Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5618
Peer-review started: April 5, 2020
First decision: September 24, 2020
Revised: October 6, 2020
Accepted: October 19, 2020
Article in press: October 19, 2020
Published online: November 26, 2020
Processing time: 233 Days and 22.5 Hours
Primary myelofibrosis (PMF) is a myeloproliferative neoplasm (MPN) characterized by recurrent mutations in the JAK2, CALR, and MPL genes. The CALR and MPL co-mutation is very rare. To our knowledge, no more than five cases have been reported. Here, we report a case of PMF in which a CALR and MPL co-mutation was detected by next-generation sequencing (NGS) technology, and a literature review was performed.
A 73-year-old woman was admitted to our hospital in 2018 due to abdominal distension. The patient had splenomegaly, lymphadenopathy, leukopenia, anemia, and immature granulocytes in peripheral blood. There were dacrocytes and atypical megakaryocytes in bone marrow, and megakaryocytic proliferation was very active, accompanied by reticulin fibrosis grade 2. By NGS analysis of the bone marrow sample, we detected mutations in CALR, MPL, and PIK3RI, while JAK2 V617F and BCR-ABL were negative. Therefore, the patient was diagnosed with PMF and received oral ruxolitinib. However, the spleen and hematologic responses were poor. We review the literature, analyze previous reports of the mutation sites in our patient and differences between our patient and other reported cases of co-mutated CALR and MPL genes, and discuss the reason why the CALR and MPL co-mutations are rare and possible mechanisms and their impact on the prognosis of patients.
CALR and MPL mutations can be concurrent in MPN, but they are rare. The use of NGS may help to identify more patients with co-mutated CALR and MPL genes. This will help to further explore the mechanism and its impact on these patients to develop appropriate treatment strategies.
Core Tip: We report a rare case of primary myelofibrosis in which a CALR and MPL co-mutation was detected by next-generation sequencing technology. It demonstrated that CALR and MPL mutations can be concurrent in myeloproliferative neoplasm. We review the literature, analyze previous reports of the mutation sites in our patient and differences between our patient and other reported cases of co-mutated CALR and MPL genes, and discuss the reason why the CALR and MPL co-mutations are rare and possible mechanisms and their impact on the prognosis of patients.
- Citation: Zhou FP, Wang CC, Du HP, Cao SB, Zhang J. Primary myelofibrosis with concurrent CALR and MPL mutations: A case report. World J Clin Cases 2020; 8(22): 5618-5624
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5618.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5618
Primary myelofibrosis (PMF) is a myeloproliferative neoplasm (MPN) characterized by recurrent mutations in the JAK2, CALR, and MPL genes. Although these mutations were initially reported to be mutually exclusive in MPN, several studies have reported the presence of JAK2-CALR or JAK2-MPL co-mutations in PMF patients[1-4], and CALR and MPL co-mutation cases are still very rarely reported. With the increasingly wide application of next-generation sequencing (NGS) technology, rare myelofibrosis cases with co-mutated CALR and MPL genes are increasingly being detected. Here, we report a patient with PMF, in which co-mutated CALR and MPL genes were detected.
Abdominal distension for 2 mo.
A 73-year-old woman was admitted to Sir Run Run Shaw Hospital, Zhejiang University School of Medicine in 2018 due to abdominal distension for 2 mo, especially after eating with no complaints of abdominal pain, diarrhea, vomiting, or reduced anal exhaust and defecation.
The patient had a history of a left kidney cyst.
She denied any other specific personal or family history of other diseases.
The patient’s conjunctiva and skin were pale, and the sclera was not yellow. Slightly swollen lymph nodes could be felt in the neck and armpit. The abdomen looked a little distended and felt soft, with no tenderness; the spleen under the ribs could be felt, and the texture was slightly hard. The rest of the abdomen did not have any obvious masses. The bowels sounded normal.
Peripheral blood tests demonstrated a leukocyte count of 2.9 × 109/L, platelet count of 126 × 109/L, and hemoglobin of 87 g/L, and a smear revealed immature granulocytes of 4%. Liver function tests showed normal levels of transaminases, alkaline phosphatase, gamma-glutamyl transferase, total bilirubin, direct bilirubin, indirect bilirubin, serum creatinine, and potassium. Lactate dehydrogenase was 570 IU/L, and total cholesterol was 2.6 mmol/L.
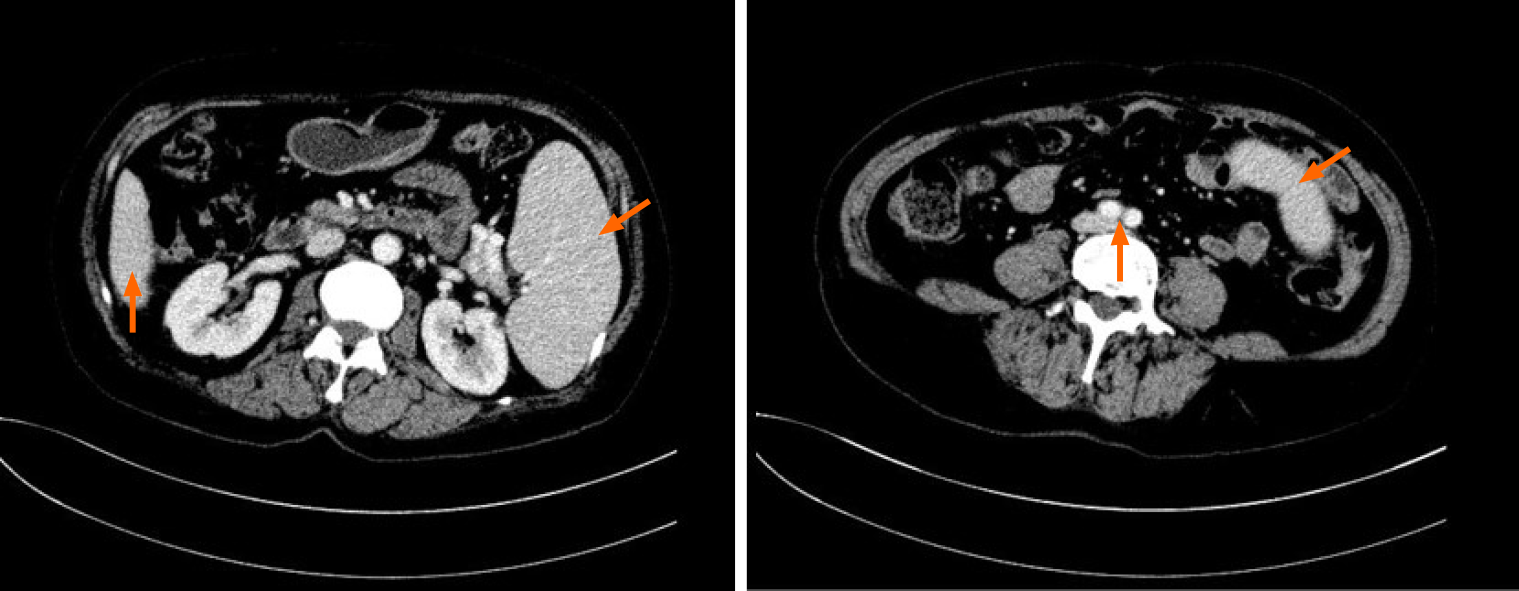
An abdominal computed tomography scan showed that the spleen was enlarged and uniform (Figure 1), with a left kidney cyst. Abdominal ultrasound showed that the spleen was enlarged, with a thickness of 5.6 cm and a length of 16.1 cm.
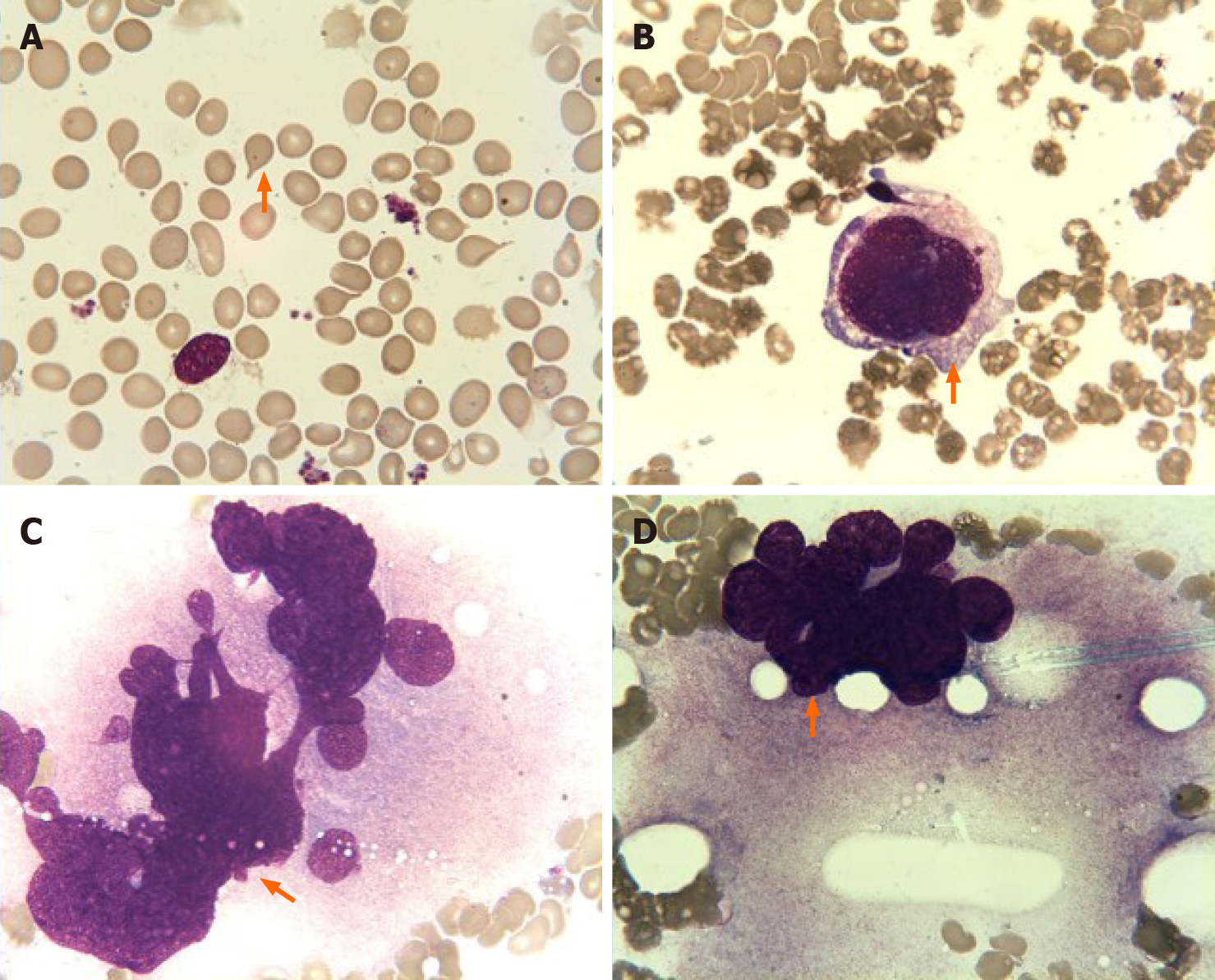
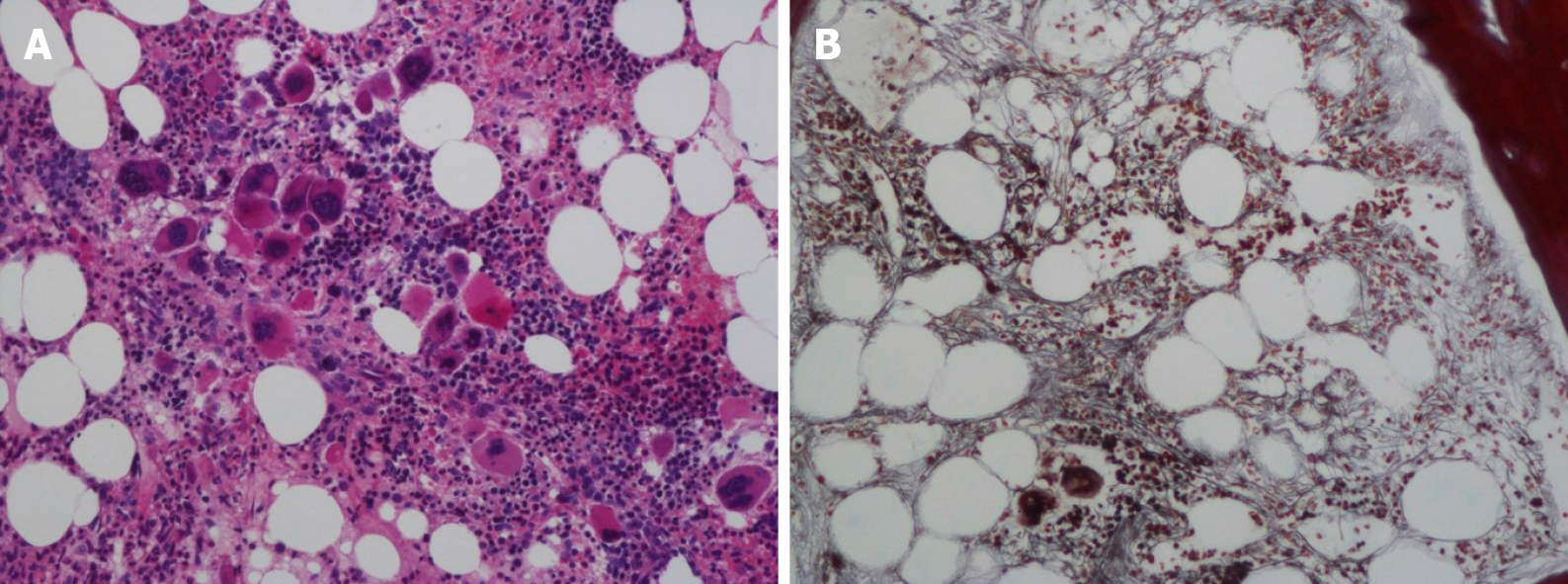
Bone marrow aspiration revealed erythroid and megakaryocytic hyperplasia; dacrocytes were easily observed, and megakaryocytes had atypia (Figure 2). Bone marrow biopsy showed megakaryocytic proliferation and atypia, accompanied by reticulin fibrosis grade 2 (Figure 3).
The bone marrow samples were sequenced by NGS, which showed mutations in CALR, MPL, and PIK3RI (Table 1), while JAK2 V617F and BCR-ABL were negative.
| Gene | VAF | HGVS | Exonic function |
| CALR | 30.04% | NM_004343: c.1092_1143del (p.364fs) | Frameshift deletion |
| MPL | 50.11% | NM_005373: c.1908A>G (p.X636W) | Nonsynonymous SNV |
| PIK3R1 | 3.18% | NM_00181523: c.683delG (p.R228fs) | Frameshift deletion |
The patient was ultimately diagnosed with PMF (International Prognostic Scoring System score = 3, high risk).
The patient received oral ruxolitinib 15 mg twice per day.
Half a year later, the spleen response was poor, and the size of the spleen increased from 16.1 × 5.6 cm to 17.7 × 6.6 cm. Anemia was progressively aggravated in the meantime and developed to blood transfusion dependence, indicating that PMF with co-mutated MPL and CALR genes cannot benefit from ruxolitinib.
Most PMF patients carry JAK2, MPL, or CALR driver mutations[5]. Mutations in MPL and CALR are drivers in the pathogenesis of MPNs, and mutant CALR can bind with MPL to activate the JAK-STAT signaling pathway. Previous studies have shown that JAK2, MPL, and CALR are mutually exclusively mutated in MPN patients[6,7]. However, studies have also reported the presence of JAK2-CALR or JAK2-MPL co-mutations in PMF patients[1-4], and cases of CALR and MPL co-mutations are still very rarely reported. Here, we report the case of a PMF patient with CALR-p.364fs and MPL-p.X636W mutations. The CALR-p.364fs mutation is usually detected in essential thrombocytosis (ET) and PMF patients, while the MPL-p.X636W mutation is not a common mutation and has only been reported in refractory anemia with ring sideroblast patients[8,9]. Furthermore, we also detected a low-burden PIK3R1 mutation in this case. The PIK3R1 gene belongs to the Akt signaling pathway and plays a direct role in regulating cell survival, growth, and differentiation. Mutated PIK3R1 can cause aberrant PI3K signaling, and the p.R228fs mutation site has already been reported in acute lymphocytic leukemia patients[10,11]. To the best of our knowledge, this is the first report of a CALR and MPL double mutant PMF patient from East Asia.
Earlier research reports show that CALR and MPL mutations are mutually exclusive in MPN patients. This case demonstrated that CALR and MPL mutations can coexist in MPN patients. Three MPN cases with co-mutated CALR and MPL genes have already been reported since 2017[12-14]. Partouche et al[12] reported that a post-ET PMF patient carried type I CALR and MPL W515R mutations, with an variant allele frequency (VAF) of 59.1% and 31.7%, respectively. Tashkandi et al[13] reported a post-ET PMF patient carrying a CALR (p.L367Tfs*46) mutation at an allele frequency of 47% and two MPL (p.S505C and p.W515L) mutations at a lower allele frequency of 4%. Bernal et al[14] detected a CALR (p.L367fs*48) mutation at an allele frequency of 30% and a MPL (p.Trip515L) mutation at an allele frequency of 11.75% in an ET patient. In contrast to these reported cases, we identified an uncommon mutation in MPL (p.X636W) in PMF patients, and its VAF was higher than that of the CALR mutation in our patient.
Patients with concurrent CALR and MPL mutations are truly rare in the clinic. In PMF patients, the frequency of CALR (20%-25%) and MPL (7%) gene mutations is low[1,6,7]. Before 2017, there were no case reports about CALR and MPL co-mutations. Currently, a few MPN patients have been detected, which is inseparable from the progress of molecular testing technology. From previous cases, we can see that in MPN patients, a MPL mutation VAF is usually low[2,3,15], and some uncommon mutations exist. Traditional Sanger sequencing may miss these mutations because of its test range and sensitivity[7,13-15]. High-throughput sequencing may cover the full loci, and the detection VAF limit was lowered to 1%. Therefore, MPN patients with concurrent CALR and MPL mutations can be accurately detected.
The MPL mutation may have been a molecular event independent of the CALR deletion or a secondary event in the CALR-containing clone. Some researchers have proven that the CALR gene mutation is an early event during disease development, and MPL is a passenger event[2,13]. It is speculated that the mutated CALR gene can trigger genome instability, which then causes mutations in the MPL gene[13]. This is a possible explanation for why CALR and MPL can co-mutate. However, in our case, the MPL mutation was improbable as a secondary event because its VAF was more than 50%, indicating the possibility of a germline mutation. Some research has already proven that MPN patients can carry germline MPN mutations[16-18]. The co-mutation mechanism is not clear and still needs more investigations.
Gene mutations can predict prognosis in PMF patients. CALR mutations indicate a good prognosis in ET and PMF patients[19,20]. Compared to JAK2 or triple-negative patients, CALR mutation-positive patients have a longer overall survival and low risk of thrombogenesis[1,7,19,21]. Patients who harbor the MPL W515L/K mutation have a moderate prognosis and higher risk of thrombogenesis than CALR mutation-positive patients[19]. The prognosis of patients with MPL and CALR co-mutations is still unknown due to the limited number of patients, but it seems that these patients have more aggressive progression based on our patient and other reported cases[12,13]. Our patient’s disease progression was rapid. In Partouche’s and Tashkandi’s patients, the CALR mutation was a pre-existing clone in the ET stage, and the MPL mutation was acquired when the disease developed into PMF. Disease acceleration may be associated with MPL-mutated clones. Because of the lack of patients with coexisting mutations, further investigations in large cohorts are needed to better quantify the presence and clinical significance.
In conclusion, we have described a rare case of PMF with concurrent CALR and MPL mutations. This demonstrates that CALR and MPL mutations are not mutually exclusive in MPN. The use of NGS may help to identify more patients with CALR and MPL co-mutations. This will help to further explore the mechanism and its impact on these patients to develop appropriate treatment strategies.
| 1. | Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, Maffioli M, Caramazza D, Passamonti F, Pardanani A. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 427] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 2. | Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, Girsberger S, Lehmann T, Passweg J, Stern M, Beisel C, Kralovics R, Skoda RC. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123:2220-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 486] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 3. | McGaffin G, Harper K, Stirling D, McLintock L. JAK2 V617F and CALR mutations are not mutually exclusive; findings from retrospective analysis of a small patient cohort. Br J Haematol. 2014;167:276-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Pardanani A, Guglielmelli P, Lasho TL, Pancrazzi A, Finke CM, Vannucchi AM, Tefferi A. Primary myelofibrosis with or without mutant MPL: comparison of survival and clinical features involving 603 patients. Leukemia. 2011;25:1834-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Takenaka K, Shimoda K, Akashi K. Recent advances in the diagnosis and management of primary myelofibrosis. Korean J Intern Med. 2018;33:679-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NC, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer GI, Bagienski K, Milanesi C, Casetti IC, Sant'Antonio E, Ferretti V, Elena C, Schischlik F, Cleary C, Six M, Schalling M, Schönegger A, Bock C, Malcovati L, Pascutto C, Superti-Furga G, Cazzola M, Kralovics R. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1420] [Cited by in RCA: 1514] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 7. | Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, Avezov E, Li J, Kollmann K, Kent DG, Aziz A, Godfrey AL, Hinton J, Martincorena I, Van Loo P, Jones AV, Guglielmelli P, Tarpey P, Harding HP, Fitzpatrick JD, Goudie CT, Ortmann CA, Loughran SJ, Raine K, Jones DR, Butler AP, Teague JW, O'Meara S, McLaren S, Bianchi M, Silber Y, Dimitropoulou D, Bloxham D, Mudie L, Maddison M, Robinson B, Keohane C, Maclean C, Hill K, Orchard K, Tauro S, Du MQ, Greaves M, Bowen D, Huntly BJP, Harrison CN, Cross NCP, Ron D, Vannucchi AM, Papaemmanuil E, Campbell PJ, Green AR. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1404] [Cited by in RCA: 1429] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 8. | Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, Steensma DP, Elliott MA, Wolanskyj AP, Hogan WJ, McClure RF, Litzow MR, Gilliland DG, Tefferi A. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472-3476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 781] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 9. | Beer PA, Campbell PJ, Scott LM, Bench AJ, Erber WN, Bareford D, Wilkins BS, Reilly JT, Hasselbalch HC, Bowman R, Wheatley K, Buck G, Harrison CN, Green AR. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008;112:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 296] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Gutierrez A, Sanda T, Grebliunaite R, Carracedo A, Salmena L, Ahn Y, Dahlberg S, Neuberg D, Moreau LA, Winter SS, Larson R, Zhang J, Protopopov A, Chin L, Pandolfi PP, Silverman LB, Hunger SP, Sallan SE, Look AT. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (1)] |
| 11. | Andersson AK, Ma J, Wang J, Chen X, Gedman AL, Dang J, Nakitandwe J, Holmfeldt L, Parker M, Easton J, Huether R, Kriwacki R, Rusch M, Wu G, Li Y, Mulder H, Raimondi S, Pounds S, Kang G, Shi L, Becksfort J, Gupta P, Payne-Turner D, Vadodaria B, Boggs K, Yergeau D, Manne J, Song G, Edmonson M, Nagahawatte P, Wei L, Cheng C, Pei D, Sutton R, Venn NC, Chetcuti A, Rush A, Catchpoole D, Heldrup J, Fioretos T, Lu C, Ding L, Pui CH, Shurtleff S, Mullighan CG, Mardis ER, Wilson RK, Gruber TA, Zhang J, Downing JR; St. Jude Children's Research Hospital–Washington University Pediatric Cancer Genome Project. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat Genet. 2015;47:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 401] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 12. | Partouche N, Conejero C, Barathon Q, Moroch J, Tulliez M, Cordonnier C, Giraudier S. Emergence of MPLW515 mutation in a patient with CALR deletion: Evidence of secondary acquisition of MPL mutation in the CALR clone. Hematol Oncol. 2018;36:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Tashkandi H, Moore EM, Tomlinson B, Goebel T, Sadri N. Co-occurrence of type I CALR and two MPL mutations in patient with primary myelofibrosis. Ann Hematol. 2017;96:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Bernal M, Jiménez P, Puerta J, Ruíz-Cabello F, Jurado M. Co-mutated CALR and MPL driver genes in a patient with myeloproliferative neoplasm. Ann Hematol. 2017;96:1399-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Boddu P, Chihara D, Masarova L, Pemmaraju N, Patel KP, Verstovsek S. The co-occurrence of driver mutations in chronic myeloproliferative neoplasms. Ann Hematol. 2018;97:2071-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129:667-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 472] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 17. | Ding J, Komatsu H, Iida S, Yano H, Kusumoto S, Inagaki A, Mori F, Ri M, Ito A, Wakita A, Ishida T, Nitta M, Ueda R. The Asn505 mutation of the c-MPL gene, which causes familial essential thrombocythemia, induces autonomous homodimerization of the c-Mpl protein due to strong amino acid polarity. Blood. 2009;114:3325-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Lombardi AM, Ferrari S, Barzon I, Navaglia F, Fabris F, Vianello F. A novel germ-line mutation of c-mpl gene in a sporadic case of essential thrombocythemia. Blood Cells Mol Dis. 2017;64:51-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martínez-Trillos A, Casetti I, Colomer D, Pieri L, Pratcorona M, Rotunno G, Sant'Antonio E, Bellini M, Cavalloni C, Mannarelli C, Milanesi C, Boveri E, Ferretti V, Astori C, Rosti V, Cervantes F, Barosi G, Vannucchi AM, Cazzola M; Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014;124:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (2)] |
| 20. | Palandri F, Latagliata R, Polverelli N, Tieghi A, Crugnola M, Martino B, Perricone M, Breccia M, Ottaviani E, Testoni N, Merli F, Aversa F, Alimena G, Cavo M, Martinelli G, Catani L, Baccarani M, Vianelli N. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia. 2015;29:1344-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, Gangat N, Fjerza R, Belachew AA, Lasho TL, Ketterling RP, Hanson CA, Rambaldi A, Finazzi G, Thiele J, Barbui T, Pardanani A, Vannucchi AM. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 554] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Barosi G, Tomizawa M S-Editor: Chen XF L-Editor: Wang TQ P-Editor: Liu JH