Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.8974
Peer-review started: April 29, 2021
First decision: June 17, 2021
Revised: June 23, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 26, 2021
Processing time: 175 Days and 7.4 Hours
Right-sided infective endocarditis is an increasingly recognized disease entity, with tricuspid valve being most frequently involved. Risk factors for tricuspid valve endocarditis (TVIE) include intravenous drug use, cardiac implantable electronic devices and indwelling catheters. Staphylococcus aureus is the pre
Core Tip: Right-sided infective endocarditis (RSIE) is an increasingly important subtype of infective endocarditis (IE), although less published literature is available regarding RSIE compared to left-sided IE. Recently, with improvements in multi
- Citation: Fava AM, Xu B. Tricuspid valve endocarditis: Cardiovascular imaging evaluation and management. World J Clin Cases 2021; 9(30): 8974-8984
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/8974.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.8974
It has been reported that right-sided infective endocarditis (RSIE) makes up 5%-10% of all cases of infective endocarditis (IE)[1-3]. RSIE involves native or prosthetic valves, any intracardiac devices within the right heart, and more rarely non-functional embryonic remnants such as Eustachian valve or Chiari network that are present in the right atrium (RA)[4]. Among patients with RSIE, the tricuspid valve is involved in approximately 90% of cases[5]. Echocardiography is the technique of choice for the diagnosis of IE. Other imaging modalities such as computed tomography and nuclear imaging have also been shown to be particularly useful for the detection of acute infection, especially in those cases of prosthetic valve IE or cardiac implantable electronic device infection[6,7].
Many patients with tricuspid valve endocarditis (TVIE) can be successfully treated with antibiotics, with a smaller proportion of patients requiring surgical intervention. Compared to left-sided IE, less data is available guiding the indications and timing for surgery[8]. As the vast majority of RSIE cases involve the tricuspid valve, in this review, we focus on TVIE and the utility of multimodality imaging for the diagnosis and practical management.
In terms of risk factors for TVIE, it is strongly associated with intravenous drug use (IVDU), presence of a cardiac implantable electronic device (CIED) or other intra
IVDU is the most common predisposing factor for TVIE, and is responsible for the increasing incidence of IE in developed countries, with an overall incidence of IE among IVDU patients ranging between 2% and 5% per year[10]. Repetitive use of injected drugs can lead to structural abnormalities of the TV detected by transthoracic echocardiography (TTE), such as focal thickening, valve prolapse, and regurgitation. These findings are likely the result of particles contaminating the illicit drugs being injected intravenously[10]. Among injection drug users presenting with fever, 13% will have echocardiographic evidence of IE and when concomitant bacteremia is present, up to 41% will show evidence of IE[10]. Blood cultures are positive in a high pro
IE related to CIED (CIED-IE) is an important subtype of RSIE that typically involves the device leads in the right heart, with frequent involvement of the adjacent tricuspid valve leaflets[11]. The infection can spread from an infected device pocket or through bacterial seeding[11]. With ageing population, its incidence has risen due to growing use of intra-cardiac devices[12].
The reported risk of infection is 0.5%-1% in the first year after implantation of a cardiac pacemaker, and rises with increasing complexity of the implanted device. For example, the infection rate for implantable cardioverter-defibrillator implantations is 1.7%, and even higher (2%) for cardiac resynchronization therapy implantations within 6 mo of hospital discharge. Compared with primary implantation, the risk of infection in the case of device replacement or revision procedures is between 2- and 4-fold higher[12].
TVIE resulting from peripheral venous lines is a relatively uncommon entity[1,6]. However, independent risk factors of TVIE have been shown include local cellulitis, use of infusion pumps, and insertion of a cannula in the lower extremity[12,13]. Dialysis patients often have a greater burden of co-morbidities, including diabetes, hypertension and atrial fibrillation, in addition to end-stage renal disease[11]. A total of 119 patients who underwent surgery to treat IE from a North American study showed that 16 patients were receiving chronic hemodialysis and approximately 20% of hemodialysis-related IE involved TV[11].
Staphylococcus aureus is the most common cause of TVIE, being responsible for up to 70% of cases[9,11]. Streptococci and Enterococci are the next most common pathogens, accounting for 5-30, and 2%-5%of cases, respectively[9]. Prosthetic valve IE and CIED-IE have a distinct distribution of causal microorganisms, as coagulase negative Staphylococcal infections are responsible for 25% of CIED-IE[14]. Fungi and gram-negative bacilli can also cause RSIE (less than 1% of cases)[9]. Increasing numbers of immunocompromised patients and the use of intravascular and intra-cardiac devices may be associated with a rise in fungal RSIE, associated with a high mortality[15]. In terms of fungal endocarditis, the in-hospital mortality is usually very high[16]. In a study of 78 patients with fungal endocarditis, 19 had isolated RSIE, and the overall mortality was 54%[16]. Infrequently, polymicrobial TVIE has been reported in a small number of TVIE cases[1,6].
The accepted diagnostic criteria for IE are based on the modified Duke criteria[9]. The diagnosis is established based on clinical manifestations, blood cultures, and the presence of valvular vegetations detected by echocardiography[9].
Echocardiography remains the first-line imaging modality for the evaluation of TVIE. However, there is emerging evidence for multimodality imaging in TVIE, potentially allowing better anatomical assessment with improved diagnosis in certain cases. A review of the relative strengths and weaknesses of the imaging modalities are shown in Table 1.
| Imaging modality | Strengths | Weaknesses |
| Transthoracic echocardiography | Good assessment of vegetation and valvular function; Reproducible and low cost; Evaluation of the hemodynamic consequences | Limited sensitivity for vegetations attached to pacemaker leads and paravalvular complications; Limited ability to evaluate PVE |
| Transesophageal echocardiography | Better evaluation in PVE and CIED; Tricuspid valve function and PHT assessment; Detection of potential residual material after device extraction | Potential procedural complications for TEE; Limited differentiation between lead vegetation vs thrombus; Limited detection of peripheral complications |
| Multislice computed tomography | Can detect abscess/pseudoaneurysms; PVE extension and fistulas; Coronary artery preoperative assessment; Identifying pulmonary diseases as abscesses; Evidence of extracardiac involvement | Radiation exposure; Lead artifacts; Limited assessment in small vegetations contrast-induced nephrotoxicity |
| Magnetic resonance | Detection of extra-cardiac embolic lesions and systemic emboli | Claustrophobia; Cannot be performed for certain CIED |
| 18F-FDG PET/CT | High sensitivity in PVE, generator/pocket and extracardiac or extravascular lead infection; Hypermetabolism + anatomic lesions (vegetations, leaflet thickening and perforation, fistulas); Better definition of the locoregional extension of the infection | Radiation exposure; Patient preparation (myocardial suppression); Visual interpretation. Non-standardized quantification analysis; False positive studies from inflammation or FDG uptake of the prosthetic materials |
| WBC-SPECT/CT | High specificity for pocket/generator or extravascular lead infection | Radiation exposure; Longer acquisition time; Lower spatial resolution |
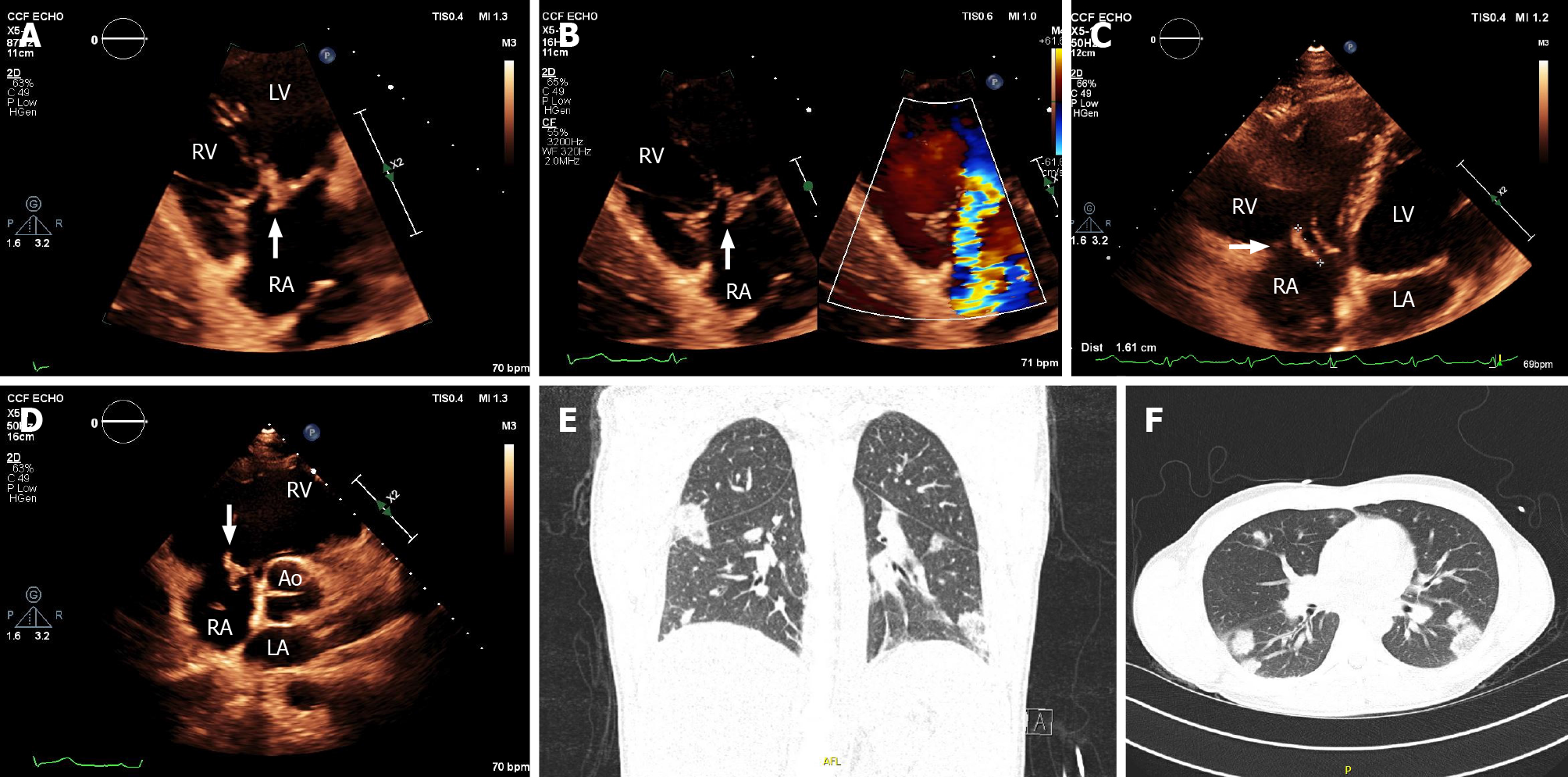
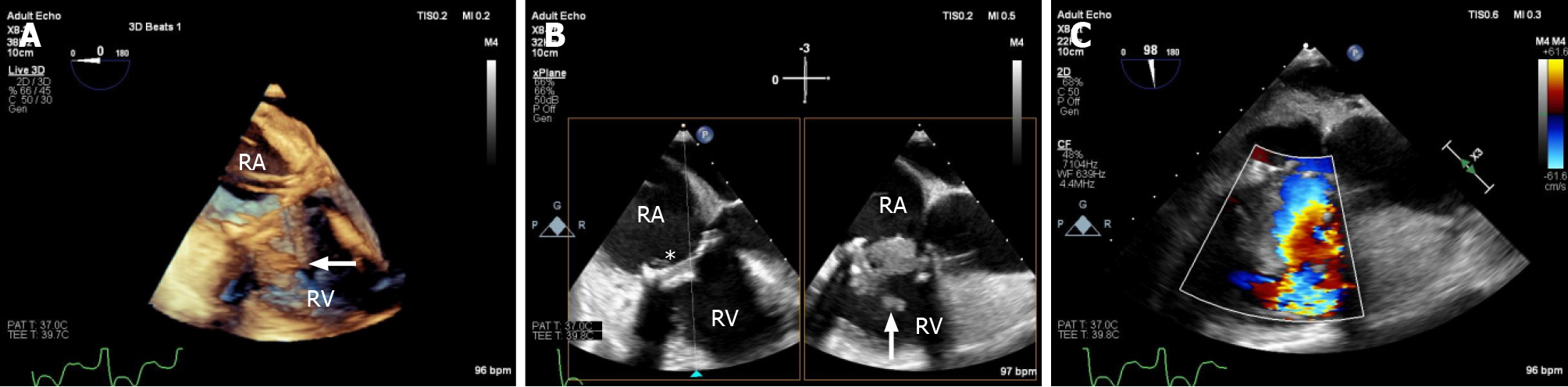
Echocardiography must be performed as soon as IE is suspected[1]. TTE is the recommended initial modality of choice[1]. Vegetations are detected by echocardiography as irregular shaped echodensities of variable size with independent oscillatory motion[6,11]. Tricuspid vegetations tend to be larger than left-sided lesions, partly due to lower pressure conditions (Figure 1). In native valve endocarditis, vegetations are typically found on the low-pressure surface, occurring usually on the atrial side of the atrioventricular valves[6]. TVIE lesions may cause valve stenosis, and valvular re
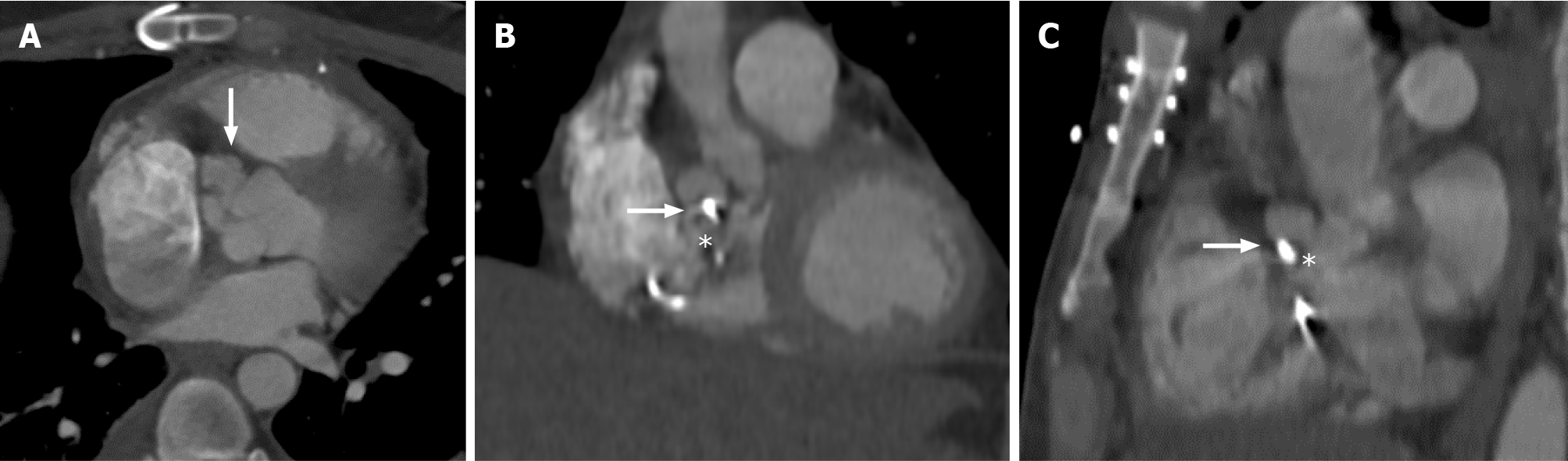
Multislice computed tomography (MSCT) should be considered when there is an absolute contraindication for TEE[21]. A gated CT study is recommended to reduce motion artifact. MSCT can be used to detect abscesses/pseudoaneurysms with a diagnostic accuracy similar to TEE, and it is excellent in evaluating the extent and consequences of any peri-valvular extension, including the anatomy of pseudoaneurysms, abscesses and fistulae (Figure 4)[22]. It should be noted, in RSIE, if cardiac CT is performed, meticulous attention must be paid to contrast timing, to maximize the ability to interrogate infective changes, such as vegetations. A recent study evaluated the associations between cardiac CT and TEE findings and adverse out
It must be noted that in RSIE, the utility of cardiac CT also lies in evaluating extra-cardiac complications. Specifically, in TVIE, there are often associated pulmonary pathologies, and MSCT can be used to support IE diagnosis. TVIE may reveal on MSCT concomitant pulmonary findings, such as septic pulmonary emboli, paren
18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) and radiolabelled white blood cell-single photon emission computed tomography (WBC-SPECT/CT) provide added diagnostic value to the Duke criteria, when the infection is related to prosthetic valves or CIEDs. The use of these alternative imaging modalities is supported by current guidelines. The European Society of Cardiology diagnostic algorithm for IE stipulate findings on both MSCT and nuclear imaging modalities (FDG PET and leukocyte SPECT) as major criteria, in the context of prosthetic valve IE[1]. In addition, the American Collage of Cardiology in the Appropriate Use Criteria for Multimodality Imaging in Valvular Heart Disease guidelines recommend FDG PET as an appropriate imaging modality to use in suspected prosthetic valve endocarditis[21]. This imaging modality has the capability to demonstrate infective changes the whole device[26]. FDG PET/CT is useful in patients with evidence of pocket infection (local signs of inflammation at the generator pocket) and negative microbiological and echocardiographic examinations, and in patients with positive blood cultures but negative echocardiographic examinations[27]. FDG PET/CT is very specific when tracer uptake is visualized, but its sensitivity is lower[11], and a negative result does not completely exclude the presence of small vegetations[27]. A recent meta-analysis[28] found FDG PET/CT to be most useful in assessing prosthetic valve IE and CIED-IE, but is unlikely to be diagnostically useful in native valve IE, with a high sensitivity for prosthetic valve IE (86%) and CIED-IE (74%), but low sensitivity for native valve IE (31%). Of note, FDG PET/CT is not recommended to evaluate the surgical region of interest within 3 mo of surgery, as it can be associated with false positive results, due to post-operative inflammation[27]. Other pathological conditions that can mimic the pattern of focally increased FDG uptake include vasculitis, atherosclerotic plaque, foreign body reactions (such as surgical adhesive used to repair the aortic root), active thrombus, Libman-Sacks endocarditis, cardiac metastasis from a non-cardiac tumors or primary cardiac tumors[29].
Radiolabeled WBC SPECT/CT is reported to be more specific for the detection of IE and infectious foci than 18F-FDG PET/CT. However, compared with PET/CT, this modality requires blood handling for radiopharmaceutical preparation and is more time-consuming, with a slightly lower spatial resolution and photon detection effi
Magnetic resonance (MR) can be used to quantify the extent of valvular regurgitation caused by IE. However, this imaging modality seems to be of most value in the identification of extra-cardiac systemic complications, such as cerebral, pulmonary, and intra-abdominal embolization[9]. MR is not associated with ionizing radiation, but has a lower spatial resolution and reduced availability[30]. MR angiography in the setting of potential mycotic aneurysms from septic embolism may be useful[31]. However, a study where the aim was to compare 12 patients with a total of 17 aneurysms demonstrated that the sensitivities of CT scanning and MR angiography were 94% and 86%, respectively, for the detection of intracranial non-infectious aneurysms 5 mm or larger, but only 57% and 35%, respectively, for aneurysms < 5 mm[31]. Thus, conventional angiography remains the gold standard, and should be performed when non-invasive techniques are abnormal[32].
Successful treatment of TVIE relies on microbial eradication with appropriate anti-microbials. Non-operative management of TVIE with antibiotics alone has been reported to clear the bacteremia in 70%-85% of cases[1]. However, medical treatment alone is not always effective, and surgical intervention may be warranted in select cases. Patients undergoing surgery for TVIE compared to left side IE presented with a higher rate of pulmonary septic emboli, more Staphylococcus aureus infections and larger vegetations[2]. The proportion of patients who are reported to undergo surgery for RSIE ranges from 5%-40%[33], with an operative mortality as high as 15% for patients with isolated TVIE[14].
Because surgery during the active phase of TVIE has considerable risk, it is important to provide accurate assessment of the extent of peri-valvular extension of IE, and to identify those patients who would not be expected to recover with medical therapy alone[34]. In other words, the decision for surgery should be based on the best possible understanding of the pathology, incorporating multimodality imaging fin
Common indications for surgery in patients with TVIE include: (1) Right heart failure secondary to severe tricuspid regurgitation, valvar obstruction or fistula[6]; (2) Uncontrolled infection (persistent bacteremia for more than 7 d despite adequate antimicrobial therapy (e.g., fungi; bacteremia due to S. aureus, P. aeruginosa); or peri
The American Association for Thoracic Surgery guidelines recommend that once a surgical indication is evident, surgery should not be delayed[19]. In the event that multiple valves are infected, the indication for surgery is of tendictated by the left-sided IE[8].
The principles of surgery for TVIE include radical debridement of vegetations/ infected tissue and valve repair whenever possible[19]. If valve replacement is per
Infections related to cardiac devices should be diagnosed early, and mandate the removal of the device and all implanted leads[8]. Special note is made of percutaneous removal for RSIE, as an alternative to surgery. The use of percutaneous aspiration devices is effective in removing large tricuspid valve vegetations in cases where the risk of tricuspid surgery is prohibitive[35]. Vacuum assisted devices (AngioVAC system, AngioDynamics, Latham, NY, United States) can be used for the removal of right sided intra-cardiac masses[35]. This percutaneous aspiration is composed of an extracorporeal circuit pump head and bubble trap, an outflow line and a reinfusion cannula[35]. The aspiration of vegetations immediately prior to, and during the lead extraction procedure may prevent septic embolization into the pulmonary circulation, and may confer improved short- and long-term survival[36]. On the other hand, it is important to mention that percutaneous vacuum-assisted devices have its own potential risks, such as disruption of the vegetation leading to pulmonary embo
The European Society of Cardiology and American Heart Association guidelines recommend a team approach for optimizing the management of patients with infective endocarditis. IE is not a single-faceted disease, but rather a very complex disease with many different aspects and factors involved, depending on the type of bacteremia, patient factors, and underlying cardiac disease.
Patients with TVIE due to IVDU are associated with a high risk of recurrence. To reduce this risk, these patients undergo comprehensive treatment, including psychotherapy, medication-assisted treatment, in order to establish a safe environment for recovery.
TVIE is generally associated with better clinical outcomes than left-side IE[38]. There is less systemic embolization, less abscess formation, and less drug-resistant infection and thus is clinically better tolerated. A retrospective study of patients with IE (n = 215), Stavi et al[39] reported that in-hospital mortality was lower among patients with right-sided IE compared with left-sided IE (2.6% vs 17%, P = 0.037). Conservative medical management may be used in many patients. However, there are some factors associated with poor prognosis. A size vegetation length of > 20 mm, increase in vegetation size despite antibiotic treatment, fungal etiology, recurrent septic pul
Imaging has a crucial role in the diagnosis and management of patient with TVIE. With increasing IVDU and intra-cardiac device implantations, the diagnostic suspicion of TVIE should be high in appropriate clinical scenarios. Echocardiography should be always performed when TVIE is suspected, complemented by TEE when TTE results are inconclusive. MSCT, FDG PET/CT, and WBC SPECT/CT may add value to improve the accuracy of IE diagnosis, where there is a high suspicion of infection and negative echocardiographic imaging, and/or in the presence of an intra-cardiac prosthesis or device. Multimodality imaging may lead to improved diagnosis and appropriate tailored treatment. Many patients with TVIE can be successfully treated with antibiotic therapy. Some patients with TVIE require surgical intervention, with tricuspid valve debridement and repair/replacement being accomplished in most cases. More data are required to establish the role of percutaneous aspiration devices in the management of TVIE and CIED-IE.
| 1. | Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL; ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2661] [Cited by in RCA: 3472] [Article Influence: 315.6] [Reference Citation Analysis (0)] |
| 2. | Akinosoglou K, Apostolakis E, Koutsogiannis N, Leivaditis V, Gogos CA. Right-sided infective endocarditis: surgical management. Eur J Cardiothorac Surg. 2012;42:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Gomes A, Glaudemans AWJM, Touw DJ, van Melle JP, Willems TP, Maass AH, Natour E, Prakken NHJ, Borra RJH, van Geel PP, Slart RHJA, van Assen S, Sinha B. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis. 2017;17:e1-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Vilesov SP. [The causes of mortality and ways of lowering it in surgery for heart wounds]. Grudn Khir. 1970;12:42-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Chahoud J, Sharif Yakan A, Saad H, Kanj SS. Right-Sided Infective Endocarditis and Pulmonary Infiltrates: An Update. Cardiol Rev. 2016;24:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 2234] [Article Influence: 203.1] [Reference Citation Analysis (1)] |
| 7. | Erba PA, Pizzi MN, Roque A, Salaun E, Lancellotti P, Tornos P, Habib G. Multimodality Imaging in Infective Endocarditis: An Imaging Team Within the Endocarditis Team. Circulation. 2019;140:1753-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8:630-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 9. | Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 705] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 10. | Wurcel AG, Anderson JE, Chui KK, Skinner S, Knox TA, Snydman DR, Stopka TJ. Increasing Infectious Endocarditis Admissions Among Young People Who Inject Drugs. Open Forum Infect Dis. 2016;3:ofw157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 293] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 11. | Habib G, Erba PA, Iung B, Donal E, Cosyns B, Laroche C, Popescu BA, Prendergast B, Tornos P, Sadeghpour A, Oliver L, Vaskelyte JJ, Sow R, Axler O, Maggioni AP, Lancellotti P; EURO-ENDO Investigators. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40:3222-3232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 552] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 12. | Shmueli H, Thomas F, Flint N, Setia G, Janjic A, Siegel RJ. Right-Sided Infective Endocarditis 2020: Challenges and Updates in Diagnosis and Treatment. J Am Heart Assoc. 2020;9:e017293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 13. | Baidya A, Ganakumar V, Jadon RS, Ranjan P, Manchanda S, Sood R. Septic pulmonary emboli as a complication of peripheral venous cannula insertion. Drug Discov Ther. 2018;12:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Hussain ST, Witten J, Shrestha NK, Blackstone EH, Pettersson GB. Tricuspid valve endocarditis. Ann Cardiothorac Surg. 2017;6:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Manoff SB, Vlahov D, Herskowitz A, Solomon L, Muñoz A, Cohn S, Willoughby SB, Nelson KE. Human immunodeficiency virus infection and infective endocarditis among injecting drug users. Epidemiology. 1996;7:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Siciliano RF, Gualandro DM, Sejas ONE, Ignoto BG, Caramelli B, Mansur AJ, Sampaio RO, Pierrotti LC, Barbosa G, Golebiovski W, Weksler C, Lamas C, Fortes NRQ, Fortes CQ, Tarasoutchi F, Strabelli TMV. Outcomes in patients with fungal endocarditis: A multicenter observational cohort study. Int J Infect Dis. 2018;77:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
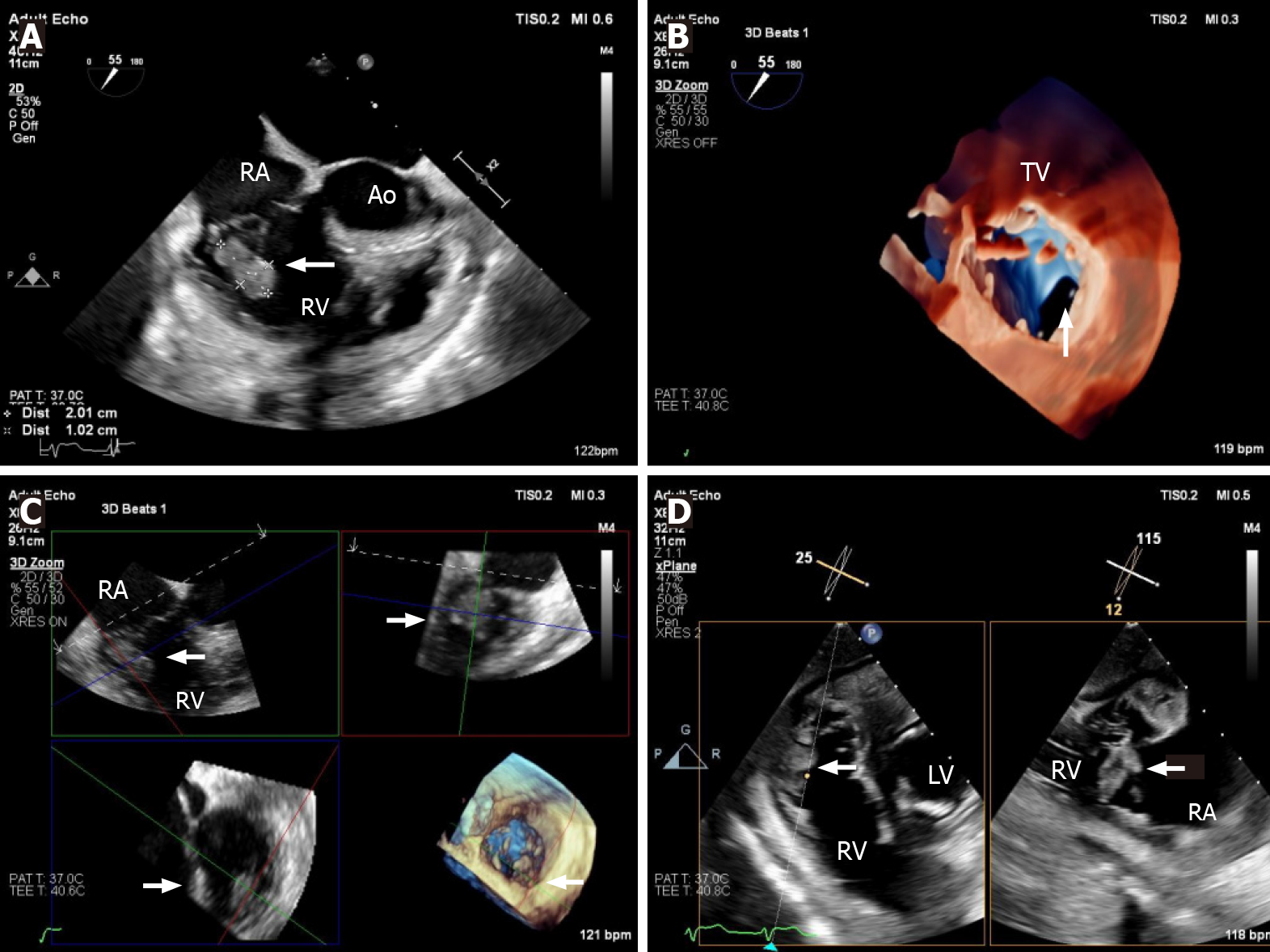
| 17. | Pérez-García CN, Olmos C, Islas F, Marcos-Alberca P, Pozo E, Ferrera C, García-Arribas D, Pérez de Isla L, Vilacosta I. Morphological characterization of vegetation by real-time three-dimensional transesophageal echocardiography in infective endocarditis: Prognostic impact. Echocardiography. 2019;36:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Berdejo J, Shibayama K, Harada K, Tanaka J, Mihara H, Gurudevan SV, Siegel RJ, Shiota T. Evaluation of vegetation size and its relationship with embolism in infective endocarditis: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. 2014;7:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, Pettersson GB, Coselli JS, Hussain ST, Griffin B, Blackstone EH, Gordon SM, LeMaire SA, Woc-Colburn LE. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg. 2017;153:1241-1258.e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 297] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 20. | Wang TKM, Bin Saeedan M, Chan N, Obuchowski NA, Shrestha N, Xu B, Unai S, Cremer P, Grimm RA, Griffin BP, Flamm SD, Pettersson GB, Popovic ZB, Bolen MA. Complementary Diagnostic and Prognostic Contributions of Cardiac Computed Tomography for Infective Endocarditis Surgery. Circ Cardiovasc Imaging. 2020;13:e011126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate Use Criteria for Multimodality Imaging in Valvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;70:1647-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 22. | Feuchtner GM, Stolzmann P, Dichtl W, Schertler T, Bonatti J, Scheffel H, Mueller S, Plass A, Mueller L, Bartel T, Wolf F, Alkadhi H. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol. 2009;53:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 23. | Jain V, Wang TKM, Bansal A, Farwati M, Gad M, Montane B, Kaur S, Bolen MA, Grimm R, Griffin B, Xu B. Diagnostic performance of cardiac computed tomography vs transesophageal echocardiography in infective endocarditis: A contemporary comparative meta-analysis. J Cardiovasc Comput Tomogr. 2021;15:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Grob A, Thuny F, Villacampa C, Flavian A, Gaubert JY, Raoult D, Casalta JP, Habib G, Moulin G, Jacquier A. Cardiac multidetector computed tomography in infective endocarditis: a pictorial essay. Insights Imaging. 2014;5:559-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Hekimian G, Kim M, Passefort S, Duval X, Wolff M, Leport C, Leplat C, Steg G, Iung B, Vahanian A, Messika-Zeitoun D. Preoperative use and safety of coronary angiography for acute aortic valve infective endocarditis. Heart. 2010;96:696-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Erba PA, Sollini M, Conti U, Bandera F, Tascini C, De Tommasi SM, Zucchelli G, Doria R, Menichetti F, Bongiorni MG, Lazzeri E, Mariani G. Radiolabeled WBC scintigraphy in the diagnostic workup of patients with suspected device-related infections. JACC Cardiovasc Imaging. 2013;6:1075-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Cautela J, Alessandrini S, Cammilleri S, Giorgi R, Richet H, Casalta JP, Habib G, Raoult D, Mundler O, Deharo JC. Diagnostic yield of FDG positron-emission tomography/computed tomography in patients with CEID infection: a pilot study. Europace. 2013;15:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Wang TKM, Sánchez-Nadales A, Igbinomwanhia E, Cremer P, Griffin B, Xu B. Diagnosis of Infective Endocarditis by Subtype Using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: A Contemporary Meta-Analysis. Circ Cardiovasc Imaging. 2020;13:e010600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 29. | Bensimhon L, Lavergne T, Hugonnet F, Mainardi JL, Latremouille C, Maunoury C, Lepillier A, Le Heuzey JY, Faraggi M. Whole body [(18) F]fluorodeoxyglucose positron emission tomography imaging for the diagnosis of pacemaker or implantable cardioverter defibrillator infection: a preliminary prospective study. Clin Microbiol Infect. 2011;17:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Smith AA, Newman RV, Thompson PA, Farkas SI, Wynne J. Mitral Valve Repair Outcomes in a Community Hospital: A Retrospective Analysis. Heart Lung Circ. 2016;25:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Tsuchiya K, Makita K, Furui S. 3D-CT angiography of cerebral aneurysms with spiral scanning: comparison with 3D-time-of-flight MR angiography. Radiat Med. 1994;12:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Rodriguez-Régent C, Edjlali-Goujon M, Trystram D, Boulouis G, Ben Hassen W, Godon-Hardy S, Nataf F, Machet A, Legrand L, Ladoux A, Mellerio C, Souillard-Scemama R, Oppenheim C, Meder JF, Naggara O. Non-invasive diagnosis of intracranial aneurysms. Diagn Interv Imaging. 2014;95:1163-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Weber C, Gassa A, Eghbalzadeh K, Merkle J, Djordjevic I, Maier J, Sabashnikov A, Deppe AC, Kuhn EW, Rahmanian PB, Liakopoulos OJ, Wahlers T. Characteristics and outcomes of patients with right-sided endocarditis undergoing cardiac surgery. Ann Cardiothorac Surg. 2019;8:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Moss R, Munt B. Injection drug use and right sided endocarditis. Heart. 2003;89:577-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Divekar AA, Scholz T, Fernandez JD. Novel percutaneous transcatheter intervention for refractory active endocarditis as a bridge to surgery-angiovac aspiration system. Catheter Cardiovasc Interv. 2013;81:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Starck CT, Schaerf RHM, Breitenstein A, Najibi S, Conrad J, Berendt J, Esmailian F, Eulert-Grehn J, Dreizler T, Falk V. Transcatheter aspiration of large pacemaker and implantable cardioverter-defibrillator lead vegetations facilitating safe transvenous lead extraction. Europace. 2020;22:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Abubakar H, Rashed A, Subahi A, Yassin AS, Shokr M, Elder M. AngioVac System Used for Vegetation Debulking in a Patient with Tricuspid Valve Endocarditis: A Case Report and Review of the Literature. Case Rep Cardiol. 2017;2017:1923505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Kamaledeen A, Young C, Attia RQ. What are the differences in outcomes between right-sided active infective endocarditis with and without left-sided infection? Interact Cardiovasc Thorac Surg. 2012;14:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Stavi V, Brandstaetter E, Sagy I, Sapunar S, Nevzorov R, Bartal C, Barski L. Comparison of Clinical Characteristics and Prognosis in Patients with Right- and Left-sided Infective Endocarditis. Rambam Maimonides Med J. 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim J, Zhao BW S-Editor: Wu YXJ L-Editor: A P-Editor: Yuan YY