Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2102
Peer-review started: February 27, 2020
First decision: April 29, 2020
Revised: May 13, 2020
Accepted: May 23, 2020
Article in press: May 23, 2020
Published online: June 6, 2020
Processing time: 102 Days and 0.7 Hours
Adolescent idiopathic scoliosis is the most common spinal deformity during puberty, especially in females. It is characterized by aberrant skeletal growth and generalized reduced bone density, which is associated with impaired bone mineral metabolism. Despite recent progress in multidisciplinary research to support various hypotheses, the pathogenesis of Adolescent idiopathic scoliosis is still not clearly understood. One of the hypothesis is to study the role of mesenchymal stem cells due to its involvement in the above-mentioned bone metabolic abnormalities. In this review, we will summarize reported literatures on the role of mesenchymal stem cells, particularly in the pathogenesis of Adolescent idiopathic scoliosis. In addition, we will describe the research on mesenchymal stem cells of Adolescent idiopathic scoliosis performed using bioinformatics tools.
Core tip: Adolescent idiopathic scoliosis (AIS) is a common spinal deformity that occurs in adolescent females. Despite many reports, the pathomechanism of this disease is still unclear. Mesenchymal stem cells (MSCs) that differentiate into multiple cell lineages are known to contribute to the initiation and progression of AIS, but the exact role has not been understood yet. In this review, we summarize a series of studies on the role of mesenchymal stem cells, with differential expression levels of gene, RNA, and protein, in the pathogenesis of AIS. Furthermore, we present the future perspectives on the role of MSCs in the clinical outcome of AIS.
- Citation: Ko DS, Kim YH, Goh TS, Lee JS. Altered physiology of mesenchymal stem cells in the pathogenesis of adolescent idiopathic scoliosis. World J Clin Cases 2020; 8(11): 2102-2110
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2102.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2102
Adolescent idiopathic scoliosis (AIS) is a complex three-dimensional deformity of the spine that is accompanied not only by coronal plane deformity but also by deformity in the sagittal and axial planes[1]. It mostly occurs in adolescent females with pubertal growth spurt[2-4]. Despite extensive research over the years, there is no established etiology for the pathogenesis of AIS[4-6]. Multiple factors such as genetics, melatonin deficiency, platelet abnormality, unique upright posture of humans, and aberrant growth of endochondral-membranous bone growth contribute to the initiation and progression of the complex three dimensional curve in AIS[4,6-13]. However, there is still a lot of controversy regarding the extent and the manner in which each of these factors contribute to the development of AIS.
Mesenchymal stem cells (MSCs) differentiate into progenitor cells of bone, cartilage, and adipose tissues[14-16]. Altered MSC proliferation, division, and differentiation could lead to abnormal physiological processes or osteogenic disease[17,18]. Functional defects and reduced cell proliferation of osteoblasts and their precursors, MSCs, cause an imbalance between bone formation and bone resorption resulting in osteoporosis, which is characterized by a reduction in bone mass[19,20]. Two major features of AIS, namely, systemic low bone mass and disproportionate endochondral-membranous bone growth, are known to be associated with abnormal differentiation of MSCs[21-26].
Since the pathogenesis of AIS is multifactorial and complex, the role of MSCs in the pathogenesis of this disease has not been reviewed yet. Herein, we will discuss the studies to date on the abnormal differentiation of MSCs in patients with AIS[27,28]. Furthermore, the expression profile of genes, proteins and noncoding RNAs of MSCs, between patients with AIS and normal individuals is different[16,29-33]. This review highlights the role of MSCs in the pathogenesis of AIS, thereby contributing not only to further research but also to improve clinical outcome.
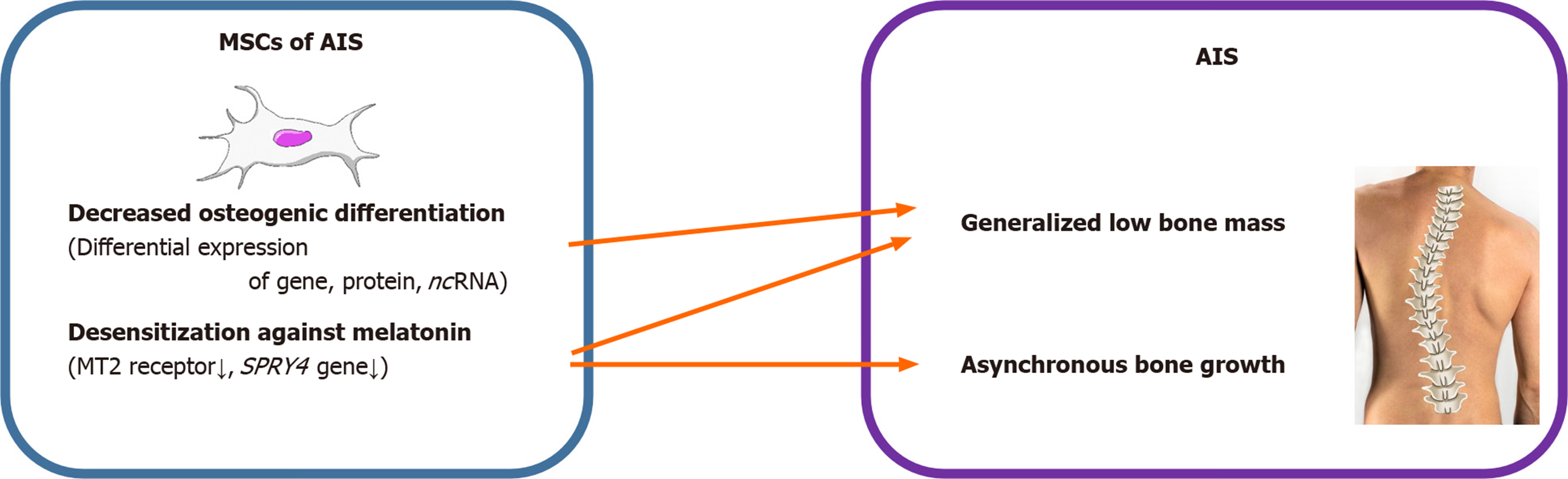
AIS is characterized by generalized osteopenia and systemic low bone mass in both axial and peripheral skeletons and disproportionate endochondral-membranous bone growth[22,34]. The exact pathomechanism for these characteristics is mostly unknown. However, it has been predicted that osteoblasts and their progenitors, MSCs, might contribute to these features of AIS. Park et al[28] conducted an age- and sex-matched comparative study of patients with AIS and patients with lower leg fracture as control group to define the relationship between bone mineral density and differentiation potential of MSCs. Compared to the control group, the mean lumbar spinal bone mineral density was significantly low in AIS patients, while mean femoral neck bone mineral density was not altered. There was no difference in the mean doubling time and adipogenic differentiation abilities of MSCs obtained from patients with AIS and that of the control group. Both the osteogenic differentiation ability and alkaline phosphatase (ALP) activity of MSCs from patients with AIS were significantly lower than those of the control group. Correlation analysis demonstrated that there was no association between osteogenic and adipogenic differentiation abilities of MSCs and bone mineral density in either group, while there was a positive correlation between osteogenic differentiation ability of MSCs and ALP activity of patients with AIS.
The pathoetiology of AIS has been reported to be associated with a dysfunctional melatonin pathway including melatonin receptor 1B gene polymorphism and melatonin deficiency[35-37]. MSCs of patients with AIS showed lower expression of melatonin receptor 2 (MT2) than those of controls, while the expression of melatonin receptor 1 had no significant difference between the two groups. Melatonin did not alter the differentiation ability in patients with AIS. However, melatonin promoted osteogenic and chondrogenic differentiation by increasing ALP activity and glycosaminoglycan synthesis and upregulating the expression of genes including ALP, osteopontin, osteocalcin, and collagen type II in normal controls. Decreased osteogenic differentiation ability, relatively lower expression of MT2, and lack of response of MSCs to melatonin might contribute to generalized low bone mass and abnormal dissociation of endochondral-membranous bone growth resulting in the development and progression of AIS.
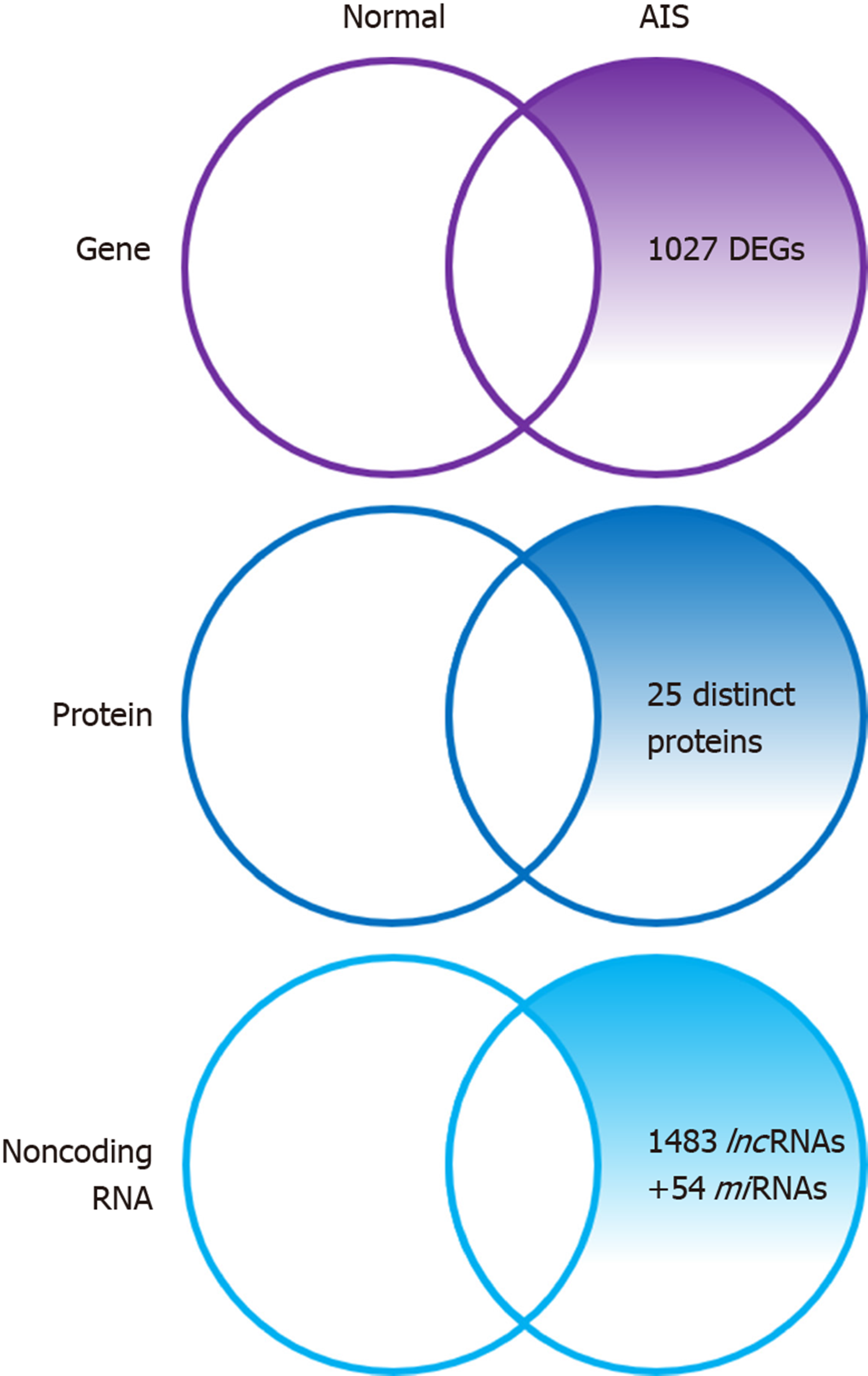
Zhuang et al[32] identified 1027 novel differentially expressed genes (DEGs) in MSCs of patients with AIS compared to normal controls. Of these 1027 DEGs, 551 genes were upregulated including SMAD3 and HOXC69 and 476 genes were downregulated including MAP2K1 and HSPA6. Gene ontology (GO) analysis confirmed that the upregulated DEGs belonged mainly to categories such as small GTPase-mediated signal transduction, DNA-dependent transcription, and cytokinesis. The downregulated DEGs included genes involved in small-molecule metabolic process, cell adhesion, etc. Pathway analysis demonstrated that several biological pathways were significantly upregulated and downregulated including mitogen-activated protein kinase (MAPK) signaling pathway, peroxisome proliferator-activated receptor signaling pathway, calcium signaling pathway, and Notch signaling pathway. All these identified pathways were known to have an important role in controlling the osteogenic and adipogenic differentiation of MSCs. Finally, 24 genes including mitogen-activated protein kinase kinase 1 (MAP2K1), SMAD family member 3 (SMAD3), homeobox C6 (HOXC6), heat shock 70 kDa protein 6 (HSPA6), and general transcription factor IIi (GTF2I), were identified as the most significant genes, which might play essential roles in the pathogenesis of AIS, through network analysis of DEGs from the above-mentioned significant pathways.
Among the 1027 DEGs, SPRY4 was the most significantly downregulated gene involved in MAPK signaling, and has been reported to be crucial for both osteogenic differentiation and melatonin response. A previous study has reported that SPRY4 expression was significantly decreased in MSCs of patients with AIS[32]. Knockdown of SPRY4 in MSCs of healthy controls using siRNAs showed impairment in osteogenic differentiation of MSCs. Moreover, ectopic expression of SPRY4 in MSCs of healthy controls by lenti-SPRY4 infection showed enhancement in osteogenic differentiation of MSCs. Melatonin treatment increased SPRY4 expression in MSCs, and upregulation of SPRY4 gene in these cells facilitated the differentiation of osteoblasts from MSCs. Collectively, impaired expression of SPRY4 in MSCs might contribute to aberrant bone metabolism and skeletal growth in AIS by desensitizing melatonin response.
Research regarding the role of MSCs in patients with AIS has been largely focused on osteogenic or chondrogenic differentiation[27,28,38,39]. Recently, abnormal adipogenesis in AIS has been considered in addition to leptin might contribute to the pathogenesis of AIS[31]. In vitro adipogenic differentiation of MSCs from AIS patients and normal controls followed by microarray analysis detected 300 DEGs. Seven genes including Spot14 (THRSP, thyroid hormone responsive protein) showed the most marked differential expression between MSCs of patients with AIS and normal controls. Spot14 mRNA and protein were highly expressed during adipogenic differentiation of MSCs in AIS patients. Immunohistochemistry indicated that the ratio of positively stained Spot14 cells in adipose tissue samples from AIS patients was higher than that of normal controls. Although the study was limited to Spot14, which plays an important role in the regulation of adipose tissue differentiation, adipogenic differentiation was found to be different in AIS patients and normal individuals. Based on this study, the exact role of adipogenic differentiation in AIS needs to be further studied.
Differential proteome analysis revealed 25 significantly distinct proteins in MSCs of AIS patients compared to that of normal controls[16]. Among the 25 distinct proteins, five were associated with bone growth and metabolism. These included pyruvate kinase M2 (PKM2), annexin A2, β-actin, γ-actin, and heat shock 27 kDa protein (HSP27). Downregulation of β-actin and γ-actin in MSCs of AIS patients indicated impaired osteogenic differentiation potential, which was consistent with previous studies. Downregulation of proteins such as β-actin, γ-actin, HSP27, WD repeat-containing protein 1, and moesin which were localized in the cytoskeleton might contribute to the initiation and progression of AIS. Downregulation of β-actin, γ-actin, HSP27, and annexin A2 and upregulation of PKM2 indicated decreased osteogenic differentiation capacity and increased proliferation ability of MSCs in AIS, and the former might contribute to low bone mass in AIS.
Non-coding RNA (ncRNA), is a functional RNA molecule, which is transcribed from DNA but not translated into protein, includes microRNA (miRNA), long non-coding RNA (lncRNA), etc. and plays a role in regulating gene expression[40]. Recently, studies regarding the roles of ncRNAs in the pathogenesis of AIS have been reported[29,33,41].
Zhuang et al[33] identified 1483 differentially expressed lncRNAs by microarray analysis of bone marrow MSCs from AIS patients and normal controls. LncAIS (gene symbol: ENST00000453347) was defined to be most significantly downregulated among the 10 lncRNAs from MSCs of patients with AIS. Silencing of lncAIS in normal MSCs in vitro leads to inhibition of osteogenic differentiation and ectopic bone formation in vivo. Inversely, overexpression of lncAIS in normal MSCs enhances osteogenic differentiation and bone formation. In normal MSCs, lncAIS interacts with NF90 to stabilize HOXD8 mRNA. Stable HODX8 promotes RUNX2 expression, resulting in osteogenic differentiation whereas downregulation of lncAIS impairs NF90 recruitment and destabilizes HOXD8 mRNA and MSCs, resulting in decreased osteogenic differentiation in AIS patients.
Hui et al[29] identified novel 54 differentially expressed miRNAs in bone marrow mesenchymal cells of AIS patients through microarray analysis. Of the 54 differentially expressed miRNAs, 42 miRNAs including miR-17-5p and miR-106a had increased expression and miRNAs such as miR-199a-3p, miR-29c-3p had decreased expression compared to that of non-AIS controls. Comprehensive bioinformatics studies including functional analysis by GO analysis, KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis, and network analysis were performed. Upregulated miRNAs were associated with transmembrane transport, small-molecule metabolic process, and immune response, while downregulated miRNAs were involved in DNA-dependent transcription, cytokinesis, and small GTPase-mediated signal transduction. Pathway analysis showed that calcium signaling, Notch signaling, and ubiquitin-mediated proteolysis pathways were significantly upregulated, while MAPK signaling and phosphatidylinositol-3-kinase-Akt signaling pathways were significantly downregulated. Network analysis of differentially expressed miRNAs, GO categories, and significant biological pathways revealed seven most significant miRNAs (miR-17-5p, miR-106a-5p, miR-106-5p, miR-16-5p, miR-93a-5p, miR-15a-5p, and miR-181b-5p). Previously, Zhuang et al[33] identified 1027 DEGs in bone marrow mesenchymal cells of AIS patients. Corresponding with the differentially expressed miRNAs, 204 target genes such as MAP2K1, SMAD3, GTF21, and DUSP were associated with the predicted miRNAs. The study uncovered several novel biological pathways that were hitherto not found to be associated with osteogenic differentiation of MSCs.
As discussed above, studies have been conducted to determine the exact role of MSCs in the pathogenesis of different diseases. Attempts have been made to treat various diseases using MSCs[42]. Previously, MSCs were solely used in the field of cell replacement such as autologous iliac bone graft in bone-related surgery[43,44]. Currently, MSCs are being used to treat diseases, by regulating the surrounding cytokine milieu, via secretion of paracrine factors[45]. Indeed, MSCs have been useful for the treatment of inflammatory diseases such as rheumatoid arthritis and psoriasis, as well as for left ventricular failure following myocardial infarction and aging frailty[46-49]. Furthermore, research has demonstrated the positive effects of MSCs-containing scaffold grafting in angular deformity after growth plate injury[50]. Recently, Brzoska et al[51] evaluated the contribution of muscle component in the initiation of AIS in various ways and examined the possibility of applying regenerative medicine using stem cells in AIS compared to the results of other diseases. Therapeutic strategy of precisely injecting myogenic precursor cells, which are reinforced through genetic modulation of MSCs, into the target muscle using imaging modality will soon begin. Collectively, after more accurately understanding the pathomechanism of AIS, treatment using MSCs will also be initiated. In the near future, it is expected that treatments to prevent AIS would be developed and widely used, possibly by regulating growth rate in the x, y, and z axes by injecting MSCs into growth plates or muscle, or by functional modulation of MSCs through ncRNAs.
AIS is a unique disease that occurs only in people walking upright. With the development of various surgical treatment techniques and the development of clinical guidelines, patient satisfaction and treatment outcomes are getting better. However, as discussed above, no clear pathogenesis is known yet, and research is being conducted in various fields. Herein, we reviewed and summarized the basic and bioinformatics-based researches regarding the expression levels of mesenchymal stem cell-derived genes, proteins, and noncoding RNAs and their role in the pathogenesis of adolescent idiopathic scoliosis (Figure 1, Table 1). This review shows that the low bone mass in the body, which is one of the major features of AIS, is closely related to decreased osteogenic differentiation of MSCs and the aberrant response of these cells to melatonin (Figure 2, Table 1). Although studies on MSCs that have been reviewed so far are related to AIS and MSCs, they are not completely definitive and can be considered as a basis for further research. Based on this, multidisciplinary research and clinical trials utilizing MSCs for AIS treatment would be attempted for advancing clinical treatment.
| Function | Gene | Protein | ncRNA |
| Decreased osteogenic differentiation | 1027 DEGs | 25 distinct proteins | 1483 differentially expressed lncRNAs 54 differentially expressed miRNAs |
| 24 central genes (MAP2K1, SMAD3, HOXC6, HSPA7, GTF2I, etc.) | β-actin, γ-actin, HSP27, etc.↓ PKM2↑ | lncAIS 204 target genes (MAP2K1, SMAD3, GTF21, etc.) | |
| Desensitization against melatonin | SPRY4↓ | MT2↓ |
We would like to express gratitude for MD Yoon Jae Cho for his working on figures of this manuscript.
| 1. | Cheng JC, Castelein RM, Chu WC, Danielsson AJ, Dobbs MB, Grivas TB, Gurnett CA, Luk KD, Moreau A, Newton PO, Stokes IA, Weinstein SL, Burwell RG. Adolescent idiopathic scoliosis. Nat Rev Dis Primers. 2015;1:15030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 418] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 2. | Cheung KM, Wang T, Qiu GX, Luk KD. Recent advances in the aetiology of adolescent idiopathic scoliosis. Int Orthop. 2008;32:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Hresko MT. Clinical practice. Idiopathic scoliosis in adolescents. N Engl J Med. 2013;368:834-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Wise CA, Gao X, Shoemaker S, Gordon D, Herring JA. Understanding genetic factors in idiopathic scoliosis, a complex disease of childhood. Curr Genomics. 2008;9:51-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Machida M. Cause of idiopathic scoliosis. Spine (Phila Pa 1976). 1999;24:2576-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Peng Y, Wang SR, Qiu GX, Zhang JG, Zhuang QY. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin Med J (Engl). 2020;133:483-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Ford DM, Bagnall KM, Clements CA, McFadden KD. Muscle spindles in the paraspinal musculature of patients with adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 1988;13:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Ford DM, Bagnall KM, McFadden KD, Greenhill BJ, Raso VJ. Paraspinal muscle imbalance in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 1984;9:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Herman R, Mixon J, Fisher A, Maulucci R, Stuyck J. Idiopathic scoliosis and the central nervous system: a motor control problem. The Harrington lecture, 1983. Scoliosis Research Society. Spine (Phila Pa 1976). 1985;10:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Kindsfater K, Lowe T, Lawellin D, Weinstein D, Akmakjian J. Levels of platelet calmodulin for the prediction of progression and severity of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1994;76:1186-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Kulis A, Goździalska A, Drąg J, Jaśkiewicz J, Knapik-Czajka M, Lipik E, Zarzycki D. Participation of sex hormones in multifactorial pathogenesis of adolescent idiopathic scoliosis. Int Orthop. 2015;39:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J, Toriyama S. Pathogenesis of idiopathic scoliosis: SEPs in chicken with experimentally induced scoliosis and in patients with idiopathic scoliosis. J Pediatr Orthop. 1994;14:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Yang Y, Wu Z, Zhao T, Wang H, Zhao D, Zhang J, Wang Y, Ding Y, Qiu G. Adolescent idiopathic scoliosis and the single-nucleotide polymorphism of the growth hormone receptor and IGF-1 genes. Orthopedics. 2009;32:411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38 Suppl 1:S26-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 15. | Chu WC, Man GC, Lam WW, Yeung BH, Chau WW, Ng BK, Lam TP, Lee KM, Cheng JC. Morphological and functional electrophysiological evidence of relative spinal cord tethering in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2008;33:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Zhuang Q, Li J, Wu Z, Zhang J, Sun W, Li T, Yan Y, Jiang Y, Zhao RC, Qiu G. Differential proteome analysis of bone marrow mesenchymal stem cells from adolescent idiopathic scoliosis patients. PLoS One. 2011;6:e18834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 315] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res. 1998;13:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 263] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 237] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Rodríguez JP, Astudillo P, Ríos S, Pino AM. Involvement of adipogenic potential of human bone marrow mesenchymal stem cells (MSCs) in osteoporosis. Curr Stem Cell Res Ther. 2008;3:208-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Cheng JC, Guo X. Osteopenia in adolescent idiopathic scoliosis. A primary problem or secondary to the spinal deformity? Spine (Phila Pa 1976). 1997;22:1716-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Cheng JC, Guo X, Sher AH. Persistent osteopenia in adolescent idiopathic scoliosis. A longitudinal follow up study. Spine (Phila Pa 1976). 1999;24:1218-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Cheng JC, Qin L, Cheung CS, Sher AH, Lee KM, Ng SW, Guo X. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res. 2000;15:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Cook SD, Harding AF, Morgan EL, Nicholson RJ, Thomas KA, Whitecloud TS, Ratner ES. Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop. 1987;7:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Suh KT, Lee SS, Hwang SH, Kim SJ, Lee JS. Elevated soluble receptor activator of nuclear factor-kappaB ligand and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 2007;16:1563-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Thomas KA, Cook SD, Skalley TC, Renshaw SV, Makuch RS, Gross M, Whitecloud TS, Bennett JT. Lumbar spine and femoral neck bone mineral density in idiopathic scoliosis: a follow-up study. J Pediatr Orthop. 1992;12:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Chen C, Xu C, Zhou T, Gao B, Zhou H, Chen C, Zhang C, Huang D, Su P. Abnormal osteogenic and chondrogenic differentiation of human mesenchymal stem cells from patients with adolescent idiopathic scoliosis in response to melatonin. Mol Med Rep. 2016;14:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Park WW, Suh KT, Kim JI, Kim SJ, Lee JS. Decreased osteogenic differentiation of mesenchymal stem cells and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 2009;18:1920-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Hui S, Yang Y, Li J, Li N, Xu P, Li H, Zhang Y, Wang S, Lin G, Li S, Qiu G, Zhao RC, Zhang J, Zhuang Q. Differential miRNAs profile and bioinformatics analyses in bone marrow mesenchymal stem cells from adolescent idiopathic scoliosis patients. Spine J. 2019;19:1584-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Li J, Li N, Chen Y, Hui S, Fan J, Ye B, Fan Z, Zhang J, Zhao RC, Zhuang Q. SPRY4 is responsible for pathogenesis of adolescent idiopathic scoliosis by contributing to osteogenic differentiation and melatonin response of bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2019;10:805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Wang Q, Yang J, Lin X, Huang Z, Xie C, Fan H. Spot14/Spot14R expression may be involved in MSC adipogenic differentiation in patients with adolescent idiopathic scoliosis. Mol Med Rep. 2016;13:4636-4642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Zhuang Q, Mao W, Xu P, Li H, Sun Z, Li S, Qiu G, Li J, Zhang J. Identification of Differential Genes Expression Profiles and Pathways of Bone Marrow Mesenchymal Stem Cells of Adolescent Idiopathic Scoliosis Patients by Microarray and Integrated Gene Network Analysis. Spine (Phila Pa 1976). 2016;41:840-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Zhuang Q, Ye B, Hui S, Du Y, Zhao RC, Li J, Wu Z, Li N, Zhang Y, Li H, Wang S, Yang Y, Li S, Zhao H, Fan Z, Qiu G, Zhang J. Long noncoding RNA lncAIS downregulation in mesenchymal stem cells is implicated in the pathogenesis of adolescent idiopathic scoliosis. Cell Death Differ. 2019;26:1700-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Guo X, Chau WW, Chan YL, Cheng JC. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis. Results of disproportionate endochondral-membranous bone growth. J Bone Joint Surg Br. 2003;85:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Lombardi G, Akoume MY, Colombini A, Moreau A, Banfi G. Biochemistry of adolescent idiopathic scoliosis. Adv Clin Chem. 2011;54:165-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Qiu XS, Tang NL, Yeung HY, Lee KM, Hung VW, Ng BK, Ma SL, Kwok RH, Qin L, Qiu Y, Cheng JC. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2007;32:1748-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Qiu Y, Wu L, Wang B, Yu Y, Zhu Z. Asymmetric expression of melatonin receptor mRNA in bilateral paravertebral muscles in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2007;32:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Burwell RG, Aujla RK, Grevitt MP, Dangerfield PH, Moulton A, Randell TL, Anderson SI. Pathogenesis of adolescent idiopathic scoliosis in girls - a double neuro-osseous theory involving disharmony between two nervous systems, somatic and autonomic expressed in the spine and trunk: possible dependency on sympathetic nervous system and hormones with implications for medical therapy. Scoliosis. 2009;4:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Xu E, Shao W, Jiang H, Lin T, Gao R, Zhou X. A Genetic Variant in GPR126 Causing a Decreased Inclusion of Exon 6 Is Associated with Cartilage Development in Adolescent Idiopathic Scoliosis Population. Biomed Res Int. 2019;2019:4678969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Jiang X, Yu H, Teo CR, Tan GS, Goh SC, Patel P, Chua YK, Hameed NB, Bertoletti A, Patzel V. Advanced Design of Dumbbell-shaped Genetic Minimal Vectors Improves Non-coding and Coding RNA Expression. Mol Ther. 2016;24:1581-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | García-Giménez JL, Rubio-Belmar PA, Peiró-Chova L, Hervás D, González-Rodríguez D, Ibañez-Cabellos JS, Bas-Hermida P, Mena-Mollá S, García-López EM, Pallardó FV, Bas T. Circulating miRNAs as diagnostic biomarkers for adolescent idiopathic scoliosis. Sci Rep. 2018;8:2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Murray IR, Chahla J, Safran MR, Krych AJ, Saris DBF, Caplan AI, LaPrade RF; Cell Therapies Communication Expert Group. International Expert Consensus on a Cell Therapy Communication Tool: DOSES. J Bone Joint Surg Am. 2019;101:904-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 43. | Park JS, Kim BC, Kim BH, Lee JI, Lee J. Up-and-Coming Mandibular Reconstruction Technique With Autologous Human Bone Marrow Stem Cells and Iliac Bone Graft in Patients With Large Bony Defect. J Craniofac Surg. 2015;26:e718-e720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38 Suppl 1:S75-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 384] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 45. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1365] [Article Influence: 195.0] [Reference Citation Analysis (0)] |
| 46. | Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, Gersh BJ, Povsic TJ, Henry TD, Alexandre B, Homsy C, Edwards C, Seron A, Wijns W, Cotter G; CHART Investigators. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail. 2017;19:1520-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 47. | Schulman IH, Balkan W, Hare JM. Mesenchymal Stem Cell Therapy for Aging Frailty. Front Nutr. 2018;5:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Luque-Campos N, Contreras-López RA, Jose Paredes-Martínez M, Torres MJ, Bahraoui S, Wei M, Espinoza F, Djouad F, Elizondo-Vega RJ, Luz-Crawford P. Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Front Immunol. 2019;10:798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 49. | Seetharaman R, Mahmood A, Kshatriya P, Patel D, Srivastava A. Mesenchymal Stem Cell Conditioned Media Ameliorate Psoriasis Vulgaris: A Case Study. Case Rep Dermatol Med. 2019;2019:8309103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Azarpira MR, Shahcheraghi GH, Ayatollahi M, Geramizadeh B. Tissue engineering strategy using mesenchymal stem cell-based chitosan scafolds in growth plate surgery: a preliminary study in rabbits. Orthop Traumatol Surg Res. 2015;101:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Brzoska E, Kalkowski L, Kowalski K, Michalski P, Kowalczyk P, Mierzejewski B, Walczak P, Ciemerych MA, Janowski M. Muscular Contribution to Adolescent Idiopathic Scoliosis from the Perspective of Stem Cell-Based Regenerative Medicine. Stem Cells Dev. 2019;28:1059-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang RL S-Editor: Wang J L-Editor: A E-Editor: Liu JH