Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8825
Peer-review started: May 28, 2021
First decision: June 14, 2021
Revised: July 5, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: October 16, 2021
Processing time: 139 Days and 23.6 Hours
Percutaneous endoscopic gastrostomy with jejunal extension (PEG-J) is often used to treat patients with neurological impairment and difficulty in swallowing. However, these patients often develop copper deficiency. This report describes a case of isolated neutropenia, which is a rare manifestation of copper deficiency.
Our patient was a 19-year-old boy with neurological impairment and gastroesophageal reflux. He received PEG-J feeding, including an enteral supplement containing copper and zinc. However, as his serum zinc level was low (53 μg/dL) at the age of 19 years and 2 mo, we changed to a zinc-rich supplement containing 22 mg/d of zinc and 1.0 mg/d of copper. The supplement comprised a mixture of isocal 1.0 junior (5 packs/d), Tezon [2 packs (250 mL)/d], and cocoa powder. Seven months later, he had neutropenia (606/mm3) with a serum copper level of 16 μg/dL. There were no other manifestations of copper deficiency, including anemia. Copper deficiency and neutropenia both improved following the administration of cocoa powder and Tezon.
In patients receiving long-term PEG-J feeds, white blood cell counts, hemoglobin, and serum levels of copper and zinc should be regularly monitored.
Core Tip: Patients with percutaneous endoscopic gastrostomy with jejunal extension (PEG-J) often develop copper deficiency. Approximately 2 mg/d of copper may be needed to prevent deficiency. The intake ratio of copper to zinc is critical for maintaining an adequate serum copper concentration. We report an isolated case of neutropenia caused by copper deficiency in a patient with neurological impairment receiving PEG-J feeding with additional supplementation of zinc. Copper deficiency improved with the addition of cocoa powder to the enteral formula.
- Citation: Ohmori H, Kodama H, Takemoto M, Yamasaki M, Matsumoto T, Kumode M, Miyachi T, Sumimoto R. Isolated neutropenia caused by copper deficiency due to jejunal feeding and excessive zinc intake: A case report. World J Clin Cases 2021; 9(29): 8825-8830
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8825.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8825
Patients with neurological impairment often experience difficulty in swallowing. They also often experience gastroesophageal reflux (GER), which induces reflux esophagitis and aspiration pneumonia[1]. Percutaneous endoscopic gastrostomy (PEG) and fundoplication are used to treat these conditions that are associated with prolonged enteral feeding in such patients[2]. Jejunal tube feeding by PEG with jejunal extension (PEG-J) is also used to treat GER in these patients[3-5]. However, patients receiving PEG-J feeding occasionally develop deficiencies in trace elements, including copper[6-9]. We report an isolated case of neutropenia caused by copper deficiency in a patient with neurological impairment receiving PEG-J feeding. The copper deficiency was improved by the addition of cocoa powder to the enteral formula.
Nutritional care.
At the age of 16 years, upper gastrointestinal examination revealed that he had complicated GER. During an examination, he accidentally suffered a cardiac-respiratory arrest. After resuscitation, he was bedridden with spastic quadriplegia and difficulty in swallowing.
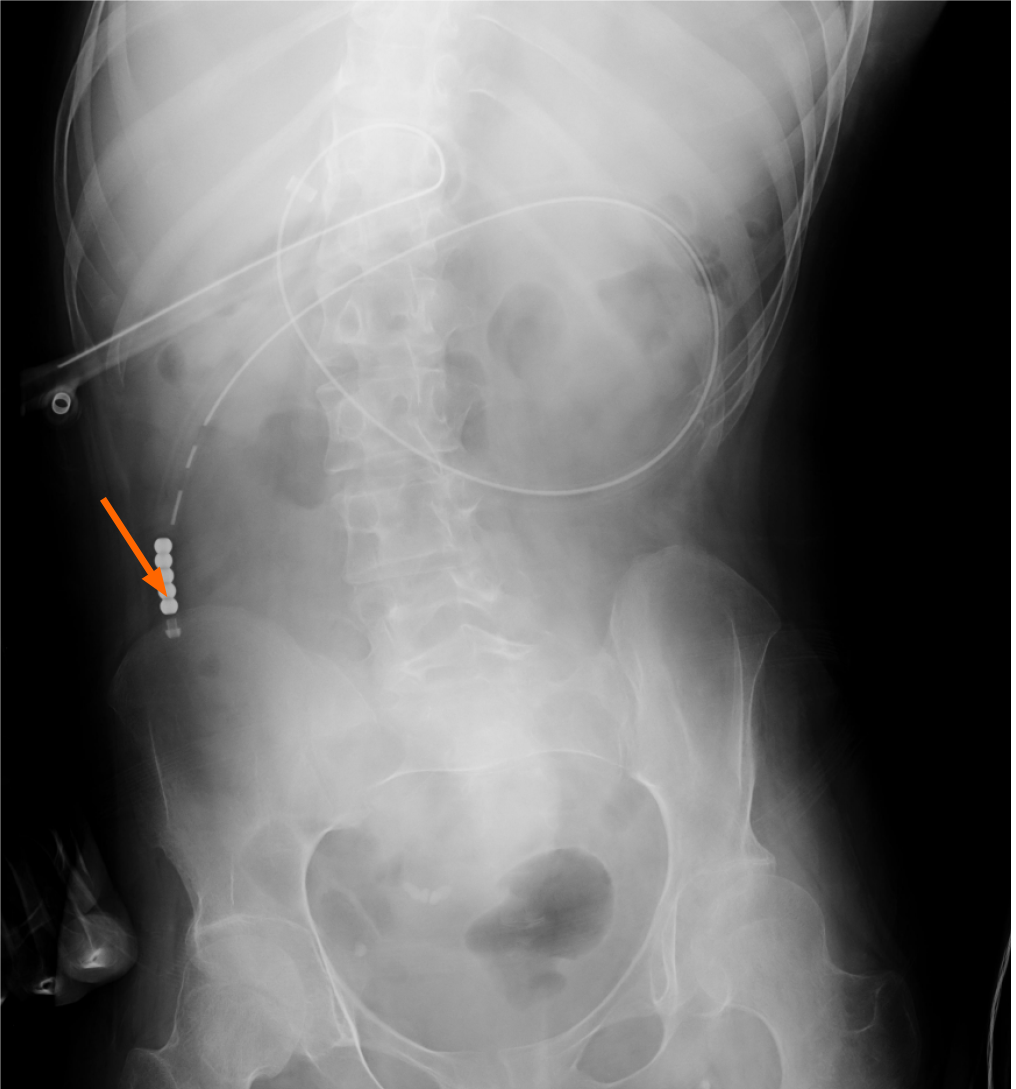
At the age of 18, a PEG-J catheter was inserted in the proximal jejunum 60 cm from the gastrostomy site for nutrition (Figures 1 and 2). The catheter tip was located 4 cm below the ligament of Treitz. An enteral formula containing Lacol (900 mL/d) and BFLUID (500 mL/d) was administered, which provided 1260 kcal, 1.1 mg of copper, and 5.8 mg of zinc per day. At the age of 19 years and 2 mo, a low serum level of zinc [53 μg/dL (normal range: 80-130 μg/dL)] was detected. Therefore, we replaced the enteral formula with a zinc-rich one containing isocal 1.0 junior (Nestle Health Science, Tokyo; 5 packs/d) and VCRESC CP-10 (NUTRI Co., Ltd., Mie; 1 pack/d), which provided 1080 kcal, 1.0 mg of copper, and 22.0 mg of zinc (zinc to copper ratio: 22:1) per day.
| Age | 19 yr 4 mo | 19 yr 6 mo | 19 yr 9 mo | 19 yr 10 mo | 19 yr 11 mo | |
| RDA1 | ||||||
| Cu intake (mg/d) | 0.9 | 1.0 | 1.0 | 1.98 | 1.98 | 1.98 |
| Zn intake (mg/d) | 11 | 22 | 22 | 18 | 18 | 18 |
| Kcal | 1080 | 1080 | 1076 | 1076 | 1076 | |
| Normal ranges | ||||||
| WBC (/mm3) | 3300-8600 | 5000 | 4800 | 2900 | 3700 | 4200 |
| Neutrocyte (/mm3) | 2475 | 1786 | 606 | 1166 | 1865 | |
| RBC × 104 (/mm3) | 435-555 | 468 | 489 | 468 | 464 | 486 |
| Hb (g/dL) | 13.7-16.8 | 13.6 | 14.6 | 14.4 | 14.5 | 15.0 |
| Platelets × 104 (/mm3) | 15.8-34.8 | 28.5 | 18.8 | 18.8 | 21.6 | 20.0 |
| Cu (μg/dL) | 66-130 | 111 | 65 | 16 | 92 | 116 |
| Zn (μg/dL) | 80-130 | 53 | 106 | 86 | 70 | |
| Se (μg/dL) | 10.6-17.4 | 92 | 99 | 109 | 84 | |
| Fe (μg/dL) | 54-181 | 64 | 93 | 80 | 93 | |
| Ferritin (ng/mL) | 25-280 | 54 | 111 | 96 | ||
| Ceruloplasmin(mg/dL) | 21-37 | 15.9 | 4.6 | 19.5 | 20.4 | |
The patient was a 19-year-old boy diagnosed with xeroderma pigmentation type A based on the clinical manifestations and analysis of XPA 9q34.1. Due to neurological impairment, his motor development was delayed. He also developed GER.
The patient did not have a remarkable family history.
At the age of 19 years and 4 mo, he was admitted to our hospital. On admission, the patient's height was 152 cm, and he was appreciably underweight at 35 kg. The patient was bedridden, non-verbal, unable to sit upright, roll over, or walk. Intermittent urine catheterization had been performed due to neurogenic bladder. Mental retardation was severe and IQ was presumed < 35. His respiratory and heart sounds were normal. The abdomen was soft and nondistended.
As shown in Table 1, at admission, his neutrocyte count was 2475/mm3, and the serum levels of copper and zinc were 111 µg/dL and 53 µg/dL, respectively. As the serum zinc level was still low, the same enteral formula was continued. However, at the age of 19 years and 9 mo, the patient developed neutropenia (606/mm3) (Table 1). His red blood cell and platelet counts, hemoglobin, and serum levels of zinc and selenium were all normal. However, the serum levels of copper and ceruloplasmin were significantly decreased to 16 µg/dL and 4.6 mg/dL (normal range: 21-37 mg/dL), respectively.
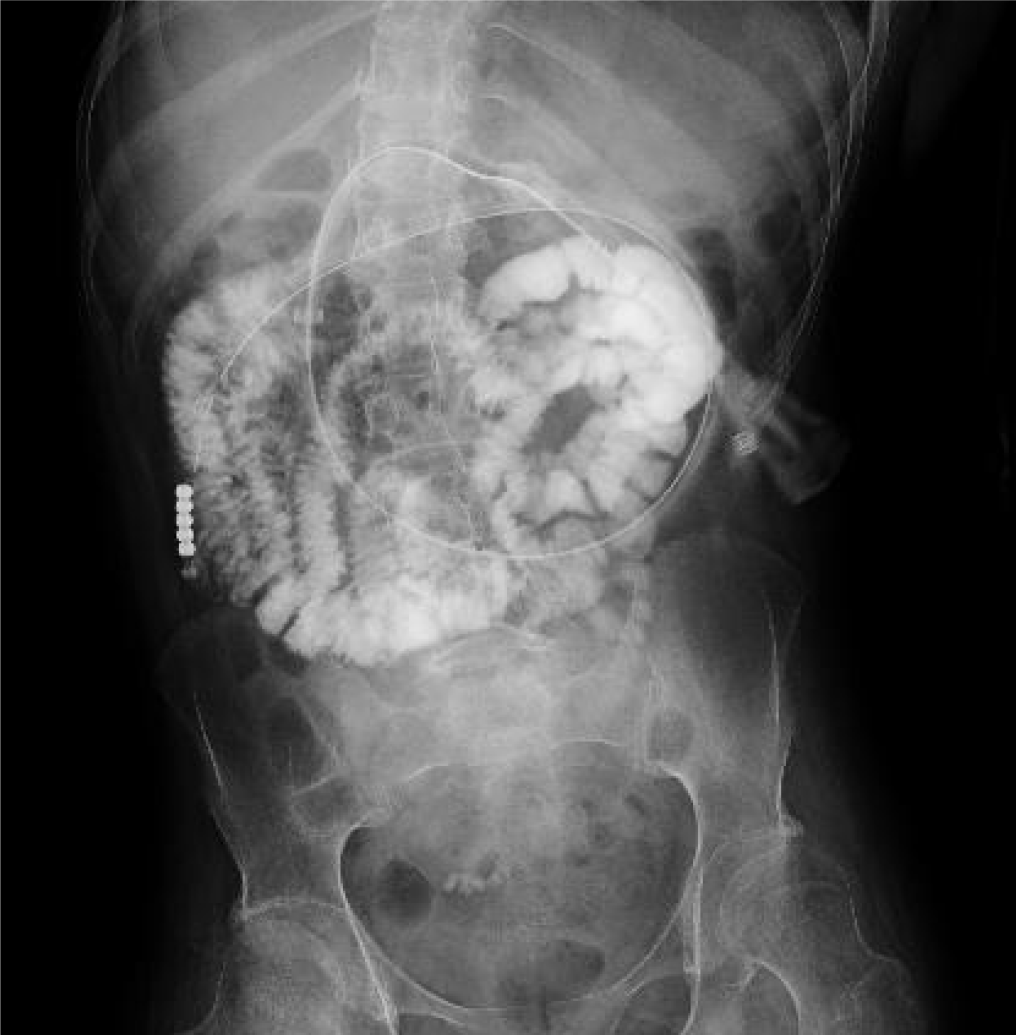
Figures 1 and 2 present the radiographs that were obtained when the PEG-J catheter was inserted at the age of 18 years; these confirm the proper placement of the catheter’s tip in the upper jejunum and the infusion of the contrast medium (gastrograffin) through the catheter, respectively. The contrast medium was noted from the upper jejunum to the lower portion of the small intestine.
He was diagnosed with neutropenia due to copper deficiency. No other symptoms of copper deficiency, such as hair abnormalities, were observed.
To treat the copper deficiency, the enteral formula was changed to a mixture of isocal 1.0 junior (5 packs/d), Tezon [TERUMO Co., Ltd., Tokyo; 2 packs (250 mL)/d], and cocoa powder [Morinaga & Co., Ltd, Tokyo; 2 spoons (10 g of cocoa)/d]. Tezon is a supplement of trace elements containing zinc (8.0 mg/250 mL) and copper (0.6 mg/250 mL). Ten grams of cocoa powder contained 0.7 mg of zinc and 0.38 mg of copper.
The new enteral formula provided 1076 kcal, 1.98 mg of copper, 18.0 mg of zinc (zinc to copper ratio: 18:1.98). Two months later, the neutrocyte count and serum levels of copper and ceruloplasmin were normal, as shown in Table 1. The serum C-reactive protein level was negative during this period.
Copper deficiency is caused by jejunal feeding, low copper intake, excessive zinc intake, the malabsorption syndrome, and parenteral nutrition[10]. When our patient developed copper deficiency, his copper intake of 1 mg/d was in line with the recommended daily allowance of 0.9 mg/d for men aged 18-29 years old[11].
Copper is absorbed in two phases: The early phase occurs in the stomach and the proximal duodenum, while the delayed phase occurs in the distal duodenum and the small intestine[6,7].
Figure 2 clearly shows the contrast flowing from the catheter into the upper jejunum and below, but no contrast was seen in the reflux to the duodenum. These findings indicated that copper from the enteral formula was not absorbed in the stomach and the duodenum, leading to its deficiency.
In our patient, the nutritional formula was changed to a zinc-rich preparation 3 mo prior to the onset of neutropenia. While the recommended ratio of zinc: copper intake is 5-10:1[12], our enteral formula had a much higher ratio of 22:1. Excessive zinc intake has been shown to induce copper deficiency through the induction of metallothionein, an endogenous metal chelator with a higher affinity to copper than to zinc. An increase in metallothionein levels further inhibits enteric copper absorption in the intestinal epithelial cells[13]. Thus, the ratio of zinc to copper is critical for preventing copper deficiency. Therefore, copper deficiency in our patient could have been caused by the combination of PEG-J feeding and excessive zinc intake.
Copper is an essential trace element that functions as a cofactor for several enzymes, including cytochrome C oxidase, dopamine β-hydroxylase, lysyl oxidase, and ceruloplasmin. Copper deficiency leads to a decrease in the activities of these enzymes, resulting in various manifestations of the deficiency, including hair abnormalities, osteoporosis, and neurological dysfunction (such as sensory ataxia and peripheral neuropathy)[14-16]. Copper deficiency also results in hematological abnormalities[8,17,18].
Halfdanarson et al[17], in a study of 40 patients, reported the hematological manifestations of copper deficiency. While 52.5% of the patients had anemia and leukopenia, 30% only had anemia, 10% had pancytopenia, 5% had anemia and thrombocytopenia, and only one patient had isolated neutropenia. Therefore, isolated neutropenia, as seen in our patient, is a rare manifestation of copper deficiency.
Neutropenia and anemia caused by copper deficiency have different etiologies. Anemia due to copper deficiency is caused by the inhibition of ferroxidase copper-dependent enzymes[18], resulting in impaired hemoglobin synthesis. In contrast, copper deficiency-induced neutropenia is speculated to be caused by the destruction of myeloid progenitor cells in the bone marrow, impaired maturation of myeloid precursors, and arrested maturation of neutrophils[16,17].
In PEG-J feeding, copper is absorbed only via the upper jejunum and below, but not via the stomach and the duodenum. Thus, a copper intake higher than the dietary reference intake of copper is required, as shown in our patient. Copper deficiency in our patient was improved by the administration of Tezon (2 packs/d) and cocoa powder (2 spoons containing 0.7 mg of zinc and 0.38 mg of copper, per day), along with the enteral formula, via the jejunal feeding tube. Cocoa powder contains both zinc and copper, in a ratio of 1.8:1. It, therefore, has sufficient copper as compared to zinc. Nishiwaki et al[6] have also reported that copper deficiency was improved by administering 10 g of cocoa powder a day to a patient with dysphagia receiving PEG-J feed; this is consistent with our findings.
Our findings indicate that approximately 2 mg/d of copper may be needed to prevent its deficiency. Besides the amount of copper intake, its ratio to zinc intake is also critical for maintaining adequate serum levels of copper. These findings are especially relevant in the treatment and management of patients receiving jejunal feeding. However, this study represents only one case report about copper deficiency due to jejunal feeding. As jejunal feeding is increasingly used to aid patients with difficulty in swallowing, it is critical to study the quantity of copper needed for this procedure.
| 1. | Lauriti G, Lisi G, Lelli Chiesa P, Zani A, Pierro A. Gastroesophageal reflux in children with neurological impairment: a systematic review and meta-analysis. Pediatr Surg Int. 2018;34:1139-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Wales PW, Diamond IR, Dutta S, Muraca S, Chait P, Connolly B, Langer JC. Fundoplication and gastrostomy vs image-guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux. J Pediatr Surg. 2002;37:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Egnell C, Eksborg S, Grahnquist L. Jejunostomy enteral feeding in children: outcome and safety. JPEN J Parenter Enteral Nutr. 2014;38:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | King M, Barnhart DC, O'Gorman M, Downey EC, Jackson D, Mundorff M, Holubkov R, Feola P, Srivastava R. Effect of gastrojejunal feedings on visits and costs in children with neurologic impairment. J Pediatr Gastroenterol Nutr. 2014;58:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Michaud L, Coopman S, Guimber D, Sfeir R, Turck D, Gottrand F. Percutaneous gastrojejunostomy in children: efficacy and safety. Arch Dis Child. 2012;97:733-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Nishiwaki S, Iwashita M, Goto N, Hayashi M, Takada J, Asano T, Tagami A, Hatakeyama H, Hayashi T, Maeda T, Saito K. Predominant copper deficiency during prolonged enteral nutrition through a jejunostomy tube compared to that through a gastrostomy tube. Clin Nutr. 2011;30:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Jayakumar S, Micallef-Eynaud PD, Lyon TD, Cramb R, Jilaihawi AN, Prakash D. Acquired copper deficiency following prolonged jejunostomy feeds. Ann Clin Biochem. 2005;42:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Camblor M, De la Cuerda C, Bretón I, Pérez-Rus G, Alvarez S, García P. Copper deficiency with pancytopenia due to enteral nutrition through jejunostomy. Clin Nutr. 1997;16:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Jacobson AE, Kahwash SB, Chawla A. Refractory cytopenias secondary to copper deficiency in children receiving exclusive jejunal nutrition. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Jaiser SR, Winston GP. Copper deficiency myelopathy. J Neurol. 2010;257:869-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Ministry of Health, Labour and Welfare. Dietary Reference Intakes for Japanese; 2020 [cited 5 Jan 2021]. Available from: https://www.mhlw.go.jp/stf/newpage_08415.html. |
| 12. | Johnson MA, Smith MM, Edmonds JT. Copper, iron, zinc, and manganese in dietary supplements, infant formulas, and ready-to-eat breakfast cereals. Am J Clin Nutr. 1998;67:1035S-1040S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Duncan A, Yacoubian C, Watson N, Morrison I. The risk of copper deficiency in patients prescribed zinc supplements. J Clin Pathol. 2015;68:723-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Kodama H, Fujisawa C, Bhadhprasit W. Inherited copper transport disorders: biochemical mechanisms, diagnosis, and treatment. Curr Drug Metab. 2012;13:237-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Choi EH, Strum W. Hypocupremia-related myeloneuropathy following gastrojejunal bypass surgery. Ann Nutr Metab. 2010;57:190-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Tan JC, Burns DL, Jones HR. Severe ataxia, myelopathy, and peripheral neuropathy due to acquired copper deficiency in a patient with history of gastrectomy. JPEN J Parenter Enteral Nutr. 2006;30:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Halfdanarson TR, Kumar N, Li CY, Phyliky RL, Hogan WJ. Hematological manifestations of copper deficiency: a retrospective review. Eur J Haematol. 2008;80:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Myint ZW, Oo TH, Thein KZ, Tun AM, Saeed H. Copper deficiency anemia: review article. Ann Hematol. 2018;97:1527-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: De Nardi P S-Editor: Wu YXJ L-Editor: A P-Editor: Wang LYT