Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3487
Peer-review started: January 14, 2021
First decision: February 10, 2021
Revised: March 7, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: May 26, 2021
Processing time: 117 Days and 3.7 Hours
Coronavirus disease 2019 (COVID-19) combined with liver injury has become a very prominent clinical problem. Due to the lack of a clear definition of liver injury in patients with COVID-19, the different selection of evaluation parameters and statistical time points, there are the conflicting conclusions about the incidence rate in different studies. The mechanism of COVID-19 combined with liver injury is complicated, including the direct injury of liver cells caused by severe acute respiratory syndrome coronavirus 2 replication and liver injury caused by cytokines, ischemia and hypoxia, and drugs. In addition, underlying diseases, especially chronic liver disease, can aggravate COVID-19 liver injury. In the treatment of COVID-19 combined with liver injury, the primary and basic treatment is to treat the etiology and pathogenesis, followed by support, liver protection, and symptomatic treatment according to the clinical classification and severity of liver injury. This article evaluates the incidence, pathogenesis and prevention and treatment of COVID-19 combined with liver injury, and aims to provide countermeasures for the prevention and treatment of COVID-19 combined with liver injury.
Core Tip: The prevention and treatment of coronavirus disease 2019 (COVID-19) combined with liver injury face many challenges. First, the definition of COVID-19 combined with liver injury is not clear, the selected parameters and the time of statistics are inconsistent, and the conclusions about the incidence rate are consistent. Second, the etiology and mechanism of COVID-19 combined liver injury are not clear and need to be studied in depth. Third, there is a lack of effective treatment methods. This article provides and additional view of the incidence of COVID-19-associated liver injury and explores the contemporary management modalities.
- Citation: Deng ML, Chen YJ, Yang ML, Liu YW, Chen H, Tang XQ, Yang XF. COVID-19 combined with liver injury: Current challenges and management. World J Clin Cases 2021; 9(15): 3487-3497
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3487.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3487
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally, resulting in an ongoing pandemic[1]. As of February 15, 2021, the World Health Organization (WHO) has reported a total of 108579352 confirmed cases of COVID-19 in 184 countries by world region, with a cumulative total of 2396408 deaths[2]. The main target organ of SARS-CoV-2 infection is not only the lung but also many extrapulmonary tissues[3]. Among them, COVID-19 combined with liver injury has become a very prominent clinical problem and has garnered great attention[4]. The author of this article once reported that about one-fifth of the 48 COVID-19 patients have abnormal liver function[5]. At present, the prevention and treatment of COVID-19 combined with liver injury face many challenges, and several key problems need to be solved. For example, the diagnosis of COVID-19 combined with liver injury. Due to the inconsistent diagnostic criteria, a series of problems have arisen in the diagnosis and treatment of COVID-19 combined with liver damage. If the diagnostic criteria are too low, it may lead to overtreatment in clinical practice[6]. Another problem is the study of pathogenesis. It is necessary to determine whether SARS-CoV-2 can directly invade the liver, especially when angiotensin-converting enzyme 2 (ACE2) appears to be negligibly expressed on liver cells[4]. In addition, the mechanisms underlying liver dysfunction in COVID-19 patients are not fully understood; it may be multifactorial and related to hyperinflammation, dysregulated immune responses, abnormal coagulation, and drugs[4].
This article provides an additional view of the incidence, pathogenesis, prevention, and treatment of COVID-19-associated liver injury and explores the contemporary management modalities.
At present, the evaluation parameters for studying the incidence of COVID-19 liver injury are different. The liver function parameters generally include alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TB)[5-14]. Some also include alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT), and so on[5]. Liver injury in COVID-19 patients lacks a clear definition[6]. Some researchers define liver damage as liver enzyme levels above the upper limit of normal (ULN)[5,7] and albumin below the lower limit of normal[5]; other researchers define it as liver enzyme levels 2 or 3 times or even 5 times higher than the ULN[8,9]. China’s “New Coronavirus Pneumonia with Liver Injury Prevention, Diagnosis and Treatment Program” defined as COVID-19 combined with liver injury in the following situations, COVID-19 patients with or without underlying liver disease, the upper limit of serum ALT or AST higher than normal is called COVID-19 with abnormal liver function; ALT or AST ≥ 3 times the ULN or TB ≥ 2 times is called COVID-19 with liver injury[10]. American College of Gastroenterology and British Society of Gastroenterology clinical guidelines define liver injury as an increase in ALT or AST by at least 3 × ULN, or an increase in alkaline phosphatase, TB, or direct bilirubin by at least 2 × ULN[11,12]. Due to the different parameters and criteria for evaluating liver function in patients with COVID-19, the incidence of liver injury varies widely across studies, from 4.8% to a striking 78%[13]. The difference in the incidence of liver injury in different studies is also related to the statistical time point. For example, Ding et al[14] calculated that the abnormal liver function rate of COVID-19 patients at hospitalization admission was 46.2% (958/2073), and the incidence of liver injury was 5.1% (105/2073); Statistics during hospitalization (during disease progression) showed that the abnormal liver function rate was 61.8% (1282/2073), and the incidence of liver injury was 14.3% (297/2073).
In short, due to the unclear definition of liver injury, the different selection of evaluation parameters, the inconsistent statistical time points (i.e. on admission or during disease progression), the incidence of COVID-19 combined with liver injury is extremely inconsistent in different research reports. Therefore, some academics suggest that researchers pay close attention to the terminology and its definition to avoid ambiguity in future analyses and overtreatment in clinical practice[6].
The mechanism of COVID-19 combined with liver injury is complex including direct injury, immune injury, ischemia and hypoxia, and drug injury. In addition, underlying diseases, especially chronic liver disease, can aggravate COVID-19 liver injury.
An increasing amount of evidence has shown that SARS-CoV-2 can directly cause liver damage in COVID-19 patients. Tian et al[15] used reverse transcription polymerase chain reaction to detect the expression of SARS-CoV-2 nucleic acid in the liver of four cases of COVID-19, and the result was positive in one case. Wang et al[1] found a large number of liver cells apoptosis in the liver tissues of two cases patients with COVID-19 during the autopsy and SARS-CoV-2 virus particles in the liver cells through transmission electron microscopy.
SARS-CoV-2 mainly replicates in type II alveolar epithelial cells, which can cause tissue cell injury and destruction. The main manifestation of cell destruction is apoptosis[16]. Wang et al[1] also observed a large number of hepatocyte apoptosis and some binuclear hepatocytes in the autopsy of two COVID-19 patients. The hepato
The new coronavirus, like SARS virus, can use ACE2 as a receptor for cell entry[17-19]. There are differences in the expression of ACE2 in different tissues and even different cells in the same tissue[20,21]. Han et al[20] combined the Genotype-Tissue Expression and The Cancer Genome Atlas databases and found that ACE2 is most highly expressed in the small intestine, and the expression level is lower in the spleen, brain, muscle, pituitary, and skin tissues. It is also expressed in other tissues such as the kidney, heart, liver, and other tissues[20]. Chai et al[21] used the single-cell RNA sequencing method to determine that the expression of ACE2 in hepatic bile duct cells was significantly higher than that of hepatocytes (59.7% vs 2.6% of cells), and the average expression level of ACE2 mRNA in bile duct cells was 20 times higher than that of hepatocytes. Thus, it is speculated that the SARS-CoV-2 virus enters the bile duct cells through ACE2 to cause liver injury. However, based on current research, ACE2-expressing organs do not equally participate in COVID-19 pathophysiology, indicating that other mechanisms are involved in orchestrating cellular infection resulting in tissue injury[22]. However, clinical data show that some patients with COVID-19 do not have a significant increase in the serum indicators of bile duct cells such as ALP and γ-GT but do have increased ALT and AST levels, reflecting liver cell injury[23]. These data suggest that the specific causes of liver cell injury caused by SARS-CoV-2 virus still need a lot of research to clarify its mechanism. Other receptors (e.g., dipeptidyl-peptidase 4, transmembrane serine protease 2) may also mediate the entry of SARS-CoV-2 virus into liver cells to cause liver injury[19,24]. It may even be caused by other mechanisms such as systemic inflammatory response, ischemia, and hypoxia[10].
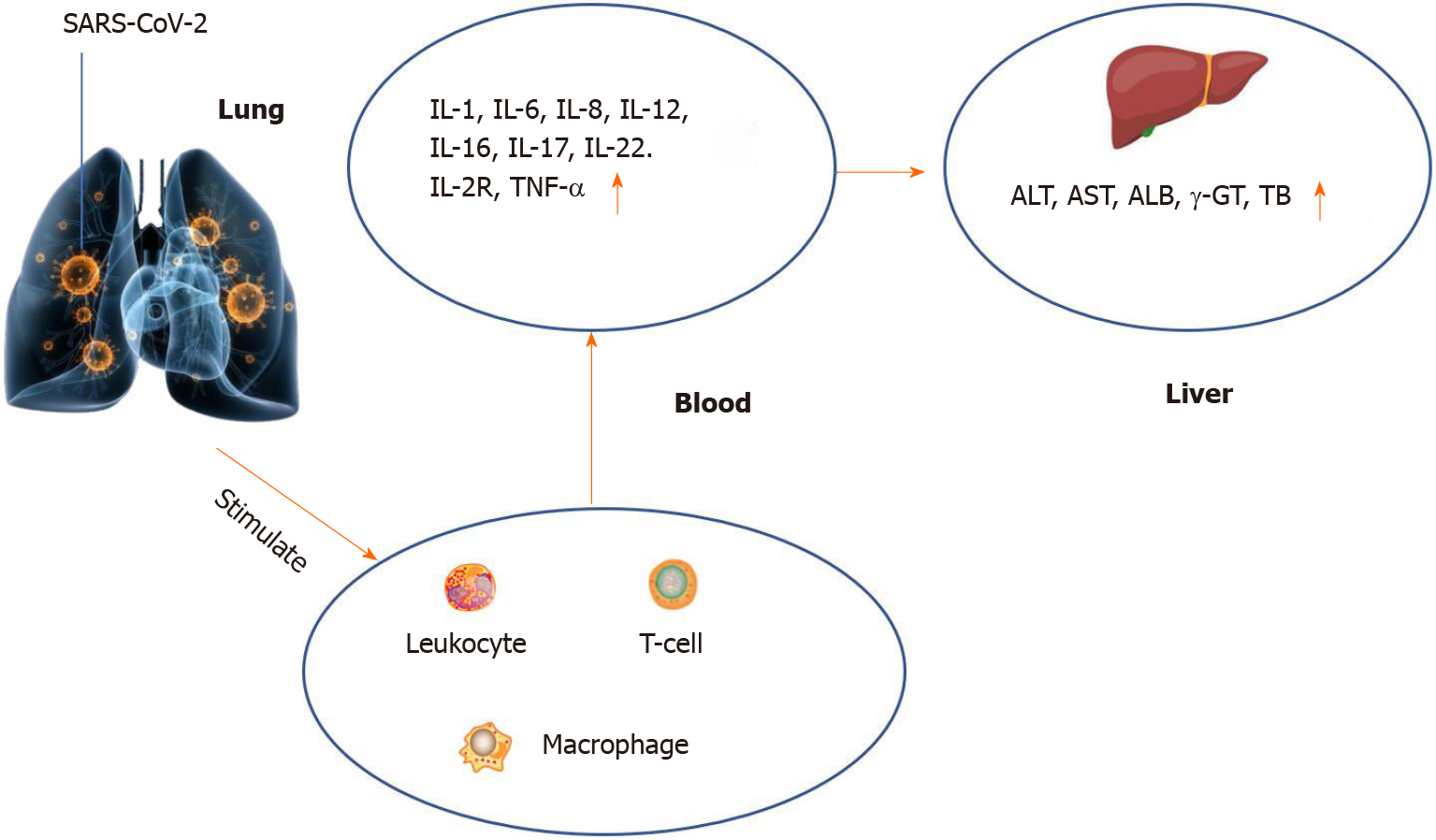
As aforementioned, the main target organ of new coronary pneumonia is the lung, and immune dysfunction is one of the leading causes of lung injury. At present, the most discussed immune injury is the “cytokine storm”, which is infection of the organism by microorganisms. Subsequent immune system-related reactions can further cause multiple organ injuries and acute respiratory distress syndrome to induce liver hypoxia and damage liver cells. Both of these reasons can lead to abnormal liver function indicators in the laboratory[25]. Studies have pointed out that in critically ill patients, the abnormal ratio and degree of cytoinflammatory factors are significantly higher than those in moderate to severe patients[26,27], accompanied by an increase in the proportion of neutrophils and lymphopenia[28]. Nevertheless, in critically ill patients, the probability and degree of liver injury are significantly higher than those of mild to moderate patients. In a large cohort of 5771 people, it was found that elevated ALT and AST were accompanied by lymphopenia and increased neutrophil count[28]. A retrospective study by Huang et al[29] found that the increase of cytoinflammatory factors, namely interleukin (IL)-1, IL-6, IL-8, and IL2R is negatively correlated with the reduction of albumin, and the liver is the main organ for albumin synthesis. This also further illustrates that the “cytokine storm” may be one of the potential causes of liver injury (Figure 1). In a recent study in the United States, it was noted that the systemic inflammatory response was excessive in patients with acute liver failure, which was manifested by significantly increased levels of inflammatory markers and cytokines, and the elevated levels of inflammatory markers were linearly related to the number of organ failures. The authors believed that it may be the inflammatory response in patients with new coronary pneumonia that triggers the occurrence of acute liver failure in patients with potential chronic liver disease[30].
Drug-induced liver injuries in patients with COVID-19 is a factor that cannot be ignored[31]. According to the “New Coronavirus Infection Pneumonia Diagnosis and Treatment Program”[32], antiviral is one of the main treatment measures, and multiple antiviral drugs such as remdesivir, arbidol, darunavir, and lopinavir are recommended. Liver injury side effects can occur in these drugs[33]. In Fan et al[7]’s case-control study, the proportion of liver dysfunction who received ribinavir or lobinavir antiviral treatment was significantly higher than that of those who did not receive these two antiviral treatments, which confirmed that ribinavir or lobinavir navir antiviral drugs can cause liver injury. It is generally believed that the pathogenesis of liver injury from antiviral drugs is related to mitochondrial toxicity, hypersensitivity, and inducing autoimmune hepatitis[34]. Chloroquine (CQ) is an antimalarial that has been used for 70 years; it and its derivative, hydroxychloroquine (HCQ), have attracted wide attention for treating COVID-19[35]. To date, it remains uncertain whether CQ and HCQ are beneficial antiviral drugs for combating COVID-19[35]. There are many reports on the cardiac toxicity of CQ and HCQ, but few reports on liver injury[35,36]. Traditional Chinese medicine (TCM) plays an essential role in treating the new coronary pneumonia, but it can also cause liver injuries[37]. According to the different chemical structures of the risk ingredients in TCM, they are divided into alkaloids, glycosides, toxic proteins, terpenoids and lactones, anthraquinones, and heavy metals[37]. Mechanisms of the hepatotoxic ingredients in TCM-induced hepatotoxicity include cytochrome P450 (CYP450) induction, mitochondrial dysfunction, oxidative damage, apoptosis, and idiosyncratic reaction[37].
Generally, chronic diseases such as hypertension, diabetes, cardiovascular disease, chronic lung disease, chronic liver disease, and chronic kidney disease are one of the reasons for the severity of coronary pneumonia[38]. Ji et al[39] also concluded that patients with chronic obstructive pulmonary disease progress faster than patients without chronic obstructive pulmonary disease. Clinical studies have shown that the abnormal rate and average value of serum ALT and AST levels of SARS-CoV-2-infected patients with hepatitis B virus (HBV) are higher than those in SARS-CoV-2 patients without HBV infection, which indicates that HBV is one of the risk factors for liver injury in COVID-19 patients[40]. Compared with COVID-19 without HBV infection group, patients with dual infection had a higher proportion of severe/critically ill disease, higher levels of ALT, AST and activated partial thromboplastin[41]. Of course, there are clinical reports that the abnormalities of liver function are not uncommon on COVID-19 patients with chronic HBV infection in a case series[42]. These contradictory results need to be further screened by big data.
The common symptoms of COVID-19 patients are fever and cough, with one-third of patients complaining of shortness of breath[43]. The author of this article reported that 9 out of 48 COVID-19 patients experienced dyspnea, accounting for about 18.8% of all patients[5]. Approximately one-third of patients may progress to acute respiratory distress syndrome requiring intensive care[43]. The results reported above suggest that after SARS-CoV-2 infection, ischemia and hypoxia in tissues and organs of COVID-19 patients is a common pathophysiological phenomenon[44]. Consequences of progressive hypoxia may include potentiation of viral proliferation, cytokine release, inflammation, intravascular coagulation, and pulmonary hypoxic vasoconstriction, which are also pathophysiologic characteristics of COVID-19 disease progression[43]. Liver ischemia and hypoxia, severe cases can cause hypoxic hepatitis[45].
COVID-19 combined with liver injury has become a very prominent clinical problem. Timely detection and treatment are particularly important for the prevention and treatment of COVID-19 combined with liver injury.
Since SARS-CoV-2 infection affects the whole body, which is necessary to monitor the body tissues and organs of all COVID-19 patients, including liver function. Routine examination items include liver function, various hepatitis virus markers, inflammatory factors (such as IL-6, C-reactive protein, procalcitonin), and bleeding coagulation function. If COVID-19 combined with liver injury is considered to be caused by basic diseases, blood glucose, blood lipids and other organ function indicators, which are such as B-type natriuretic peptides, N-terminal fragment brain natriuretic peptides or high-sensitivity troponin T must be monitored. Due to hypoxia progression may also be insidious in that patients may not be short of breath despite relatively low blood oxygen levels[43]. Therefore, whether patients with COVID-19 have breathing difficulties, blood oxygen saturation should be routinely monitored.
When the liver function of COVID-19 patients is normal before hospitalization admission, and abnormalities gradually appear during the treatment process, the drug-induced liver injury should be considered[10]. The diagnosis of drug-induced liver injury is exclusive. It needs to be combined with a medical history and related examinations to rule out other liver diseases, then causality assessment is used to determine the degree of correlation between liver injury and suspected drugs. For patients with suspected drug-induced liver injury, consideration should be given to discontinuing or reducing the use of suspicious drugs. For more details, please refer to the 2015 version of the “Guidelines for the diagnosis and treatment of drug-induced liver injury” for treatment[46].
If patients with chronic hepatitis B receive long-term antiviral therapy, the drug should not be stopped; those who need hormone therapy should also receive high-efficiency and low-resistance anti-hepatitis B drugs (e.g., entecavir, tenofovir dipivoxil or propofol tenofovir) inhibit HBV replication from preventing HBV replication reactivation or hepatitis B attack[10]. For patients with hypertension, blood pressure needs to be closely monitored and maintained at a stable level[47]. For patients with coronary heart disease, it is recommended to actively control heart rate, stabilize hemodynamics, and protect the heart and other related treatments[47]. When COVID-19 patients have diabetes at the same time, for mild COVID-19 patients, if the patient's blood sugar is stable, the original hypoglycemic regimen can be used to control blood glucose. For critically severe COVID-19 patients, insulin pump is recommended to lower blood sugar, and the blood glucose range. It is recommended that fasting blood glucose be 7.8-10.0 mmol/L, and blood glucose should be controlled at 7.8-13.9 mmol/L 2 h after meal[47,48].
There is currently no evidence that hepatoprotective drugs can improve the prognosis of patients, and patients with mild liver biochemical abnormalities generally do not need to use hepatoprotective drugs[10]. For patients with acute liver injury, the changes in liver function should be closely monitored, and 1-2 kinds of liver protection drugs with less side effects should be selected as appropriate[10]. Specific liver protection drugs include anti-oxidant hepatoprotective drugs and detoxification liver protection drugs such as reduced glutathione, glycyrrhizic acid liver protection drugs such as diammonium glycyrrhizinate capsules, magnesium magnesium isoglycyrrhizinate injection, liver cell membrane protection liver protection drugs such as polyene phosphatidyl choline, anti-oxidant hepatoprotective drugs such as silibinin and bicyclol, and cholinergic hepatoprotective drugs such as ursodeoxycholic acid and S-adenosylmethionine[49]. For patients with acute liver failure, actively carry out etiological treatment and symptomatic and supportive treatment. For details, please refer to China’s “Guidelines for Diagnosis and Treatment of Liver Failure (2018 Edition)”[50].
COVID-19 patients have different degrees of hypoxemia, and many of them need to be given effective oxygen therapy in time[32]. Hypoxic hepatitis mostly occurs in severe or critical patients. At this time, liver injury is mostly caused by multiple organ dysfunction, ischemia and hypoxia. For patients with hypoxia, hypoxemia can be corrected by oxygen inhalation, mechanical ventilation, and airway management or extracorporeal membrane oxygenation; For patients with circulatory failure, vasoactive drugs can be used on the basis of fluid resuscitation to improve tissue perfusion. Used to improve oxygenation, reduce myocardial oxygen consumption, correct internal environment, remove inflammatory factors, and promote liver function recovery[10,32,44,45].
Antiviral therapy is a basic treatment. If the liver injuries associated with COVID-19 is not caused by antiviral drugs, antiviral treatment should be given as soon as possible to inhibit virus replication and accelerate virus clearance.
The eighth edition of the diagnosis and treatment plan recommends that the following drugs can continue to be tried and further evaluated in clinical applications[32]: α-interferon, ribavirin, CQ phosphate, and arbidol.
The current consensus is that antiviral drugs with potential antiviral effects should be used within 10 d after the onset of disease, because the virus is in the replication stage at this stage[51], and the combination use of two antiviral drugs is advocated[52].
Due to the unclear efficacy, some antiviral drugs are not recommended or cannot be used alone[53,54]. The eighth edition of the diagnosis and treatment plan does not recommend the use of lopinavir/ritonavir and ribavirin alone, nor the use of HCQ or the combined use of azithromycin[32].
The efficacy of some drugs needs to be screened. Remdesivir is a drug officially approved by the United States Food and Drug Administration for the treatment of hospitalized patients with COVID-19, but the clinical efficacy results are con
When using antiviral drugs, pay attention to drug adverse reactions, con
If there are intolerable side effects such as liver injury, the relevant drugs should be stopped, and the convalescent plasma antiviral can be used when conditions permit. According to the currently available treatment data, plasma therapy for recovered patients is very effective for patients who still have viruses in their bodies[57,58]. In view of limited source of plasma sources in recovery patients from COVID-19, the development and use of therapeutic monoclonal antibodies against the new coronavirus is of great significant[59,60].
Currently, treatments for cytokine storm caused by new coronavirus infection include: the IL-6 and receptor antagonists, blood purification, glucocorticoids, etc. Studies by Shruti Gupta and others found that among critically ill patients with new coronavirus pneumonia, patients treated with IL-6 receptor antagonist-cilizumab had a lower risk of in-hospital death during the first 2 d of admission to the intensive care unit. Patients treated with cilizumab early[61]. Stutuzumab is a drug that directly targets IL-6, which can directly bind to IL-6 to neutralize the biological effects caused by IL-6[62]. The efficacy of these two drugs on the cytokine storm caused by SARS-CoV-2 still needs further clinical trials to verify[63]. Since both cilizumab and stutuzumab can cause liver damage[63], it is recommended to avoid severe and critical COVID-19 patients with liver damage as much as possible. Glucocorticoids can be used to treat cytokine storm within a short period of time (generally recommended 3-5 d, no more than 10 d). For patients with drug-induced liver injury without contraindications, especially patients with severe liver injury, the early use of hormones is effective and safe[64]. On September 2, 2020, WHO also published a guidance document on the role of corticosteroids in the treatment of COVID-19[65]. In addition, blood purification can also be used for the early and mid-term treatment of cytokine storm in severe and critical COVID-19 patients with liver injury[10,32].
The prevention and treatment of COVID-19 combined with liver injury faces many challenges. First, the definition of COVID-19 combined with liver injury is not clear, the selected parameters and the statistics time are inconsistent, and the final conclusions on the incidence rate are consistent. Second, the etiology and mechanism of COVID-19 combined liver injury are not clear and need to be studied in depth. Third, there is a lack of effective treatment methods. The development and use of therapeutic monoclonal antibodies against the new coronavirus is of great significance.
| 1. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 2. | World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard Data. [cited 2 February 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/Home/Emergencies/Diseases/Coronavirus disease (COVID-19). |
| 3. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2114] [Article Influence: 352.3] [Reference Citation Analysis (7)] |
| 4. | Li D, Ding X, Xie M, Tian D, Xia L. COVID-19-associated liver injury: from bedside to bench. J Gastroenterol. 2021;56:218-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Zhong ZF, Huang J, Yang X, Peng JL, Zhang XY, Hu Y, Fu N, Lin HL, Jiang B, Tian YY, Yao HY, Deng LP, Tang XQ, Zhou JC, Tang J, Xie X, Liu Q, Liu J, Dou CY, Dai RJ, Yan B, Yang XF. Epidemiological and clinical characteristics of COVID-19 patients in Hengyang, Hunan Province, China. World J Clin Cases. 2020;8:2554-2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Ye Z, Song B. COVID-19 Related Liver Injury: Call for International Consensus. Clin Gastroenterol Hepatol. 2020;18:2848-2851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 7. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 560] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 9. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 10. | Chinese Digestion Association; Chinese Medical Doctor Association. ; Chinese Society of Hepatology, Chinese Medical Association. [The protocol for prevention, diagnosis and treatment of liver injury in coronavirus disease 2019]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 11. | Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112:18-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 789] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 12. | Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, Hall R, Harrower U, Hudson M, Langford A, Mackie A, Mitchell-Thain R, Sennett K, Sheron NC, Verne J, Walmsley M, Yeoman A. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67:6-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 361] [Article Influence: 45.1] [Reference Citation Analysis (1)] |
| 13. | Bin Arif T, Khalid S, Siddiqui MS, Hussain H, Sohail H. Incidence, patterns, risk factors, and histopathological findings of liver injury in coronavirus disease 2019 (COVID-19): a scoping review. Hong Kong Med J. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, Liang JN, Zhu P, Zhu W, Li Y, Zhang BH, Feng H, Zhang WG, Yin ZY, Yu WK, Yang Y, Zhang HQ, Tang ZP, Wang H, Hu JB, Liu JH, Yin P, Chen XP, Zhang B; Tongji Multidisciplinary Team for Treating COVID-19 (TTTC). Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 15. | Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 663] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 16. | Huang J, Hume AJ, Abo KM, Werder RB, Villacorta-Martin C, Alysandratos KD, Beermann ML, Simone-Roach C, Lindstrom-Vautrin J, Olejnik J, Suder EL, Bullitt E, Hinds A, Sharma A, Bosmann M, Wang R, Hawkins F, Burks EJ, Saeed M, Wilson AA, Mühlberger E, Kotton DN. SARS-CoV-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response. Cell Stem Cell 2020; 27: 962-973. e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 257] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 17. | Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1334] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 18. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14355] [Article Influence: 2392.5] [Reference Citation Analysis (10)] |
| 19. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14593] [Article Influence: 2432.2] [Reference Citation Analysis (3)] |
| 20. | Han T, Kang J, Li G, Ge J, Gu J. Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann Transl Med. 2020;8:1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. 2020 Preprint. Available from: bioRxiv:2020.02.03.931766. [DOI] [Full Text] |
| 22. | Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 769] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 23. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30487] [Article Influence: 5081.2] [Reference Citation Analysis (13)] |
| 24. | Strollo R, Pozzilli P. DPP4 inhibition: Preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab Res Rev. 2020;36:e3330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 25. | Li X, Zhang ZC, Zhang PL. Severe COVID-19 patients with liver injury: a seven-case series. Eur Rev Med Pharmacol Sci. 2020;24:7855-7860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1858] [Cited by in RCA: 1990] [Article Influence: 331.7] [Reference Citation Analysis (0)] |
| 27. | Zhao Y, Zhou J, Pan L, Zhang Y, Wang H, Wu W, He J, Chen J, Huang H. Detection and analysis of clinical features of patients with different types of coronavirus disease 2019. J Med Virol. 2021;93:401-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Skevaki C, Fragkou PC, Cheng C, Xie M, Renz H. Laboratory characteristics of patients infected with the novel SARS-CoV-2 virus. J Infect. 2020;81:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Huang W, Li C, Wang Z, Wang H, Zhou N, Jiang J, Ni L, Zhang XA, Wang DW. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci China Life Sci. 2020;63:1678-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Umair M, Mushtaq K, Alkaabi SR. Acute-on-Chronic Liver Failure: Possibly the Main Culprit of Increased Mortality in COVID-19 Patients with Liver Disease. Gastroenterology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1307] [Article Influence: 217.8] [Reference Citation Analysis (8)] |
| 32. | Ming Y, Yun Y, Yong G. The clinical significance of COVID-19 Diagnosis and Treatment Guidelines (Interim version 8). Redaibing Yu Jishengchongxue. 2020;18:243-246. [DOI] [Full Text] |
| 33. | Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, Scarlata S, Agrò FE. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288:192-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 746] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 34. | Sun LN, Zhang XX. Hepatotoxicity of antiviral drugs. Ganzang Zazhi. 2012;17:350-353. [DOI] [Full Text] |
| 35. | Ho TC, Wang YH, Chen YL, Tsai WC, Lee CH, Chuang KP, Chen YA, Yuan CH, Ho SY, Yang MH, Tyan YC. Chloroquine and Hydroxychloroquine: Efficacy in the Treatment of the COVID-19. Pathogens. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Doyno C, Sobieraj DM, Baker WL. Toxicity of chloroquine and hydroxychloroquine following therapeutic use or overdose. Clin Toxicol (Phila). 2021;59:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 37. | Pan X, Zhou J, Chen Y, Xie X, Rao C, Liang J, Zhang Y, Peng C. Classification, hepatotoxic mechanisms, and targets of the risk ingredients in traditional Chinese medicine-induced liver injury. Toxicol Lett. 2020;323:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 38. | Razonable RR, Pennington KM, Meehan AM, Wilson JW, Froemming AT, Bennett CE, Marshall AL, Virk A, Carmona EM. A Collaborative Multidisciplinary Approach to the Management of Coronavirus Disease 2019 in the Hospital Setting. Mayo Clin Proc. 2020;95:1467-1481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Ji D, Zhang D, Yang T, Mu J, Zhao P, Xu J, Li C, Cheng G, Wang Y, Chen Z, Qin E, Lau G. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int. 2020;14:701-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Lin Y, Yuan J, Long Q, Hu J, Deng H, Zhao Z, Chen J, Lu M, Huang A. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Wu J, Yu J, Shi X, Li W, Song S, Zhao L, Zhao X, Liu J, Wang D, Liu C, Huang B, Meng Y, Jiang B, Deng Y, Cao H, Li L. Epidemiological and clinical characteristics of 70 cases of coronavirus disease and concomitant hepatitis B virus infection: A multicentre descriptive study. J Viral Hepat. 2021;28:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 42. | Li Y, Li C, Wang J, Zhu C, Zhu L, Ji F, Liu L, Xu T, Zhang B, Xue L, Yan X, Huang R, Wu C. A case series of COVID-19 patients with chronic hepatitis B virus infection. J Med Virol. 2020;92:2785-2791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Somers VK, Kara T, Xie J. Progressive Hypoxia: A Pivotal Pathophysiologic Mechanism of COVID-19 Pneumonia. Mayo Clin Proc. 2020;95:2339-2342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 45. | Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol. 2016;4:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Yu YC, Mao YM, Chen CW, Chen JJ, Chen J, Cong WM, Ding Y, Duan ZP, Fu QC, Guo XY, Hu P, Hu XQ, Jia JD, Lai RT, Li DL, Liu YX, Lu LG, Ma SW, Ma X, Nan YM, Ren H, Shen T, Wang H, Wang JY, Wang TL, Wang XJ, Wei L, Xie Q, Xie W, Yang CQ, Yang DL, Yu YY, Zeng MD, Zhang L, Zhao XY, Zhuang H; Drug-induced Liver Injury (DILI) Study Group; Chinese Society of Hepatology (CSH); Chinese Medical Association (CMA). CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11:221-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 47. | Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2333] [Cited by in RCA: 2090] [Article Influence: 348.3] [Reference Citation Analysis (0)] |
| 48. | Wang X, Wang S, Sun L, Qin G. Prevalence of diabetes mellitus in 2019 novel coronavirus: A meta-analysis. Diabetes Res Clin Pract. 2020;164:108200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Duvignaud A, Lhomme E, Pistone T, Onaisi R, Sitta R, Journot V, Nguyen D, Peiffer-Smadja N, Crémer A, Bouchet S, Darnaud T, Poitrenaud D, Piroth L, Binquet C, Michel JF, Lefèvre B, Lebeaux D, Lebel J, Dupouy J, Roussillon C, Gimbert A, Wittkop L, Thiébaut R, Orne-Gliemann J, Joseph JP, Richert L, Anglaret X, Malvy D; COVERAGE study group. Home Treatment of Older People with Symptomatic SARS-CoV-2 Infection (COVID-19): A structured Summary of a Study Protocol for a Multi-Arm Multi-Stage (MAMS) Randomized Trial to Evaluate the Efficacy and Tolerability of Several Experimental Treatments to Reduce the Risk of Hospitalisation or Death in outpatients aged 65 years or older (COVERAGE trial). Trials. 2020;21:846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Liver Failure and Artificial Liver Group; Chinese Society of Infectious Diseases; Chinese Medical Association. ; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. [Guideline for diagnosis and treatment of liver failure]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (1)] |
| 51. | Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4682] [Cited by in RCA: 4853] [Article Influence: 808.8] [Reference Citation Analysis (0)] |
| 52. | Arabi YM, Asiri AY, Assiri AM, Balkhy HH, Al Bshabshe A, Al Jeraisy M, Mandourah Y, Azzam MHA, Bin Eshaq AM, Al Johani S, Al Harbi S, Jokhdar HAA, Deeb AM, Memish ZA, Jose J, Ghazal S, Al Faraj S, Al Mekhlafi GA, Sherbeeni NM, Elzein FE, Al-Hameed F, Al Saedi A, Alharbi NK, Fowler RA, Hayden FG, Al-Dawood A, Abdelzaher M, Bajhmom W, AlMutairi BM, Hussein MA, Alothman A; Saudi Critical Care Trials Group. Interferon Beta-1b and Lopinavir-Ritonavir for Middle East Respiratory Syndrome. N Engl J Med. 2020;383:1645-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 53. | RECOVERY Collaborative Group. , Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse T, Felton T, Williams J, Faccenda J, Underwood J, Baillie JK, Chappell LC, Faust SN, Jaki T, Jeffery K, Lim WS, Montgomery A, Rowan K, Tarning J, Watson JA, White NJ, Juszczak E, Haynes R, Landray MJ. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383:2030-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 874] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 54. | RECOVERY Collaborative Group. . Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 475] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 55. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5829] [Cited by in RCA: 5229] [Article Influence: 871.5] [Reference Citation Analysis (0)] |
| 56. | Dyer O. Covid-19: Remdesivir has little or no impact on survival, WHO trial shows. BMJ. 2020;371:m4057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 57. | Gul MH, Htun ZM, Shaukat N, Imran M, Khan A. Potential specific therapies in COVID-19. Ther Adv Respir Dis. 2020;14:1753466620926853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 58. | Perotti C, Baldanti F, Bruno R, Del Fante C, Seminari E, Casari S, Percivalle E, Glingani C, Musella V, Belliato M, Garuti M, Meloni F, Frigato M, Di Sabatino A, Klersy C, De Donno G, Franchini M, Covid-Plasma Task Force. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020;105:2834-2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 59. | Pelegrin M, Naranjo-Gomez M, Piechaczyk M. Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents? Trends Microbiol. 2015;23:653-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 60. | Zhang B, Teng W, Zhong H, Cao K, Zhang X. Analysis of related patents on coronavirus monoclonal antibody therapy. Sci Bull. 2020;34:3979-3983. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, Reiser J, Bansal A, Srivastava A, Zhou Y, Finkel D, Green A, Mallappallil M, Faugno AJ, Zhang J, Velez JCQ, Shaefi S, Parikh CR, Charytan DM, Athavale AM, Friedman AN, Redfern RE, Short SAP, Correa S, Pokharel KK, Admon AJ, Donnelly JP, Gershengorn HB, Douin DJ, Semler MW, Hernán MA, Leaf DE; STOP-COVID Investigators. Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19. JAMA Intern Med. 2021;181:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 371] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 62. | Palanques-Pastor T, López-Briz E, Poveda Andrés JL. Involvement of interleukin 6 in SARS-CoV-2 infection: siltuximab as a therapeutic option against COVID-19. Eur J Hosp Pharm. 2020;27:297-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Cortegiani A, Ippolito M, Greco M, Granone V, Protti A, Gregoretti C, Giarratano A, Einav S, Cecconi M. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2021;27:52-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 64. | Hu PF, Xie WF. Corticosteroid therapy in drug-induced liver injury: Pros and cons. J Dig Dis. 2019;20:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 65. | World Health Organization. Corticosteroids for COVID-19. [cited 2 September 2020]. In: World Health Organization [Internet]. Available from: https://apps.who.int/Home/News/Listings of WHO’s response to COVID-19. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Omar AS S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH