Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4753
Peer-review started: May 21, 2020
First decision: June 7, 2020
Revised: June 22, 2020
Accepted: September 1, 2020
Article in press: September 1, 2020
Published online: October 26, 2020
Processing time: 157 Days and 10.6 Hours
Horseshoe kidney (HK) with renal stones is challenging for urologists. Although both retroperitoneal and transperitoneal laparoscopic approaches have been reported in some case reports, the therapeutic outcome of retroperitoneal compared with transperitoneal laparoscopic lithotripsy is unknown.
To assess the efficacy of laparoscopic lithotripsy for renal stones in patients with HK.
This was a retrospective study of 12 patients with HK and a limited number (n ≤ 3) of 20-40 mm renal stones treated with either retroperitoneal or transperitoneal laparoscopic lithotripsy (June 2012 to May 2019). The perioperative data of both groups were compared including operation time, estimated blood loss, postoperative fasting time, perioperative complications and stone-free rate (SFR).
No significant difference was observed for age, gender, preoperative symptoms, body mass index, preoperative infection, hydronephrosis degree, largest stone diameter, stone number and isthmus thickness. The mean postoperative fasting time of the patients in the retroperitoneal group and the transperitoneal group was 1.29 ± 0.49 and 2.40 ± 0.89 d, respectively (P = 0.019). There was no significant difference in operation time (194.29 ± 102.48 min vs 151.40 ± 39.54 min, P = 0.399), estimated blood loss (48.57 ± 31.85 mL vs 72.00 ± 41.47 mL, P = 0.292) and length of hospital stay (12.14 ± 2.61 d vs 12.40 ± 3.21 d, P = 0.881) between the retroperitoneal and transperitoneal groups. All patients in both groups had a complete SFR and postoperative renal function was within the normal range. The change in estimated glomerular filtration rate (eGFR) from the preoperative stage to postoperative day 1 in the retroperitoneal group and the transperitoneal group was -3.86 ± 0.69 and -2.20 ± 2.17 mL/(min·1.73 m2), respectively (P = 0.176). From the preoperative stage to the 3-mo follow-up, the absolute change in eGFR values for patients in the retroperitoneal group and the transperitoneal group was -3.29 ± 1.11 and -2.40 ± 2.07 mL/(min·1.73 m2), respectively (P = 0.581).
Both retroperitoneal and transperitoneal laparoscopic lithotripsy seem to be safe and effective for HK patients with a limited number of 20-40 mm renal stones.
Core Tip: This was a retrospective study of 12 patients with horseshoe kidney (HK) and a limited number (n ≤ 3) of 20-40 mm renal stones treated by either retroperitoneal or transperitoneal laparoscopic lithotripsy. The mean postoperative fasting time of patients in the retroperitoneal group and the transperitoneal group was 1.29 ± 0.49 and 2.40 ± 0.89 d, respectively (P = 0.019). All patients in both groups had a complete SFR and postoperative renal function was within the normal range. Both retroperitoneal and transperitoneal laparoscopic lithotripsy seem to be safe and effective for HK patients with a limited number of 20-40 mm renal stones.
- Citation: Chen X, Wang Y, Gao L, Song J, Wang JY, Wang DD, Ma JX, Zhang ZQ, Bi LK, Xie DD, Yu DX. Retroperitoneal vs transperitoneal laparoscopic lithotripsy of 20-40 mm renal stones within horseshoe kidneys. World J Clin Cases 2020; 8(20): 4753-4762
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4753.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4753
Horseshoe kidney (HK) is a major congenital kidney anomaly which occurs in approximately 0.25% of the general population[1]. Pathological fusion of the lower kidney moiety gives rise to ureteropelvic junction obstruction (UPJO) in up to 35% of HK patients[2,3], and distortion of the ureteropelvic junction naturally leads to renal stones. For large renal stones (largest stone diameter > 20 mm), flexible ureteroscopy, laparoscopic lithotripsy, percutaneous nephrolithotomy (PCNL) and extracorporeal shock wave lithotripsy (ESWL) have been successfully performed in selected specific cases[4,5]. PCNL is the preferred procedure for large renal stones (largest stone diameter > 40 mm), while ESWL and flexible ureteroscopy are feasible and safe for small renal stones (largest stone diameter < 20 mm). However, no studies have investigated the best surgical approach for HK with a limited number of 20-40 mm renal stones, which is commonly found.
Laparoscopic lithotripsy was proposed as a promising technique for renal stones in HK patients[4-10]. However, in most of the reported cases treatment was performed using the transperitoneal approach[6-9]. Comparisons between retroperitoneal and transperitoneal laparoscopic approaches for HK with renal stones have not yet been performed. The present study aimed to report our single center experience of laparoscopic stone management via both retroperitoneal and transperitoneal approaches in HK patients with a limited number (n ≤ 3) of 20-40 mm renal stones.
Patients with HK and renal stones in our clinic who were treated laparoscopically between July 2012 and May 2019 were retrospectively reviewed. The exclusion criteria included bilateral renal stones, staghorn stones, small (< 20 mm) or large (> 40 mm) stones, stone number > 3, other abnormal upper urinary tract anatomies including duplex kidney and ectopic kidney, severe obstruction of the urinary tract, history of open surgery of the upper abdomen, and concomitant renal tumor. The study was approved by the Ethics Committee of Anhui Medical University, Hefei, China and carried out in accordance with the Helsinki Declaration.
Perioperative data were reviewed including operation time, estimated blood loss, perioperative complications, and postoperative fasting time. Rates of minor and major complications were evaluated according to the Clavien-Dindo classification[11]. Perioperative total renal function was calculated as the estimated glomerular filtration rate (eGFR) using the Modified Diet in Renal Disease equation[12] preoperatively and on postoperative day 1 and 30, respectively. All patients underwent X-ray kidney-ureter-bladder radiography as the imaging examination preoperatively on postoperative day 30, to evaluate the stone-free rate (SFR). Isthmus thickness was determined by routine preoperative three-dimensional computed tomography (CT) and CT angiography in all patients. Perioperative hydronephrosis degree was evaluated by renal ultrasound and classified according to the Society for Fetal Urology guideline (Grade 0: No renal pelvic dilatation; Grade 1: Renal pelvic dilatation with only visualized small calyces; Grade 2: Renal pelvic dilatation with some visualized large calyces; Grade 3: Renal pelvic dilatation with all visualized calyces and a normal parenchyma; Grade 4: Renal pelvic dilatation with all visualized calyces and a thin parenchyma)[13].
All patients received laparoscopic isthmusectomy concomitantly with lithotripsy. Depending on patient status and surgeon preference, a retroperitoneal or transperitoneal approach was performed.
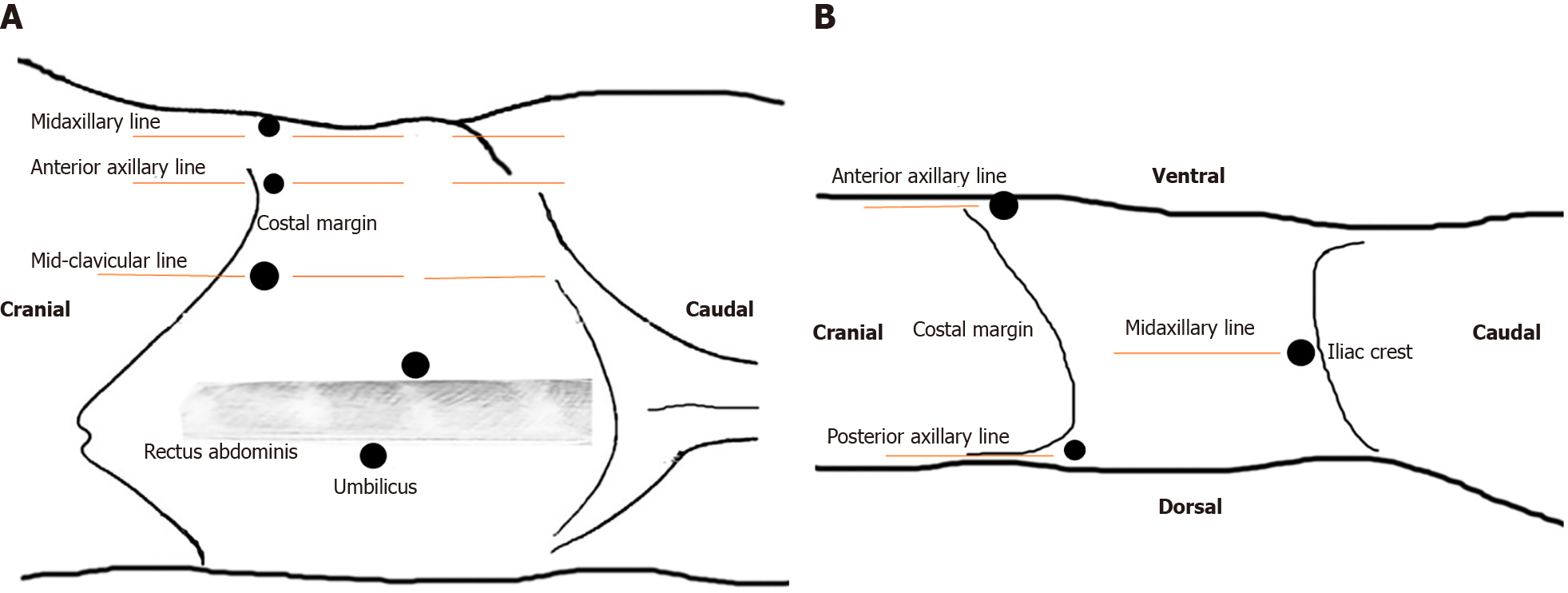
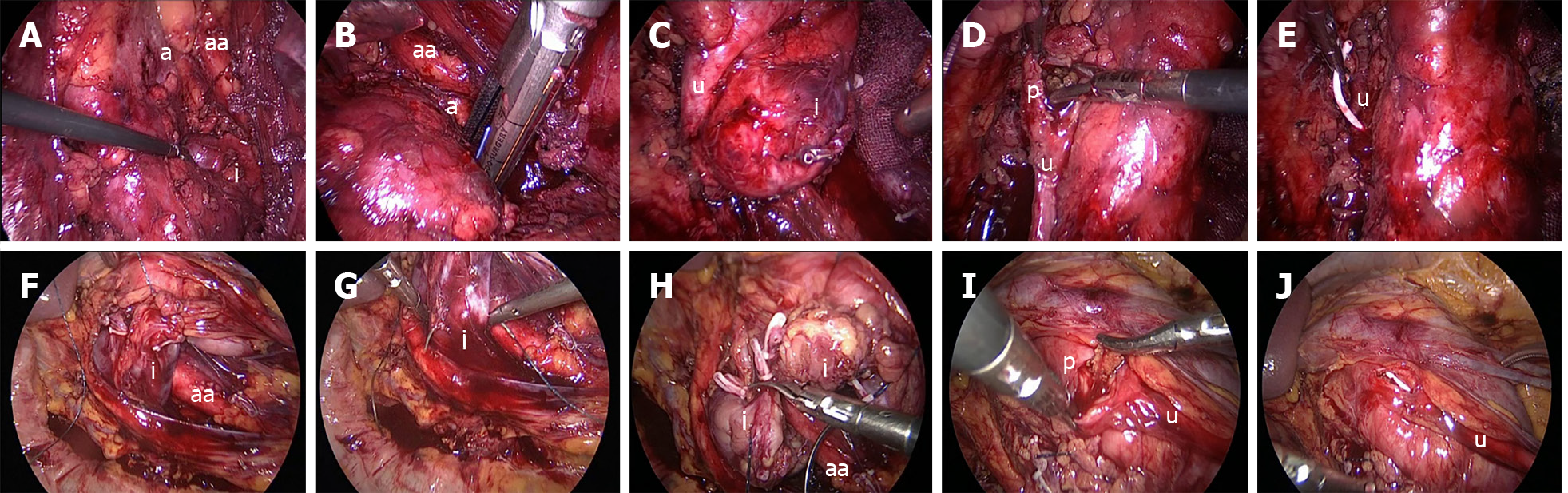
Under general anesthesia, patients were placed in the semi-lateral decubitus position with the ipsilateral side up. The laparoscopic port placements are shown in Figure 1A. After entering the retroperitoneal space, the retroperitoneal fat was separated first, the Gerota fascia was then uncovered at the lower pole, and the perinephric fat was eliminated to expose the renal hilum. As the isthmus existed in the lower poles in all our cases, we released the lower pole of the kidney to expose the isthmus (Figure 2A). When the isthmus was isolated with a stapler (Endopath, Ethicon Endo-Surgery, United States) (Figure 2B), we dissected the renal pelvis to extract the stones (Figure 2C and D). Distilled water was applied to flush the renal pelvis to promote stone removal. After a Double-J ureteral stent was inserted into the ureter (Figure 2E), renal pelvic anastomoses were performed with a 4-0 absorbable V-LOC suture. A flexible cystoscope was used in one patient to view all small calyces to guarantee complete clearance of renal stones.
Patients were placed in the supine position and the laparoscopic ports were located as shown in Figure 1B. The Toldt line was incised first, and the colon was mobilized medially to expose the aorta and Gerota fascia. The Gerota fascia was dissected and the isthmus was completely released (Figure 2F). Following exposure of the isthmus, a 3-0 absorbable V-LOC suture was applied on both sides of the narrowest isthmus moiety for renal parenchymal anastomoses and the isthmus was isolated using Mayo scissors (Figure 2G and H). The nephrolithotomy procedure was performed as described in the retroperitoneal approach (Figure 2I and J).
Statistical analyses were performed with SPSS Statistics v. 22.0 (SPSS Inc., Chicago, IL, United States). All continuous data are shown as mean ± SD and were compared by the Student’s t-test. The Wilcoxon signed-rank test was used to compare perioperative eGFR changes. Categorical data were compared by the Chi-square test. A P value < 0.05 was considered statistically significant.
Twelve patients (seven adult females, four adult males, and one boy) were enrolled into our cohorts. Demographic data of these patients were systematically reviewed and are summarized in Table 1. The mean patient age was 35.25 ± 13.69 years (range, 12-55 years). Nine patients (75.0%) presented with a history of moderate to severe flank pain, two patients (16.7%) presented with abdominal pain and the remaining patient (8.3%) complained of moderate hematuria (Table 1). Five patients (41.7%) with preoperative urinary infection recovered after antibiotic treatment for three days. Nine patients (75.0%) had renal stones on the left side, while three (25.0%) had renal stones on the right side. All patients had an extra-renal pelvis.
| Retroperitoneal group (n = 7) | Transperitoneal group (n = 5) | P value | |
| Age (yr) | 37.29 ± 13.30 | 32.40 ± 15.24 | 0.567 |
| Gender | 1.000 | ||
| Male, n (%) | 3 (42.9) | 2 (40.0) | |
| Female, n (%) | 4 (57.1) | 3 (60.0) | |
| Laterality | 1.000 | ||
| Left, n (%) | 5 (71.4) | 4 (80.0) | |
| Right, n (%) | 2 (28.6) | 1 (20.0) | |
| BMI (kg/m2) | 21.69 ± 1.86 | 22.65 ± 2.43 | 0.457 |
| Preoperative symptoms | 0.559 | ||
| Flank pain, n (%) | 5 (71.4) | 4 (80.0) | |
| Abdominal pain, n (%) | 1 (14.3) | 1 (20.0) | |
| Hematuria, n (%) | 1 (14.3) | 0 (0.0) | |
| Preoperative infection | 0.558 | ||
| No, n (%) | 5 (71.4) | 2 (40.0) | |
| Yes, n (%) | 2 (28.6) | 3 (60.0) | |
| Hydronephrosis degree | 1.000 | ||
| ≤ 1, n (%) | 3 (42.9) | 3 (60.0) | |
| 2, n (%) | 4 (57.1) | 2 (40.0) | |
| Maximal stone diameter (mm) | 26.71 ± 4.27 | 28.40 ± 4.04 | 0.507 |
| Stone number | 0.125 | ||
| 1, n (%) | 4 (57.1) | 2 (40.0) | |
| 2, n (%) | 3 (42.9) | 1 (20.0) | |
| 3, n (%) | 0 (0.0) | 2 (40.0) | |
| Isthmus thickness (cm) | 4.07 ± 1.10 | 4.18 ± 0.75 | 0.853 |
| Operation time (min) | 194.29 ± 102.48 | 151.40 ± 39.54 | 0.399 |
| Estimated blood loss (mL) | 48.57 ± 31.85 | 72.00 ± 41.47 | 0.292 |
| Postop fasting time (d) | 1.29 ± 0.49 | 2.40 ± 0.89 | 0.019 |
| Hospital stay (d) | 12.14 ± 2.61 | 12.40 ± 3.21 | 0.881 |
| Minor complications, n (%) | 1 (14.3) | 1 (20.0) | 1.000 |
| Major complications, n (%) | 0 (0.0) | 0 (0.0) | N/A |
| eGFR [mL/(min·1.73 m2)] | |||
| Preoperative | 143.00 ± 24.34 | 126.00 ± 40.48 | 0.383 |
| Postoperative day 1 | 139.14 ± 23.97 | 123.80 ± 40.67 | 0.428 |
| Postoperative day 30 | 139.71 ± 26.23 | 123.60 ± 39.42 | 0.398 |
| Mean follow-up | 29.42 ± 23.87 | 27.79 ± 21.75 | 0.906 |
| SFR, n (%) | 7 (100.0) | 5 (100.0) | 1.000 |
Seven of 12 patients (58.3%) were included in the retroperitoneal group while the other five (41.7%) were included in the transperitoneal group. There were no statistically significant differences between the retroperitoneal and transperitoneal groups regarding age, gender, body mass index, side of renal stones, and preoperative infection (Table 1). The mean stone diameter in the retroperitoneal group (26.71 ± 4.27 mm) was smaller than that in the transperitoneal group (28.40 ± 4.04 mm) without reaching statistical significance (P = 0.507). Stone number in the retroperitoneal group was comparable to that in the transperitoneal group (P = 0.125). Isthmus thickness in the retroperitoneal group and transperitoneal group was 4.07 ± 1.10 vs 4.18 ± 0.75 cm, respectively (P = 0.853) (Table 1).
Mean postoperative fasting time in patients in both groups was 1.29 ± 0.49 and 2.40 ± 0.89 d, respectively (P = 0.019). No significant differences were found in terms of operation time (194.29 ± 102.48 min vs 151.40 ± 39.54 min, P = 0.399), estimated blood loss (48.57 ± 31.85 mL vs 72.00 ± 41.47 mL, P = 0.292) and length of hospital stay (12.14 ± 2.61 d vs 12.40 ± 3.21 d, P = 0.881) between the two groups (Table 1).
eGFR values in patients in both groups were recorded: preoperative period: 143.00 ± 24.34 mL/(min·1.73 m2) vs 126.00 ± 40.48 mL/(min·1.73 m2); postoperative day 1: 139.14 ± 23.97 mL/(min·1.73 m2) vs 123.80 ± 40.67 mL/(min·1.73 m2), postoperative day 30: 139.71 ± 26.23 mL/(min·1.73 m2) vs 123.60 ± 39.42 mL/(min·1.73 m2) (Table 1). The eGFR values assessed at each time point were comparable between the two groups (P = 0.383, P = 0.428 and P = 0.398, respectively) (Table 1). Although the postoperative eGFR values in both groups on postoperative day 1 and 30 were worse than the preoperative values, these differences were not statistically significant (P = 0.176 and P = 0.581, respectively) (Table 2).
| eGFR [mL/(min·1.73 m2)] | Retroperitoneal group (n = 7) | Transperitoneal group (n = 5) | P value |
| Postoperative day 1 | -3.86 ± 0.69 | -2.20 ± 2.17 | 0.176 |
| Postoperative day 30 | -3.29 ± 1.11 | -2.40 ± 2.07 | 0.581 |
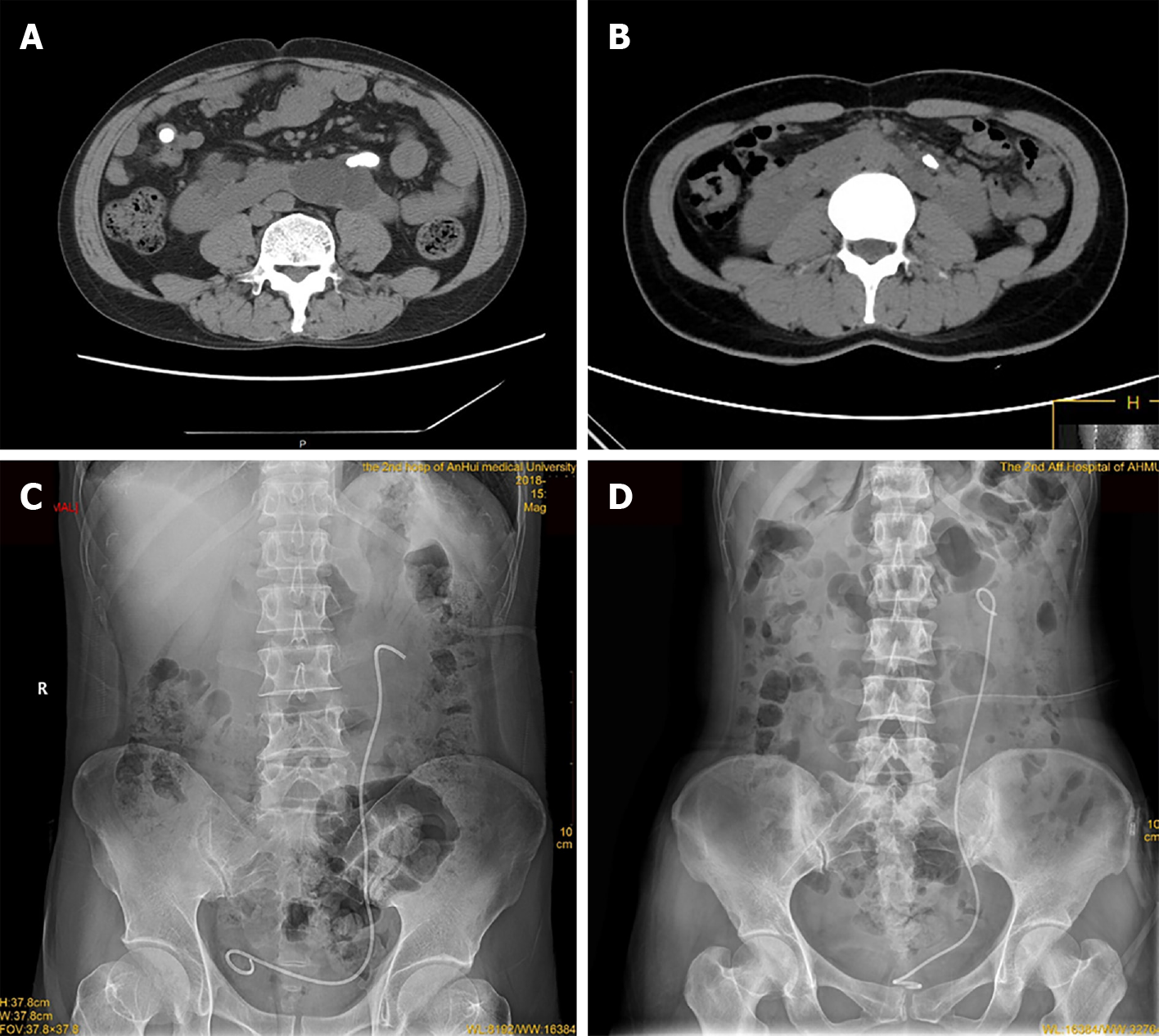
None of the patients received a perioperative transfusion, and no major complications (grade III-V) occurred according to the Clavien-Dindo classification. Only one patient in the retroperitoneal group had low blood pressure, which improved after sufficient liquid compensation, while one patient in the transperitoneal group experienced postoperative fever, which subsided after antibiotic treatment for three days. Postoperative drainage, a catheter and a Double-J stent were placed for 3 d, 3 d and one month, respectively. No statistically significant differences were observed between the two groups in terms of the mean follow-up period (29.42 ± 23.87 mo vs 27.79 ± 21.75 mo, P = 0.906). The SFR in both groups was 100% (Table 1 and Figure 3).
HK is the most common renal fusion malformation which develops within 7-9 gestational weeks. An isthmus of the two lower renal poles exists in almost 90% of patients and is generally located anterior to the aorta and vena cava[3]. Due to the connection of the lower or upper poles of two individual kidneys, the isthmus induces renal malrotation and renal pelvic anteposition. As a result of kidney rotation, the ureter atypically passes through the anterior surface of the isthmus and contributes to UPJO, which further promotes renal stone formation. Furthermore, metabolic abnormalities may also contribute to the high incidence (21.0%-60.0%) of renal stones in HK[14,15].
Although flexible ureteroscopy is recommended by the European Association of Urology guideline for small renal stones in HK patients[16], large and/or multiple renal stones are still challenging for urologists. PCNL has been reported to be applicable for large or multiple renal stones in HK patients, including pediatric patients, with a SFR of 75.0%-89.0%[5,17-20]. However, the complication rate varied from 13.0% to 29.2%, whilst the complications included significant hematuria requiring blood transfusion and colonic injury. Furthermore, renal stones in specific locations (such as the lower calyx and isthmus) cannot be reached by the nephroscope. These renal stones can potentially be treated by laparoscopy. In addition, laparoscopic lithotripsy may also be effective for renal stones with a limited stone number (n ≤ 3) and stone size (stone largest diameter within the range of 20-40 mm).
The present study revealed that both the retroperitoneal and transperitoneal approach might provide a satisfactory outcome for renal stones in patients with HK. Use of the retroperitoneal approach may avoid entering the peritoneal cavity and minimally affect intestinal function. In our cohort, patients in the retroperitoneal group had a shorter postoperative fasting time than patients in the transperitoneal group. Furthermore, urologists are more familiar with the retroperitoneal space and identifying the isthmus safely after exposing the ureter. According to our experience, this approach is more appropriate for a non-isthmus stone. However, the transperitoneal approach offers considerable space, direct access to vasculature and feasible suturing for renal parenchymal anastomoses. Therefore, it is more cost-effective than the retroperitoneal approach. In addition, the operation time was shorter than that in the retroperitoneal group, although the difference was not significant. Thus, it was theoretically feasible for treating bilateral renal stones. We consider that the operation time was more dependent on the surgeon’s experience rather than the surgical approach. The limited retroperitoneal space did not result in a significant difference in operation time (Table 1). Both approaches avoided a high complication rate (14.3% vs 20.0%, P = 1.000).
Although isthmusectomy is not recommended by European and United States surgeons for HK patients[21], Chinese surgeons prefer isthmusectomy as they consider that it may reset the anatomic location of abnormal kidneys and resolve the UPJO-induced hydronephrosis[4]. All patients in our cohort underwent isthmusectomy. This was due to the fact that hydronephrosis in our cohort was caused by isthmic compression on the ureter, and hydronephrosis grades were comparably low (n ≤ 2). Our decision on technical choice was consistent with the recommendation by Jarzemski et al[22]. Both parenchymal suturing and stapling can avoid hemorrhage. A stapler was recommended for isthmus division in the retroperitoneal approach, as the contralateral kidney is free and complex to complete renal parenchymal anastomoses, and the estimated blood loss can also be reduced.
Improvement in the SFR is still a vital part of renal stone treatment in HK patients. In our study, the SFR did not differ between the two groups (100% vs 100%, P = 1.000). Renal pelvic stones could be removed directly after pelvis dissection due to the dilated renal pelvis. In cases with lower calyceal stones, it was difficult to reach the stones by laparoscopy, and distilled water was applied to flush the pelvis. In one patient with three renal stones in the lower calyx, a flexible cystoscope was used for the renal stones. Our results indicated that the flexible cystoscope was effective for aiding complete stone removal, which was in accordance with the study by Kramer et al[6]. Similarly, a flexible ureteroscope has also been proposed to improve the renal stone removal rate in lithotripsy[23].
Several drawbacks of this study should be acknowledged. First, this is a retrospective study and the patient number was limited. The rigorous exclusion criteria in our study determined the limited therapeutic spectrum of this laparoscopic approach. However, the SFR in both groups was satisfactory (100% vs 100%, P = 1.000), and was attributed to the limited number of renal stones (n ≤ 3) and the extra-renal pelvis. More prospective clinical trials are needed to identify whether the laparoscopic technique is suitable for a greater number (n > 3) of renal stones. In cases with an intra-renal pelvis, a complete exploration of all calyces is challenging. A flexible cystoscope or flexible ureteroscope may be beneficial for stone removal within the intra-renal pelvis. Second, as a higher degree of hydronephrosis (> 2) and renal stones are found in HK patients, lithotripsy concomitant with pyeloplasty can be performed. More experience of laparoscopic lithotripsy and pyeloplasty should be accumulated. Third, the laparoscopic technique for stone removal in HK patients is a demanding procedure that requires considerable surgical expertise.
Both retroperitoneal and transperitoneal laparoscopic lithotripsy seem to be safe and effective for HK patients with a limited number of 20-40 mm renal stones.
Horseshoe kidney (HK) with renal stones is challenging for urologists. No studies have investigated the best surgical approach for HK with a limited number of 20-40 mm renal stones, which is commonly found.
Laparoscopic treatment was reported in some case reports; however, the therapeutic outcome of the retroperitoneal compared with the transperitoneal approach is unknown. The possible therapeutic differences between these two approaches was worthy of study.
This study aimed to investigate the clinical characteristics and outcomes of patients with HK and renal stones, who underwent retroperitoneal or transperitoneal laparoscopic treatment.
We performed a retrospective study of 12 patients treated with retroperitoneal or transperitoneal laparoscopy for HK and a limited number of 20-40 mm renal stones. The baseline characteristics and postoperative outcomes of these patients were summarized and analyzed. Statistical analyses were performed with SPSS Statistics v. 22.0.
The mean postoperative fasting time of patients in the retroperitoneal group was shorter than that in the transperitoneal group. There was no significant difference in operation time, estimated blood loss and length of hospital stay between the retroperitoneal group and transperitoneal group. All patients in both groups achieved a complete stone-free rate and postoperative renal function was within the normal range. The change in estimated glomerular filtration rate from the preoperative stage to postoperative day 1 and 3-mo in the retroperitoneal group and transperitoneal group was not statistically significant.
Both retroperitoneal and transperitoneal laparoscopic lithotripsy are safe and effective surgical approaches for HK patients with a limited number (n ≤ 3) of 20-40 mm renal stones. Laparoscopic lithotripsy is an effective clinical alternative to percutaneous nephrolithotomy and flexible ureteroscopy.
More attention should be paid to the treatment of HK patients with a limited number of medium-sized renal stones. The ideal choice of retroperitoneal or transperitoneal laparoscopy should be patient-individualized and further elucidated by more prospective clinical trials.
| 1. | Allen AC. Horseshoe kidney. In: The Kidney: Medical and surgical diseases. New York Grune; 1951: 94-96. |
| 2. | Yohannes P, Smith AD. The endourological management of complications associated with horseshoe kidney. J Urol. 2002;168:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Khan A, Myatt A, Palit V, Biyani CS, Urol D. Laparoscopic heminephrectomy of a horseshoe kidney. JSLS. 2011;15:415-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Wang P, Xia D, Ma Q, Wang S. Retroperitoneal laparoscopic management of ureteropelvic junction obstruction in patients with horseshoe kidney. Urology. 2014;84:1351-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 5. | Symons SJ, Ramachandran A, Kurien A, Baiysha R, Desai MR. Urolithiasis in the horseshoe kidney: a single-centre experience. BJU Int. 2008;102:1676-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Kramer BA, Hammond L, Schwartz BF. Laparoscopic pyelolithotomy: indications and technique. J Endourol. 2007;21:860-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Myint M, Luke S, Louie-Johnsun M. Laparoscopic pyelolithotomy and pyeloplasty in a horseshoe kidney. ANZ J Surg. 2015;85:492-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Shadpour P, Akhyari HH, Maghsoudi R, Etemadian M. Management of ureteropelvic junction obstruction in horseshoe kidneys by an assortment of laparoscopic options. Can Urol Assoc J. 2015;9:E775-E779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Nambirajan T, Jeschke S, Albqami N, Abukora F, Leeb K, Janetschek G. Role of laparoscopy in management of renal stones: single-center experience and review of literature. J Endourol. 2005;19:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 10. | Nadu A, Schatloff O, Morag R, Ramon J, Winkler H. Laparoscopic surgery for renal stones: is it indicated in the modern endourology era? Int Braz J Urol. 2009;35:9-17; discussion 17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26161] [Article Influence: 1189.1] [Reference Citation Analysis (2)] |
| 12. | Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, Poggio ED, Schmid CH, Steffes MW, Zhang YL, Van Lente F, Coresh J. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993;23:478-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 730] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 14. | Evans WP, Resnick MI. Horseshoe kidney and urolithiasis. J Urol. 1981;125:620-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Gambaro G, Fabris A, Puliatta D, Lupo A. Lithiasis in cystic kidney disease and malformations of the urinary tract. Urol Res. 2006;34:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Türk TKC, Petrik A, Sarica K, Skolarikos A, Straub M, Seitz C. Guidelines on Urolithiasis. 2015. |
| 17. | Al-Otaibi K, Hosking DH. Percutaneous stone removal in horseshoe kidneys. J Urol. 1999;162:674-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Purkait B, Sankhwar SN, Kumar M, Patodia M, Bansal A, Bhaskar V. Do Outcomes of Percutaneous Nephrolithotomy in Horseshoe Kidney in Children Differ from Adults? J Endourol. 2016;30:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Shokeir AA, El-Nahas AR, Shoma AM, Eraky I, El-Kenawy M, Mokhtar A, El-Kappany H. Percutaneous nephrolithotomy in treatment of large stones within horseshoe kidneys. Urology. 2004;64:426-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Raj GV, Auge BK, Weizer AZ, Denstedt JD, Watterson JD, Beiko DT, Assimos DG, Preminger GM. Percutaneous management of calculi within horseshoe kidneys. J Urol. 2003;170:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Kölln CP, Boatman DL, Schmidt JD, Flocks RH. Horseshoe kidney: a review of 105 patients. J Urol. 1972;107:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Jarzemski P, Listopadzki S. Laparoscopic horseshoe kidney isthmusectomy: four case reports. Wideochir Inne Tech Maloinwazyjne. 2014;9:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Tsuru N, Mugiya S, Kurita Y, Sato S, Hirano Y. Laparoscopic Pyeloplasty for Ureteropelvic Junction Obstruction in an Incompletely Duplicated Collecting System in a Patient with a Horseshoe Kidney. Urol Case Rep. 2016;9:55-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta N S-Editor: Yan JP L-Editor: Webster JR P-Editor: Li X