Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4958
Peer-review started: March 31, 2020
First decision: August 23, 2020
Revised: August 29, 2020
Accepted: September 10, 2020
Article in press: September 10, 2020
Published online: October 26, 2020
Processing time: 208 Days and 21.5 Hours
Amiodarone is the drug most commonly used to manage arrhythmias. Long-term amiodarone administration causes hepatotoxicity due to iodine accumulation in the liver. Here, we present three cases of amiodarone-induced hepatotoxicity in patients on long-term oral amiodarone therapy who underwent dual-energy computed tomography (DECT).
We report the clinical and iodine density in the liver using DECT in three patients with amiodarone-induced hepatotoxicity. Liver enzymes were increased in these three patients, and abdominal DECT without contrast medium showed highly increased attenuation in the liver. Furthermore, the iodine concentration in the liver was increased. The first patient with amiodarone-induced reversible hepatotoxicity, showed a reversible course of liver function and a decrease in CT values after discontinuation of amiodarone. The second patient on long-term oral amiodarone had increased iodine concentration in the liver and liver damage, the patient eventually developed rapidly progressive pneumonia and died of multiple organ failure. The third patient, showed an increased iodine concentration in the liver and elevated liver enzymes. However, the patient refused radiofrequency ablation for atrial fibrillation and continued oral amiodarone to control atrial fibrillation, and routine liver function tests were required every 3-6 mo in this patient.
DECT is a potentially noninvasive diagnostic tool for quantifying iodine concentration in the liver and monitoring adverse reactions due to amiodarone.
Core Tip: Amiodarone is the drug most commonly used to manage arrhythmias. Long-term amiodarone administration causes hepatotoxicity due to iodine accumulation in the liver. Our study investigated three patients receiving low-dose oral amiodarone therapy for the long-term prevention of paroxysmal atrial fibrillation in whom quantitative measurement of the iodine concentration in the liver was measured using dual-energy computed tomography. Dual-energy computed tomography is a potentially noninvasive diagnostic tool for quantifying iodine concentration in the liver and monitoring adverse reactions due to amiodarone.
- Citation: Lv HJ, Zhao HW. Amiodarone-induced hepatotoxicity — quantitative measurement of iodine density in the liver using dual-energy computed tomography: Three case reports. World J Clin Cases 2020; 8(20): 4958-4965
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4958.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4958
Amiodarone is the drug most commonly used to manage arrhythmias[1]. Amiodarone has a long list of potential adverse effects. Hepatotoxicity and elevated transaminase levels are commonly observed with long-term amiodarone use and occur in 15% to 50% of patients[2]. Increases in liver density on computed tomography (CT) are also seen with long-term usage of amiodarone[3]. To the best of our knowledge, the measurement of iodine concentrations in patients with amiodarone-induced liver damage has not previously been reported. Here, we present three cases in which the quantitative measurement of iodine concentration in the liver was performed using dual-energy computed tomography (DECT) in patients receiving low-dose oral amiodarone therapy for the long-term prevention of paroxysmal atrial fibrillation.
Case 1: A 66-year-old male was admitted to the hospital with a 2-wk history of episodic palpitations.
Case 2: An 85-year-old male presented with a 4-wk history of progressively worsening dyspnea on exertion and fatigue.
Case 3: A 71-year-old female was admitted to our hospital due to worsening palpitations.
Case 1: The patient was admitted to hospital with a 2-wk history of episodic palpitations. He noted that each episode was abrupt in onset and lasted approximately 2 h to 1 d before gradually abating.
Case 2: The patient presented with a 4-wk history of progressively worsening dyspnea on exertion and fatigue. He also suffered from a dry cough at night. He denied fevers, chills, night sweats, and chest pain.
Case 3: The patient had episodic palpitations for years, which worsened in the previous 3 d. She denied dyspnea and chest pain.
Case 1: The patient had been diagnosed with paroxysmal atrial fibrillation when he was 56 years old. Since then, he had been treated with amiodarone (200 mg/d) for 10 years. He had no apparent history of liver disease. He reported no nausea, vomiting, or diarrhea. He had no history of smoking-related chronic obstructive pulmonary disease.
Case 2: The patient’s medical history included hypertension and atrial fibrillation, for which he took a thiazide diuretic and amiodarone (200 mg/d). The antiarrhythmic drug had been started 6 years earlier for rhythm control.
Case 3: The patient’s past medical history revealed atrial fibrillation for approximately 6 years that was managed with amiodarone. She used 200 mg of amiodarone in tablet form per day.
Case 1: He had no abnormal findings on abdominal examination.
Case 2: His pulmonary exam revealed significant bibasilar inspiratory crackles. His abdominal examination was unremarkable.
Case 3: She presented with a blood pressure of 145/88 mmHg and a regular pulse of 62 bpm. Holter monitoring confirmed the presence of paroxysmal atrial fibrillation but no signs of ischemic injury. During her examination, she reported decreased appetite without significant weight loss. There were no abnormal findings on abdominal examination.
Case 1: Direct bilirubin, 11.2 μmol/L (reference range, 0-4 μmol/L); γ-glutamyl transpeptidase, 85 U/L (reference range, 10-60 U/L); alanine aminotransferase, 127 IU/L (reference range, 9-50 IU/L); aspartate aminotransferase, 101 IU/L (reference range, 15-40 IU/L); and γ-glutamyl transferase, 1080 IU/L (reference range, 120-250 IU/L). Elevations of these enzymes indicated liver damage and hepatotoxicity.
Case 2: Direct bilirubin, 9.3 μmol/L (reference range, 0-4 μmol/L); γ-glutamyl transpeptidase, 112 U/L (reference range, 10-60 U/L); alanine aminotransferase, 144 IU/L (reference range, 9-50 IU/L); and aspartate aminotransferase, 240 IU/L (reference range, 15-40 IU/L). Elevations of these enzymes indicated drug-induced liver damage.
Case 3: Direct bilirubin, 3.3 μmol/L (reference range, 0-4 μmol/L); γ-glutamyl transpeptidase, 58 U/L (reference range, 10-60 U/L); alanine aminotransferase, 112 IU/L (reference range, 9-50 IU/L), and aspartate aminotransferase, 50 IU/L (reference range, 15-40 IU/L).
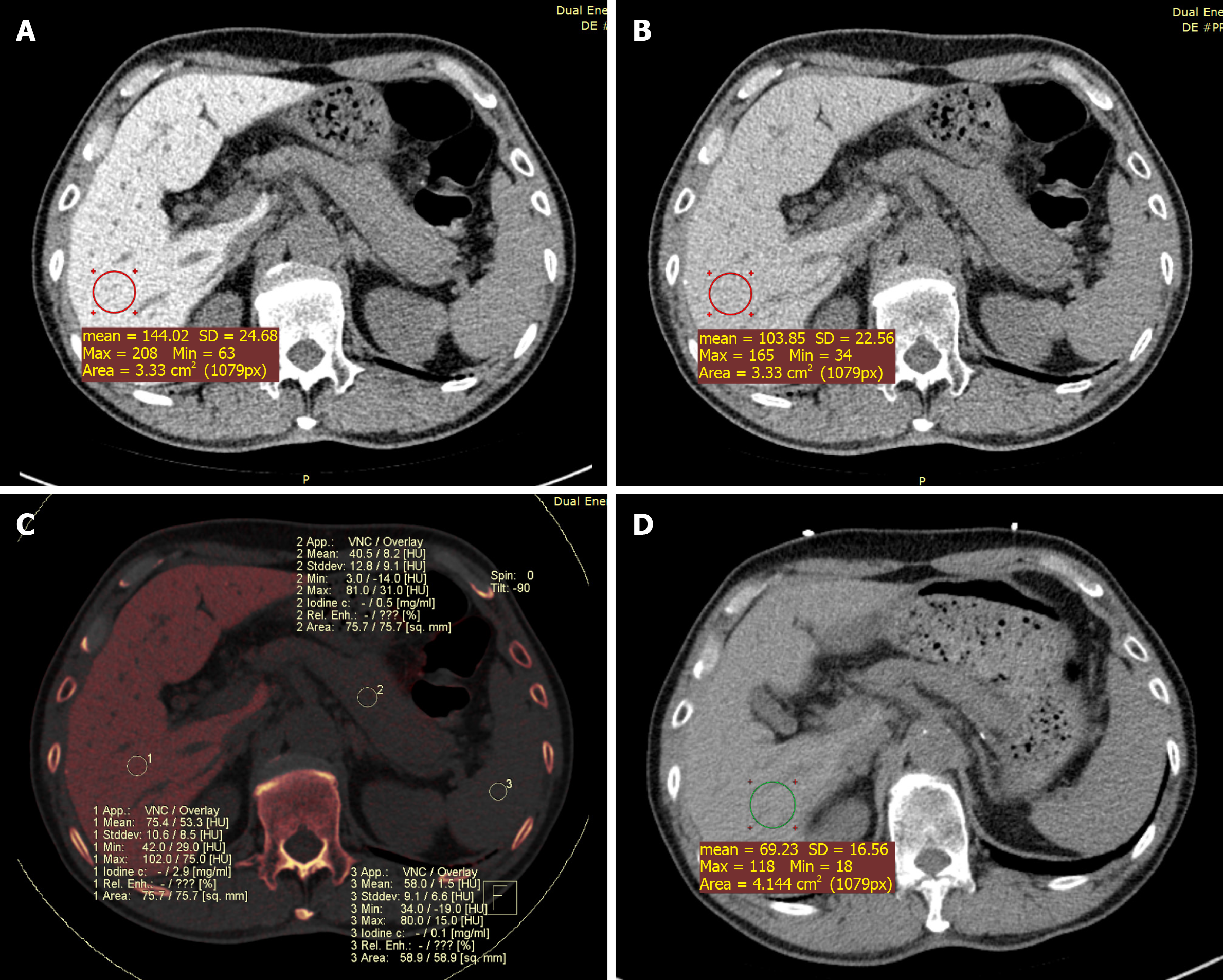
Case 1: Due to abnormal liver enzyme levels, a plain abdominal CT scan was performed which revealed increased liver density. We measured the Hounsfield unit (HU) values in four different locations of the liver. Regions of interest were manually delineated on the liver parenchyma, avoiding major vessels, bile ducts, and liver edges. The average CT value of the liver at 120 kVp was 118 HU (the normal range for the liver is 55-70 HU).
An additional DECT scan was performed to assess the status of amiodarone-induced iodine accumulation in the liver. The CT values of the liver were 144 HU and 104 HU at 100 kVp and 140 kVp (Figure 1A and B), respectively. No additional lesions were found. In the fused iodine image, an increased iodine concentration in the liver was observed relative to the iodine concentrations in the spleen and pancreas. The measured iodine concentration in the liver was 2.9 mg/mL (Figure 1C). In the VNC image, the liver density was normal (68 HU). The imaging findings were compatible with the diagnosis of intrahepatic iodine deposition associated with hepatic dysfunction.
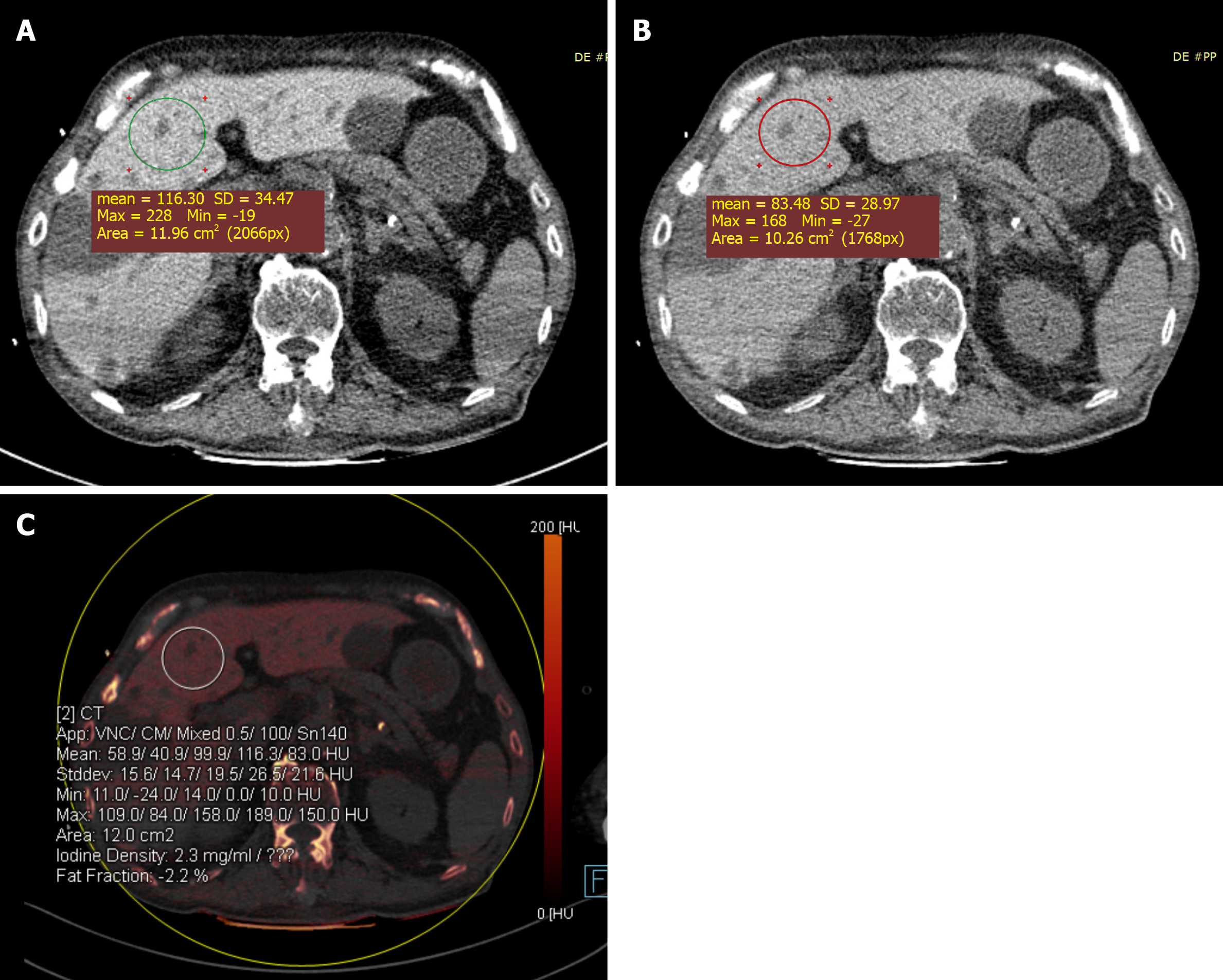
Case 2: DECT imaging of the abdomen demonstrated a significant increase in the density of the liver: the mean liver attenuations were 116 HU and 83 HU at 100 kVp and 140 kVp, respectively; the iodine concentration in the liver was 2.3 mg/mL (Figure 2A-C). CT images also showed multiple liver cysts throughout the entire liver.
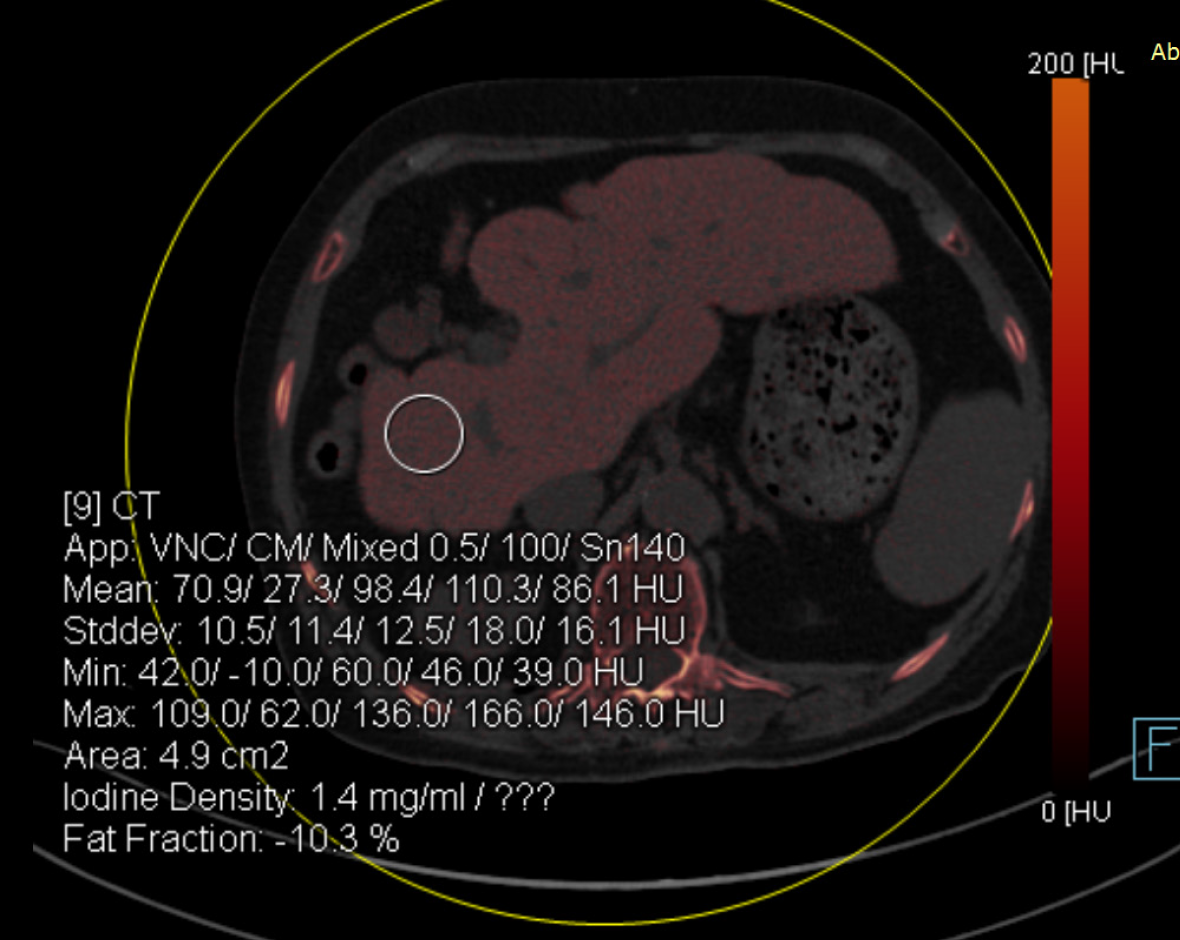
Case 3: An abdominal DECT scan showed that the liver was small, and the proportions were altered, with diffusely increased attenuation; the mean liver attenuations were 110 HU and 86 HU at 100 kVp and 140 kVp, respectively. In addition, the color-coded iodine map revealed high iodine deposition in the liver; the iodine concentration in the liver reached 1.4 mg/mL (Figure 3). These findings were determined to be attributable to the side effects of amiodarone.
Paroxysmal atrial fibrillation.
Severe pneumonia, respiratory failure and heart failure.
Paroxysmal atrial fibrillation.
Treatment with amiodarone was discontinued. Radiofrequency ablation was performed for atrial fibrillation.
Amiodarone was discontinued.
The patient refused radiofrequency ablation for atrial fibrillation. The treating physician and patient decided to continue amiodarone to control atrial fibrillation. She was also informed of the potential hazard to the liver; routine liver function tests were required every 3-6 mo in this patient.
A chest non-enhanced CT scan performed 1 year later showed a significant decrease in hepatic attenuation with 69 HU at 120 kVp (Figure 1D). Furthermore, the patient’s liver enzyme activities returned to reference ranges.
Over the next 5 wk, the patient suffered from rapidly progressive pneumonia. Eventually, he died in the intensive care unit of multiple organ failure.
The patient was discharged with further cardiology and hepatology follow-up, but refused an abdominal DECT scan follow-up.
Amiodarone is the most commonly used antiarrhythmic drug. Due to severe and potentially life-threatening adverse drug reactions, careful use is essential to derive optimal benefits from the drug with the least risk[1].
Kim et al[3] reported a case of amiodarone toxicity in a long-term amiodarone user who showed an abnormally high liver density on a non-enhanced CT scan but normal liver enzyme results and serum amiodarone levels. Previous reports have also shown that plasma amiodarone levels may be poorly correlated to amiodarone toxicity[4]. In light of the present data, it is unclear if amiodarone-induced hepatotoxicity may occur without any abnormalities in liver enzymes[5]. In a previous case report of a patient who was administered the low dose of 200 mg amiodarone once daily for 2 years, the symptoms progressed to hepatic cirrhosis[6]. In conclusion, for those cases that show normal liver function test results and amiodarone plasma levels but symptoms of nausea, vomiting and tremor, which correspond to the side effects of amiodarone, clinicians should be reminded that amiodarone toxicity can be a cause of marked hyper-density in the liver on CT scan. To date, there are no evidence-based standards for monitoring amiodarone-induced hepatotoxicity.
Amiodarone has two iodine atoms that make up 37.3% of its molecular weight. This drug is metabolized in the liver to produce the active metabolite desethylamiodarone. Iodine is highly attenuated on CT; therefore, high attenuation on non-enhanced CT might represent iodine accumulation in the liver of chronic amiodarone users[7]. The levels of amiodarone and its major metabolite desethylamiodarone are correlated with liver CT density[8]. Studies have shown that high liver density observed on CT, which is secondary to increased iodine content due to amiodarone and reflects the tissue levels of amiodarone, may be useful in detecting amiodarone toxicity, but the radiological appearance of these effects is nonspecific[3].
Liver histology shows amiodarone-induced nonalcoholic steatohepatitis in patients who are long-term users of amiodarone[9]. Moreover, conventional, single-energy CT fails to detect and quantify iodine concentration in the liver in the presence of coexisting fat, which has an inverse effect on attenuation by lowering the CT values; the values of single-energy CT are also influenced by different tube voltages, beam hardening artifacts, and other factors. We speculated that the conventional CT appearance of amiodarone hepatotoxicity may not yet be accurate enough to provide useful results.
Traditionally, liver biopsy has been the gold standard for monitoring the side effects of amiodarone, although this type of measure is difficult to obtain. Overall, amiodarone hepatotoxicity is underrecognized and is often diagnosed too late. Hepatic disease may progress despite discontinuation of the drug; this progression may be due to the slow elimination of amiodarone[10]. However, in our first case, the return of liver enzyme activities and hepatic CT density to within the reference ranges after amiodarone discontinuation supports that it is the cumulative dose of amiodarone that has liver toxicity effects in patients with continual low-dose therapy.
In contrast to single-energy CT, DECT using an iodine-specific, three-tissue decomposition algorithm enables accurate quantification of the absolute liver iodine concentration, irrespective of the confounding effects of normal variation in liver attenuation and coexisting fat[11]. Many studies have been conducted to examine the accuracy of DECT-derived iodine concentrations[12]. A DECT scan without contrast media showed an increased iodine concentration in the liver, which provided information to analyze the extent of liver damage. In our cases, the diagnosis of hepatotoxicity was made based on liver enzyme elevations and high iodine deposition in the liver, and these patients had no history of transfusion.
Several studies have shown that CT attenuation of the liver is significantly correlated with the plasma levels of amiodarone and desethylamiodarone and can be used to estimate their deposition in the liver[7,13]. Researchers have suggested that serum amiodarone levels less than 1.5 mg/L are associated with a minimal risk of hepatotoxicity, whereas a level above 2.5 mg/L may be associated with a risk of up to 6% of hepatotoxicity[5]. Amiodarone concentrations in the liver may be up to 500-fold higher than serum levels[14]. Additional experiments have confirmed that amiodarone levels in the liver were 175- to 500-fold higher than the corresponding serum levels[15]. Amiodarone contains 37.3% inorganic iodine, after conversion to liver iodine concentration, liver function damage occurs in 6% of patients when the concentration is greater than 0.47 mg/mL. We used DECT to quantitatively measure the liver iodine concentrations in our patients; the iodine concentrations ranged from 1.4-2.9 mg/mL, far exceeding the iodine concentration that can cause liver damage. According to our research, iodine is evenly distributed in the liver. In the future, DECT examination of the liver in a small field of view will determine the iodine concentration in the liver, reduce radiation exposure and improve the clinical operability.
Although two related side effects of amiodarone therapy, increased CT liver density and secondary phospholipidosis, were reported 34 years ago[16], the relationship between iodine concentration in the liver and the severity of liver damage remains unclear. The threshold of hepatic iodine concentration that leads to hepatotoxicity is not well defined. Further studies are necessary to determine the relationship between histologic progression and hepatic iodine concentration in patients treated with amiodarone.
There are some limitations in our case series. First, we do not have histopathology or biopsy results. Second, the patient sample was small; it is not clear whether the degree of elevation in liver enzymes is correlated with liver iodine concentration; this finding requires further investigation with a larger sample size in the future.
Based on the preliminary findings described above, DECT is an accurate, noninvasive diagnostic tool for quantifying iodine concentration in the liver, and may be more suitable for monitoring adverse reactions to amiodarone. Iodine quantification with liver function tests might be advantageous to establish an early diagnosis of amiodarone-induced liver toxicity before an irreversible stage is reached and to differentiate amiodarone-induced liver toxicity from other common diseases, such as hemochromatosis and hemosiderosis.
| 1. | Epstein AE, Olshansky B, Naccarelli GV, Kennedy JI Jr, Murphy EJ, Goldschlager N. Practical Management Guide for Clinicians Who Treat Patients with Amiodarone. Am J Med. 2016;129:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Gayam V, Khalid M, Dahal S, Garlapati P, Gill A, Alex R, Mansour M. Fatal Acute Liver Failure With Intravenous Amiodarone: A Case Report and Literature Review. Gastroenterology Res. 2018;11:62-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Kim BB, Kim DM, Choi DH, Chung JW, Koh YY, Chang KS, Hong SP. Amiodarone toxicity showing high liver density on CT scan with normal liver function and plasma amiodarone levels in a long-term amiodarone user. Int J Cardiol. 2014;172:494-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Goldschlager N, Epstein AE, Naccarelli G, Olshansky B, Singh B. Practical guidelines for clinicians who treat patients with amiodarone. Practice Guidelines Subcommittee, North American Society of Pacing and Electrophysiology. Arch Intern Med. 2000;160:1741-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Atiq M, Davis JC, Lamps LW, Beland SS, Rose JE. Amiodarone induced liver cirrhosis. Report of two cases. J Gastrointestin Liver Dis. 2009;18:233-235. [PubMed] |
| 6. | Puli SR, Fraley MA, Puli V, Kuperman AB, Alpert MA. Hepatic cirrhosis caused by low-dose oral amiodarone therapy. Am J Med Sci. 2005;330:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (2)] |
| 7. | Kang IS, Kim KJ, Kim Y, Park SH. The diagnostic utility of chest computed tomography scoring for the assessment of amiodarone-induced pulmonary toxicity. Korean J Intern Med. 2014;29:746-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Markos J, Veronese ME, Nicholson MR, McLean S, Shevland JE. Value of hepatic computerized tomographic scanning during amiodarone therapy. Am J Cardiol. 1985;56:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Tsuda T, Tada H, Tanaka Y, Nishida N, Yoshida T, Sawada T, Sakata K, Hayashi K, Kawashiri MA, Oyama T, Sasaki M, Kurose N, Yamagishi M. Amiodarone-induced reversible and irreversible hepatotoxicity: two case reports. J Med Case Rep. 2018;12:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Snir Y, Pick N, Riesenberg K, Yanai-Inbar I, Zirkin H, Schlaeffer F. Fatal hepatic failure due to prolonged amiodarone treatment. J Clin Gastroenterol. 1995;20:265-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Fischer MA, Reiner CS, Raptis D, Donati O, Goetti R, Clavien PA, Alkadhi H. Quantification of liver iron content with CT-added value of dual-energy. Eur Radiol. 2011;21:1727-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Pelgrim GJ, van Hamersvelt RW, Willemink MJ, Schmidt BT, Flohr T, Schilham A, Milles J, Oudkerk M, Leiner T, Vliegenthart R. Accuracy of iodine quantification using dual energy CT in latest generation dual source and dual layer CT. Eur Radiol. 2017;27:3904-3912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 13. | Hirakawa K, Abe K, Ayabe Y, Nishimura M. [Analysis of increased hepatic density during chronic amiodarone therapy]. Nihon Igaku Hoshasen Gakkai Zasshi. 2003;63:221-224. [PubMed] |
| 14. | Brien JF, Jimmo S, Brennan FJ, Ford SE, Armstrong PW. Distribution of amiodarone and its metabolite, desethylamiodarone, in human tissues. Can J Physiol Pharmacol. 1987;65:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Goldman IS, Winkler ML, Raper SE, Barker ME, Keung E, Goldberg HI, Boyer TD. Increased hepatic density and phospholipidosis due to amiodarone. AJR Am J Roentgenol. 1985;144:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Patrick D, White FE, Adams PC. Long-term amiodarone therapy: a cause of increased hepatic attenuation on CT. Br J Radiol. 1984;57:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ho HK S-Editor: Gao CC L-Editor: Webster JR P-Editor: Li JH