Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.370
Peer-review started: October 11, 2019
First decision: December 4, 2019
Revised: December 6, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: January 26, 2020
Processing time: 92 Days and 15 Hours
Central nervous system (CNS) metastases are a catastrophic complication of non-small cell lung cancer (NSCLC), including brain and leptomeningeal carcinomatosis, and are always accompanied by a poor prognosis. Despite the continuous development of existing treatments, the therapy of CNS metastases remains challenging.
We report a patient who was definitively diagnosed with brain and leptomeningeal metastases from NSCLC with a targeted mutation in epidermal growth factor receptor (EGFR). A standard dosage of icotinib (125 mg three times daily) was implemented but ineffective. CNS lesions developed despite stable systemic control, so pulsatile icotinib (1125 mg every 3 d) was administered. This new strategy for administration has lasted 25 mo so far, and resulted in complete remission of neurological symptoms, almost vanished lesions, and longer survival with no notable side effects.
This is the first successful example of pulsatile icotinib for treating isolated CNS progression from EGFR mutation-positive NSCLC, providing a new alternative for the local treatment of CNS metastases.
Core tip: Central nervous system (CNS) metastases are a catastrophic complication of non-small cell lung cancer (NSCLC) and always accompanied by a poor prognosis. We report a patient who was diagnosed with CNS metastases from NSCLC with a targeted mutation in epidermal growth factor receptor. A standard dosage of icotinib (125 mg three times daily) was implemented but ineffective. So pulsatile icotinib (1125 mg every 3 d) was administered. This new strategy for administration resulted in complete remission of neurological symptoms, almost vanished lesions, and longer survival without notable side effects, providing a new alternative for the treatment of CNS metastases.
- Citation: Li HY, Xie Y, Yu TT, Lin YJ, Yin ZY. Durable response to pulsatile icotinib for central nervous system metastases from EGFR-mutated non-small cell lung cancer: A case report. World J Clin Cases 2020; 8(2): 370-376
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/370.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.370
Central nervous system (CNS) metastases are a catastrophic complication of non-small cell lung cancer (NSCLC), including brain and leptomeningeal carcinomatosis. The incidence of brain metastases (BM) is 10%-40% in NSCLC patients, and the median overall survival (OS) time is estimated to be 7 mo[1,2]. Leptomeningeal metastases (LM) are relatively rare, with an approximate incidence of 3%–5% among patients with NSCLC, but always imply a poor prognosis, with a median OS of only 3 mo[3,4].
The main treatment options for CNS metastases include tyrosine kinase inhibitors (TKIs), systemic chemotherapy, whole-brain radiotherapy (WBRT), intrathecal chemotherapy, and combined therapies. For epidermal growth factor receptor (EGFR) mutation-positive patients, TKIs are a standard treatment and have a high response rate. Icotinib hydrochloride, a highly specific EGFR-TKI, has shown reliable antitumour activity and has been approved as the firstline choice in advanced EGFR+ NSCLC patients by the State Food and Drug Administration of China[5,6]. Herein, we report the first case of a patient with CNS metastases from EGFR-mutant NSCLC who benefited from “pulsatile” administration of high-dose icotinib after the invalidity of standard daily dosage.
A 68-year-old Asian male non-smoker presented with paroxysmal cough in December 2014.
He had no history of fever, hemoptysis, or weight loss.
He had no history of surgery, chronic diseases, or allergies.
He had no smoking or drinking history. There was no specific family history of cancer or cancer related disease.
Physical examination was basically normal.
Carcinoembryonic antigen level was 12.5 ng/mL (0-10 ng/mL), neuron-specific enolase was 15.9 ng/mL (0-16.3 ng/mL), and cytokeratin 19 fragment (CYFRA21-1) was 15.5 ng/mL (0-3.3 ng/mL).
Chest computed tomography (CT) showed a space-occupying lesion in the right upper lobe of the lung.
Lung cancer.
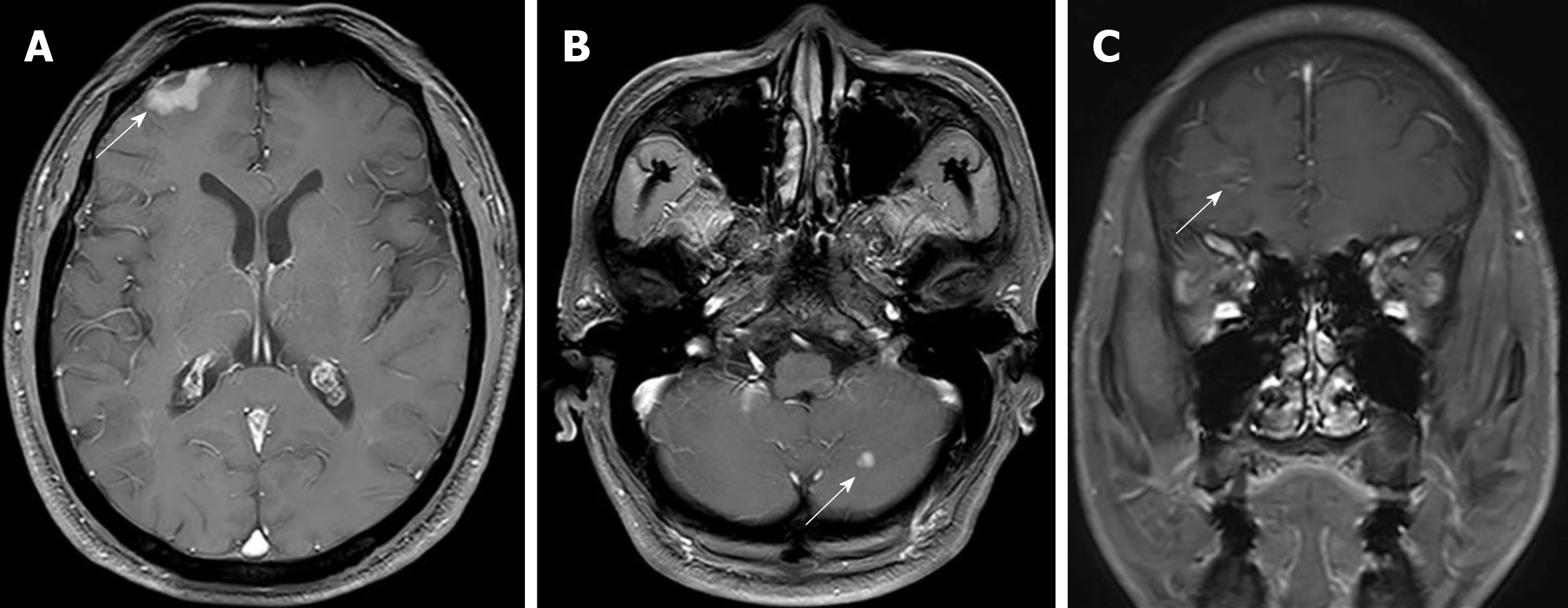
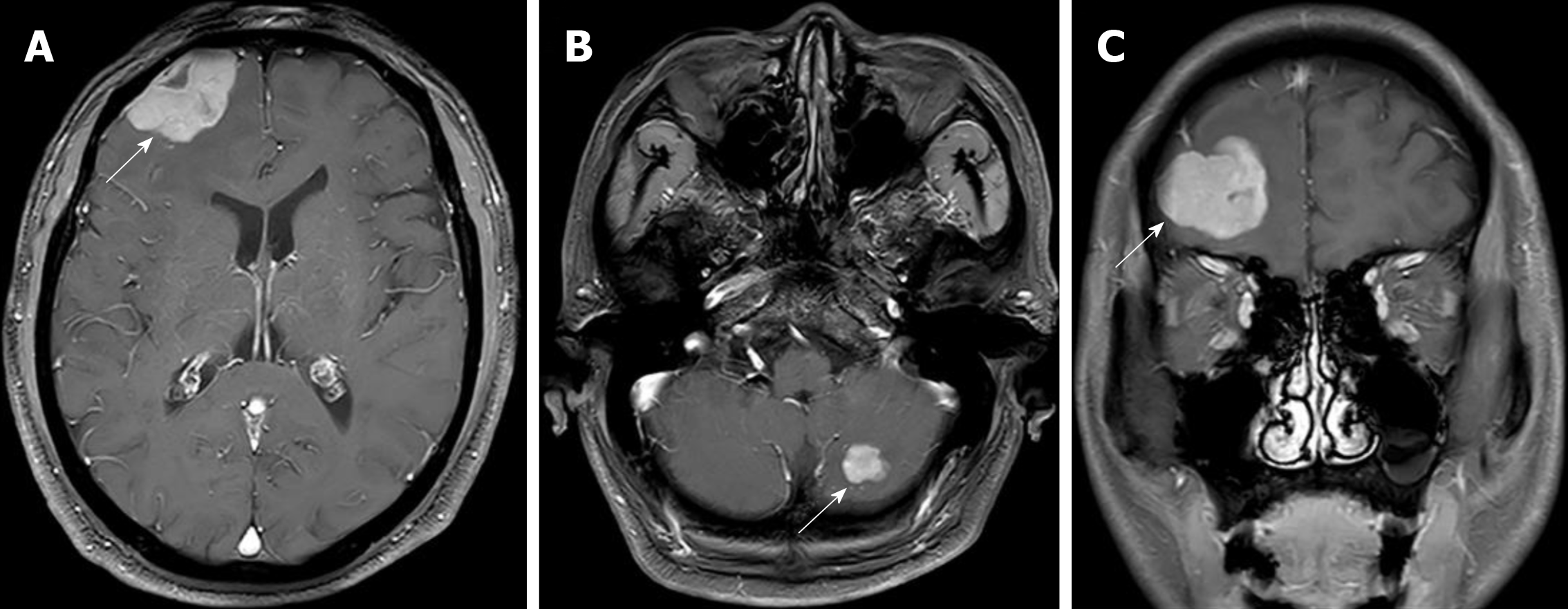
Radical surgery by video-assisted thoracoscopic surgery (VATS) was carried out in January 2015. The clinical stage was pT2N2M0 IIIA according to the postsurgical pathology. Adjuvant chemotherapy with pemetrexed and oxaliplatin was applied for six cycles. The disease was controlled for over a year until August 2016, when the patient showed symptoms of headaches, nausea, and vomiting. The brain and leptomeningeal metastases were diagnosed by magnetic resonance imaging (MRI) (Figure 1). EGFR testing revealed an exon 21 L858R point mutation. Icotinib 125 mg (three times daily) was administered orally, which decreased the CEA levels, relieved neurological symptoms, and stabilized the disease for 12 mo. However, a repeat MRI scan revealed enlarged brain lesions in August 2017 (Figure 2). After obtaining written informed consent, icotinib was adjusted to 1125 mg every 3 d.
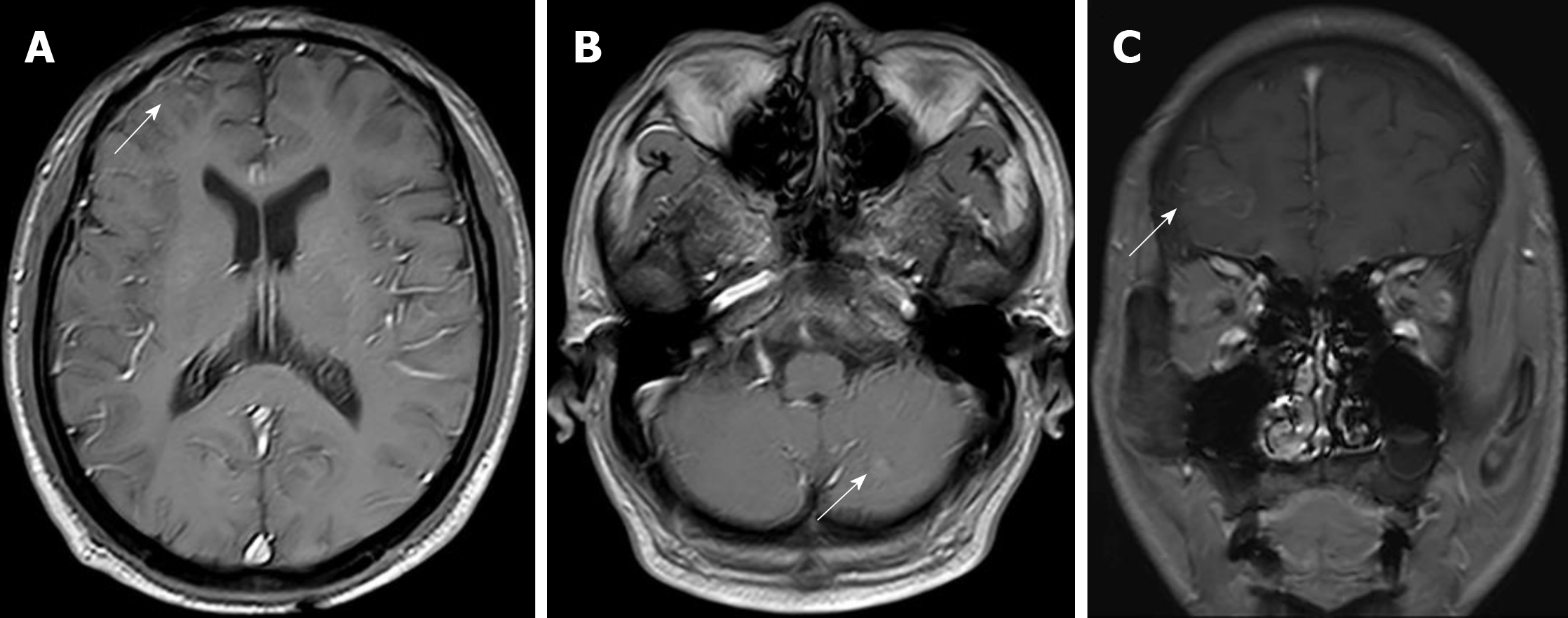
The patient tolerated this treatment well, and the clinical symptoms of nausea, vomiting, and headaches were gradually alleviated. The value of his CEA remained in the normal range, and MRI findings exhibited significantly shrunken CNS lesions (Figure 3) without any notable side effects during treatment. At the last follow-up in September 2019, a repeat MRI scan of the brain revealed further reduced lesions (Figure 4).
At present, it is generally recognized that EGFR-TKIs are a standard first-line therapy for NSCLC patients with targeted EGFR mutations, and they could significantly prolong the survival time[7]. New-generation TKIs, such as osimertinib, have better CNS permeability and control rates[8]. However, the therapy of CNS (brain and/or leptomeningeal) metastases from NSCLC is still challenging.
The main reason for progressive systemic disease in patients treated with TKIs is acquired TKI resistance, such as the T790M mutation, which is detected in two-thirds of all TKI tumour samples[9]. It is worth noting that there is different expression of the T790M mutation in the CNS and extra-CNS sites. Some studies have shown that the T790M mutation rate is lower in intracranial lesions than in extracranial lesions[10,11]. Therefore, it appears that the cause of CNS progression is different from the mechanisms of systemic progression.
Due to the restriction of the blood brain barrier (BBB), generations of TKIs have shown limited clinical efficacy within the CNS, especially in non-selected patients. Pharmacologic studies suggest that the cerebrospinal fluid (CSF) concentrations are higher in patients with CNS tumours than those in normal primates because of the disruption of the BBB caused by brain metastasis[12]. Antitumour drugs still cannot reach sufficient drug concentrations in the brain parenchyma for BM or in CSF for LM. Thus, improving the penetration into the protected space is considered the key for successful CNS metastasis treatment.
Icotinib hydrochloride is the first small-molecule EGFR-TKI exploited by the Chinese company[5,6]. The phase 4 ICOGEN trial has confirmed the efficacy and safety of icotinib in patients with advanced NSCLC. In EGFR-mutated patients, the objective response rate (ORR) and disease control rate (DCR) were 49.2% (327/665) and 92.3% (614/655), respectively[13]. Another two studies showed that in patients with advanced EGFR+ NSCLC, progression-free survival (PFS) associated with icotinib was longer than that with gefitinib and cisplatin/pemetrexed plus pemetrexed maintenance therapy[14,15]. In addition, icotinib showed positive efficacy in the patients with acquired EGFR Leu792H mutation after becoming resistant to the third-generation TKIs[16]. For CNS disease treatment, some studies have indicated that icotinib has definite efficacy and could provide survival benefits[17,18]. A phase 3 trial (BRAIN) was conducted to compare the antitumour activity between icotinib and WBRT in patients with multiple CNS metastases from EGFR-mutant NSCLC. The median intracranial PFS of the icotinib group (10.0 mo) was much longer than that of the WBRT group (4.8 mo)[19]. Furthermore, the combined treatment of icotinib and WBRT could further prolong the survival of the patients without increasing the occurrence of toxicity[20,21]. However, drug resistance inevitably develops after 6-12 mo of treatment[22,23]. A new therapeutic strategy is desperately in demand to overcome resistance.
Pulsatile administration, applying a high dose of medicine in a short time, has been effective for patients with TKI secondary drug resistance[24,25]. Several studies demonstrated that pulsatile erlotinib had a proven impact on CNS metastasis in EGFR+ patients, with a median PFS of 2.7 mo, but median OS had no apparent difference from that of patients treated with standard-dose erlotinib[25-27]. Liu et al[28] reported that higher-dose icotinib has good tolerability, practicability of long-period treatment, remarkable antitumour activity, and a favourable pharmacological profile. The present case obtained stable clinical remission for 12 mo with a standard dosage of icotinib (125 mg three times daily). Then, CNS lesions developed despite stable systemic control. He could not afford to try other expensive TKI. Thus, after full consultation with the patient, off-label pulsatile icotinib (1125 mg every 3 d) was administered. Until now, this new usage has lasted 25 mo and achieved a complete remission of neurological symptoms and almost vanished lesions without notable side effects.
This new mode for administration has the advantage that drug concentration in CSF and blood accumulates rapidly and effectively, which ensures adequate CSF penetration and better antitumour activity. When systemic disease is steady, pulsatile icotinib at an increased dose could overcome the drug resistance and is worth considering for isolated CNS progression.
In summary, pulsatile high-dose icotinib was well-tolerated, and achieved durable symptomatic remission, reduced CEA levels, and almost vanished lesions in this case. More clinical cases and further prospective studies are warranted to assess its safety and validity for treating CNS metastases from refractory EGFR-mutant NSCLC.
| 1. | Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 1091] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 2. | Goncalves PH, Peterson SL, Vigneau FD, Shore RD, Quarshie WO, Islam K, Schwartz AG, Wozniak AJ, Gadgeel SM. Risk of brain metastases in patients with nonmetastatic lung cancer: Analysis of the Metropolitan Detroit Surveillance, Epidemiology, and End Results (SEER) data. Cancer. 2016;122:1921-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017;53:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 4. | Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, Guo WB, Yan HH, Wu YL. Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol. 2016;11:1962-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 5. | Tan F, Shen X, Wang D, Xie G, Zhang X, Ding L, Hu Y, He W, Wang Y, Wang Y. Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung Cancer. 2012;76:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Shi Y, Sun Y, Yu J, Ding C, Wang Z, Wang C, Wang D, Wang C, Wang Z, Wang M, Zhi X, Lu Y, Feng J, Liu Y, Liu X, Liu W, Wu G, Li X, Li K, Li E, Li W, Chen G, Chen Z, Yu P, Wu N, Wu M, Xiao W, Zhang L, Zhang Y, Zhang S, Yang S, Song X, Lin D, Luo R, Shan L, Zhou C, Zhou Z, Zhao Q, Hu C, Hu Y, Guo Q, Chang J, Huang C, Zeng X, Han B, Han X, Jia B, Han Y, Huang Y. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 version). Asia Pac J Clin Oncol. 2017;13:87-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Reckamp KL. Targeted Therapy for Patients With Metastatic Non-Small Cell Lung Cancer. J Natl Compr Canc Netw. 2018;16:601-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, Pickup K, Jordan A, Hickey M, Grist M, Box M, Johnström P, Varnäs K, Malmquist J, Thress KS, Jänne PA, Cross D. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res. 2016;22:5130-5140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 579] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 9. | Su KY, Chen HY, Li KC, Kuo ML, Yang JC, Chan WK, Ho BC, Chang GC, Shih JY, Yu SL, Yang PC. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 443] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 10. | Nanjo S, Arai S, Wang W, Takeuchi S, Yamada T, Hata A, Katakami N, Okada Y, Yano S. MET Copy Number Gain Is Associated with Gefitinib Resistance in Leptomeningeal Carcinomatosis of EGFR-mutant Lung Cancer. Mol Cancer Ther. 2017;16:506-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, Ladanyi M, Miller VA, Pao W. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494-6501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 646] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 12. | Meany HJ, Fox E, McCully C, Tucker C, Balis FM. The plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite (OSI-420) after intravenous administration of erlotinib in non-human primates. Cancer Chemother Pharmacol. 2008;62:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Hu X, Han B, Gu A, Zhang Y, Jiao SC, Wang CL, He J, Jia X, Zhang L, Peng J, Wu M, Ying K, Wang J, Ma K, Zhang S, You C, Tan F, Wang Y, Ding L, Sun Y. A single-arm, multicenter, safety-monitoring, phase IV study of icotinib in treating advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2014;86:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Shi Y, Zhang L, Liu X, Zhou C, Zhang L, Zhang S, Wang D, Li Q, Qin S, Hu C, Zhang Y, Chen J, Cheng Y, Feng J, Zhang H, Song Y, Wu YL, Xu N, Zhou J, Luo R, Bai C, Jin Y, Liu W, Wei Z, Tan F, Wang Y, Ding L, Dai H, Jiao S, Wang J, Liang L, Zhang W, Sun Y. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 356] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 15. | Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, Ding CM, Song X, Ma ZY, Ren XL, Feng JF, Zhang HL, Chen GY, Han XH, Wu N, Yao C, Song Y, Zhang SC, Song W, Liu XQ, Zhao SJ, Lin YC, Ye XQ, Li K, Shu YQ, Ding LM, Tan FL, Sun Y. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28:2443-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 261] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 16. | Wang J, Chen J. Positive response to Icotinib in metastatic lung adenocarcinoma with acquiring EGFR Leu792H mutation after AZD9291 treatment: a case report. BMC Cancer. 2019;19:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Zhou L, He J, Xiong W, Liu Y, Xiang J, Yu Q, Liang M, Zhou X, Ding Z, Huang M, Ren L, Zhu J, Li L, Hou M, Ding L, Tan F, Lu Y. Impact of whole brain radiation therapy on CSF penetration ability of Icotinib in EGFR-mutated non-small cell lung cancer patients with brain metastases: Results of phase I dose-escalation study. Lung Cancer. 2016;96:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Fan Y, Huang Z, Fang L, Miao L, Gong L, Yu H, Yang H, Lei T, Mao W. A phase II study of icotinib and whole-brain radiotherapy in Chinese patients with brain metastases from non-small cell lung cancer. Cancer Chemother Pharmacol. 2015;76:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Yang JJ, Zhou C, Huang Y, Feng J, Lu S, Song Y, Huang C, Wu G, Zhang L, Cheng Y, Hu C, Chen G, Zhang L, Liu X, Yan HH, Tan FL, Zhong W, Wu YL. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017;5:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 20. | Jiang AY, Zhang J, Luo HL, Gao F, Lv YF. Icotinib and whole-brain radiotherapy for the treatment in patients with brain metastases from EGFR-mutant nonsmall cell lung cancer: A retrospective study. Medicine (Baltimore). 2018;97:e0312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Fan Y, Xu Y, Gong L, Fang L, Lu H, Qin J, Han N, Xie F, Qiu G, Huang Z. Effects of icotinib with and without radiation therapy on patients with EGFR mutant non-small cell lung cancer and brain metastases. Sci Rep. 2017;7:45193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Jänne PA, Lynch T, Johnson BE, Miller VA. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 663] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 23. | Guan YS, He Q, Li M. Icotinib: activity and clinical application in Chinese patients with lung cancer. Expert Opin Pharmacother. 2014;15:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Zhu Y, Du Y, Liu H, Ma T, Shen Y, Pan Y. Study of efficacy and safety of pulsatile administration of high-dose gefitinib or erlotinib for advanced non-small cell lung cancer patients with secondary drug resistance: A single center, single arm, phase II clinical trial. Thorac Cancer. 2016;7:663-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | How J, Mann J, Laczniak AN, Baggstrom MQ. Pulsatile Erlotinib in EGFR-Positive Non-Small-Cell Lung Cancer Patients With Leptomeningeal and Brain Metastases: Review of the Literature. Clin Lung Cancer. 2017;18:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Kawamura T, Hata A, Takeshita J, Fujita S, Hayashi M, Tomii K, Katakami N. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol. 2015;75:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, Holodny AI, Clarke JL, Lassman AB. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 279] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 28. | Liu J, Wu L, Wu G, Hu X, Zhou H, Chen J, Zhu M, Xu W, Tan F, Ding L, Wang Y, Shentu J. A Phase I Study of the Safety and Pharmacokinetics of Higher-Dose Icotinib in Patients With Advanced Non-Small Cell Lung Cancer. Oncologist. 2016;21:1294-1295d. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Taheri S, Yorioka N S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Qi LL