paparo F, Filauro M. Gastric glomus tumor: Endoscopy, MD-CT and pathologic features. Gastroenterol Hepatol. 2021;44:35-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Eun Young (Ann) Kim. Subepithelial Lesions. In: Haws RH FP, Varadarajulu S. Endosonography. 3rd ed Elsevier 2015: 122. |
| 10. | Tang M, Hou J, Wu D, Han XY, Zeng MS, Yao XZ. Glomus tumor in the stomach: computed tomography and endoscopic ultrasound findings. World J Gastroenterol. 2013;19:1327-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Lin J, Shen J, Yue H, Li Q, Cheng Y, Zhou M. Gastric Glomus Tumor: A Clinicopathologic and Immunohistochemical Study of 21 Cases. Biomed Res Int. 2020;2020:5637893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sato H, Shrestha UK, Zhang H S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Wu YXJ