Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2394
Peer-review started: December 15, 2020
First decision: December 28, 2020
Revised: January 6, 2021
Accepted: January 28, 2021
Article in press: January 28, 2021
Published online: April 6, 2021
Processing time: 104 Days and 20.9 Hours
Chimeric antigen receptor T cell (CART) therapy has benefited many refractory lymphoma patients, but some patients experience poor effects. Previous studies have shown that programmed cell death protein-1 (PD-1) inhibitors can improve and prolong the therapeutic effect of CAR-T cell treatment.
A 61-year-old male presented with 15-d history of diarrhea and lower-limb edema. A large mass was detected in the pelvis, and pathology indicated non-Hodgkin diffuse large B-cell lymphoma. After three cycles of the R-CHOP chemotherapeutic regimen, the patient showed three subcutaneous nodules under the left armpit and both sides of the cervical spine. Pathological examination of the nodules indicated DLBCL again. The patient was diagnosed with relapsed and refractory diffuse large B-cell lymphoma. We recommended CAR-T cell treatment. Before treatment, the patient’s T cell function and expression of immune detection points were tested. Expression of PD-1 was obviously increased (52.7%) on cluster of differentiation (CD)3+ T cells. The PD-1 inhibitor (3 mg/kg) was infused prior to lymphodepleting chemotherapy with fludarabine and cyclophosphamide. CAR-CD19 T cells of 3 × 106/kg and CAR-CD22 T cells 1 × 106/kg were infused, respectively. The therapeutic effect was significant, and the deoxyribonucleic acid copy numbers of CAR-CD19 T cells and CAR-CD22 T cells were stable. Presently, the patient has been disease-free for more than 12 mo.
This case suggests that the combination of PD-1 inhibitors and CAR-T cells improved therapeutic efficacy in B-cell lymphoma.
Core Tip: The mechanism of early loss of chimeric antigen receptor T (CAR-T) cells may be the depletion of activated T cells due to stimulation of the immune checkpoint pathway [such as programmed cell death protein-1 (PD-1)] of lymphoma cells. Immune checkpoints have a critical role in the immune system. This case suggests that PD-1 expression may affect the therapeutic effect of CAR-T cell therapy, and combination CAR-T cells and a PD-1 inhibitor may be a viable treatment option for relapsed and refractory non-Hodgkin lymphoma.
- Citation: Niu ZY, Sun L, Wen SP, Song ZR, Xing L, Wang Y, Li JQ, Zhang XJ, Wang FX. Programmed cell death protein-1 inhibitor combined with chimeric antigen receptor T cells in the treatment of relapsed refractory non-Hodgkin lymphoma: A case report. World J Clin Cases 2021; 9(10): 2394-2399
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2394.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2394
With the development of new targeted drugs, the remission rate of non-Hodgkin lymphoma (NHL) has significantly improved, but some patients still experience relapse and refractory disease. In addition to targeted therapy, immunotherapy plays a leading role in the treatment of lymphoma. In particular, chimeric antigen receptor T (CART) cell immunotherapy has been beneficial for many refractory lymphoma patients. However, some B-cell lymphoma patients experience poor effects on this therapy. The efficacy of CAR-T cell therapy in patients with relapsed/refractory NHL has been less impressive compared to that in patients with acute lymphoid leukemia[1]. After 12 mo of CAR-T cell therapy, the relapse-free survival rate of patients is reduced to 60%, and the main cause of relapse is the early loss of CAR-T cells. The mechanism of loss may be the depletion of activated T cells due to stimulation of the immune checkpoint pathway [such as programmed cell death protein-1 (PD-1)] of lymphoma cells. Immune checkpoints have a critical role in the immune system. Based on the above theory, we boldly tried a combination of CAR-T cell therapy and a PD-1 inhibitor to treat a patient with relapsed and refractory NHL, which led to satisfactory results[2].
A 61-year-old male patient was admitted to the Gastrointestinal Surgery Department and subsequently transferred to the Hematology Department, Second Hospital of Hebei Medical University. He presented with diarrhea and edema of the lower limbs that had lasted for 15 d.
The patient’s symptoms of diarrhea and edema had started 15 d previously and had worsened over the last 2 d.
The patient had no previous medical history.
The patient had no personal or family history.
The patient’s temperature was 36.6°C, heart rate was 88/bpm, respiratory rate was 18 breaths/min, blood pressure was 110/80 mmHg, and oxygen saturation in room air was 99%. Physical examination of hypogastria found a pelvis mass 15 cm in the maximum dimension. Our first clinical consideration was the possibility of sigmoid colorectal cancer.
After admission, the blood work showed the following: white blood cells, 5.5 × 109/L; hemoglobin, 96 g/L; platelets, 358 × 109/L. His biochemical results were as follows: lactate dehydrogenase, 317 U/L; protein, 54.1 g/L; albumin, 29.5 g/L; β-2 microglobulin, 3.6 mg/L; creatinine, 118 μmol/L. A rectal biopsy of the large mass in the pelvis was performed, and the pathology results indicated non-Hodgkin diffuse large B-cell lymphoma [(DLBCL) non-germinal center B-cell like]. The results of immunohistochemistry revealed the following: BCL-2 (–); BCL-6 (+);CD10 (+); CD20 (+); CD21 (–); CD3 (scattered +); CD30 (–); CD5 (scattered +); CD56 (–); chromogranin A (–); pan-cytokeratin (–); cyclinD1 (–); LCA (+); multiple myeloma 1 (–); paired box gene 5 (+); synaptophysin (–); vimentin (–); Ki-67 (> 80%); EBER (–).
Enhanced pelvic computed tomography (CT) examination revealed a large mass in the pelvis. Positron emission tomography-CT examination suggested that there was a high-metabolism mass in the pelvis; in addition, there were many high-metabolism shadows in the liver, pancreas, kidneys and bones. There were multiple high-metabolism nodules in the subcutaneous region, muscle, and right paracolon ditch. The results of flow cytometry showed that there was no lymphoma cell infiltration in the bone marrow.
The final diagnosis indicated non-Hodgkin DLBCL (non-germinal center B-cell like). According to Ann Arbor staging classification, the lymphoma was categorized as stage IV.
After two sessions of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine and prednisolone) chemotherapy, the tumor disappeared completely. Two months later, the patient showed three subcutaneous nodules under the left armpit and both sides of the cervical spine. Pathological examination of the nodules also indicated DLBCL. The patient then underwent R-CHOP regimen chemotherapy again and received lenalidomide (25 mg/d, orally) for maintenance treatment.
One month later, the nodules were obviously enlarged. The patient was diagnosed with relapsed and refractory DLBCL. We recommended that he should receive anti-CD19 and anti-CD22 CAR-T cell treatment. Before treatment, the patient’s T cell function and expression of immune detection points were tested. The expression of PD-1 was obviously increased (52.7%) on the CD3+ T cells. Therefore, he was given a PD-1 inhibitor before CAR-T cell treatment. The PD-1 inhibitor carrizumab (3 mg/kg) was infused into the patient before fludarabine and cyclophosphamide pretreatment was administered. The numbers of CAR-CD19 T cells and CAR-CD22 T cells were 3 × 106 /kg and 1 × 106/kg, respectively.
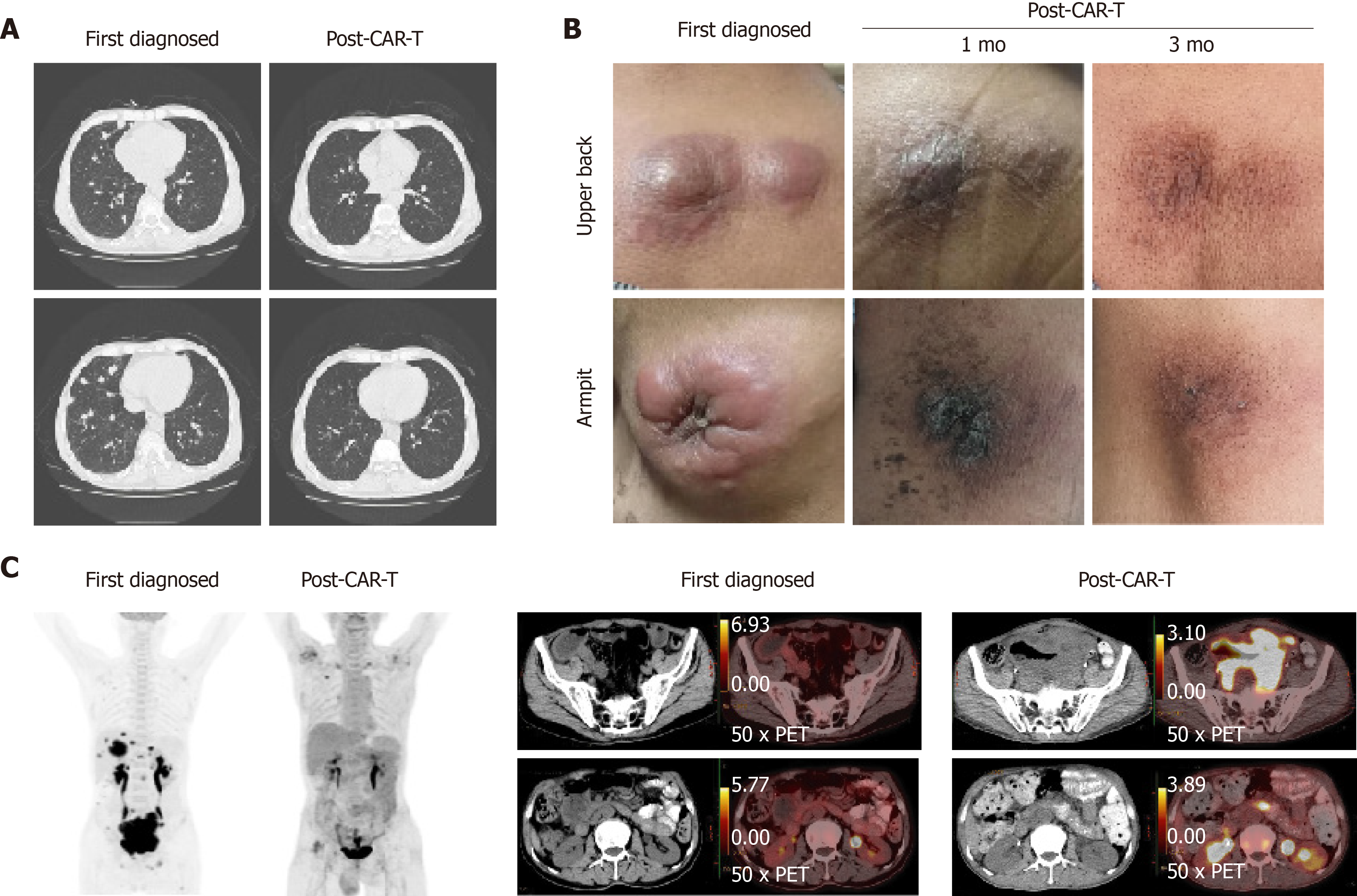
Eight day after CAR-T infusion, the lung CT showed that there were more nodules in both lungs. We considered the pulmonary nodules to be lymphoma. After the infusion of CAR-T cells for 10 d, the deoxyribonucleic acid (DNA) copy numbers of CAR-CD19 T cells and CAR-CD22 T cells were 7.98 × 103/µg and 3.07 × 103/µg, respectively. However, the peripheral blood cell counts decreased significantly, and many hemophagocytes were seen in the bone marrow. This systemic inflammatory response syndrome was related to CAR-T cell treatment, and the patient was treated with dexamethasone for 5 d.
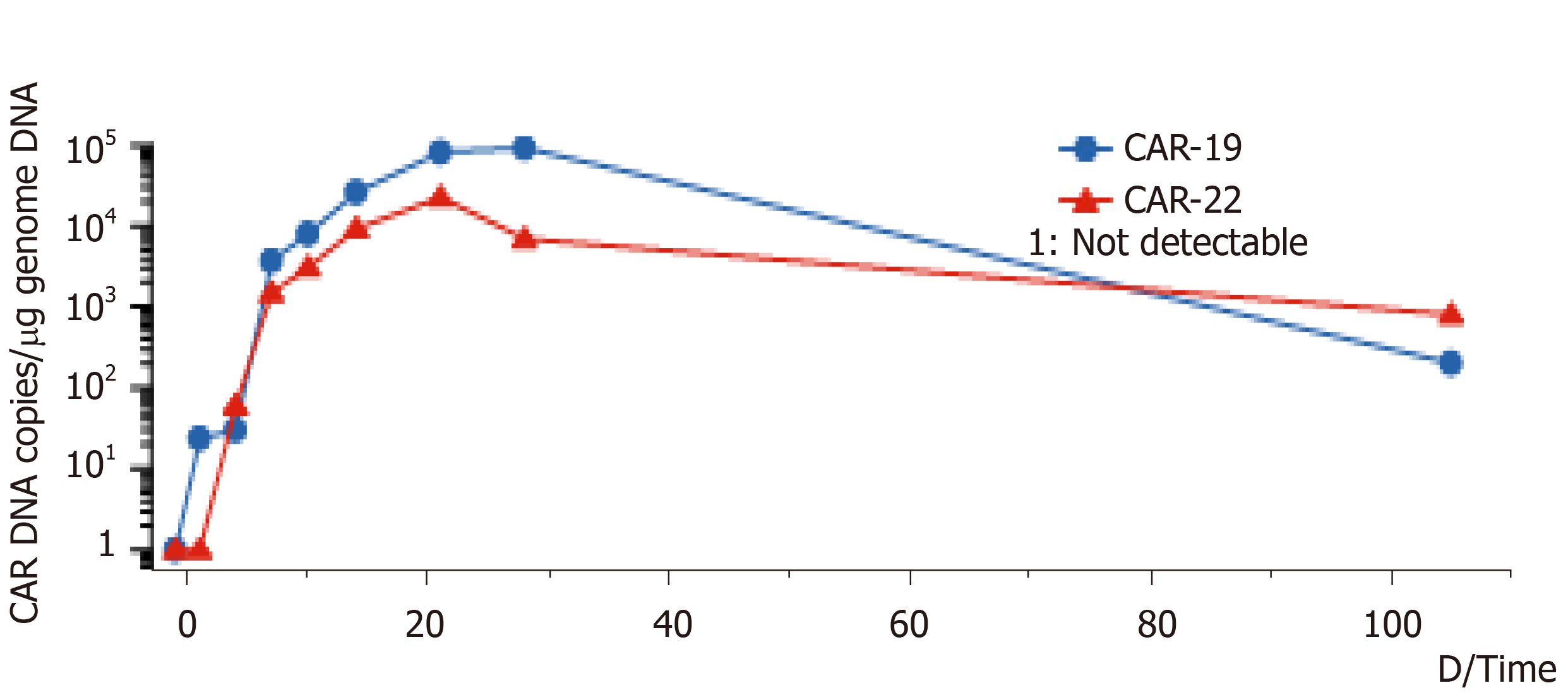
On the 14th d after CAR-T cell treatment, the subcutaneous nodules began to shrink and were smaller. On the 19th d, PD-1 expression decreased to within the normal range (2.04%). On day 20 after combination therapy, the highest copy numbers of anti-CD19 and anti-CD22 CAR DNA detected were 8.13 × 104/µg and 2.26 × 104/µg, respectively. The changes in DNA copy numbers of CAR-CD19 T and CAR-CD22 T cells for 2 mo are shown in Figure 1. On the 21st day after initiating combination therapy, the results of lung CT showed that the multiple nodules in the two lungs had reduced in size (Figure 2A) and that the peripheral blood cell counts returned to normal levels. After 28 d of combination therapy, the skin nodules were reduced, and had disappeared by the 3rd mo (Figure 2B). In the 5th month, the DNA copy numbers of CAR-CD19 T cells and CAR-CD22 T cells were 1.82 × 102/µg and 1.92 × 103/µg, respectively. The patient’s positron emission tomography-CT results at this time showed complete metabolic remission of the lymphoma (Figure 2C). At present, the patient has been disease-free for more than 12 mo.
Emerging evidence has identified many types of inhibitory molecules, including immunosuppressive cells and cytokines, in the microenvironment of tumors; autoimmune inhibition may inhibit the activity of CAR-T cells and weaken its therapeutic effect[3]. Through the active interaction between checkpoint molecules and ligands, inhibitory signals can escape immune surveillance, leading to T cell failure and tumor tolerance[4]. The expression of PD-1 in CAR-T cells is significantly up-regulated, which reduces the level of anti-tumor immune response and allows tumor cells to escape the immune system[5]. In vitro experiments have shown that blocking the PD-1/PD-1 ligand (PD-L1) pathway not only increases the number of T cells but also enhances the anti-tumor effect of T cells by changing the tumor inhibitory microenvironment[6]. Previous studies have shown that PD-1 inhibitors can improve and prolong the therapeutic effect of CAR-T cells treatment[7]. Blocking the signal transduction between PD-L1 and PD-1 can improve the function of CAR-T cells and make their effect longer lasting[8]. CAR-T cell therapy combined with PD-1 inhibitors or cytokine inhibitors can improve the overall or local immune environment, which can significantly enhance the overall anti-tumor effect[9-11].
Clinical research has proven that combination CAR-T cell therapy and PD-1 checkpoint blocker is a very effective treatment for solid tumors, and this combination has yielded good results in the treatment of hematologic tumors[12,13]. Another way to combine CAR-T cell therapy with an immunosuppressant is genetic engineering. T cells expressing the single-chain variable fragment of PD-1 antibody can block the interaction between immune cell PD-1 and tumor cell PD-L1, thus negating the immunosuppression[14]. Therefore, using genetic engineering to knock out the PD-1 gene or "graft" PD-1 antibody into CAR-T cells is one direction for new research in CAR-T cell therapies[10].
Our research has confirmed once again that PD-1 blockade plays a key role in the regulation of T cell-mediated anti-tumor therapy. The combination not only improved the therapeutic efficacy in B-cell lymphoma but also provided a novel treatment option for relapsed/refractory instances of the disease. In addition to PD-1, there are other immune checkpoints on lymphoma cells. Whether blocking them can also improve the efficacy of CAR-T cell therapy remains to be determined.
In summary, the effect of the standard treatment regimen in this patient with relapsed/refractory B-cell lymphoma was poor. Before CART cell therapy, we detected a high expression of PD-1 in the T cells of the patient. We predicted that PD-1 expression may affect the therapeutic effect of CAR-T cell therapy and chose to combine it with a PD-1 inhibitor. This method can solve the problems of poor efficacy of CAR-T cells and the short-term efficacy of PD-1 inhibitors as well as improve the therapeutic effect of CAR-T cell therapy. The combination not only improved the therapeutic efficacy in B-cell lymphoma but also provided the basis for a new treatment for relapsed/refractory instances of the disease.
| 1. | Zhang R, Deng Q, Jiang YY, Zhu HB, Wang J, Zhao MF. Effect and changes in PD1 expression of CD19 CART cells from T cells highly expressing PD1 combined with reduceddose PD1 inhibitor. Oncol Rep. 2019;41:3455-3463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Song W, Zhang M. Use of CAR-T cell therapy, PD-1 blockade, and their combination for the treatment of hematological malignancies. Clin Immunol. 2020;214:108382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Parihar R, Rivas C, Huynh M, Omer B, Lapteva N, Metelitsa LS, Gottschalk SM, Rooney CM. NK Cells Expressing a Chimeric Activating Receptor Eliminate MDSCs and Rescue Impaired CAR-T Cell Activity against Solid Tumors. Cancer Immunol Res. 2019;7:363-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 4. | Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, Lewis ID, Brenner MK, Brown MP. GD2-specific CAR T Cells Undergo Potent Activation and Deletion Following Antigen Encounter but can be Protected From Activation-induced Cell Death by PD-1 Blockade. Mol Ther. 2016;24:1135-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 5. | McGowan E, Lin Q, Ma G, Yin H, Chen S, Lin Y. PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges. Biomed Pharmacother. 2020;121:109625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 6. | Shi X, Zhang D, Li F, Zhang Z, Wang S, Xuan Y, Ping Y, Zhang Y. Targeting glycosylation of PD-1 to enhance CAR-T cell cytotoxicity. J Hematol Oncol. 2019;12:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Wang H, Kaur G, Sankin AI, Chen F, Guan F, Zang X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J Hematol Oncol. 2019;12:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 8. | Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130-3144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 860] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 9. | Simon B, Harrer DC, Schuler-Thurner B, Schaft N, Schuler G, Dörrie J, Uslu U. The siRNA-mediated downregulation of PD-1 alone or simultaneously with CTLA-4 shows enhanced in vitro CAR-T-cell functionality for further clinical development towards the potential use in immunotherapy of melanoma. Exp Dermatol. 2018;27:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, Ma X, Wei F. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother. 2019;68:365-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (1)] |
| 11. | Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, Liu H, Wu MF, Mei Z, Gee A, Mehta B, Zhang H, Mahmood N, Tashiro H, Heslop HE, Dotti G, Rooney CM, Brenner MK. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol Ther. 2017;25:2214-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 429] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 12. | Chen N, Morello A, Tano Z, Adusumilli PS. CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy. Oncoimmunology. 2017;6:e1273302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Wang J, Deng Q, Jiang YY, Zhang R, Zhu HB, Meng JX, Li YM. CAR-T 19 combined with reduced-dose PD-1 blockade therapy for treatment of refractory follicular lymphoma: A case report. Oncol Lett. 2019;18:4415-4420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 14. | Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, Yan S, Xiang J, Ma X, Seshan VE, Hendrickson RC, Liu C, Brentjens RJ. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36:847-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 657] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bao Y S-Editor: Zhang L L-Editor: Webster JR P-Editor: Xing YX