Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4797
Peer-review started: January 20, 2021
First decision: March 25, 2021
Revised: March 29, 2021
Accepted: April 20, 2021
Article in press: April 20, 2021
Published online: June 26, 2021
Processing time: 142 Days and 7 Hours
Management of chronic refractory wounds is one of the toughest clinical challenges for surgeons. Because of poor blood supply, less tissue coverage, and easy exposure, the lower leg is a common site for chronic refractory wounds. The current therapeutic regimens often lead to prolonged hospital stay and higher healthcare costs. Concentrated growth factor (CGF) is a novel blood extract that contains various growth factors, platelets, and fibrins to promote wound healing process. However, there has been little research reported on the treatment of lower extremity wounds with CGF.
A 37-year-old man, without any past medical history, presented an ulcerated chronic wound on his right lower leg. The skin defect exhibited clear boundaries, with a size of 2.0 cm × 3.5 cm. The depth of wound was up to the layer of deep fascia. Staphylococcus aureus was detected by bacterial culture. The final diagnosis was right lower extremity ulcers with infection. Cefathiamidine, silver sulfadiazine, and mupirocin cream were applied to control the infection. CGF gel was prepared from the patient’s blood sample, and was used to cover the wound after thorough debridement. The skin wound was successfully healed after three times of CGF treatment.
CGF displays an excellent wound healing promoting effect in patients with lower-extremity chronic refractory wounds.
Core Tip: The effects of the current interventions, such as flap reconstruction, artificial dermis grafting, and negative pressure wound therapy, on chronic refractory wounds may vary among different patients. Concentrated growth factor (CGF) is obtained from the patient’s blood sample, and has a significant amount of growth factors. Here, we report a male patient with lower-extremity wound defects who received CGF treatment. The patient recovered well after three times of CGF treatment. This case highlights the beneficial effect of CGF on chronic wound and provides a novel therapeutic option for lower-extremity refractory wound repair.
- Citation: Liu P, Liu Y, Ke CN, Li WS, Liu YM, Xu S. Therapeutic effect of autologous concentrated growth factor on lower-extremity chronic refractory wounds: A case report. World J Clin Cases 2021; 9(18): 4797-4802
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4797.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4797
Chronic refractory wounds are termed as the wounds that do not proceed through an orderly and timely repair to form anatomic and functional integrity within 3 mo. The main causes of refractory wounds include vascular insufficiency, diabetes mellitus, and local pressure effects. Besides, systemic factors such as poor nutritional condition, infection, and altered immunological status also delay the wound healing process[1]. Currently, the management strategies for skin defects, including flap reconstruction, artificial dermis grafting, and negative pressure wound therapy[2,3], still need further optimization. Therefore, it is of utmost urgency to develop new therapeutic options for achieving better clinical outcomes.
As a new-generation biomaterial, concentrated growth factor (CGF) is derived from autologous fresh whole blood by differential centrifugation[4]. CGF is known to contain abundant platelets, fibrins, and growth factors, such as epidermal growth factor, fibroblast growth factor (FGF), insulin-like growth factor, platelet-derived growth factor, transforming growth factor beta 1, and vascular endothelial growth factor[5]. Increasing evidence has demonstrated the multiple effects of CGF on cell growth, migration, vascularization, and tissue transplantation. Previous studies have reported that CGF exhibits the potential for Schwann cell proliferation, dental pulp stem cell regeneration, and bone reconstruction[6-8]. Besides, CGF could increase the success rate of fat grafting by improving the vascularization of the transplanted fat tissue[5]. Therefore, CGF is a promising agent for promoting the growth and regeneration of skin tissue, but very few adequate studies have been conducted to determine its effects on lower-extremity chronic refractory wounds.
A 37-year-old man, who was admitted to our hospital on July 2, 2017, presented an ulcerated wound on his right lower leg.
Two weeks before admission, the patient was exposed to trauma by accident. The improper treatment led to an ulcerated wound on his right lower leg.
The patient was free from other illnesses.
The patient had no special personal and family history.
Physical examination showed no significant abnormalities. His blood pressure was 125/74 mmHg, pulse was 93 beats per minute, and temperature was 36.3 °C.
Upon admission, a routine blood test revealed the following values: Hemoglobin, 167 g/L; white blood cell count, 8.69 × 109/L; red blood cell count, 5.30 × 1012/L; and thrombocytes, 185 × 109/L. Drug susceptibility testing showed that the wound was infected with Staphylococcus aureus.
No imaging examinations were performed as the patient displayed good conditions except the wound on his lower leg.
The patient was diagnosed as having a right lower-extremity chronic refractory wound, along with Staphylococcus aureus infection.
Wound secretion culture and drug sensitivity test were carried out after admission. Transparent graph paper was employed to measure the wound size. Vessel forceps was used to assess the depth of the wound. A thorough mechanical debridement accompanying with Moist Exposed Burn Ointment (MEBO) was performed to remove necrotic tissue. All surgical procedures were conducted under aseptic conditions according to the local policy. Povidone iodine (0.5%) or H2O2 (3%) solution was used to rinse the wound once a day, while mupirocin or silver sulfadiazine ointment was employed after wound dressing until the necrotic tissue and wound exudate were decreased significantly, which were essential for the following procedures. The dressing materials, such as gauze with or without vaseline, were replaced once a day. The interval of wound refilling was dependent on CGF dissolution, typically ranging from 5 d to 7 d.
Autologous venous blood (9 mL) was collected into a sterile Vacuette tube with no additives, and immediately centrifuged using a fixed-program device (Silfradent, Italy). The detailed centrifugation settings are as follows: Acceleration (30 s), 2700 rpm for 2 min, 2400 rpm for 4 min, 2700 rpm for 2 min, and deceleration (30 s). CGF gel was suspended in the middle layer in the Vacuette tube and placed on gauze by forceps. The gel was applied onto the wound, and the dressing was replaced daily. The CGF clots were pressed gently in order to fill the wound space and prevent wound fluid effusion.
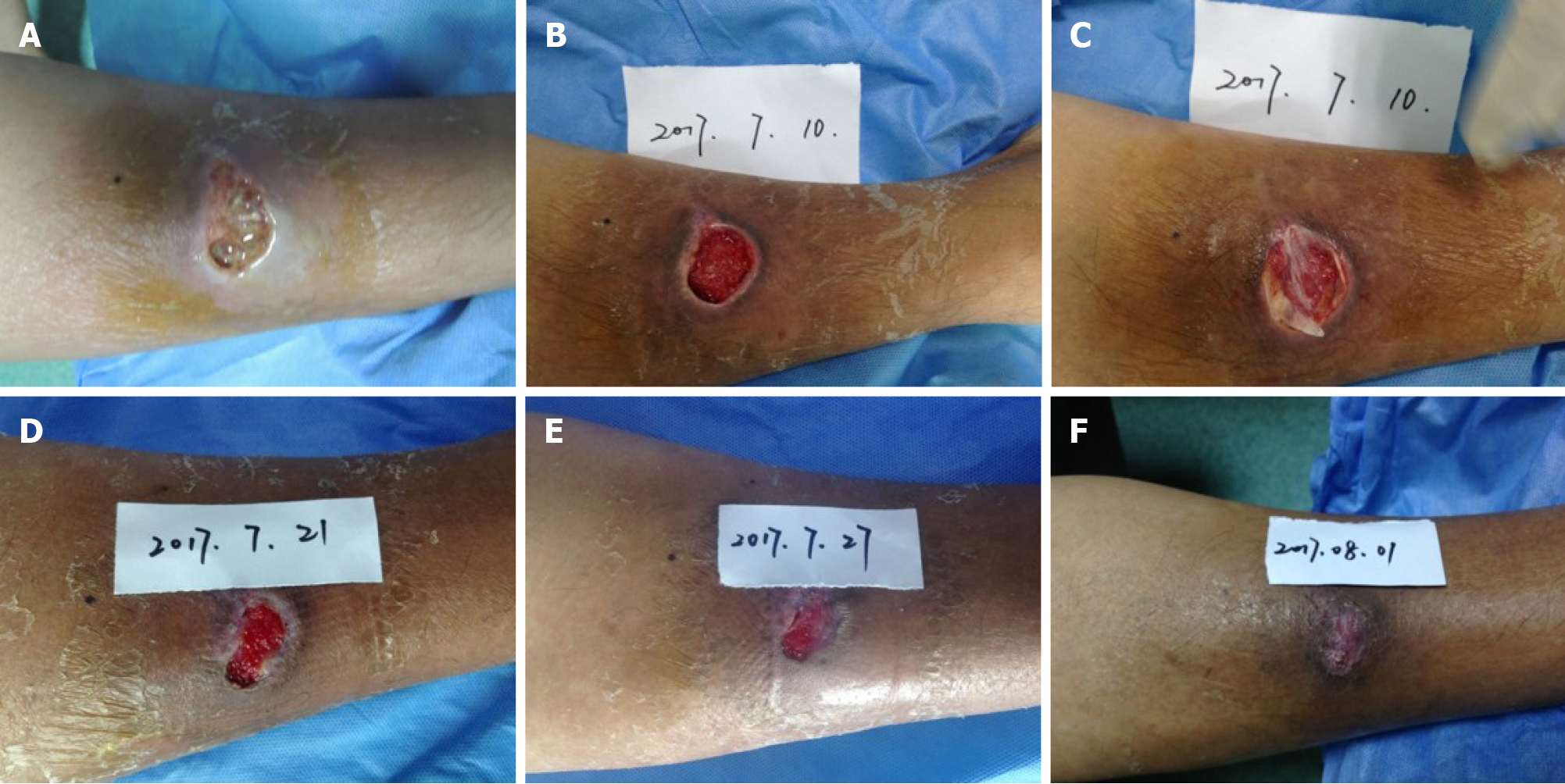
The ulcerated wound exhibited clear boundaries, with a size of 2.0 cm × 3.5 cm. The depth of wound was up to the layer of deep fascia. A sinus tract, around 2 cm in length, was extended to the periosteum of the tibia. Besides, necrotic tissues and light yellow liquid effusions were observed inside the wound bed during admission (Figure 1A). Moreover, the condition of the wound bed was poor, and a small amount of granulation tissue was found. Symptoms such as slight redness, swelling, and pain were also present near the wound site. Cefathiamidine, silver sulfadiazine, and mupirocin cream were applied to control infection, as Staphylococcus aureus was detected by bacterial culture. The periosteum was exposed after thorough debridement and MEBO application (Figure 1B). The formation of granulation tissue was enhanced by recombinant bovine basic FGF. The ulcerated wound was successfully covered by CGF gel (Figure 1C), and the wound closure showed an encouraging trend after CGF treatment. The wound cavity was thinner and wound bed was smaller on postoperative days 11 and 17 after CGF treatment, respectively (Figure 1D and E). The wound site was refilled once the CGF gel was degraded. After three times of CGF treatment, the skin wound was healed (Figure 1F).
Encouraging results were obtained by CGF intervention, even in the infected wound. As mentioned above, the skin defect was successfully healed after three times of CGF treatment. The patient was discharged on August 1 and no medication was needed throughout the whole follow-up period.
Wound healing is a complex process involving the interactions among cells, extracellular matrix, and cytokines. The normal skin repair process is characterized by inflammatory responses, granulation tissue formation, re-epithelialization, stromal cell production, and tissue remodeling[9]. Currently, there are no well-established and widely accepted guidelines for the clinical management for chronic wound due to its complicated pathogenesis and no satisfactory treatment strategies in the clinical settings. Several factors have been identified to affect the healing process of a chronic wound. Insufficient growth factors generally retard cell proliferation, thereby leading to chronic or non-healing wounds[10]. Therefore, improving the growth factor content may be a promising approach for chronic wound management.
CGF is a new-generation whole blood extraction following the platelet-rich plasma and platelet-rich fibrin[11]. As a bio-scaffold and reservoir of growth factors, CGF has been applied in bone regeneration and dental implantation[12]. The abundance of growth factors is beneficial for cell proliferation, vascularization, and tissue remodeling. It has been reported that CD34 positive cells, which play essential roles in mediating inflammation and revascularization, were enriched in CGF gel[13]. Besides, the unique three-dimensional structure of fibrin network provides excellent conditions for the slow release of growth factors and cell proliferation. The characteristics of soft texture and elastic shape are the distinguishing features for CGF application.
Chronic refractory wound on the lower leg is a common physical trauma that occurs after surgical procedures. Although only one representative case was reported in this study, we collected the data of 56 cases with non-healing wounds on their lower extremity and good outcomes were achieved after CGF treatment. Several clinical experiences with respect to the management of wound care are warranted. First, aggressive treatment is essential for primary disease. For example, nutritional support therapy is favorable for those patients with poor physical health, while patients with lower extremity varicose veins should pay more attention to venous stasis. Patients with nodular vasculitis need to receive hormone systemic therapy in order to reduce autoimmune response. For patients with limited physical mobility, suspended bed is helpful to relieve the compression in order to reduce local tissue damage. Second, preparing the wound bed is the foundation of treatment. Most of the wounds showed local infection, therefore, antibiotics should be used cautiously during CGF treatment. Topical ointment was sufficient to control the disease progression if there are no symptoms and signs of systemic infection. Mupirocin ointment is preferred for Staphylococcus aureus infection, while silver sulfadiazine ointment is preferred for Pseudomonas aeruginosa infection. Due to the thin subcutaneous tissue on lower extremity, excessive debridement should be avoided otherwise it may lead to wound bed deterioration and bone exposure. Therefore, proper wound treatment including debridement and antibiotics indeed facilitated wound healing in clinical practice. Third, the appropriate dose of CGF gel is the key of treatment. For patients with bone or periosteum exposure, the CGF gel should be thick enough to maintain a moist wound environment and prevent tissue necrosis. For patients with a sinus tract, CGF gel should be firmly filled in the cavity to minimize the risk of infection. Dressing is also an important factor in successful CGF application. Vaseline gauze can serve as the outer layer of dressing to prevent CGF shifting. During the dressing change, removal of wound exudate may help promote the wound healing process.
CGF is a novel biomaterial that holds great promise for applications in tissue repair and regeneration, but several shortcomings exist as well. Lack of negative control, proper wound treatment might play an important role in CGF therapy on lower-extremity chronic refractory wounds. Moreover, the high demand for blood sample is the main limitation of CGF application in clinical practice, especially for skin defect patients with poor physical health. Besides, the storage of CGF is another challenging issue for its application, as a specific device is needed and CGF has to be used freshly to ensure its biological activity. Therefore, it is necessary to develop CGF-related products for overcoming these problems.
In conclusion, our study reveals that CGF has an excellent curative effect on lower-extremity chronic refractory wounds. Thus, CGF treatment is worthy of clinical promotion and popularization.
| 1. | Werdin F, Tenenhaus M, Rennekampff HO. Chronic wound care. Lancet. 2008;372:1860-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Attia A, Elmenoufy T, Atta T, Harfoush A, Tarek S. Combination of negative pressure wound therapy (NPWT) and integra dermal regeneration template (IDRT) in the lower extremity wound; Our experience with 4 cases. JPRAS Open. 2020;24:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Seth AK, Friedstat JS, Orgill DP, Pribaz JJ, Halvorson EG. Microsurgical Burn Reconstruction. Clin Plast Surg. 2017;44:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Topkara A, Özkan A, Özcan RH, Öksüz M, Akbulut M. Effect of Concentrated Growth Factor on Survival of Diced Cartilage Graft. Aesthet Surg J. 2016;36:1176-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Hu Y, Jiang Y, Wang M, Tian W, Wang H. Concentrated Growth Factor Enhanced Fat Graft Survival: A Comparative Study. Dermatol Surg. 2018;44:976-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Qin J, Wang L, Sun Y, Sun X, Wen C, Shahmoradi M, Zhou Y. Concentrated growth factor increases Schwann cell proliferation and neurotrophic factor secretion and promotes functional nerve recovery in vivo. Int J Mol Med. 2016;37:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Xu F, Qiao L, Zhao Y, Chen W, Hong S, Pan J, Jiang B. The potential application of concentrated growth factor in pulp regeneration: an in vitro and in vivo study. Stem Cell Res Ther. 2019;10:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Mirković S, Djurdjević-Mirković T, Pugkar T. Application of concentrated growth factors in reconstruction of bone defects after removal of large jaw cysts--the two cases report. Vojnosanit Pregl. 2015;72:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 562] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 10. | Qing C. The molecular biology in wound healing & non-healing wound. Chin J Traumatol. 2017;20:189-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 11. | Kim TH, Kim SH, Sándor GK, Kim YD. Comparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF) in rabbit-skull defect healing. Arch Oral Biol. 2014;59:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (7)] |
| 12. | Pirpir C, Yilmaz O, Candirli C, Balaban E. Evaluation of effectiveness of concentrated growth factor on osseointegration. Int J Implant Dent. 2017;3:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Rodella LF, Favero G, Boninsegna R, Buffoli B, Labanca M, Scarì G, Sacco L, Batani T, Rezzani R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech. 2011;74:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jun YM S-Editor: Liu M L-Editor: Wang TQ P-Editor: Liu JH