Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2302
Peer-review started: October 1, 2020
First decision: December 28, 2020
Revised: January 10, 2021
Accepted: January 25, 2021
Article in press: January 25, 2021
Published online: April 6, 2021
Processing time: 180 Days and 3.9 Hours
Chondrosarcoma, a cartilage matrix producing tumor, is the second most commonly observed primary bone tumor after osteosarcoma, accounting for 15% of all chest wall malignancies. We herein report the case of a patient with chondrosarcoma of the sternum and our management of the chest wall defects that presented following radical tumor resection.
A 31-year-old patient presented to our hospital with dull pain and a protruding mass overlying the chest for 3 mo. The presence of nocturnal pain and mass size progression was reported, as were overhead arm elevation-related limitations. Computed tomography showed a focal osteoblastic mass in the sternum with bony exostosis and adjacent soft tissue calcification. Positron emission tomography-computed tomography revealed hypermetabolic activity with a mass located over the upper sternum. Magnetic resonance imaging showed a focal ill-defined bony mass of the sternum with cortical destruction and periosteal reaction. Preoperative biopsy showed a consistent result with chondrosarcoma with immunohistochemical positivity for S100 and focal positivity for IDH-1. The grade II chondrosarcoma diagnosis was confirmed by postoperative pathology. The patient underwent radical tumor resection and chest wall reconstruction with a locking plate and cement spacer. The patient was discharged 1 wk after surgery without any complications. At the 1-year follow-up, there was no local recurrence on imaging. The functional scores, including Constant Score, Nottingham Clavicle Score, and Oxford Shoulder Score, showed the absence of pain in the performance of daily activities or substantial functional disabilities.
The diagnosis of chondrosarcoma must be considered when chest wall tumors are encountered. The surgical reconstructive materials, with a locking plate and cement spacer, used in our study are cost-effective and readily-available for the sternum defect.
Core Tip: Chondrosarcoma is the second most commonly observed primary bone tumor after osteosarcoma, accounting for 15% of all chest wall malignancies. Radical tumor excision is deemed the gold standard treatment. However, reconstruction of chest wall defect following tumor resection remains challenging. Our clinical case presents an innovative surgical procedure in managing chest wall defect. The surgical reconstructive materials including a locking plate and cement spacer are cost-effective and readily-available. We believe this technique, which yielded promising results, may serve as an alternative in cases such as ours.
- Citation: Lin CW, Ho TY, Yeh CW, Chen HT, Chiang IP, Fong YC. Innovative chest wall reconstruction with a locking plate and cement spacer after radical resection of chondrosarcoma in the sternum: A case report. World J Clin Cases 2021; 9(10): 2302-2311
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2302.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2302
Chondrosarcomas are the second most commonly observed primary bone tumors after osteosarcoma, and the majority of these are observed in middle-aged populations[1,2]. The characteristics of chondrosarcoma include slow tumor growth, low-grade disease, extremely low rates of metastasis, and slight male predominance. Primary bone tumors located over the chest wall are rarely observed, and account for less than 1% of all primary bone neoplasms[3,4]. However, 15% of chondrosarcomas are located over the chest wall, with an annual incidence of 0.5 million/year[5,6]. Since chondrosarcomas are resistant to radiotherapy and chemotherapy, wide tumor excision followed by anterior chest wall reconstruction is deemed the gold standard for treatment[7].
Generally, chest wall defects larger than 5 cm require surgical reconstruction for functional and cosmetic purposes[8-10]. Reconstructive procedures are performed with the aim of restoring the stability and rigidity of the thoracic cage, eliminating thoracic dead space, preserving pulmonary function, protecting the major intra-thoracic organs, providing adequate soft tissue coverage, and optimizing the patient’s cosmetic appearance. The reconstruction is considered challenging regarding the proximity of major vital organs and difficulties in finding suitable materials. Previous studies with variable tumors located on the sternum reported various methods of sternal reconstruction, including those performed using a methyl methacrylate Marlex mesh sandwich plate, titanium mesh, titanium plate and stainless steel plate, allograft transplantation, and three-dimensional custom-made prosthesis[4,11-15]. Ideally, the materials used for these purposes must be readily-available, durable, and cost-effective. The lack of flexibility of metal prosthesis could lead to unexpected breakage or potential dislocation if malpositioned. Other issues include inaccurate sizing or restriction of movement after surgery[4]. Allograft transplantation takes the major advantage in the ability to incorporate into native tissue and revascularization. However, bone allograft comes in limited source and bears the risk of disease transmission[10]. Three-dimensional custom-made prosthesis achieves a perfect shape in chest wall reconstruction process but is deemed time-consuming and costly[4].
The advantages of using cement spacer for chest wall reconstruction include low cost and being durable, readily-available, and most importantly moldable based on individual body size. Accordingly, we sought to present the case of a patient who underwent bilateral sternoclavicular joint fixation and cement spacer augmentation following radical tumor resection.
A 31-year-old man presented to our hospital with dull and nocturnal pain. A protruding mass overlying the left side chest wall with progressive growing was noted.
According to the patient’s statement, the mass gradually increased in size over these 3 mo. The pain interfered with the patient’s performance of daily activities. There were no symptoms related to airway compression or respiratory disturbance.
The patient had a past history of left lower lobe lung adenocarcinoma in situ and underwent wedge resection 3 mo ago.
The patient had no family history.
Physical examination revealed tenderness on palpation and the presence of a firm, non-movable, well-defined 5 cm mass. The motion of the left shoulder and overhead arm elevation was interfered by this mass. There was no other palpable mass over the head, neck, or axillary lymph nodes.
The results of the laboratory tests, including those for complete blood count, biochemical profiling, and coagulation function, were all within the normal range.
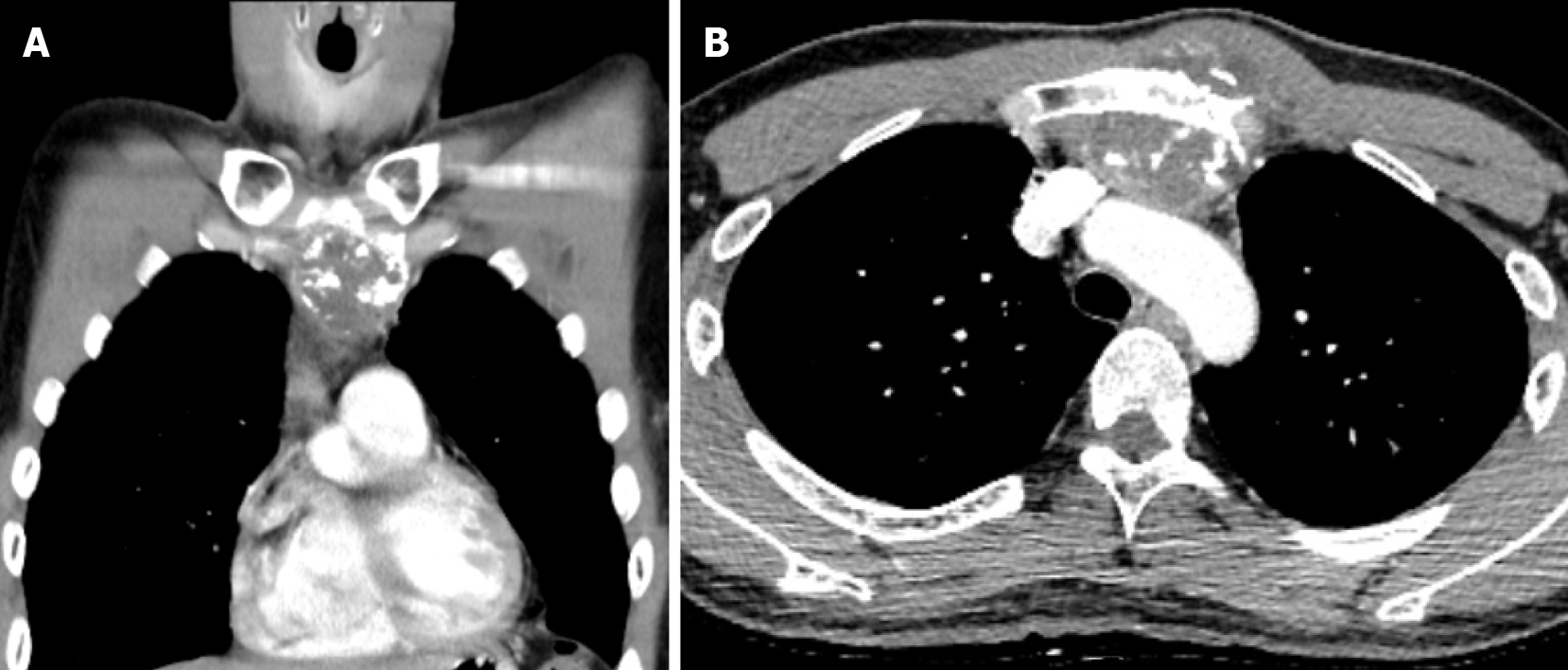
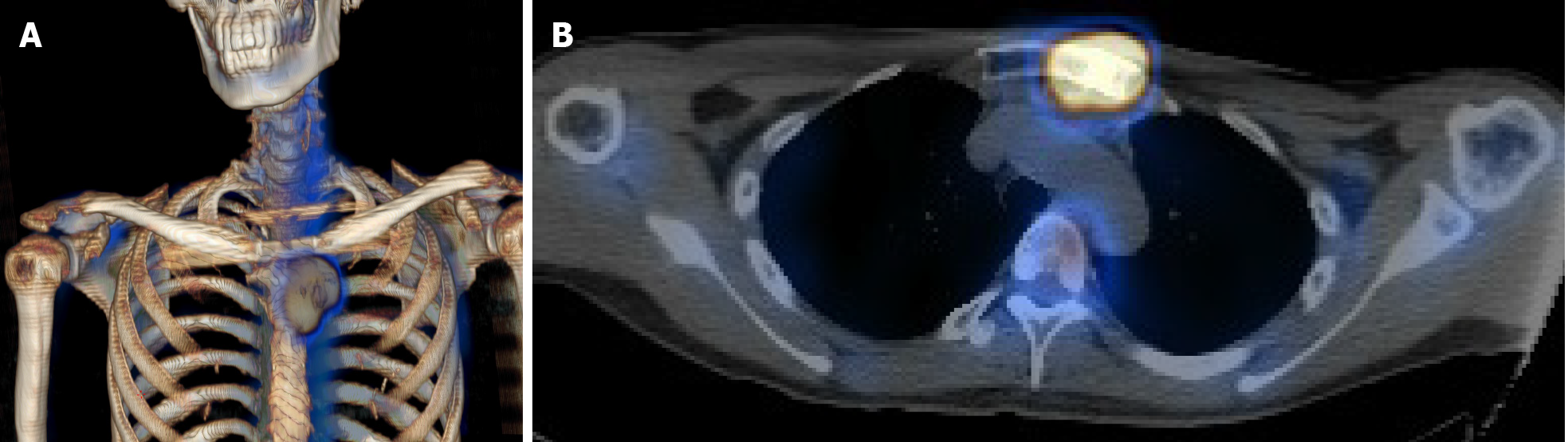
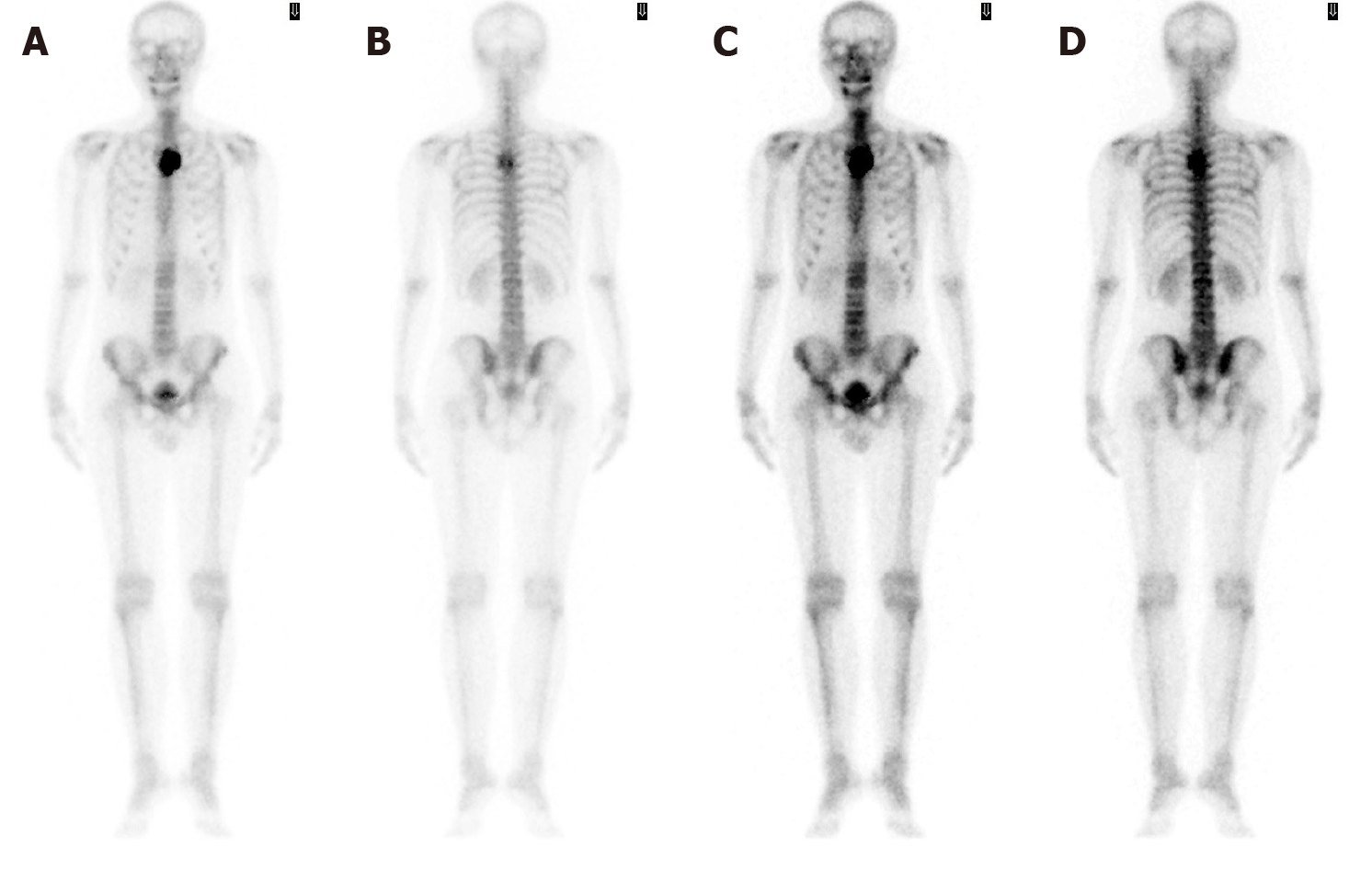
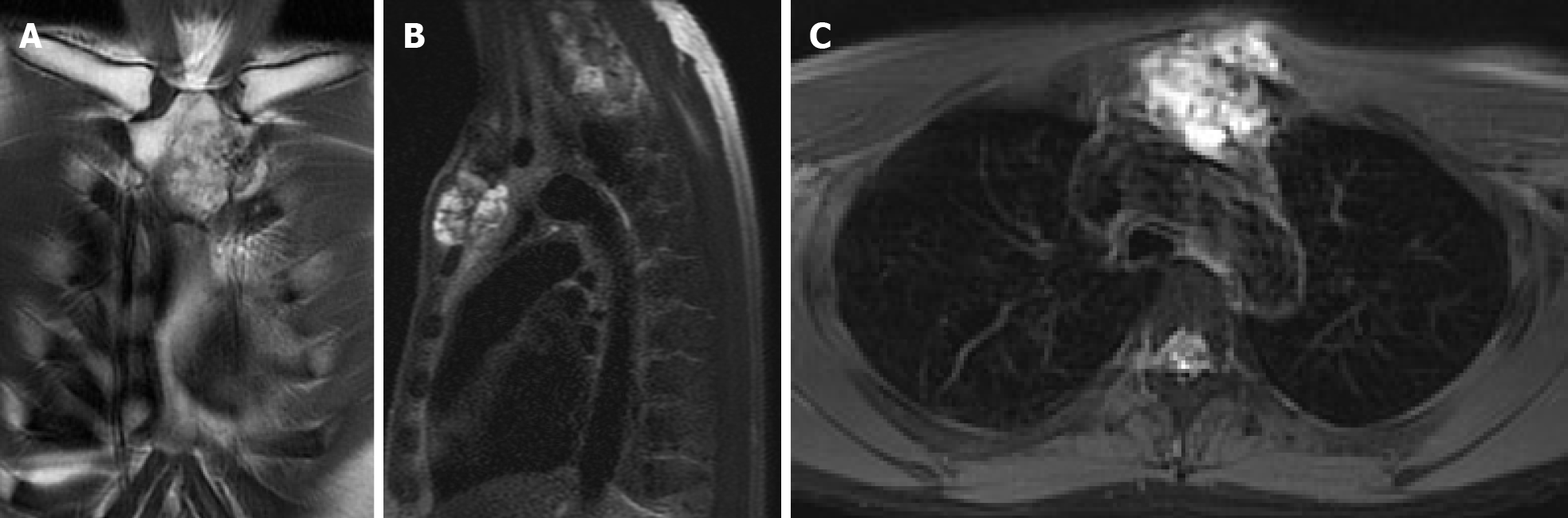
Computed tomography (CT) with contrast showed a focal osteoblastic mass in sternum manubrium region with bony exostosis and adjacent soft tissue calcification (Figure 1). Positron emission tomography-computed tomography (PET-CT) revealed the presence of hypermetabolic activity with a mass located over the upper sternum (Figure 2). Bone scintigraphy also revealed increased focal uptake in the area, indicating a strong suspicion of a primary sternal tumor (Figure 3). Magnetic resonance imaging (MRI) showed a focal ill-defined bony mass of the sternum with cortical destruction and periosteal reaction. The tumor size was approximately 5.0 cm × 4.2 cm × 5.2 cm, with an edge abutting the aortic arch (Figure 4). The presence of malignancies including chondrosarcoma was suspected in the differential diagnosis.
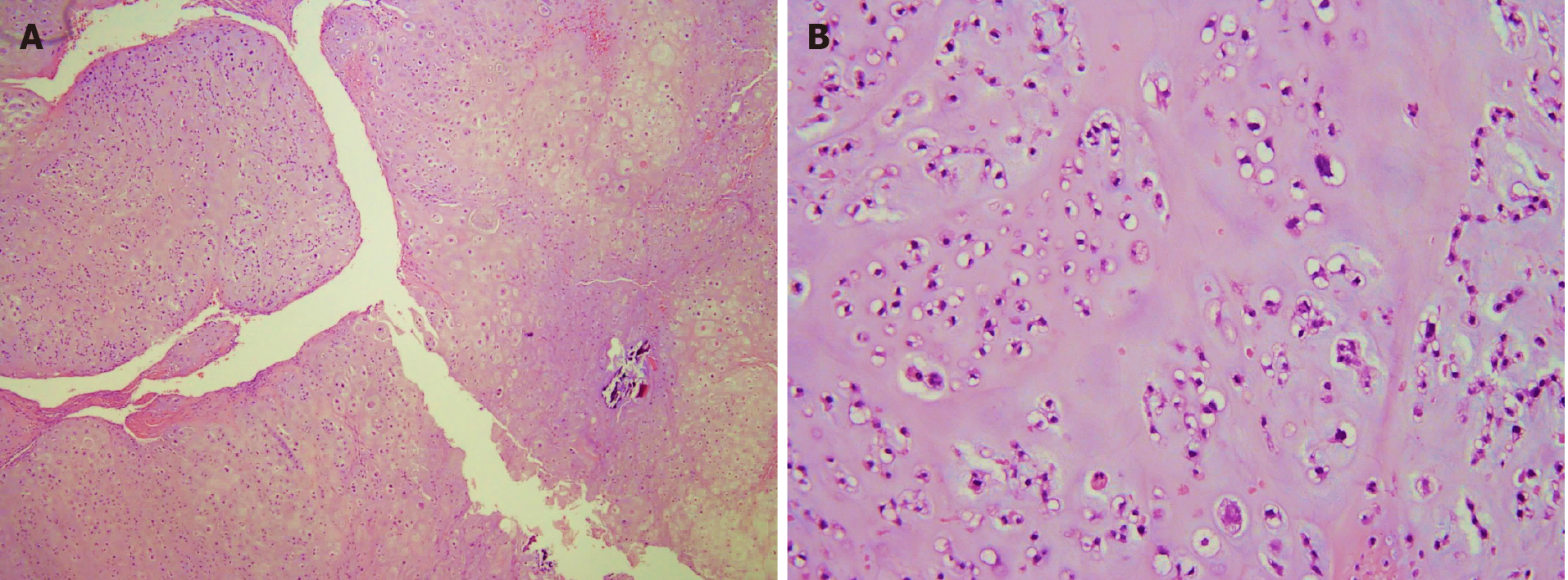
Section through the tumor at the sternum showed hypercellularity and contained groups of neoplastic chondrocytes with extensive chondroid matrix component. The tumor cells had enlarged, hyperchromatic nuclei and occasional binucleation. Some bony fragments were also present. The immunostaining of the tumor cell revealed positivity for S100 and focal positivity for IDH-1. It was consistent with grade II chondrosarcoma.
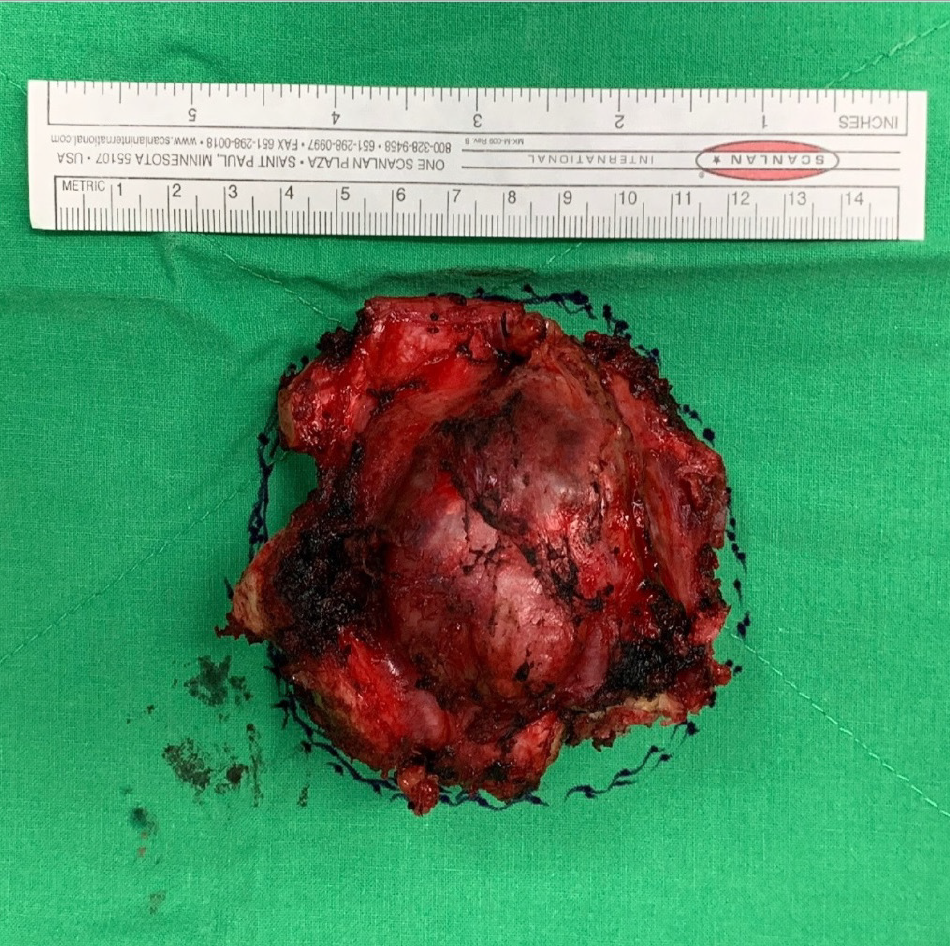
The gross specimen showed a 6.5 cm × 6.0 cm × 5.0 cm gelatinous and elastic to firm tumor mass composed of hyaline cartilage (Figure 5). Pathology suggested grade II moderately differentiated chondrosarcoma with clear and uninvolved margins. Hematoxylin and eosin (HE) staining indicated less cellularity without atypia and a lobulated architecture with abundant cartilaginous matrix separated by the fibrovascular bands. The spread trabecular bone filled the lacunar space, which was surrounded by the hyaline cartilaginous matrix (Figure 6).
Preoperative evaluation and preparation were performed over the first few days following admission. A multidisciplinary tumor board, comprising chest surgeons, orthopedic surgeons, anesthesiologists, and radiologists, was established. After a detailed discussion, the performance of radical resection and chest wall reconstruction, with a goal of complete tumor eradication, was decided on. The advantages and disadvantages associated with the procedure, surgical morbidities, and surgical-related risks were explained clearly to the patient and his family members.
The final diagnosis was grade II moderately differentiated chondrosarcoma.
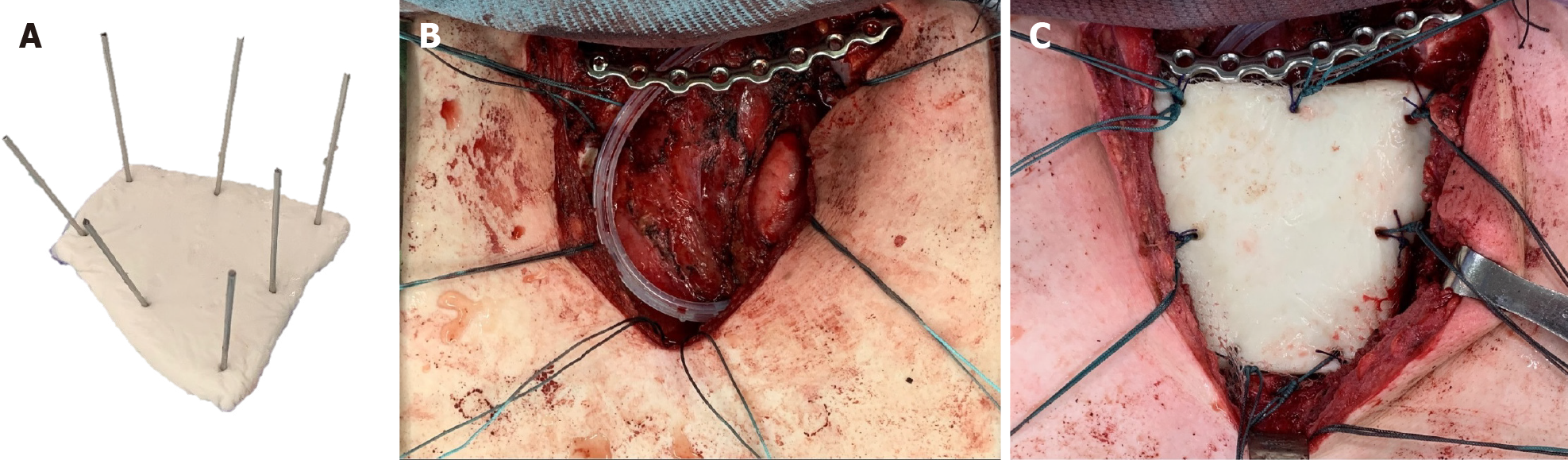
The patient was kept in a supine position and surgery was performed under general anesthesia. After a routine disinfection procedure, a T-shaped incision was made so as to expose the sternum and bilateral sternoclavicular joint. Radical en bloc resection of the medial part of the left clavicle, manubrium, and medial part of the left first and second ribs were performed. Safe margins were confirmed by intraoperative frozen section. Reconstruction began with bilateral clavicle fixation, which was performed using a J-shaped locking plate (DePuy Synthes Comp., Zuchwil, Switzerland) with three screws placed on either side of the clavicle. Then, polymethyl methacrylate (PMMA) medium viscosity bone cement (DePuy Inc., Warsaw, IN, United States) was introduced for sternum reconstruction. The cement curing process was divided into four stages: Mixing, sticky/waiting, working, and hardening[16]. After the defect size was carefully measured, the PMMA was remodeled to meet the shape of the manubrium during the working stage. Kirschner wires (K wires) were inserted into the cement spacer, creating holes for further suture anchoring (Figure 7), following which 1-0 Ethibond sutures (Ethicon, Somerville, NJ, United States) were passed through the pectoralis major fascia, medial portion of the clavicle, the J-shaped locking plate, and sternum body (Figure 7). The polypropylene mesh (Marlex, Bard Cardiosurgery, Billerica, MA, United States) was first placed over the mediastinum for protection, and the cement spacer was then introduced into the desire location. The Ethibond sutures passing through the spacer were tightened, ensuring the stability of the thoracic cage. Eventually, the soft tissue and skin were sutured layer by layer. The estimated blood loss was 150 mL.
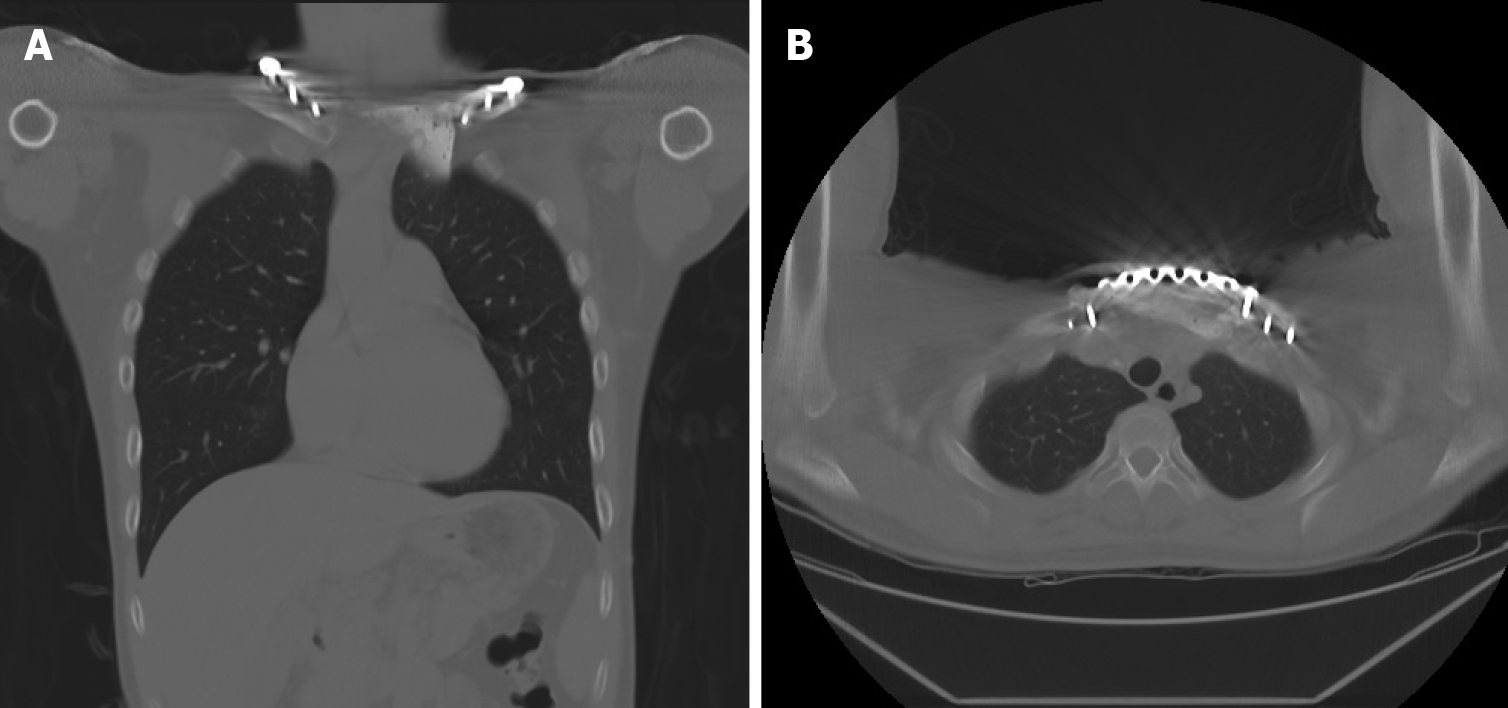
The postoperative course was uneventful, and the patient was discharged 1 wk after surgery without any skin-related or respiratory complications. At the 1-year follow-up, chest radiography showed a solid chest with a good shape. CT revealed the absence of local tumor recurrence and stable fixation of the sternoclavicular joint (Figure 8). There was no evidence of infection, peri-implant fracture, screw loosening, or foreign body rejection. The patient’s functional scores, including the Constant Score (CS), Nottingham Clavicle Score (NCS), and Oxford Shoulder Score (OSS), were well-documented[17]. The CS was 66 before surgery and 85 at the 1-year postoperative follow-up. The NCS improved from 74 to 94, and the OSS showed some advancement, from 44 to 46. The clinical results demonstrated improvements in the patient’s quality of life, with the absence of pain or substantial functional disabilities. Table 1 summarizes the treatment timeline of the patient.
| Complaint/investigations | Details |
| Presenting symptoms | Dull pain and protruding mass for 3 mo; Nocturnal pain; Mass gradually increased in size; Overhead arm elevation limitation |
| CT | Focal osteoblastic change in sternum manubrium region with bony exostosis with adjacent soft tissue calcification mass |
| PET-CT | Mass with hypermetabolic activity over the upper sternum |
| Bone scintigraphy | Focal increased uptake over the upper sternum |
| MRI | Focal ill-defined bony mass of the sternum with cortical destruction and periosteal reaction |
| Biopsy | Hypercellular mass with groups of neoplastic chondrocytes and extensive chondroid matrix component; Tumor cells with enlarged hyperchromatic nuclei and occasional binucleation; Some bony fragments are also present; Immunohistochemical positivity for S100 positive and focal positivity for IDH-1 |
| Surgical intervention | Radical tumor resection followed by chest wall reconstruction with a locking plate and cement spacer; Pathology suggested grade II chondrosarcoma |
| Admission course | The patient was discharged 1 wk after the surgery without complication |
| Postoperative 1-year follow-up | 1-yr follow-up: Improved CS, NSS and OSS; No evidence of local recurrence; Further consecutive follow-up needed |
To the best of our knowledge, our report presents a rare case of chondrosarcoma invading the manubrium and bilateral sternoclavicular joint. The patient underwent tumor resection and chest wall reconstruction using a cement spacer with improved clinical and radiological results.
Chondrosarcomas are hyaline-producing tumors that commonly arise in the axial and proximal appendicular skeleton. They are commonly found in the pelvis, proximal femur, proximal humerus and proximal tibia, but rare cases of chondro-sarcoma in the patella and scapula have also been reported[1,18]. The disease’s clinical presentation may vary depending on the tumor grade. Patients with low-grade chondrosarcoma may present with large tumors before medical assistance is sought, while those with high-grade chondrosarcoma tend to experience worsening pain or pathologic fractures in association with scalloping and cortical destruction[19].
As chondrosarcomas are resistant to chemotherapy and radiotherapy, wide surgical resection is the gold standard for treatment. Local recurrence is more commonly observed than distant metastasis in such settings, while inadequate excision margins are important risk factors for local recurrence development. Tumor histological grade, extracompartmental spread, and local recurrence are important factors that affect the survival associated with chondrosarcoma. The 10-year survival rates were reported to be 89% for grade 1, 53% for grade 2, and 38% for grade 3 chondrosarcoma[20]. Another population-based Scandinavian Sarcoma Group study revealed a 10-year survival rate of 67% for treated patients (92% after wide resection and 47% following intralesional resection)[6].
Chondrosarcoma of the sternum is rarely encountered by orthopedic surgeons. The performance of complete tumor resection without damage to the surrounding major organs and vessels, and reconstruction following tumor resection is challenging. Our study sought to report the preliminary clinical results associated with reconstruction performed using familiar, easily-available, and durable materials. PMMA bone cement is known for its strength against compression and bending, which is vital in the construction of a solid and stable thoracic cage.
Currently, there is no guideline regarding the absolute indication for chest wall reconstruction. However, most previous studies indicated that a chest wall defect larger than 5 cm in size or containing four or more ribs would benefit from chest wall reconstruction[8-10,21]. Several studies have demonstrated various chest wall reconstruction methods in the fields of cardiac and thoracic surgery. Gao et al[13] reported on the use of titanium sternal fixation systems for reconstruction, while Ma et al[4] performed sternal reconstruction with 3-dimensional (3D) custom-made prostheses. Metal prosthesis carries the advantage of establishing adequate chest wall stability, but there are some limitations regarding the use of metal prosthesis including prosthesis dislocation, infection, or inappropriate sizing. The 3D custom made prosthesis provides a reliable option for reconstruction with perfect shape matching but is time consuming, expensive, and not readily-available in every institution. Allograft transplantation is also discussed in the literature with better biological effect in reconstruction but the source is limited and the risk of disease transmission should also be taken into consideration[10].
Some reports also advocate the performance of biological reconstruction using the rectus abdominus flap, omental flap, latissimus dorsi flap, or free flap for soft tissue coverage[22-25]. The advantage of using flap coverage is its ability to incorporate into a patient’s native tissue by revascularization and tissue regeneration. However, there are some surgery-related complications that need to be addressed. Ventral herniation and prolonged ileus were previously found after abdominus flap or omental flap surgery[22]. Shoulder extension endurance and adduction deficits and short-term reductions in the arm strength following latissimus dorsi flap transfer have been noted[23]. Free flap reconstruction, which is associated with a relatively low rate of donor site complications, is technically demanding and requires frequent inspections and anticoagulation therapy administration after surgery[24,25].
The strengths of our reconstruction technique are as follows. It is reliable and provides adequate chest wall stability. In addition, the low cost is better tolerated by the patient. Furthermore, the procedure is not time-consuming and most orthopedic surgeons are familiar with. Finally, cement is readily-available and the moldability also offers the advantage of better size-matching, providing personalized treatment. We believe the technique, which yielded promising results, may serve as an alternative in cases such as ours.
Our study has some limitations. First, the shape of handmade cement spacer may not be superior to 3D custom-made prostheses and this is not a biological reconstruction. Second, the maximal strength of PMMA bone cement against chest wall trauma requires further biomechanical study. Moreover, it is a case report with a 1-year follow-up period. Longer follow-up durations, for the monitoring of possible local recurrence or distal metastasis, are still required. Finally, there is a need for large series with control groups to ensure the reproducibility of our findings, and to confirm the radiological and functional outcomes associated with the technique that we used as well.
Bilateral sternoclavicular joint fixation with a locking plate along with PMMA cement reconstruction of the sternum is a safe and cost-effective method through which chest cavity stabilization can be achieved. The study provides a surgical alternative, from the orthopedic perspective, in the management of chest wall chondrosarcoma, with good clinical functional scores and the absence of local recurrence on imaging.
| 1. | Qiang S, Ma XN, Wang HW, Lv SC. Scapula chondrosarcoma: A case report. Medicine (Baltimore). 2019;98:e15388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Liu S, Zhou X, Song A, Huo Z, Wang Y, Liu Y. Surgical treatment of metastatic mesenchymal chondrosarcoma to the spine: A case report. Medicine (Baltimore). 2020;99:e18643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Liu ZC, Zhao H. Titanium internal fixation system used for sternum reconstruction after resection of chondrosarcoma. Chin Med J (Engl). 2010;123:2621-2622. [PubMed] |
| 4. | Ma XL, Wang DB, Ma JX, Wang Y, Sun L, Lu B, Zhao XW, Li F, Fan ZR, Han B, Bai HH, Yang BC, Jiang X, Tian AX, Dong BC, Du YR. Custom-made Prosthesis for Reconstruction after Radical Resection for Chondrosarcoma of Manubrium. Orthop Surg. 2018;10:272-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Burt M, Fulton M, Wessner-Dunlap S, Karpeh M, Huvos AG, Bains MS, Martini N, McCormack PM, Rusch VW, Ginsberg RJ. Primary bony and cartilaginous sarcomas of chest wall: results of therapy. Ann Thorac Surg. 1992;54:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Widhe B, Bauer HC; Scandinavian Sarcoma Group. Surgical treatment is decisive for outcome in chondrosarcoma of the chest wall: a population-based Scandinavian Sarcoma Group study of 106 patients. J Thorac Cardiovasc Surg. 2009;137:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Lequaglie C, Massone PB, Giudice G, Conti B. Gold standard for sternectomies and plastic reconstructions after resections for primary or secondary sternal neoplasms. Ann Surg Oncol. 2002;9:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Chapelier AR, Missana MC, Couturaud B, Fadel E, Fabre D, Mussot S, Pouillart P, Dartevelle PG. Sternal resection and reconstruction for primary malignant tumors. Ann Thorac Surg. 2004;77:1001-1006; discussion 1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Girotti P, Leo F, Bravi F, Tavecchio L, Spano A, Cortinovis U, Nava M, Pastorino U. The "rib-like" technique for surgical treatment of sternal tumors: lessons learned from 101 consecutive cases. Ann Thorac Surg. 2011;92:1208-1215; discussion 1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Seder CW, Rocco G. Chest wall reconstruction after extended resection. J Thorac Dis. 2016;8:S863-S871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Chaudhry Iu, Alhajji Z, Aldulaijan F, Mutairi H, Amr SS. Manubrioclavicular and Manubriosternal Reconstruction After Radical Resection for Chondrosarcoma of Manubriosternum: A Modified Surgical Technique. Ann Thorac Surg. 2015;99:e137-e139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Aprile V, Korasidis S, Crisci R, Ambrogi MC. Chest wall reconstruction with a novel titanium mesh after partial sternectomy for chondrosarcoma. Interact Cardiovasc Thorac Surg. 2020;30:149-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Gao E, Li Y, Zhao T, Guo X, He W, Wu W, Zhao Y, Yang Y. Reconstruction of anterior chest wall: a clinical analysis. J Cardiothorac Surg. 2018;13:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Chen C, Huang X, Chen M, Yu F, Yin B, Yuan Y. Surgical management of a giant sternal chondromyxoid fibroma: a case report. J Cardiothorac Surg. 2015;10:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Dell'Amore A, Cassanelli N, Dolci G, Stella F. An alternative technique for anterior chest wall reconstruction: the sternal allograft transplantation. Interact Cardiovasc Thorac Surg. 2012;15:944-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Vaishya R, Chauhan M, Vaish A. Bone cement. J Clin Orthop Trauma. 2013;4:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 17. | Charles ER, Kumar V, Blacknall J, Edwards K, Geoghegan JM, Manning PA, Wallace WA. A validation of the Nottingham Clavicle Score: a clavicle, acromioclavicular joint and sternoclavicular joint-specific patient-reported outcome measure. J Shoulder Elbow Surg. 2017;26:1732-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Ye C, Luo Z, Zeng J, Dai M. Chondrosarcoma of the patella: A case report. Medicine (Baltimore). 2017;96:e8049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Riedel RF, Larrier N, Dodd L, Kirsch D, Martinez S, Brigman BE. The clinical management of chondrosarcoma. Curr Treat Options Oncol. 2009;10:94-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Fiorenza F, Abudu A, Grimer RJ, Carter SR, Tillman RM, Ayoub K, Mangham DC, Davies AM. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Mahabir RC, Butler CE. Stabilization of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg. 2011;25:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Daigeler A, Simidjiiska-Belyaeva M, Drücke D, Goertz O, Hirsch T, Soimaru C, Lehnhardt M, Steinau HU. The versatility of the pedicled vertical rectus abdominis myocutaneous flap in oncologic patients. Langenbecks Arch Surg. 2011;396:1271-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Glassey N, Perks GB, McCulley SJ. A prospective assessment of shoulder morbidity and recovery time scales following latissimus dorsi breast reconstruction. Plast Reconstr Surg. 2008;122:1334-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Fischer S, Klinkenberg M, Behr B, Hirsch T, Kremer T, Hernekamp F, Kolbenschlag J, Lehnhardt M, Kneser U, Daigeler A. Comparison of donor-site morbidity and satisfaction between anterolateral thigh and parascapular free flaps in the same patient. J Reconstr Microsurg. 2013;29:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Klinkenberg M, Fischer S, Kremer T, Hernekamp F, Lehnhardt M, Daigeler A. Comparison of anterolateral thigh, lateral arm, and parascapular free flaps with regard to donor-site morbidity and aesthetic and functional outcomes. Plast Reconstr Surg. 2013;131:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Grignani G, Shimada S, Zhu Y S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Yuan YY