Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10116
Peer-review started: July 6, 2021
First decision: July 26, 2021
Revised: August 8, 2021
Accepted: August 27, 2021
Article in press: August 27, 2021
Published online: November 26, 2021
Processing time: 139 Days and 1.2 Hours
Epilepsy is a syndrome characterized by transient, rigid, paroxysmal, and repetitive central nervous system dysfunction. Prevention, control, and im
To determine the clinical and EEG characteristics and treatment results of benign epilepsy in spiking children.
A total of 106 cases of benign epilepsy in children with myocardial spines treated at our hospital from January 2017 to January 2020 were selected. Differences in clinical data and EGG characteristics between treatment-effective/-ineffective patients were analyzed, and children’s intellectual development before and after treatment evaluated using the Gesell Development Diagnostic Scale.
EEG showed that the discharge proportion in the awake and sleep periods was 66.04%, and the peak/peak discharge was mainly single-sided, accounting for 81.13%, while the discharge generalization accounted for 31.13%. There was no significant difference in any of these variables between sexes and ages (P > 0.05). The proportion of patients with early onset (< 5 years old) and seizure frequency > 3 times/half a year was 40.00% and 60.00%, respectively; the incidence rate and seizure frequency in the younger age group (< 5 years old) were significantly higher than those in the treatment-effective group (P < 0.05), while the discharge index was significantly lower than that in the treatment-effective group (P < 0.05). The discharge index was negatively correlated with fine motor skill and language development (r = -0.274 and -0.247, respectively; P < 0.05), but not with the rest (P > 0.05). Logistic regression analysis showed that low age onset (< 5 years old) and seizure frequency were the factors affecting ineffective-treatment of benign epilepsy in children (odds ratio = 11.304 and 5.784, respectively; P < 0.05). The discharge index of the responsive group after treatment was significantly lower than that of the unresponsive group (P < 0.05). However, there was no significant difference between groups after treatment in gross and fine motor skills, adaptability, language, and personal social development (P > 0.05).
The EEG of children with benign epilepsy due to spinal wave in central time zone has characteristic changes, and the therapeutic effect is influenced by age of onset and attack frequency.
Core Tip: The electroencephalogram of children with benign epilepsy with centrotemporal spikes has characteristic changes, and the therapeutic effect is affected by the age and attack frequency of the children at the time of onset.
- Citation: Chen RH, Li BF, Wen JH, Zhong CL, Ji MM. Clinical and electroencephalogram characteristics and treatment outcomes in children with benign epilepsy and centrotemporal spikes. World J Clin Cases 2021; 9(33): 10116-10125
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10116.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10116
Epilepsy is a syndrome characterized by transient, rigid, paroxysmal, and repetitive central nervous system dysfunction, generally caused by excessive neuron synchronization in the brain and self-limited abnormal discharge caused by various etiological factors. As a common disease among children, the incidence rate of epilepsy has shown an increasing trend in recent years. Benign epilepsy in children with centrotemporal spike waves is an age-dependent epileptic syndrome, which generally peaks between 6–8 years old, with normal mental and motor development[1,2]. At present, the primary goal of antiepileptic treatments is to completely control epileptic seizures, while simultaneously considering prevention, control, and improvement of cognitive and behavioral dysfunction is of great significance for improving the patients’ intellectual development and quality of life. Electroencephalogram (EEG) is an important external validator of normal brain structure and function and useful to detect some brain alterations. Cognitive function, an important aspect of brain function, is also based on brain morphology and/or function. Studies have shown that abnormal EEG can predict an accelerated decline in cognitive function[3,4]. In this study, the clinical and EEG characteristics of children with benign epilepsy and centrotemporal spikes were analyzed, and the children’s treatment and outcomes also discussed.
A total of 106 cases of benign epilepsy, including 66 males and 40 females, in children with spinous waves in the central temporal region were treated at our hospital from January 2017 to January 2020. Their ages ranged from 3 to 12 years old, and their average age was 7.15 ± 1.82 years old. The inclusion criteria were: (1) The diagnosis met the criteria for the epilepsy diagnosis and treatment guidelines of the International League Against Epilepsy; (2) First-time treatment; (3) Aged 3–12 years old; (4) Complete clinical, EEG, and follow-up data; (5) Had intelligence tests; and (6) Informed consent from the child’s guardian. The exclusion criteria were: (1) A history of encephalitis, meningitis, brain developmental malformation, and other brain diseases; (2) Other diseases such as connective tissue disease, nephrotic syndrome, and immunodeficiency; and (3) Patients with a history of glucocorticoid and other treatments within 6 mo before treatment in our hospital.
The children were treated with levetiracetam and lamotrigine. The levetiracetam dose was 10 mg/kg/d, and the final therapeutic dose was 10-30 mg/kg/d. Lamotrigine was administered at doses from 0.3-0.6 mg/kg/d, gradually increasing to 3-6 mg/kg/d. Each child was followed for 1 year after treatment, and the treatment was considered ineffective if there were clinical epileptic seizures during the follow-up period, and effective if there were no clinical epileptic seizures.
We employed an EEG-1200C manufactured by Japan Optoelectronics Co., Ltd with the following parameters: Gain, 50 µV; high-pass filtering conducted at 45 Hz; time constant, 0.3 s; accuracy, 16 bit; frequency, 200 Hz; and scalp resistance, ≤ 5000 MΩ. Reference electrodes were placed on the bilateral earlobes and we used 16 recording electrodes in total. EEG signals were recorded in a quiet and eye-closed state for 5 min, and the complexity was calculated. The human electroencephalogram frequency was (0.5-30) Hz, including β (13.5-30.0) Hz, α (8.0–13.0) Hz, θ (4.0-7.5) Hz, and δ (1.0-3.5) Hz.
The development of children’s intelligence was assessed using the Gesell Development Diagnostic Scale, which included five domains, namely, gross motor, fine motor, adaptability, language, and personal-social ability. The test result of each domain is expressed as development quotient (DQ), and a DQ > 85 was considered normal.
SPSS22.0 software was used for data analyses. Measurement data with a normal distribution are expressed as the mean ± SD, and t test was used for comparison between groups. Count data are expressed as n (%), and inter-group comparisons were performed by the chi-square test. Pearson correlation analysis for correlation, and logistic regression analysis for multivariate analysis were also performed. P values < 0.05 were considered statistically significant.
Among the 106 children, the EEG showed a discharge proportion in the awake and sleep periods of 66.04%, a spike/sharp wave discharge rate of 81.13% (mainly unilateral discharge), and a discharge generalization rate of 31.13%, as shown in Table 1.
| Electroencephalogram characteristic | Number of cases | Proportion (%) |
| Discharge period | ||
| Awake and sleep periods | 70 | 66.04 |
| Sleep period | 36 | 33.96 |
| Spine/spike discharge | ||
| Unilateral | 86 | 81.13 |
| Bilateral | 20 | 18.87 |
| Discharge generalization | 33 | 31.13 |
There was no significant difference in the proportion of discharge, spike/sharp wave unilateral discharge, and discharge generalization between the sexes and among all age groups either during the awake or asleep period (P > 0.05), as shown in Table 2.
| Group | Number of cases | Awake and sleep discharge (%) | Spike/sharp wave unilateral discharge (%) | Discharge generalization (%) |
| Gender | ||||
| Man | 66 | 42 (63.64) | 55 (83.33) | 19 (28.79) |
| Woman | 40 | 28 (70.00) | 31 (77.50) | 14 (35.00) |
| χ2 | 0.450 | 0.554 | 0.448 | |
| P value | 0.502 | 0.457 | 0.503 | |
| Age | ||||
| ≤ 7 yr | 34 | 25 (73.53) | 26 (76.47) | 11 (32.35) |
| > 7 yr | 72 | 45 (62.50) | 60 (83.33) | 22 (30.56) |
| χ2 | 1.253 | 0.711 | 0.035 | |
| P value | 0.263 | 0.399 | 0.852 | |
The proportion of children with young-age onset (< 5 years old) and attack frequency > 3 times/half a year in the treatment unresponsive group was significantly higher than that in the treatment responsive group (P < 0.05), and the discharge index significantly lower (P < 0.05). There was no significant difference in sex, age, or discharge period between the treatment responsive/unresponsive groups (P > 0.05, Table 3).
| Clinical data | Treatment ineffective (n = 20) | Treatment effective (n = 86) | t/χ2 | P value |
| Gender | 0.554 | 0.457 | ||
| Man | 11 (55.00) | 55 (63.95) | ||
| Woman | 9 (45.00) | 31 (36.05) | ||
| Age (yr) | 7.15 ± 1.98 | 7.15 ± 1.80 | 0.000 | 1.000 |
| Discharge period | 0.883 | 0.347 | ||
| Awake and sleep periods | 15 (75.00) | 55 (63.95) | ||
| Sleep period | 5 (25.00) | 31 (36.05) | ||
| Spike/sharp wave discharge | 2.081 | 0.149 | ||
| Unilateral | 19 (95.00) | 67 (77.91) | ||
| Bilateral | 1 (5.00) | 19 (22.09) | ||
| Discharge generalization | 8 (40.00) | 25 (29.07) | 0.904 | 0.342 |
| Low age onset (< 5 yr) | 8 (40.00) | 6 (6.98) | 12.690 | 0.000 |
| Seizure frequency | 9.582 | 0.002 | ||
| > 3 times/half a year | 12 (60.00) | 21 (24.42) | ||
| ≤ 3 times/half a year | 8 (40.00) | 65 (75.58) | ||
| Discharge index (%) | 65.05 ± 7.74 | 73.28 ± 9.17 | -3.714 | 0.000 |
| Gesell scale | ||||
| Gross motor (points) | 85.70 ± 6.62 | 85.28 ± 7.29 | 0.236 | 0.814 |
| Fine motor (points) | 88.60 ± 5.99 | 86.62 ± 8.00 | 1.040 | 0.301 |
| Adaptability (points) | 87.60 ± 7.02 | 86.08 ± 7.20 | 0.854 | 0.395 |
| Language (points) | 88.15 ± 7.13 | 86.33 ± 7.92 | 0.942 | 0.348 |
| Individual-social ability (points) | 85.40 ± 8.61 | 85.99 ± 8.22 | -0.287 | 0.775 |
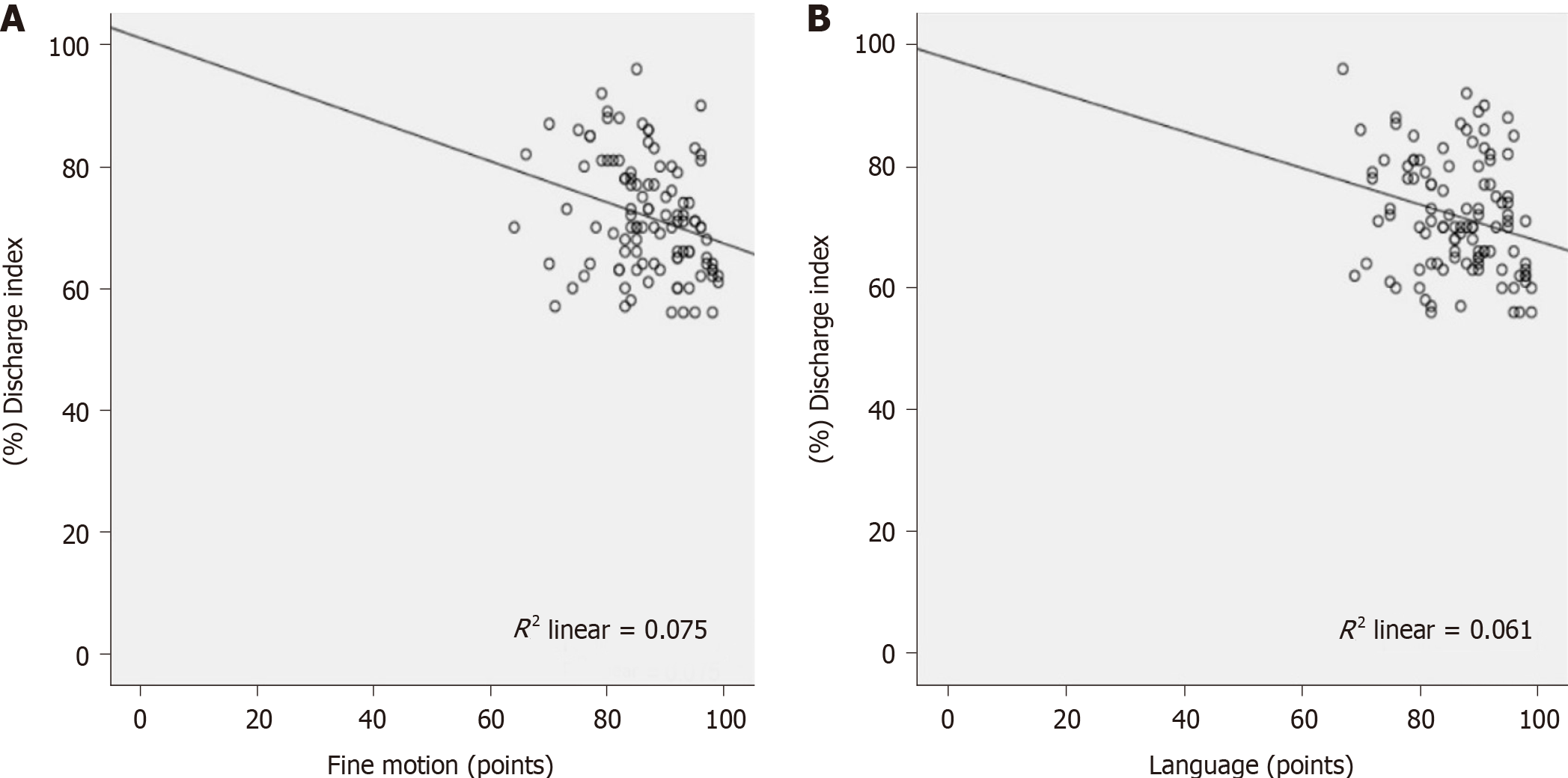
Pearson correlation analysis showed a negative correlation between the discharge index and fine motor skills and the language development quotient (r = − 0.274 and − 0.247, respectively; P < 0.05), but no significant correlation was observed in any other parameters (P > 0.05), as shown in Table 4 and Figure 1.
| Gesell scale | Discharge index | |
| r | P value | |
| Gross motor | -0.014 | 0.887 |
| Fine motor | -0.274 | 0.005 |
| Adaptability | -0.068 | 0.488 |
| Language | -0.247 | 0.011 |
| Individual-social ability | 0.098 | 0.316 |
Logistic regression analysis was performed using the above statistically significant indicators as independent variables and treatment effectiveness as the dependent variable. The results showed that low age (< 5 years old) and seizure frequency were the factors affecting the lack of treatment response in children with benign epilepsy and centrotemporal spike wave (Odds ratio = 11.304 and 5.784, respectively; P < 0.05), as shown in Table 5.
| Index | β | SE | Wals | P value | OR (95%CI) |
| Incidence at a young age (< 5 yr) | 2.425 | 0.696 | 12.131 | 0.000 | 11.304 (2.888-44.251) |
| Attack frequency | 1.755 | 0.593 | 8.760 | 0.003 | 5.784 (1.809-18.490) |
| Constant term | -2.686 | 0.480 | 31.254 | 0.000 | - |
The discharge index after treatment in the treatment responsive group was 34.47 ± 10.02%, significantly lower than that in the unresponsive group (P < 0.05). In the treatment responsive group, fine motor skills, adaptability, and language development quotient improved after treatment (P < 0.05). There was no significant difference in gross and fine motor skills, adaptability, language, or personal-social ability development quotient between the treatment responsive/unresponsive group after treatment (P > 0.05, Table 6).
| Index | Treatment ineffective (n = 20) | Treatment effective (n = 86) | t | P value |
| Discharge index (%) | ||||
| Before treatment | 65.05 ± 7.74 | 73.28 ± 9.17 | -3.714 | 0.000 |
| After treatment | 40.15 ± 5.36 | 34.47 ± 10.02 | 2.449 | 0.016 |
| t | 11.828 | 26.498 | ||
| P value | 0.000 | 0.000 | ||
| Gross motor (points) | ||||
| Before treatment | 85.70 ± 6.62 | 85.28 ± 7.29 | 0.236 | 0.814 |
| After treatment | 85.90 ± 5.47 | 86.20 ± 6.47 | -0.192 | 0.848 |
| t | -0.104 | -0.875 | ||
| P value | 0.918 | 0.383 | ||
| Fine motor (points) | ||||
| Before treatment | 88.60 ± 5.99 | 86.62 ± 8.00 | 1.040 | 0.301 |
| After treatment | 91.20 ± 2.69 | 89.24 ± 5.29 | 1.605 | 0.111 |
| t | -1.771 | -2.533 | ||
| P value | 0.085 | 0.012 | ||
| Adaptability (points) | ||||
| Before treatment | 87.60 ± 7.02 | 86.08 ± 7.20 | 0.854 | 0.395 |
| After treatment | 88.50 ± 5.04 | 88.26 ± 5.38 | 0.182 | 0.856 |
| t | -0.466 | -2.249 | ||
| P value | 0.644 | 0.026 | ||
| Language (points) | ||||
| Before treatment | 88.15 ± 7.13 | 86.33 ± 7.92 | 0.942 | 0.348 |
| After treatment | 89.35 ± 6.02 | 88.55 ± 5.99 | 0.537 | 0.592 |
| t | -0.575 | -2.073 | ||
| P value | 0.569 | 0.040 | ||
| Personal-social ability (points) | ||||
| Before treatment | 85.40 ± 8.61 | 85.99 ± 8.22 | -0.287 | 0.775 |
| After treatment | 86.95 ± 6.78 | 87.91 ± 6.27 | -0.607 | 0.545 |
| t | -0.633 | -1.722 | ||
| P value | 0.531 | 0.087 |
Epilepsy is a brain disease mainly characterized by transient central nervous system dysfunction caused by abnormal neuron discharge. Repeated epileptic seizures are often accompanied by a variety of neurobiological, cognitive, psychological, and social dysfunctions. Benign epilepsy with spinous waves in the central temporal region is the most common partial epilepsy in childhood, with an onset age between 3-13 years and accounting for 15%-24% of all kinds of epilepsy in children[5]. Several studies have shown that children with epilepsy and centrotemporal spikes have various degrees of cognitive and behavioral damage[6,7], while other reports have found that the cognitive impairment in these children is not caused by seizures but related to frequent clinical discharge. Neuropsychological and sociological problems exist in half of these children after adulthood[8,9]. Although the primary goal of antiepileptic treatment is to completely control epileptic seizures, prevention, control, and improvement of cognitive and behavioral dysfunction must be simultaneously considered. Therefore, clinicians must achieve a balance between controlling the epileptic seizures as much as possible and preserving cognitive and behavioral functions[10]. Long-term outbreaks of spike-and-slow wave rhythm and bilateral asynchronous spike-and-slow wave distribution cause more severe cognitive impairment than single spike waves[11]. EEG monitoring, a common modern auxiliary examination method for the clinical diagnosis of mild cognitive impairment diseases, induces no physical trauma and has confirmed value in the diagnosis of brain diseases. However, comprehensive analyses, as well as other experimental and auxiliary examinations, need to be conducted based on specific symptoms and signs; therefore, it is of great clinical significance to explore chemical markers of brain damage[12]. EEG represents the waveforms formed by the brain spontaneous potential; these waveforms can be divided into α, β, γ, θ, and δ waves according to their frequencies, with different waveforms being shown at different ages, in various consciousness states, and at various brain function levels[13,14]. Some studies have shown that abnormal EEGs can predict an accelerated decline in cognitive function. In children with epilepsy, EEGs accompanied by spinous waves in the central temporal region during the attack stage are often characterized by tonic-clonic seizures, where the initial fast wave activity of low amplitude in the central or middle temporal region on one side gradually increases in amplitude and decreases in frequency, gradually evolving into the alternating appearance of spinous and slow waves, which can be generalized to the ipsilateral hemisphere or even spread to the contralateral one[15]. At present, EEGs are considered to be highly related to epilepsy with spinous waves in the central temporal region. And compared with those of healthy peers, an increase in extremely high-amplitude spinous and slow waves in the high Rolandic region can be observed in the awake period, along with a widespread rhythmic outbreak of 2-3 Hz high-amplitude spinous and slow waves in the awake period. However, the discharge is significantly increased in the sleep period, and the spinous and slow wave discharge index is > 50% during the non-rapid-eye-movement sleep period[16]. In epilepsy accompanied by centrotemporal spikes, the presence of a status epilepticus EEG during sleep is known to cause nerve damage and cognitive changes; the higher the abnormal discharge index in the EEG, the more severe the cognitive damage in children. Therefore, we should actively diagnose, treat, and observe the therapeutic effects in children with epilepsy accompanied by centrotemporal spikes[17,18]. In this study, correlation analysis revealed that the discharge index was negatively correlated with fine motor performance and the language development quotient. Logistic regression analysis showed that an early age of onset (< 5 years old) and seizure frequency were influencing factors for the unresponsive treatment of benign epilepsy with centrotemporal spikes in children, indicating that monitoring the EEG discharge index could be used to preliminarily determine the children’s fine motor skills and language development quotient. During treatment, great attention should be given to children with early-onset and frequent seizures in whom clinical treatment has a poor effect. Frequent attacks can lead to delayed reaction time or even reaction loss in children, suggesting that abnormal discharges may be accompanied by transient cognitive function changes under clinical conditions, which reminds us that seizure control should not be the target of clinical treatment but the inhibition of clinical discharge and subsequent improvement in patients’ cognitive function[19,20]. An early age of onset is an important factor leading to poor treatment effect in children with benign epilepsy and centrotemporal spikes. Given the lack of clear clinical data on the specific scope of early-onset benign epilepsy with centrotemporal spikes, an early age at onset can be used as a relevant factor to predict treatment prognosis in these children. In this study, early age of onset was < 5 years old.
The analysis of the results of this study showed that the two antiepileptic drugs levetiracetam and lamotrigine could effectively control epileptic seizures and inhibit epileptic discharge, thus improving children’s cognitive function. However, this study has various limitations. Due to the limited number of enrolled children, there may be some deviation and error in the evaluation of discharge index, which may lead to a lack of generalizability. Therefore, further research expanding the sample size and extending follow-up time is needed.
In summary, the EGG of children with benign epilepsy and centrotemporal spikes has characteristic changes, and therapeutic effects are affected by the age and attack frequency at the time of onset.
The primary goal of antiepileptic treatments is to completely control epileptic seizures, while simultaneously considering prevention, control, and improvement of cognitive and behavioral dysfunction is of great significance for improving the patients’ intellectual development and quality of life.
In this study, the clinical and electroencephalograms (EEG) characteristics of children with benign epilepsy and centrotemporal spikes were analyzed, and the children’s treatment and outcomes also discussed.
This study aimed to determine the clinical and EEG characteristics and treatment results of benign epilepsy in spiking children.
A total of 106 benign epilepsy children with myocardial spines were included. Differences in clinical data and EGG characteristics between treatment-effective/-ineffective patients were analyzed, and children’s intellectual development before and after treatment evaluated using the Gesell Development Diagnostic Scale.
EEG showed that the discharge proportion in the awake and sleep periods was 66.04%, and the peak/peak discharge was mainly single-sided, accounting for 81.13%, while the discharge generalization accounted for 31.13%. The discharge index was negatively correlated with fine motor skill and language development, but not with the rest. The discharge index of the responsive group after treatment was significantly lower than that of the unresponsive group.
The EGG of children with benign epilepsy and centrotemporal spikes has characteristic changes, and therapeutic effects are affected by the age and attack frequency at the time of onset.
Further research expanding the sample size and extending follow-up time is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kuczynska J S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Serafini A, Gerard E, Genton P, Crespel A, Gelisse P. Treatment of Juvenile Myoclonic Epilepsy in Patients of Child-Bearing Potential. CNS Drugs. 2019;33:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Stowe RC, Glaze DG. Electroencephalographic Patterns During Routine Polysomnography in Childhood and Association With Future Epilepsy Diagnosis. J Clin Sleep Med. 2019;15:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Pearl PL. Epilepsy Syndromes in Childhood. Continuum (Minneap Minn). 2018;24:186-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | van Win OA, Barnes JG, Ferrier CF, Booth F, Prasad AN, Kasteleijn-Nolst Trenite DGA. A study of the significance of photoparoxysmal responses and spontaneous epileptiform discharges in the EEG in childhood epilepsy. Epilepsy Behav. 2020;107:107046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Piro E, Nardello R, Gennaro E, Fontana A, Taglialatela M, Donato Mangano G, Corsello G, Mangano S. A novel mutation in KCNQ3-related benign familial neonatal epilepsy: electroclinical features and neurodevelopmental outcome. Epileptic Disord. 2019;21:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Sathyanarayana A, El Atrache R, Jackson M, Alter AS, Mandl KD, Loddenkemper T, Bosl WJ. Nonlinear Analysis of Visually Normal EEGs to Differentiate Benign Childhood Epilepsy with Centrotemporal Spikes (BECTS). Sci Rep. 2020;10:8419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Nardello R, Mangano GD, Miceli F, Fontana A, Piro E, Salpietro V. Benign familial infantile epilepsy associated with KCNQ3 mutation: a rare occurrence or an underestimated event? Epileptic Disord. 2020;22:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 8. | Elhady M, Elattar RS, Elaidy AMA, Abdallah NA, Elmalt HA. Role of inflammation in childhood epilepsy and ADHD comorbidity. Appl Neuropsychol Child. 2020;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Aaberg KM, Bakken IJ, Lossius MI, Lund Søraas C, Håberg SE, Stoltenberg C, Surén P, Chin R. Comorbidity and Childhood Epilepsy: A Nationwide Registry Study. Pediatrics. 2016;138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Curnow SR, Vogrin SJ, Barton S, Bailey CA, Harvey AS. Focal cortical hypermetabolism in atypical benign rolandic epilepsy. Epilepsy Res. 2020;161:106288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Yang H, Wu D, Yang C, Yang Y, Zhou W, Zhang X, Sun W. Electroencephalographic abnormalities are correlated with cognitive deficits in children with benign childhood epilepsy with centrotemporal spikes: A clinical study of 61 cases. Epilepsy Behav. 2020;106:107012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Michel CM, He B. EEG source localization. Handb Clin Neurol. 2019;160:85-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Emmady PD, Murr N. EEG Triphasic Waves. Treasure Island (FL): StatPearls Publishing LLC, 2021. |
| 14. | Ahmed S, Alam ST, Rahman MM, Akhter S. Clinical Profile of Early Childhood Epilepsy: A Cross Sectional Study in a Tertiary Care Hospital. Mymensingh Med J. 2016;25:96-101. [PubMed] |
| 15. | Ticci C, Luongo T, Valvo G, Ferrari AR, Brovedani P, Masi G, Pellacani S, Sicca F. Clinical and electroencephalographic correlates of psychiatric features in children with frontal lobe epilepsy. Epilepsy Behav. 2019;92:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Hsu DA, Rayer K, Jackson DC, Stafstrom CE, Hsu M, Ferrazzano PA, Dabbs K, Worrell GA, Jones JE, Hermann BP. Correlation of EEG with neuropsychological status in children with epilepsy. Clin Neurophysiol. 2016;127:1196-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Ouyang CS, Chiang CT, Yang RC, Wu RC, Wu HC, Lin LC. Quantitative EEG findings and response to treatment with antiepileptic medications in children with epilepsy. Brain Dev. 2018;40:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Kessler SK, Shinnar S, Cnaan A, Dlugos D, Conry J, Hirtz DG, Hu F, Liu C, Mizrahi EM, Moshé SL, Clark P, Glauser TA; Childhood Absence Epilepsy Study Group. Pretreatment seizure semiology in childhood absence epilepsy. Neurology. 2017;89:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Lee SY, Park JH, Park SJ, Kim Y, Lee KY. Cognitive Function and Neuropsychological Comorbidities in Children with Newly Diagnosed Idiopathic Epilepsy. J Korean Med Sci. 2018;33:e17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Zhou S, Zhan Q, Wu X. Effect of Levetiracetam on Cognitive Function and Clonic Seizure Frequency in Children with Epilepsy. Curr Mol Med. 2019;19:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |