Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7251
Peer-review started: April 25, 2021
First decision: May 24, 2021
Revised: June 2, 2021
Accepted: June 15, 2021
Article in press: June 15, 2021
Published online: August 26, 2021
Processing time: 120 Days and 22.9 Hours
The simultaneous occurrence of schwannoma and meningioma in the absence of neurofibromatosis (NF) or a previous history of irradiation is exceedingly rare, as only 10 intracranial cases have been reported to date. Herein, we report a case of a coexistent cavernous sinus meningioma and ipsilateral vestibular schwannoma (VS) in a female patient without NF or a history of exposure to irradiation.
A 63-year-old woman presented with progressive left-side hearing loss and tinnitus over the previous year. In the past 6 mo, she developed facial numbness and intermittent headaches. Magnetic resonance imaging showed two lesions that were located on the left side of the cerebellopontine angle and parasellar region. Both lesions were totally resected via the left retrosigmoid approach. Histopathological examination revealed a VS and a meningioma. The patient did not have a family history or clinical or radiological signs of NF.
The coincident occurrence of VS and meningioma within close vicinity is very rare, and the pathogenesis is unclear. A careful whole-body examination needs to be conducted to exclude NF. Surgical treatment with the goal of total tumor resection is the best therapy. Additional studies are needed for a better under
Core Tip: Coexistent schwannoma and meningioma in the absence of neurofibromatosis or a history of irradiation are extremely rare. To the best of our knowledge, only 10 intracranial cases have been reported to date. Herein, we present the case of a coexistent cavernous sinus meningioma and ipsilateral vestibular schwannoma (VS) in a 63-year-old woman. This is the first report of a concurrent meningioma outside of the cerebellopontine angle, along with a coexisting VS. The medical literature was reviewed and the typical histopathological and radiological features of this rare tumor are described, with a detailed discussion of the diagnosis, treatment, and prognosis.
- Citation: Zhao LY, Jiang YN, Wang YB, Bai Y, Sun Y, Li YQ. Coexistent vestibular schwannoma and meningioma in a patient without neurofibromatosis: A case report and review of literature. World J Clin Cases 2021; 9(24): 7251-7260
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7251.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7251
Vestibular schwannoma (VS) is a benign intracranial tumor that originates from Schwann cells that reside along the vestibular portion of the eighth cranial nerve[1]. VS accounts for 10% of all the central nervous system (CNS) tumors and 80% of the tumors in the cerebellopontine angle (CPA)[2,3]. Meningioma derives from the meningothelial cells of the arachnoid layer and is the most common benign CNS tumor, accounting for 13%-26% of all brain tumors overall. More than 90% of all meningiomas are solitary[1]. However, in some extremely rare situations, VS can coexist with a meningioma in an adjacent anatomical position. This situation is most often reported in patients with neurofibromatosis (NF) or those who have a history of previous irradiation[4-7]. With the exception of those circumstances, the coexistence of these tumors is exceedingly rare. Herein, we present an unusual case of concomitant VS and meningioma that developed within a close anatomic location in a woman without NF or a history of irradiation exposure. There have been only ten reports of coexistent VS and meningioma in patients without NF or irradiation history[2-4,8-14]. The patient described in this report was the first to develop meningioma outside of the CPA, with concurrent, coexisting VS.
A 63-year-old woman presented with left-side hearing loss and tinnitus of 1 year duration had worsened over the recent 6 mo.
The patient presented with progressive left-side hearing loss and tinnitus for 1 year. She had developed facial numbness and intermittent headaches over the previous 6 mo.
The patient had no previous medical history or a family history of NF or menin
No positive personal or family history.
Neurological examination of the patient found no positive signs. A general exami
Preoperative laboratory examination, electrocardiogram, Doppler echocardiography, and lung computerized tomography (CT) were normal.
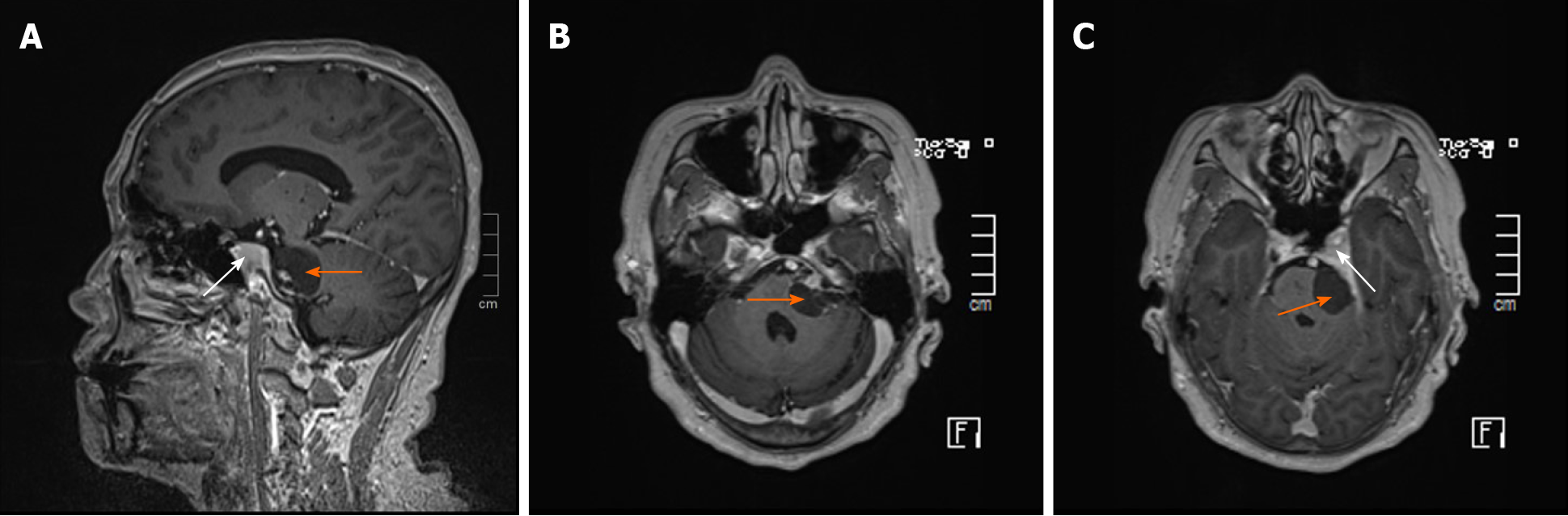
Magnetic resonance imaging (MRI) of the brain (Figure 1) showed a 1.97 cm × 2.61 cm × 2.80 cm abnormal well-defined signal in the left CPA, with a hypointense signal on T1-weighted imaging (T1WI), a hyperintense signal on T2-weighted imaging (T2WI), and a slightly hypointense signal on fluid-attenuated inversion recovery (FLAIR). On contrast enhancement, the lateral border and acoustic nerve were enhanced (Figure 1). In addition, an irregular abnormal signal was observed by the left saddle area, with slight hypointensity on T1WI, and slight hyperintensity on T2WI and FLAIR. After gadolinium administration, the lesion and adjoining dura were significantly enhanced (Figure 1A and C).
Based on the findings described above, the preliminary diagnosis was concomitant VS and left cavernous sinus meningioma, the nature of which is difficult to determine.
A craniotomy using a left retrosigmoid approach was performed under preoperative and intraoperative neuro-navigation and electrophysiological monitoring. The CPA lesion was soft, and contained a large cystic component. The mass in the cavernous sinus was grey, tough, and attached to the dura. Both tumors were totally resected, and the intraoperative diagnosis was consistent with initial diagnosis.
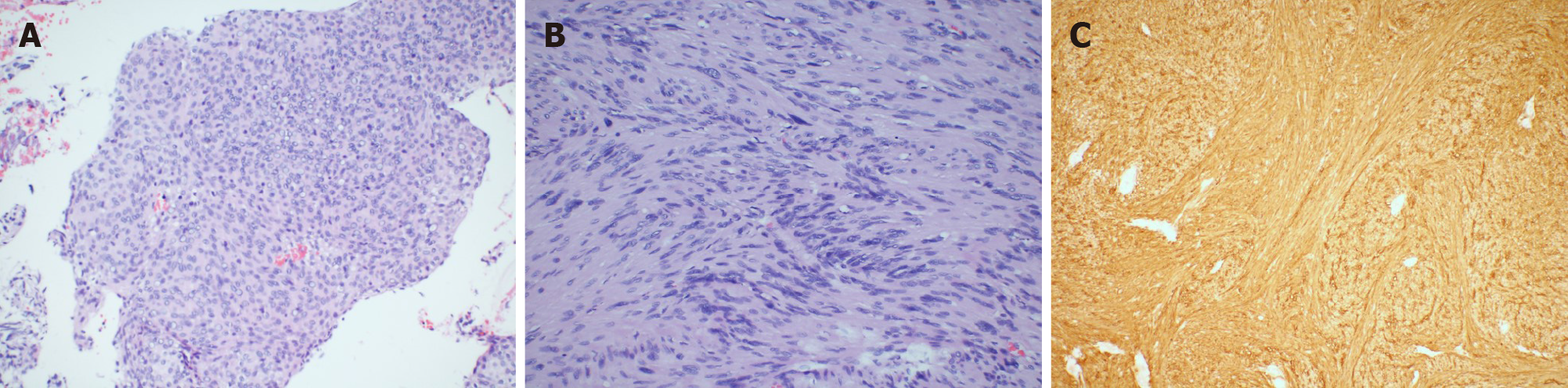
Postoperative pathological findings indicated a diagnosis of meningioma (Figure 2A), World Health Organization Grade I and VS (Figure 2B). Immunohistochemical staining of the meningioma was positive for progesterone receptor (PR), negative for epithelial membrane antigen (EMA) and S-100, and the Ki-67 index was 1%-2%. The VS was positive for S-100 (Figure 2C) and the Ki-67 index was 3%. The patient had mild House and Brackmann grade II facial paralysis, and her hearing was slightly improved after surgery. The patient was carefully re-evaluated for any evidence of NF by physical examination, review of family history, whole-body CT and positron emission tomography-CT (PET-CT). We did not find any signs or symptoms of NF. Immediate postoperative cranial CT and a follow-up MRI 3 mon after surgery demonstrated total excision and no signs of recurrence. The postoperative course was uneventful, and the patient was discharged on the 10th day. At the last telephone follow-up in April 2020, the patient reported having no issues and that her facial paralysis had improved (House and Brackmann grade II). We believe that her condition is stable, with routine follow-up MRIs.
Meningioma and VS are the first and second most common benign CNS tumors. The coexistence of these tumors is seen relatively often in patients with NF, which is an autosomal dominant genetic disease with a high mutation rate and a high risk of tumorigenesis[2,15]. A previous history of irradiation is another known cause of the occurrence of multiple primary brain tumors[16]. However, the development of two separate, histologically different tumors in a patient in the absence of the aforementioned conditions is extremely rare, with only 10 previously reported cases (Table 1)[2-4,8-14]. The patient described in this report had neither evidence of NF nor a history of irradiation exposure. Concomitant tumors have been called “collision”[17], “concurrent”[9,11], “coexisting”[9,11,13], or “coincidental” tumors[2]. Frassanito et al[8] recommended a more accurate classification of tumors with a close anatomical relationship that aimed to clarify their pathogenesis and treatment and used the term “contiguous” to describe two neoplasms that arise separately and become closely located because of their growth. Concomitant, contiguous and collision tumors most likely represent the subsequent development of tumors having a close anatomical position.
| Ref. | Age (yr)/ sex | Main symptoms and signs | Whether differentiate from preoperative MRI | MRI appearance | Surgical findings | Extend of resection | Operative approach | The histological type of meningioma | Postoperative facial nerve | Follows up (mo) |
| Present case | 63/F | Progressive left side hearing loss, tinnitus and giddiness 1 yr. Facial numbness and intermittent headache for 6 mo | Y | VS: A 1.97 cm × 2.61 cm × 2.80 cm abnormal well-defined signal at the left CPA, with hypo- seen on T1WI and hyper- on T2WI. Slightly hypo- was seen on FLAIR. The lateral border and acoustic nerve were enhanced on contrast enhanced MRI. Meningioma: Slightly hypo- was on T1WI, slightly hyper- on T2WI and FLAIR. The lesion and adjoining dura were significantly enhanced after gadolinium administration | The tumor locating at the CPA was soft, and contained a large cystic component. The tumor located the cavernous sinus was greyish and was attached to the dura | Both GTR | Retrosigmoid craniotomy | Meningothelial meningioma | The anatomical continuity of VII nerve was maintained | Follow-up 6 mo with stable condition, without recurrence or residual tumor. The facial paralysis was gradually improved |
| Verma et al[4] | 40/F | Progressive hearing loss left ear, tinnitus, and giddiness for 18 mo and developed gait ataxia since last 9 mo. Tightness of bilateral lower limbs for 6 mo. Progressively headache with intermittent projectile vomiting, and visual obscuration for 15 d | Y | A well-defined extra-axial 4.5 cm × 4.9 cm × 4.1 cm lesion in left CPA originating from IAC. The lesion was hypo- in T1WI, heterogeneously hyper- on T2WI with heterogeneous contrast enhancement. Another well-defined extra-axial 10 mm × 12 mm × 9 mm lesion posterolateral to first mass which was iso- on T1WI, mildly hyper- on T2WI, and homogenous post-contrast enhancement | A smaller tumor broad-based on dura was encountered initially after retraction of the cerebellum posterolaterally to the larger lesion noted to be arising from the IAC which was occupying the left CPA | Both GTR | Retrosigmoid craniotomy | Meningothelial meningioma | The anatomical continuity of 7th nerve was maintained. Worsening of the VII nerve palsy (House and Brackmann Grade V) | At follow-up of 11 mo, patient was ambulant without support, taking oral feeds with improving VII nerve paresis |
| Frassanito et al[8] | 72/M | 5-yr history of hearing loss | Y, the radiological features suggested the co-presence of two different tumors within the same CPA | A tumor in the left CPA with two continuous components differently enhancing after gadolinium administration | Two spatially continuous lesions were found. The rostral component shows a strict relationship with 8th and 7th cranial nerves | N/A | Retrosigmoid craniotomy | Meningothelial meningioma | N/A | N/A |
| Grauvogel et al[9] | 46/F | A history of acute hearing loss and intermittent tinnitus on left side as well as slight gait ataxia for several weeks | N, could not be clearly separated | A homogenous contrast-enhancing tumor in the IAC with extension in the CPA. The tumor portion in the CPA showed broad attachment to the dura of the petrous bone. There were no differences in contrast enhancement of the tumor | It revealed two distinct tumors, a small VS and a meningioma with clear broad attachment to the dura of the petrous bone. The meningioma located a little above and anterior of the VII and VIII cranial nerves | Both GTR | Lateral suboccipital approach | Fibromatous meningioma | Facial nerve function was completely preserved (House and Brackmann grade I) | Follow-up MRI 3 mo after surgery showed complete removal of both tumors and no signs of tumor recurrence |
| Jain et al[10] | 33/M | Redness of the right eye, episodic diplopia, and right-side face numbness for 2 mo | N | Bilateral parasellar masses abutting bilateral body and lower wing of sphenoid and extending to the anterior aspect of the petrous apex. The masses were hypo- on T1WI and hyper- in T2 with contrast enhancement | Intraoperatively, a tumor of variable color and consistency was identified arising from the Vth nerve. The color ranged from creamy white to grayish with areas of fibrosis and old hemorrhage, and a soft to firm consistency | N/A | Right pterional craniotomy and subtemporal intradural approach | Transitional meningioma | By the time of discharge, the patient had up to 50% recovery of the right-side face sensory loss | N/A |
| Kutz et al[11] | 43/F | Severe bifrontal headaches for 18 mo; right-sided hearing loss and imbalance one month before | N, could not be clearly separated | On enhanced MRI, a less intensely enhancing lesion filled the IAC and another more intensely enhancing concurrent lesion located more medially. The enhancing mass extended inferiorly to contact the jugular tubercle and extended laterally toward the jugular fossa | Two distinct tumors were found. The tumor involving the IAC was more typical for VS with soft consistency and little bleeding. The tumor adjacent to the brainstem and petrous temporal bone was fibrous and vascular. The petrous temporal bone around the IAC and inferior toward the jugular foramen was grossly involved with tumor | STR (no other detailed information available) | Translabyrinthine craniotomy | Angiomatous meningioma | Facial nerve function was grade I on the House-Brackmann scale | N/A |
| Izci et al[2] | 57/F | 3-mo history of headache, left facial numbness, speech disturbance and deafness in the left ear | Y, MRI revealed two different mass in the left CPA | A heterogeneously contrast-enhancing 3 cm × 3.1 cm × 3.2 cm lesion in the left CPA with obvious edema, compressing pons, mesencephalon, 4th ventricle and cerebella, and showed mild extension into the IAC. Another homogenous contrast-enhancing locating laterally to the first lesion with 12 mm in diameter and attached to the left tentorium cerebelli | Two distinct tumors were totally resected, respectively. (No other detailed information available) | Both GTR | Lateral suboccipital approach | Fibromatous meningioma | The 7th and 8th were observed intact but were serious compressed. Neurological status was not changed after surgery | Follow-up for 9 yr and no recurrence was observed |
| Chen et al[3] | 48/M | A sudden bilateral hearing loss 16 yr ago. His hearing loss resolved on the right side, but his hearing loss in the left ear had persisted, with some progression | Y, a partly enhancing and nonenhancing left CPA tumor | A mixed enhancing and nonenhancing mass within the left CPA that extended along the 7th and 8th cranial nerves | The posterolateral portions of the tumor were cystic and calcified. The tumor appeared to infiltrate the facial nerve and a portion of the brainstem. It was also noted adhering to the facial nerve | STR except for a portion of cystic | Translabyrinthine craniotomy | Fibromatous meningioma | The facial nerve was stimulated at 0.1 mA at the end of the surgery. (no other detailed information available) | N/A |
| Lüdemann et al[12] | 59/F | Progressive loss of hearing for 3 yr | N, a typical VS was assumed preoperatively | A cystic mass in the right CPA of approximately 20 mm × 20 mm × 10mm, which was in close relation to 7th and 8th nerves. On enhanced MRI, the mass was prominent enhanced and was apparently attached to the nerves entering the IAC with irregular margins | The tumor presented with invasion of the surrounding arachnoid membrane and 7th and 8th nerves | N/A | Lateral suboccipital approach | Meningothelialmeningioma | Facial nerve reconstruction was performed using an autologous sural nerve graft. (no other detailed information available) | N/A |
| Chandra et al[13] | 35/M | Progressive right-side hearing loss, tinnitus and giddiness for 3 yr. Developed gait ataxia and increasingly severe headaches in the last 6 mo | N, no other detailed information available | A “dumb-bell” well defined extra-axial tumor in the right CPA with iso- to mild hyper- on T1WI and hyper- on T2WI. The ventral component was cystic-solid component. The lesion was enhancing markedly with gadolinium contrast except the cystic part | Two separate tumors were encountered. The dorsally placed tumor was greyish, vascular and was attached to the petrous bone. Another ventrally placed portion could be completely separated from the dorsal part. This was soft, and contained a large cystic component | Both GTR | Retromastoid suboccipital craniectomy | Angiomatous meningioma | The facial nerve was preserved intraoperative. (The detailed postoperative information could not available) | N/A |
| Wilms et al[14] | 47/F | A sudden onset of the right-side hearing loss. Perioral and perinasal numbness on the right for a few weeks | Y, enhanced MRI showed two distinct components. | Iso on T1WI; two distinct parts was shown on T2WI: The part that was broadly implanted on the petrous bone was iso-; the part around the IAC was inhomogeneously hyper-. On enhanced MRI, two different components of the tumor were clearly shown: An anterior, strongly enhancing part extended into the IAC, and a posterior, less enhancing part was broadly attached to the petrous bone | At surgery, it was confirmed that both a VS and a meningioma arising from the petrous apex were present in this patient | N/A | N/A | Fibromatous meningioma | N/A | N/A |
Several hypotheses have been proposed to explain the formation of collision tumors. One is the neoplasm microenvironment theory, which speculates that the presence of the first tumor alters the surrounding microenvironment and promotes the occurrence of the second primary tumor[2,15,18-23]. Some theories propose that schwannomas influence the local microenvironment and promote the occurrence of meningiomas[5,23-25]. Another hypothesis considers genetic causes. The merlin gene, located on chromosome 22q, has been identified by next-generation sequencing as a critical suppressor gene of meningioma[26], and mutations are thought to be associated with the occurrence of meningioma. The development of sporadic VS also involves the absence or inactivation of merlin[27]. Consequently, concurrent meningioma and VS may have a common origin in the merlin gene on chromosome 22q. The third hypothesis involves a common origin of both neoplasms from the same mesenchymal progenitor cells that go on to differentiate into different types of tumor cells[18-21,23,28]. The fourth hypothesis involves the exposure of two different tumor cell types at the same site in response to the same oncogenic stimulus, with subsequent development as a collision tumor[2,20,28]. The last hypothesis involves the independent development of two neoplasms such that the concurrent appearance at the location is simply a coincidence[2,28,29]. These hypotheses explain why schwannomas are prone to coexist with meningiomas. In this patient, the influence of the neoplastic microenvironment and genetics are the most likely cause of the tumors. Firstly, the contribution of merlin gene mutation on chromosome 22q to the development of both meningioma and sporadic VS has been widely accepted[26,27,30-32]. Secondly, some studies of the neoplasm microenvironment theory have found that focal reactive meningothelial cell hyperplasia can occur adjacent to a schwannoma[5,24,25]. Moreover, meningothelial-like nodules with whorl patterns have been described in cellular schwannomas[33], which is consistent with a histology of schwannoma in our paper. Furthermore, meningotheliomatous cells with infiltrating schwannoma can also trigger autocrine/paracrine growth stimulation[34].
We reviewed the histology of 11 reported meningioma cases, including our patient (Table 1). Four of the 11 were fibromatous meningioma (36.36%)[2,3,9,14], four were meningothelial meningioma (36.36%)[4,8,12], two were angiomatous meningioma (2/11, 18.18%)[11,13], and one was a transitional meningioma (1/11, 9.09%)[10]. Our patient had a meningothelial meningioma coexisting with a schwannoma. No obvious susceptibility was observed, and the histologies of the meningioma and VS were not related.
The most important preoperative evaluation of meningiomas and VSs is an MRI with gadolinium contrast[11]. Visual examination of an MRI is not sufficient to make a diagnosis, but it is valuable for assessing the location and demonstrating tumor vascularity and nerve displacement, all of which are needed for surgical planning. In five of 10 case reports (50.0%), preoperative MRI had revealed the presence of two distinct component neoplasms[2,4,8,14,16], either because the tumors were located close to each other (2/5, 40.0%)[2,4] or because of different contrast enhancement patterns (3/5, 60.0%)[8,14,16]. In some cases, the MRI was not sufficiently sensitive to reveal the coexistence of multiple tumor types (5/10, 50.0%)[9-13]. VSs tend to invade the internal auditory canal and expand, with cystic changes and reduced internal homogeneity[2]. Meningiomas tend to present with a dural tail sign and calcification[18,35], which are absent in VSs[14]. Both VS and meningiomas usually present with intense to slight hypointensity relative to the brain parenchyma on T1WI[2,14,35]. On T2WI, VSs always have higher intensity than meningiomas and are more heterogeneous[2,18,35]. On contrast enhancement, schwannomas are significantly heterogeneously enhanced, and frequently extend into the internal auditory canal[2], while meningiomas are homogenous and moderately enhanced[29].
Pathological examination typically finds that schwannomas are for S-100 protein and anti-Leu 7[5,9,36]. They tend to be negative for EMA, but focal EMA reactivity may be encountered[5]. Meningiomas are always positive for EMA and negative for S-100 and anti-Leu 7[2,5,36]. Immunohistochemical diagnosis of VS and meningioma is not difficult, but the diagnosis of NF needs further clarification. The presence of multiple schwannomas and/or meningioma should prompt screening for NF, which requires a detailed physical examination, review of the family history, whole-body CT, spinal MRI, PET-CT, and possible genetic testing.
Surgical resection is the primary treatment of choice, with meticulous dissection and total tumor resection without injuring the brainstem and with preservation of cranial nerves[28,37]. In eight of the 11 reported cases (72.72%), the facial nerve was preserved, and was reconstructed by autologous sural nerve grafting in one case. Retrosigmoid craniotomy was the most common approach, and was used in four of the 11 cases (36.36%)[4,8,13]. Three cases (75.0%) underwent total resection[4,13], including the patient described in our report. Three (27.27%) underwent a lateral suboccipital approach[2,9,12], with total resection in two (66.67%)[2,9]. Two of the 11 patients underwent translabyrinthine craniotomy (18.18%)[3,11], but total resection was not achieved. There were two more patients. One underwent right pterional craniotomy with a subtemporal intradural approach. The approach was not available for the other patient. In our patient, total resection was achieved by retrosigmoid craniotomy. Intraoperative frozen section histology is useful for surgical decision-making, especially when the continuity between the two components is ambiguous[29]. Adib et al[37] reported that early resection avoided tumor interaction that triggers more aggressive behavior. When MRI reports are inconsistent with intraoperative findings, the possibility of multiple tumors should be considered, particularly for patients with NF. No postoperative radiotherapy/chemotherapy was reported in these cases. Some studies recommended radiosurgery as an alternative treatment option, particularly for smaller VSs that did not have a significant mass effect that compressed the brainstem or caused hydrocephalus[4,38]. Patient prognosis is related to the preoperative neurological status, and most patients experienced postoperative improvement of neurological function[9]. Recurrence was not reported on follow-up of any of the reported cases.
In summary, we report a case of concomitant VS and meningioma, which is exceedingly rare, especially in patients without NF or history of irradiation. Because of the rarity and lack of long-term follow-up data in the current literature, the pathogenesis and recurrence rate are not known, recommended treatment is not available, and requires further validation. Additional cases reports and long-term follow-up studies are needed to fully understand this disease.
The authors thank the patient for agreeing to provide his medical history. All the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
| 1. | Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7079] [Cited by in RCA: 8140] [Article Influence: 428.4] [Reference Citation Analysis (0)] |
| 2. | Izci Y, Secer HI, Gönül E, Ongürü O. Simultaneously occurring vestibular schwannoma and meningioma in the cerebellopontine angle: case report and literature review. Clin Neuropathol. 2007;26:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Chen AF, Samy RN, Gantz BJ. Cerebellopontine angle tumor composed of Schwann and meningeal proliferations. Arch Otolaryngol Head Neck Surg. 2001;127:1385-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Verma SK, Kumar S, Deb P, Yadav KK. Rare case of radiologically distinct but pathologically admixed vestibular schwannoma and meningioma in the cerebellopontine angle: A case report. J Cancer Res Ther. 2015;11:1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Matyja E, Kunert P, Grajkowska W, Marchel A. Coexistence of meningioma and schwannoma in the same cerebellopontine angle in a patients with NF2. Folia Neuropathol. 2012;50:166-172. [PubMed] |
| 6. | Elizabeth J, Menon G, Nair S, Radhakrishnan VV. Mixed tumour of schwannoma and meningioma in a patient with neurofibromatosis-2 : a case report. Neurol India. 2001;49:398-400. [PubMed] |
| 7. | Kim DG, Paek SH, Chi JG, Chun YK, Han DH. Mixed tumour of schwannoma and meningioma components in a patient with NF-2. Acta Neurochir (Wien). 1997;139:1061-4; discussion 1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 8. | Frassanito P, Montano N, Lauretti L, Pallini R, Fernandez E, Lauriola L, Novello M, Maira G. Simultaneously occurring tumours within the same cerebello-pontine angle: refining literature definitions and proposal for classification. Acta Neurochir (Wien). 2011;153:1989-1993; discussion 1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Grauvogel J, Grauvogel TD, Taschner C, Baumgartner S, Maier W, Kaminsky J. A Rare Case of Radiologically Not Distinguishable Coexistent Meningioma and Vestibular Schwannoma in the Cerebellopontine Angle - Case Report and Literature Review. Case Rep Neurol. 2010;2:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Jain A, Suri V, Sharma BS, Sharma MC. Mixed schwannoma with meningioma of the trigeminal nerve. Indian J Pathol Microbiol. 2010;53:769-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Kutz JW, Barnett SL, Hatanpaa KJ, Mendelsohn DB. Concurrent vestibular schwannoma and meningioma mimicking a single cerebellopontine angle tumor. Skull Base. 2009;19:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Lüdemann W, Stan AC, Tatagiba M, Samii M. Sporadic unilateral vestibular schwannoma with islets of meningioma: case report. Neurosurgery. 2000;47:451-452; discussion 452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Chandra PS, Hegde T. A case of coexisting cerebellopontine angle meningioma and schwannoma. Neurol India. 2000;48:198. [PubMed] |
| 14. | Wilms G, Plets C, Goossens L, Goffin J, Vanwambeke K. The radiological differentiation of acoustic neurinoma and meningioma occurring together in the cerebellopontine angle. Neurosurgery. 1992;30:443-445; discussion 445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 316] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 16. | Chen JC, Tseng SH, Chen Y, Tzeng JE, Lin SM. Cervical dumbbell meningioma and thoracic dumbbell schwannoma in a patient with neurofibromatosis. Clin Neurol Neurosurg. 2005;107:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Muzumdar DP, Goel A. Acoustic schwannoma and petroclival meningioma occurring as collision tumours: a case report. J Clin Neurosci. 2004;11:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Porčnik A, Žele T, Prestor B. Concurrent intradural meningioma and schwannoma at the same lumbar level in a patient without neurofibromatosis: a case report. Br J Neurosurg. 2020;34:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Matsuda S, Kajihara Y, Abiko M, Mitsuhara T, Takeda M, Karlowee V, Yamaguchi S, Amatya VJ, Kurisu K. Concurrent Schwannoma and Meningioma Arising in the Same Spinal Level: A Report of Two Cases. NMC Case Rep J. 2018;5:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Liebelt BD, Haider AS, Steele WJ, Krishna C, Blacklock JB. Spinal Schwannoma and Meningioma Mimicking a Single Mass at the Craniocervical Junction Subsequent to Remote Radiation Therapy for Acne Vulgaris. World Neurosurg. 2016;93:484.e13-484.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Oichi T, Chikuda H, Morikawa T, Mori H, Kitamura D, Higuchi J, Taniguchi Y, Matsubayashi Y, Oshima Y, Tanaka S. Concurrent spinal schwannoma and meningioma mimicking a single cervical dumbbell-shaped tumor: case report. J Neurosurg Spine. 2015;23:784-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Chen KY, Wu JC, Lin SC, Huang WC, Cheng H. Coexistence of neurofibroma and meningioma at exactly the same level of the cervical spine. J Chin Med Assoc. 2014;77:594-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Nakamizo A, Suzuki SO, Shimogawa T, Amano T, Mizoguchi M, Yoshimoto K, Sasaki T. Concurrent spinal nerve root schwannoma and meningioma mimicking single-component schwannoma. Neuropathology. 2012;32:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 24. | Sobel RA. Vestibular (acoustic) schwannomas: histologic features in neurofibromatosis 2 and in unilateral cases. J Neuropathol Exp Neurol. 1993;52:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Geddes JF, Sutcliffe JC, King TT. Mixed cranial nerve tumors in neurofibromatosis type 2. Clin Neuropathol. 1995;14:310-313. [PubMed] |
| 26. | Lee S, Karas PJ, Hadley CC, Bayley V JC, Khan AB, Jalali A, Sweeney AD, Klisch TJ, Patel AJ. The Role of Merlin/NF2 Loss in Meningioma Biology. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Sass H, Cayé-Thomasen P. Contemporary Molecular Biology of Sporadic Vestibular Schwannomas: A Systematic Review and Clinical Implications. J Int Adv Otol. 2018;14:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Nehete L, Nandeesh BN, Bharath RD, Rao MB, Arimappamagan A. Cerebellopontine Angle Schwannoma and Meningioma in Contiguity: Surgical Implications in Neurofibromatosis. J Neurol Surg A Cent Eur Neurosurg. 2018;79:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Zhan Z, Yan X, Nie W, Ding Y, Xu W, Huang H. Neurofibroma and Meningioma within a Single Dumbbell-Shaped Tumor at the Same Cervical Level without Neurofibromatosis: A Case Report and Literature Review. World Neurosurg. 2019;130:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Coy S, Rashid R, Stemmer-Rachamimov A, Santagata S. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol. 2020;139:643-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 31. | Petrilli AM, Fernández-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene. 2016;35:537-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 333] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 32. | Blakeley JO, Plotkin SR. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol. 2016;18:624-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Santi R, Franchi A, Veltri M, Valeri A, Nesi G. Meningothelial-like whorls in a retroperitoneal cellular schwannoma: potential diagnostic pitfall. Pathol Int. 2010;60:62-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Pallini R, Tancredi A, Casalbore P, Mercanti D, Larocca LM, Consales A, Lauretti L, Fernandez E. Neurofibromatosis type 2: growth stimulation of mixed acoustic schwannoma by concurrent adjacent meningioma: possible role of growth factors. Case report. J Neurosurg. 1998;89:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Lalwani AK, Jackler RK. Preoperative differentiation between meningioma of the cerebellopontine angle and acoustic neuroma using MRI. Otolaryngol Head Neck Surg. 1993;109:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018;14:2161-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 335] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 37. | Adib SD, Tatagiba M. Surgical management of collision-tumors between vestibular schwannoma and meningioma in the cerebellopontine angle in patients with neurofibromatosis type 2. Acta Neurochir (Wien). 2019;161:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Kondziolka D, Flickinger JC, Lunsford LD. The principles of skull base radiosurgery. Neurosurg Focus. 2008;24:E11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ren M S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH