Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.4245
Peer-review started: April 13, 2020
First decision: July 25, 2020
Revised: July 31, 2020
Accepted: August 15, 2020
Article in press: August 15, 2020
Published online: September 26, 2020
Processing time: 161 Days and 18.2 Hours
Massive pulmonary haemorrhage can spoil the entire lung and block the airway in a short period of time due to severe bleeding, which quickly leads to death. Alveolar lavage is an effective method for haemostasis and airway maintenance. However, patients often cannot tolerate alveolar lavage due to severe hypoxia. We used extracorporeal membrane oxygenation (ECMO) to overcome this limitation in a patient with massive pulmonary haemorrhage due to severe trauma and succeeded in saving the life by repeated alveolar lavage.
A 22-year-old man sustained multiple injuries in a motor vehicle accident and was transferred to our emergency department. On admission, he had a slight cough and a small amount of bloody sputum; computed tomography revealed multiple fractures and mild pulmonary contusion. At 37 h after admission, he developed severe chest tightness, chest pain, dizziness and haemoptysis. His oxygen saturation was 68%. Emergency endotracheal intubation was performed, and a large amount of bloody sputum was suctioned. After transfer to the intensive care unit, he developed refractory hypoxemia and heparin-free venovenous ECMO was initiated. Fibreoptic bronchoscopy revealed diffuse and profuse blood in all bronchopulmonary segment. Bleeding was observed in the trachea and right bronchus, and repeated alveolar lavage was performed. On day 3, the patient’s haemoptysis ceased, and ECMO support was terminated 10 d later. Tracheostomy was performed on day 15, and the patient was weaned from the ventilator on day 21.
Alveolar lavage combined with ECMO can control bleeding in trauma-induced massive pulmonary haemorrhage, is safe and can be performed bedside.
Core Tip: Massive pulmonary haemorrhage due to trauma is a rare but life-threatening form of pulmonary contusion. In this work, we report a patient who presented with progressive massive pulmonary haemorrhage that was successfully treated with repeated alveolar lavage combined with extracorporeal membrane oxygenation. Our study indicates the efficacy of a novel option for the treatment of massive pulmonary haemorrhage. Moreover, we discuss the medication regimen of alveolar lavage and the management of heparin-free extracorporeal membrane oxygenation.
- Citation: Zhang BY, Chen XC, You Y, Chen M, Yu WK. Massive pulmonary haemorrhage due to severe trauma treated with repeated alveolar lavage combined with extracorporeal membrane oxygenation: A case report. World J Clin Cases 2020; 8(18): 4245-4251
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/4245.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.4245
Severe trauma is a leading cause of death among young adults worldwide and is often complicated by pulmonary contusion (PC)[1], which is defined as the destruction of the lung parenchyma by blunt force trauma to the chest and pulmonary haemorrhage due to pulmonary vascular injury. Approximately 10%-35% cases of trauma are complicated by PC[2,3]. Massive pulmonary haemorrhage due to trauma is a rare but life-threatening form of PC that often manifests as marked dyspnoea, tachypnoea and haemoptysis within hours of the trauma. Massive pulmonary haemorrhage can destroy the entire lung, and the severe bleeding can cause rapid blockage of the airway resulting in severe hypoxia and shock or even death. Therefore, simultaneous respiratory maintenance and bleeding control is the main principle of treatment for massive pulmonary haemorrhage[4].
Flexible bronchoscopes have become more advanced over the last two decades. Because of its relative safety and bedside availability, flexible bronchoscopy has become a common method for localizing the bleeding sites and controlling bleeding for mild to moderate haemoptysis[5]. However, the use of flexible bronchoscopes in the treatment of massive pulmonary haemorrhage in severe trauma patients, to our knowledge, has never been reported. One main reason is that such patients cannot tolerate alveolar lavage due to severe hypoxemia. Therefore, we used repeated alveolar lavage combined with extracorporeal membrane oxygenation (ECMO) to overcome this limitation and successfully treat a patient with massive pulmonary haemorrhage.
A 22-year-old man was admitted to our emergency department following a road traffic accident and presented with a slight cough and a small amount of bloody sputum. Approximately 37 h after admission, he developed severe chest tightness, chest pain, dizziness and haemoptysis. His was 178 cm in height and 82 kg in weight.
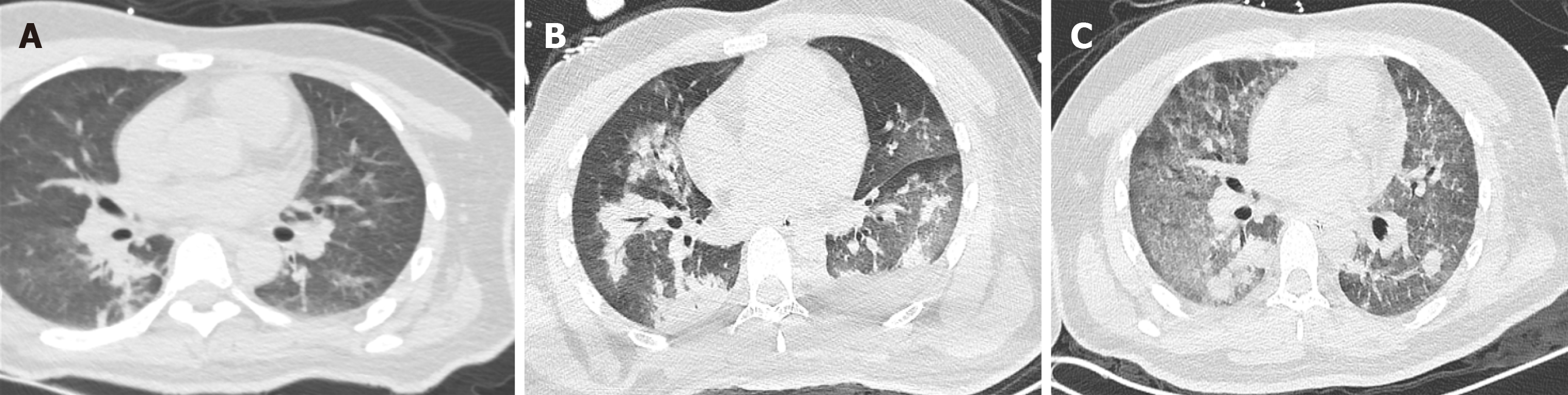
The patient experienced multiple traumas when his motorcycle collided with a truck. On admission to our emergency department, he was conscious and had a slight cough and a small amount of bloody sputum. His blood pressure and breathing were stable, with an oxygen saturation (SpO2) of 95%-97% at 5 L/min of mask O2. Whole-body computed tomography (CT) showed a left fifth rib fracture and mild PC (Figure 1A) as well as fractures to the left clavicle and scapula, pubis, left femoral shaft and right ulna. After plaster fixation of his right upper arm, he was admitted 18 h after the onset of mild PC to the orthopaedic ward for further surgical treatment. There he received an analgesic, antibiotics and left tibial tuberosity traction treatment. However, he developed severe chest tightness, chest pain, dizziness and haemoptysis 19 h later. Clinical examination revealed severe hypoxia with an SpO2 of 68%, tachypnoea (respiratory rate: 36 breaths/min) and tachycardia (heart rate: 157 bpm). Endotracheal intubation was subsequently performed, and a large amount of bloody sputum was suctioned from the endotracheal tube. The patient was transferred to the intensive care unit (ICU) 15 min later.
The patient was free of relevant medical history.
When transferred to the ICU, the patient received volume-controlled ventilation and the following parameters were set: Fraction of inspired oxygen, 100%; positive end-expiratory pressure, 15 cm H2O; tidal volume, 420 mL (6 mL/kg); and respiratory rate, 20 breaths/min. The patient was maintained under deep sedation (Richmond Agitation-Sedation Scale: -3 to -4) to reduce respiratory distress, and a lung recruitment manoeuvre was performed to open his airways. Nevertheless, he developed refractory hypoxemia (SpO2: 78%) and hypotension (invasive blood pressure: 10.5/5.5 kPa). Physical examination revealed coarse, moist rales that pervaded the lung. No apparent abnormalities were found in the heart or abdomen. Both legs were deformed and swollen, and multiple skin abrasions were observed all over his body.
Arterial blood gas analysis revealed a pH of 7.44, partial pressure of CO2 of 5.2 kPa and partial pressure of O2 of 7.9 kPa. Routine blood examination indicated mild leucocytosis (15.3 × 109 cells/L) and a reduction in haemoglobin (from 12.3 g/dL at admission to 10.3 g/dL after transfer to the ICU). Coagulation function test results showed a normal prothrombin time, activated partial thromboplastin time and thrombin time; however, his fibrinogen level was low (1.4 g/L). Biochemical examination revealed a glutamate transaminase level of 74.2 U/L and glutamate transaminase level of 44.9 U/L, indicating slightly abnormal liver function.
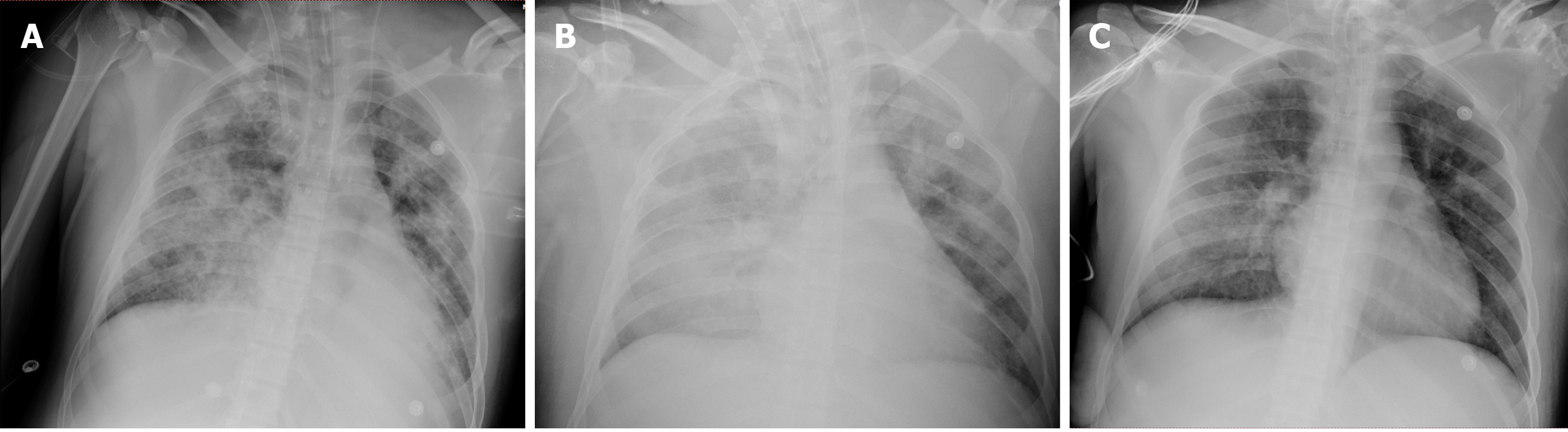
Bedside chest X-ray showed diffuse infiltration on both sides (Figure 2A). After ECMO was established, chest CT and pulmonary artery imaging were performed, at which point there was no evidence of a pulmonary embolism, but substantial consolidation was noted in both lungs (Figure 1B). Fibreoptic bronchoscopy revealed diffuse and profuse blood in all bronchopulmonary segments and fresh bleeding was observed in the trachea and right bronchus.
Massive pulmonary haemorrhage, acute respiratory distress syndrome, hypovolemic shock, multiple trauma, PC, left rib fracture, left clavicle fracture, left scapula fracture, pubis fracture, left femoral shaft fracture, right ulna fracture and mild liver injury.
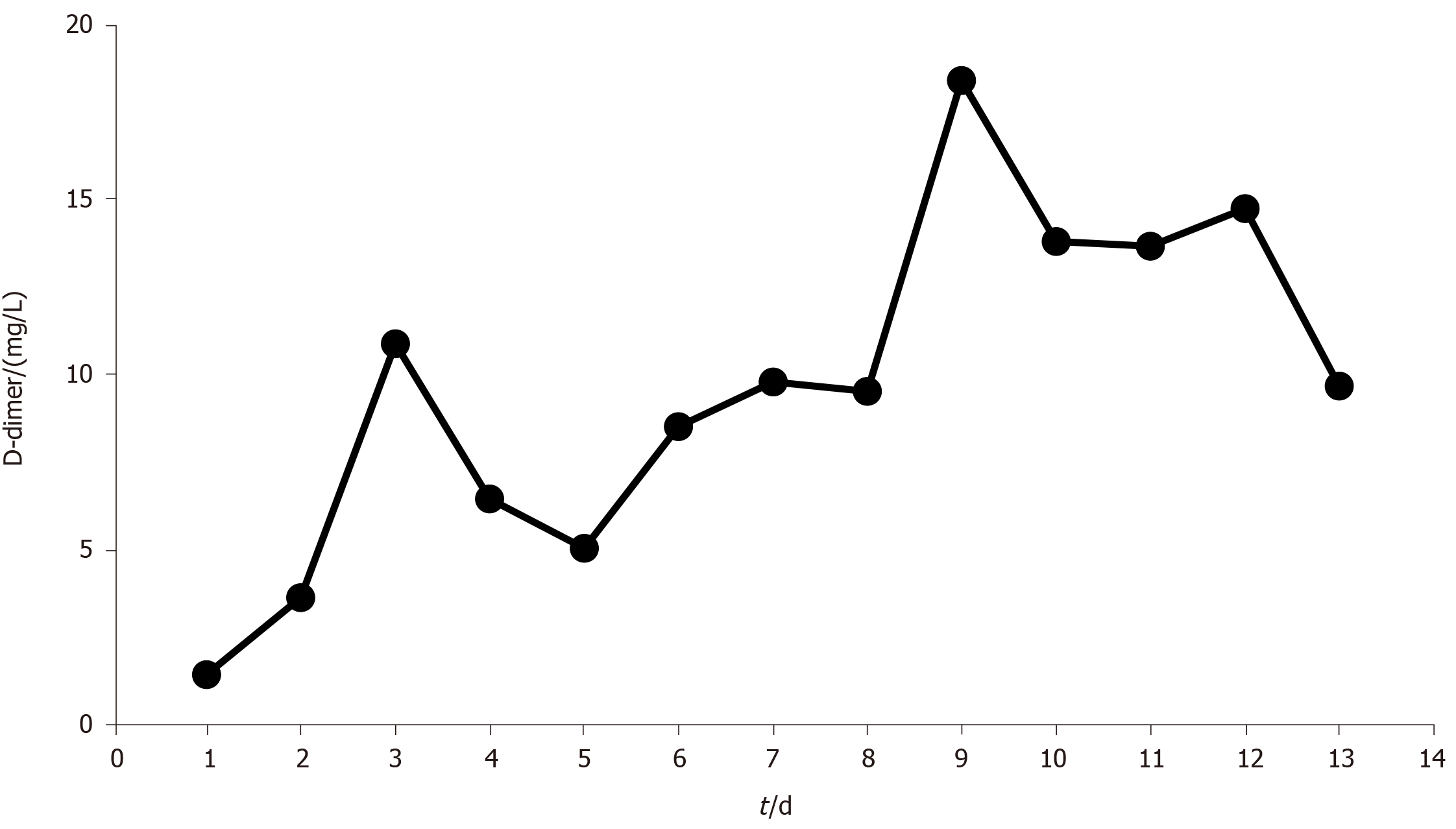
After the patient was transferred to the ICU, we established venovenous ECMO and performed cannulation via the right jugular vein (17-Fr cannula for inflow, Rastatt, Germany) and the right femoral vein (21-Fr cannula for outflow, Rastatt, Germany) simultaneously. After we established heparin-free venovenous ECMO (3500 rpm; pump flow, 4 L/min; O2 flow, 4 L/min), the patient’s SpO2 increased to 99%. The ventilator parameters were readjusted (fraction of inspired oxygen: 30%; positive end-expiratory pressure: 8 cm H2O; tidal volume: 380 mL; respiratory rate: 12/min) to reduce the risk of barotrauma. After chest CT and pulmonary artery imaging, therapeutic fibreoptic bronchoscopy was performed via endotracheal tube; this revealed diffuse and profuse blood in all bronchopulmonary segments. We suctioned the blood and performed alveolar lavage. Fresh bleeding was observed in the trachea and right bronchus. We repeated the cold saline lavage together with topical application of epinephrine (1:20000) and allowed the cold saline to remain within the bronchopulmonary segments on the right side for a few seconds. We continued to administer repeated alveolar lavage (once per day with 150 mL cold saline each time), antibiotic therapy, glucocorticoids and enteral nutrition. Bloody sputum was alleviated, and norepinephrine was discontinued on day 3. However, bedside chest X-ray revealed persistent, severe diffuse infiltration of both lungs (Figure 2B). We reduced the frequency of alveolar lavage (to every second day), performed negative fluid balance therapy and continued to perform ECMO without heparin. The patient’s D-dimer level gradually increased to 18.4 mg/L (Figure 3). On day 9, a large blood clot was found inside the microporous membrane oxygenator, and the O2 pressure of the blood drawn from behind the oxygenator was 11.2 kPa; hence, ECMO was replaced.
On day 13, fibreoptic bronchoscopy revealed blood clots on the bronchial wall but no active bleeding. Bedside chest X-ray showed significantly decreased lung infiltration (Figure 2C); thus, ECMO support was terminated on day 13. On the second day after ECMO termination (day 15), we performed another chest CT scan, which revealed further dissipation of lung consolidation (Figure 1C). Tracheostomy was performed on day 15, and the patient was weaned from the ventilator on day 21. He then returned to the orthopaedic ward and underwent surgery for his left clavicle, left femur and right ulna. The patient was discharged 1 wk later. The changes in PC and the course of treatment are presented in Table 1.
| Date | April 2, 2019 | April 3, 2019 | April 15, 2019 | April 23, 2019 |
| Diagnosis and treatment of PC | Chest CT scan showed mild PC | MV combined with ECMO to maintain oxygenation; Chest CT scan showed substantial consolidation; Bronchoscopy revealed diffuse pulmonary haemorrhage; Alveolar lavage combined with ECMO were performed | Fibreoptic bronchoscopy found no active bleeding; ECMO support was terminated; Chest CT scan indicated consolidation dissipation | The patient was weaned from the ventilator |
This is the first report describing a case of severe PC (presenting as massive pulmonary haemorrhage) that was successfully treated with repeated alveolar lavage combined with ECMO. PC caused by severe trauma is a major clinical challenge and may be fatal; trauma patients with PC have a significantly higher mortality rate than trauma patients without PC[6]. The pathological findings range from radiographic findings without clinical manifestations to respiratory failure requiring intubation and mechanical ventilation depending on the extent of the contusion and affected alveolar area. Massive pulmonary haemorrhage due to trauma is a rare but life-threatening form of PC. Severe hypovolemic shock from uncontrolled bleeding and respiratory failure due to airway obstruction caused by blood clots are the main causes of death. Maintenance of adequate oxygenation and bleeding control should be performed simultaneously to rescue life in cases of massive pulmonary haemorrhage.
Fibreoptic bronchoscopy plays an important role in the management of mild to moderate haemoptysis because it is relatively safe and can be performed at the patient’s bedside[5]. However, fibreoptic bronchoscopy is rarely applied in patients with massive pulmonary haemorrhage due to poor tolerance. Bronchoscopy can be used to localize the bleeding sites and clear the airways of blood to prevent infection and maintain adequate ventilation, particularly in the non-bleeding lung. More importantly, it supports the implementation of other endobronchial techniques such as alveolar lavage to control bleeding. Multiple agents, including cold saline, diluted epinephrine, ornipressin, terlipressin and tranexamic acid, are used to obtain haemostasis in alveolar lavage. Conlan et al[7] used alveolar lavage with cold saline to treat 12 patients with severe haemoptysis within 24 h and achieved haemostasis in all 12 patients. However, one patient experienced transient bradycardia, and two experienced recurrent bleeding. Epinephrine has been used in cases of massive haemoptysis to control bleeding; however, different doses and dilutions have been reported, from a 1:100000 solution to 20 mL of 1:20000 solution[8]. Unfortunately, the haemostatic effect of diluted epinephrine (1:20000) is not effective because the drug is easily flushed out with bleeding. Even at dilutions as low as 1:20000, arrhythmia, ventricular fibrillation and hypotension have been reported to occur with the use of epinephrine[9]. Tüller et al[10] reported the endobronchial application of ornipressin and terlipressin in 30 patients with mild to moderate airway bleeding following transbronchial lung biopsy. Terlipressin caused significant changes in heart rate and blood pressure; however, ornipressin did not result in any adverse events. Tranexamic acid, a synthetic antifibrinolytic agent, can also be applied endobronchially to treat massive haemoptysis following transbronchial lung biopsy, but few case reports have described its use[11,12].
Venovenous ECMO serves as the final recourse when conventional ventilation strategies fail to maintain oxygenation. This makes it possible to treat massive pulmonary haemorrhage with bronchoscopy. There have been a few case reports and small case series on the use of ECMO in trauma patients with various injury patterns, but mixed outcomes were reported[13-16]. However, ECMO is not often used in trauma patients, largely because of concerns about the risk of haemorrhage. In recent years, with advances in ECMO equipment, including centrifugal pumps, heparin-coated circuits and polymethylpentene oxygenators, the need for anticoagulants and the risk of thrombosis have decreased. This has led to a growing body of evidence on the use of heparin-free ECMO in trauma patients[17-20]. The longest reported duration of heparin-free venovenous ECMO in trauma patients is 11 d[19]. To maintain heparin-free ECMO for as long as possible, there are three recommendations: (1) The blood flow should be set at > 1.5 L/min; most reports state that the blood flow was set at 4-5 L/min to prevent blood clots; (2) All clotting factors should be administered via a peripheral vein or postoxygenator rather than via a central vein to preserve the membrane oxygenator; and (3) Coagulation indicators (activated partial thromboplastin time, prothrombin time, activated clotting time of the whole blood and D-dimer) need to be continuously monitored. D-dimer monitoring is important because it is a more sensitive indicator of thrombus formation in the membrane oxygenator[21]. Although the highest D-dimer level in our patient was lower than the cut-off value reported in the literature, his D-dimer levels steadily increased, and a large thrombus developed inside the membrane oxygenators on day 9. After the membrane oxygenators were exchanged, his D-dimer levels decreased. Regardless, heparin-free venovenous ECMO is not a first-line treatment option for respiratory failure in trauma patients with pulmonary haemorrhage due to the higher risk of thrombosis, bleeding and embolism. However, for patients with intractable hypoxemia due to trauma combined with massive pulmonary haemorrhage, ECMO is a highly effective rescue treatment option.
Our findings were merely a single case. In addition, ECMO requires an experienced team for rapid establishment, and this may be a challenge for some hospitals.
Massive pulmonary haemorrhage is a rare but life-threatening form of PC in trauma patients. Timely and effective control of bleeding and respiratory support therapy are vital to saving the patient’s life. Applying alveolar lavage combined with ECMO could be an alternative and effective treatment in patients with massive pulmonary haemorrhage; moreover, this strategy is relatively safe and can be performed at the patient’s bedside.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Pereira-Vega A S-Editor: Yan JP L-Editor: Filipodia P-Editor: Li JH
| 1. | Krug EG, Sharma GK, Lozano R. The global burden of injuries. Am J Public Health. 2000;90:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 767] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 2. | Hamrick MC, Duhn RD, Ochsner MG. Critical evaluation of pulmonary contusion in the early post-traumatic period: risk of assisted ventilation. Am Surg. 2009;75:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Jin H, Tang LQ, Pan ZG, Peng N, Wen Q, Tang YQ, Su L. Ten-year retrospective analysis of multiple trauma complicated by pulmonary contusion. Mil Med Res. 2014;1:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Crocco JA, Rooney JJ, Fankushen DS, DiBenedetto RJ, Lyons HA. Massive hemoptysis. Arch Intern Med. 1968;121:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 155] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Gavelli F, Patrucco F, Statti G, Balbo PE. Mild-to-moderate hemoptysis: a diagnostic and clinical challenge. Minerva Med. 2018;109:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Gayzik FS, Martin RS, Gabler HC, Hoth JJ, Duma SM, Meredith JW, Stitzel JD. Characterization of crash-induced thoracic loading resulting in pulmonary contusion. J Trauma. 2009;66:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Conlan AA, Hurwitz SS. Management of massive haemoptysis with the rigid bronchoscope and cold saline lavage. Thorax. 1980;35:901-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Radchenko C, Alraiyes AH, Shojaee S. A systematic approach to the management of massive hemoptysis. J Thorac Dis. 2017;9 Suppl 10:S1069-S1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Steinfort DP, Herth FJ, Eberhardt R, Irving LB. Potentially fatal arrhythmia complicating endobronchial epinephrine for control of iatrogenic bleeding. Am J Respir Crit Care Med. 2012;185:1028-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Tüller C, Tüller D, Tamm M, Brutsche MH. Hemodynamic effects of endobronchial application of ornipressin versus terlipressin. Respiration. 2004;71:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Zamani A. Bronchoscopic intratumoral injection of tranexamic acid: a new technique for control of biopsy-induced bleeding. Blood Coagul Fibrinolysis. 2011;22:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Hurley M, Bhatt J, Smyth A. Treatment massive haemoptysis in cystic fibrosis with tranexamic acid. J R Soc Med. 2011;104 Suppl 1:S49-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Ried M, Bein T, Philipp A, Müller T, Graf B, Schmid C, Zonies D, Diez C, Hofmann HS. Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: a 10-year institutional experience. Crit Care. 2013;17:R110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 677] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Madershahian N, Wittwer T, Strauch J, Franke UF, Wippermann J, Kaluza M, Wahlers T. Application of ECMO in multitrauma patients with ARDS as rescue therapy. J Card Surg. 2007;22:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Wu MY, Lin PJ, Tseng YH, Kao KC, Hsiao HL, Huang CC. Venovenous extracorporeal life support for posttraumatic respiratory distress syndrome in adults: the risk of major hemorrhages. Scand J Trauma Resusc Emerg Med. 2014;22:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Lorini FL, Grazioli L, Manfredi R, Rausa E, Ghitti D, Poli G, Peck M, Cattaneo S. A prolonged and successful heparin-free extracorporeal membrane oxygenation run in isolated thoracic trauma: A case report. Int J Artif Organs. 2020;43:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Ryu KM, Chang SW. Heparin-free extracorporeal membrane oxygenation in a patient with severe pulmonary contusions and bronchial disruption. Clin Exp Emerg Med. 2018;5:204-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Park JM, Kim CW, Cho HM, Son BS, Kim DH. Induced airway obstruction under extracorporeal membrane oxygenation during treatment of life-threatening massive hemoptysis due to severe blunt chest trauma. J Thorac Dis. 2014;6:E255-E258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Wen PH, Chan WH, Chen YC, Chen YL, Chan CP, Lin PY. Non-heparinized ECMO serves a rescue method in a multitrauma patient combining pulmonary contusion and nonoperative internal bleeding: a case report and literature review. World J Emerg Surg. 2015;10:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Dornia C, Philipp A, Bauer S, Stroszczynski C, Schreyer AG, Müller T, Koehl GE, Lehle K. D-dimers Are a Predictor of Clot Volume Inside Membrane Oxygenators During Extracorporeal Membrane Oxygenation. Artif Organs. 2015;39:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |