Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.2001
Peer-review started: February 10, 2020
First decision: March 5, 2020
Revised: March 26, 2020
Accepted: April 17, 2020
Article in press: April 17, 2020
Published online: May 26, 2020
Processing time: 104 Days and 19.8 Hours
Both Gilbert's syndrome (GS) and hereditary spherocytosis (HS) are common genetic disorders. However, comorbidity of GS with HS has always been considered a rare phenomenon, and it can impede accurate diagnoses in the presence of isolated unconjugated hyperbilirubinemia.
In a study on Levitt’s carbon monoxide (CO) breath test for the differential diagnosis of isolated hyperbilirubinemia, we found six GS patients with HS in 6 mo. The patients, including five males and one female, aged 25-58 years, were from four families and generally in good health. Their chronic fluctuating jaundice and/or hyperbilirubinemia had been diagnosed as simple constitutional jaundice for 6-30 years. Liver function tests showed isolated unconjugated hyperbilirubinemia with serum total bilirubin ranging from 20.7-75.4 μmol/L. Blood hemoglobin was normal in five cases, and slightly decreased in one (11.5 g/dL). Overt hemolytic signs were absent, while erythrocyte lifespan determined by the newly developed Levitt’s CO breath test was significantly short (15-50 d), definitely demonstrating the presence of hemolysis. Given that their unconjugated hyperbilirubinemia compared inappropriately with hemolytic severity, as indicated by the hemoglobin level, further combined genetic tests for both UGT1A1 and hereditary erythrocyte deficiencies were conducted. These tests confirmed, at last, the coexistence of GS with HS.
Comorbidity of GS and HS might not be uncommon in isolated unconjugated hyperbilirubinemia. While CO breath test would sensitively detect the hemolysis, the discordance between the hyperbilirubinemia and hemoglobin level could strongly indicate the coexistence of GS and HS.
Core tip: The coexistence of Gilbert’s syndrome with a hemolytic disease can impede diagnosis. Gilbert’s syndrome-hemolysis disease comorbidity should be suspected in patients who present with isolated unconjugated hyperbilirubinemia when serum bilirubin levels are discordant with hemoglobin levels, especially if there are even subtle signs of possible hemolysis. Levitt’s CO breath test for erythrocyte lifespan determination and broadly available genetic tests can be used for rapid diagnosis of these conditions.
- Citation: Kang LL, Liu ZL, Zhang HD. Gilbert’s syndrome coexisting with hereditary spherocytosis might not be rare: Six case reports. World J Clin Cases 2020; 8(10): 2001-2008
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/2001.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.2001
Gilbert’s syndrome (GS) and hereditary spherocytosis (HS) are hereditary disorders of similar etiology, the presence of isolated unconjugated hyperbilirubinemia. The pathogenesis of GS has been linked to a mutation in the gene that encodes UGT1A1, the enzyme that conjugates bilirubin in hepatocytes[1-3]. Thus, in GS, the ability of hepatocytes to transport and conjugate serum bilirubin is impaired. Conversely, HS is a hemolytic disease that is secondary to an erythrocyte (RBC) membrane abnormality due to genetic mutations, most commonly for ANK1 and Band 3 (SLC4A1)[4,5].
Although the distinct pathogenesis of these two diseases make differential diagnosis between them straightforward when each is present alone, hemolytic disease comorbidity and compensatory mechanisms can obscure their diagnoses, leading to missed and delayed diagnosis[6-11]. Here, we report six cases in which we were able to diagnose comorbidity of GS and HS quickly based on the results of a simple carbon monoxide (CO) breath test for RBC lifespan determination and broadly available genetic tests.
Cases 1 and 2: A 30-year-old male marathon runner complained of chronic jaundice for 4 years.
Case 3: A 33-year-old man presented to our gastroenterology clinic with chronic jaundice on June 3, 2019.
Case 4: On 9 July 2019, a 35-year-old male hepatologist of our hospital presented to our gastroenterology clinic seeking a CO breath test due to his chronic jaundice.
Cases 5 and 6: On 7 October 2019, a 25-year-old man complaining of dizziness and pale for 3 wk was referred to our hematology department.
Cases 1 and 2: He was diagnosed with constitutional jaundice by another hospital in a routine health medical examination 4 years ago and was told there was no need for treatment. On March 20, 2019, he was referred to our department to explore the pathogeny of his hyperbilirubinemia from the Center of Employment Medical Examination of our hospital.
Case 3: The patient exhibited jaundice from childhood. He had undergone numerous blood tests, but the cause of jaundice had not been identified.
Case 4: The doctor exhibited since childhood fluctuating jaundice, which had always been considered a constitutional jaundice by doctors, including himself.
Cases 5 and 6: The patient felt dizzy and unwell for 3 wk. He was told by his family that he was a little pale. The patient denied fever and black stool.
Cases 1 and 2: The patient had a free previous medical history.
Case 3: He had a free previous medical history.
Case 4: Small, asymptomatic gallstones were reported by abdominal ultrasonography in the past.
Cases 5 and 6: The patient was diagnosed with a “non-harmful jaundice and splenomegaly” by his university clinic in 2013 and suffered from a lithic cholecystitis in 2015.
Cases 1 and 2: He did not smoke or drink a lot and denied any persistent jaundice in his family history.
Case 3: The patient had always been in good health apart from jaundice. His parents had passed away.
Case 4: He had no bad habits and denied any persistent jaundice in his family history.
Cases 5 and 6: There was no special discovery in his personal history. However, family history revealed his father had mild jaundice.
Cases 1 and 2: A physical examination revealed only mild icterus of the sclera and a seemly palpable spleen just below the left costal.
Case 3: A physical examination revealed only mild icterus of the sclera.
Case 4: Normal.
Cases 5 and 6: A physical examination revealed mild icterus of the sclera and a palpable spleen at 1.0 cm below the left costal.
Cases 1 and 2: A routine blood test showed a slightly increased reticulocyte ratio (7.17%) with normal hemoglobin (15.8 g/dL) and RBC indexes. A peripheral blood smear was reported normal. Glucose-6-phosphate dehydrogenase activity of RBC was normal, and the Coomb’s test was negative. Liver function tests revealed an isolated unconjugated hyperbilirubinemia as indicated by an increased total bilirubin (56.6 μmol/L) and indirect bilirubin (46.7 μmol/L) with normal albumin, globin, and hepatic enzymes (Table 1). The RBC lifespan determined by an automated Levitt’s breath CO test was 28 d, far below the average normal value of 120 d[12].
| Variable | Proband | Father | Mother |
| Age, yr | 30.0 | 58.0 | 54.0 |
| Hemoglobin, g/dL | 15.8 | 14.5 | 13.9 |
| Reticulocyte, % | 7.17 | 2.03 | 3.82 |
| Blood smear | Spherocytes | Spherocytes, acanthocytes | Spherocytes |
| Total BIL, μmol/L | 56.6 | 22.1 | 12.5 |
| Direct BIL, μmol/L | 9.9 | 3.4 | 2.6 |
| Albumin/globulin, g/L | 48.7/24.0 | 51.2/22.7 | 48.3/23.7 |
| ALT/AST, U/L | 6.0/12.0 | 8.7/13.7 | 6.1/14.0 |
| ALP/GGT, U/L | 62.0/19.0 | 46.0/25.0 | 84.0/22.0 |
| RBC lifespan, d | 28 | 59 | 35 |
| Genetic mutations | |||
| UGT1A1 | c.211G>A/- | c.211G>A/- | -/- |
| ANK1 | c.1801-17G>A/- | c.925A>G/- | c.1801-17G>A /- |
Case 3: Initial routine blood test was normal with hemoglobin 15.4 g/dL and reticulocyte ratio of 2.55%. A peripheral blood smear was reported normal. Liver function test indicated isolated unconjugated hyperbilirubinemia with serum total bilirubin 75.3 μmol/L, direct bilirubin 9.3 μmol/L, and indirect bilirubin 66.0 μmol/L, respectively. CO breath test showed an abnormally shortened RBC lifespan (50 d).
Case 4: The routine blood test on the day of the breath test was completely normal with hemoglobin 12.3 g/dL and reticulocyte ratio 1.7%. Meanwhile, liver function test revealed isolated unconjugated hyperbilirubinemia as his repeated past results, with serum total bilirubin 25.7 μmol/L, direct bilirubin 8.5 μmol/L, and indirect bilirubin 17.2 μmol/L. Surprisingly, his RBC lifespan measured by breath test was only 29 d.
Cases 5 and 6: Initial routine blood test showed a mild anemia (hemoglobin 11.6 g/dL) and significantly elevated reticulocyte ratio (10.45%). The peripheral blood smear revealed easily seen spherocytes. Liver function test indicated isolated unconjugated hyperbilirubinemia with total bilirubin 75.4 μmol/L and direct bilirubin 6.7 μmol/L. His RBC lifespan measured by CO breath test was 13 d.
Cases 1 and 2: Abdominal ultrasonography revealed a slight splenomegaly.
Case 3: Abdominal ultrasonography was normal.
Case 4: Abdominal ultrasonography reported normal without gallstone.
Cases 5 and 6: Abdominal ultrasonography displayed an 8 mm × 4 mm gallstone and mild splenomegaly (199 mm × 73 mm).
Cases 1 and 2: Given the patient’s increased reticulocyte ratio and a significantly short RBC lifespan, we suspected that his jaundice was consequent to a hemolytic disease, despite there being no signs of anemia. However, his hyperbilirubinemia was inconsistent with the level of hemoglobin in a simple hemolytic disease, with the potential exception of GS. Thus, an oral phenobarbital test (40 mg/t.i.d. for 1 wk) and genetic test for GS were carried out. Serum total bilirubin did not decrease after phenobarbital induction for 1 wk, while the GS genetic test was positive (Table 1).
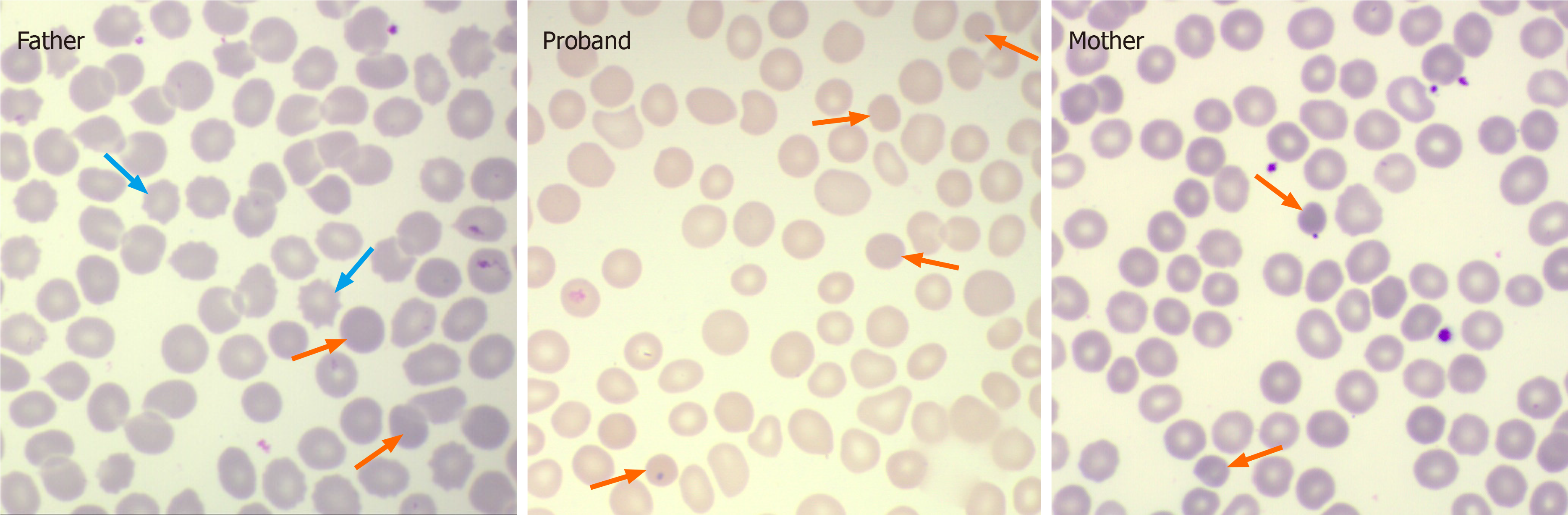
Because long-distance runners are known to exhibit relatively short RBC lifespans related to sports hemolysis[13], the patient was required to stop running or engaging in other strenuous exercise for 1 mo. The RBC lifespan re-test showed a still shortened RBC lifespan (32 d). Subsequently, a hematologist (Dr. Ze-Lin Liu, MD), whom we consulted, examined the patient’s peripheral blood smear and found a small number of small-bodied globular shaped RBCs with hyperchromia, suggesting that there might be HS (Figure 1).
To clarify the diagnosis, the patient’s parents also were evaluated. As shown in Table 1, his father (case 2) had mild isolated unconjugated hyperbilirubinemia with serum total bilirubin 22.1 μmol/L and indirect bilirubin 18.7 μmol/L, a significant shortened RBC lifespan, and a few spherocytes and easily seen acanthocytes, whereas his mother had a slightly high reticulocyte ratio, a few spherocytes, and a significantly shortened RBC lifespan with a completely normal liver function test. Next generation genetic sequence analysis showed that the patient was heterozygous for both the UGT1A1 c.211G>A (a.k.a. UGT1A1*6) mutation, which he shared with his father, and the ANK1 c.1801-17G>A mutation, which he shared with his mother. Surprisingly, another ANK1 locus mutation (c.925A>G) was found to be present only in his father. Finally, both the patient and his father were diagnosed with comorbidity of GS and HS, while his mother was diagnosed as HS.
Case 3: This case reminded us of case 1 and case 2, so the same hematologist was invited to review the peripheral blood smear. A small number of spherocytes was identified. The patient’s genotypes were as follows: Homozygous mutation for UGT1A1 c.-3279 T>G (a.k.a. UGT1A1*60) and TATA box [A(TA)7TAA] (a.k.a. UGT1A1*28) combined with heterozygous mutation for the ANK1 c.694G>A, which is consistent with comorbidity of GS and HS.
Case 4: The same hematologist was invited once more to view the peripheral blood smear, and a few atypical spherocytes were found. Next, the genetic tests revealed, not surprisingly, heterozygous mutations both for UGT1A1 (UGT1A1*60/-; UGT1A1*28/-) and SLC4A1 (c.31C>A).
Cases 5 and 6: A diagnosis of HS was soon made based on the typical clinical features. Because of the patient’s inappropriately high serum bilirubin level in comparison with the degree of hemolysis, a coexisting GS was highly suspected by the hematologists who had already encountered four such cases. Genetic analysis confirmed the speculation, with heterozygous mutations for both UGT1A1*6 and SLC4A1 (c.14A>T) identified.
The patient’s parents kindly accepted our invitation to be evaluated. The results showed that his mother (case 6) was also comorbid for GS and HS, while his father was a GS patient. The results of the mother’s main laboratory tests were as follows: Hemoglobin 13.3 g/dL, reticulocyte ratio 1.9%, typical spherocytes on the peripheral blood smear, a 28-d RBC lifespan, and an isolated unconjugated hyperbilirubinemia (total bilirubin 20.7 μmol/L, direct bilirubin 7.0 μmol/L). She had identical genetic mutations to her son. His father had UGT1A1*6/UGT1A1*6 mutation and was characterized by mild isolated unconjugated hyperbilirubinemia (total bilirubin 23.6 μmol/L, direct bilirubin 7.8 μmol/L).
GS coexisting with HS was confirmed in all six patients with isolated unconjugated hyperbilirubima after genetic analysis, and this was the final diagnosis.
No further treatment was given after diagnosis, but an annual routine health medical examination was recommended. The exception was case 5, who was suggested to have gallbladder-preserving colecystolithotomy and splenectomy in order to treat and prevent the gallstones. He was informed that splenectomy might not completely alleviate his hyperbilirubinemia owing to GS. The patient, however, refused our advice.
All six patients were in general good health during the follow-up period of 6 mo to 1 year.
Previously, GS combined with HS was considered to be rare, because only single cases had been reported. Indeed, from the individual prevalence rates (GS, approximately 2% to 20%; HS, 1 per 2000 persons), the calculated rate of coexistence of GS and HS is low (10 to 100 per million births). However, we confirmed six cases in just half a year, strongly indicating that this phenomenon might not be uncommon in a specific subset of population like isolated hyperbilirubinemia, which mainly includes GS and chronic hemolysis. Perhaps a number of cases were missed because of a lack of awareness and available techniques.
In the present cases, all patients had been presumed previously to have constitutional jaundice without further investigation because they were not anemic and did not exhibit signs of abnormal liver function. There were subtle signs of hemolysis that were not attended to, especially in case 1 and case 5, where they had significantly elevated reticulocyte ratio and splenomegaly. Although the other four patients did not show overt signs of hemolysis, a few RBCs were later shown to have a spheroid morphology, and this sign had been missed previously. Fortunately, hemolysis was demonstrated in all six cases by the CO breath test results, which showed markedly shortened RBC lifespans.
GS is characterized by mild to moderate, chronic unconjugated hyperbilirubinemia in the absence of overt hemolysis or structural liver disease[14]. The reported prevalence is as high as approximately 2% to 20%[3]. Patients with GS typically present when isolated hyperbilirubinemia is detected as an incidental finding on routine multiphasic biochemical screening, and clinical jaundice is uncommon. Except for the finding of icterus or jaundice, patients are usually asymptomatic. Jaundice often worsens in response to fasting, stress, dehydration, menstruation, or overexertion[3,15]. Family history is often positive. Low calorie intake test and phenobarbital stimulation test were the common diagnostic tests used in the past, and they have been gradually replaced by simple and reliable detection of UGT1A1 gene mutation[16,17]. Although GS has generally been thought to be an entirely benign condition with no necessary treatment, persons with this disorder may be at increased risk for gallstones and for the toxicity of selected drugs like irinotecan that require glucuronidation for metabolic disposal[18-20].
HS is a common congenital hemolytic disease. Its etiology involves intrinsic abnormalities of RBC membrane proteins that alter RBC morphology, resulting in spheroid, rather than disc-shaped RBCs, which are thus less deformable than normal RBCs and more vulnerable to splenic sequestration and destruction. Typically, patients with HS have icterus, anemia, and splenomegaly due to chronic hemolysis. Most patients diagnosed with HS have a positive family history. The most common complication of HS is the formation of bilirubinate gallstones, which occurs in about half of HS-diagnosed patients. A substantial number of spherocytes in peripheral blood smears is a key diagnostic indicator of HS. Splenectomy can be performed to alleviate anemia and prevent gallstone formation. Genetic mutations that affect RBC membrane proteins [e.g., ANKs, spectrins, band 3, and/or (rarely) protein 4.2] cause HS. The clinical presentation and severity of HS symptoms are highly variable across patients depending upon which of these mutations, and how many of them, are present. HS diagnoses may be missed in mild cases due to there being few spherocytes in peripheral blood smears. Furthermore, in patients with well-compensated hemolysis, signs of HS may only be detected when complications occur. Currently, a definitive diagnosis of HS requires RBC membrane protein electrophoresis or genetic testing[5,21,22].
Although GS and HS can both present as isolated unconjugated hyperbilirubinemia, they can be distinguished because the former is caused by decreased conjugation in hepatocytes. In contrast, the latter is the result of hemolysis. Patients with both diseases, however, may remain undiagnosed for long periods despite the presence of chronic jaundice. GS has been suspected in some patients already diagnosed with HS because of high serum bilirubin levels in the absence of pronounced anemia[6,7]. In other cases, poor responses to hemolytic therapies, such as persistent hyperbilirubinemia after splenectomy or ineffective phototherapy for neonatal jaundice, have provided important diagnostic clues[8,9]. Some patients with GS are examined for comorbidity of hemolytic disease (including HS) based on an elevated reticulocyte ratio, low hemoglobin, or splenomegaly[10,11]. However, a few perceptive doctors suspected the coexistence of GS and HS when they encountered an isolated hyperbilirubinemia with hemolytic signs by carefully evaluating inappropriately elevated bilirubin level compared with the degree of hemoglobin[23].
An abnormally short RBC lifespan is a defining characteristic of hemolysis. However, standard RBC lifespan measurement techniques (e.g., isotope or biotin labeling quantitation) are not amenable to routine clinical practice because they are cumbersome and time-consuming[24-27]. Based on hemoglobin degradation dynamics, Levitt and colleagues[28,29] developed a simple and accurate CO breath test method for determining RBC lifespan, which was subsequently adapted to an automated instrument that can report RBC lifespan within 15 min[12]. Thus, attending to the clues of a high serum bilirubin level without an abnormal hemoglobin level, we administered the aforementioned automated CO breath test to confirm the diagnoses of our patients.
Comorbidity of GS and HS might not be uncommon, particular in patients with isolated unconjugated hyperbilirubinemia. Clinicians should be vigilant to test for hemolysis disease comorbidity in patients with GS who exhibit isolated unconjugated hyperbilirubinemia with even subtle signs of possible hemolysis. While the simple and rapid Levitt’s CO breath test could be used as a reliable tool for detecting hemolysis, the discordance between the significant unconjugated hyperbilirubinemia and the less severity of hemolysis seems to be a valuable clue to the coexistence.
The authors thank Drs. Dan-Yu Wang and Zhao-Gui Zhou for their enthusiastic participation and thank Mr. Jun-Feng Luo for his technical support for erythrocyte lifespan determination.
| 1. | Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995;333:1171-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1012] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 2. | Ehmer U, Kalthoff S, Fakundiny B, Pabst B, Freiberg N, Naumann R, Manns MP, Strassburg CP. Gilbert syndrome redefined: a complex genetic haplotype influences the regulation of glucuronidation. Hepatology. 2012;55:1912-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Wagner KH, Shiels RG, Lang CA, Seyed Khoei N, Bulmer AC. Diagnostic criteria and contributors to Gilbert's syndrome. Crit Rev Clin Lab Sci. 2018;55:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Maruo Y, Ohta S. Gilbert syndrome and blood diseases. Jpn J Pediatr Hematol. 2004;18:601-608. [DOI] [Full Text] |
| 5. | Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372:1411-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 436] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 6. | Garg PK, Kumar A, Teckchandani N, Hadke NS. Hereditary spherocytosis coexisting with Gilbert's syndrome: a diagnostic dilemma. Singapore Med J. 2008;49:e308-e309. [PubMed] |
| 7. | Lee JH, Moon KR. Coexistence of gilbert syndrome and hereditary spherocytosis in a child presenting with extreme jaundice. Pediatr Gastroenterol Hepatol Nutr. 2014;17:266-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Iijima S, Ohzeki T, Maruo Y. Hereditary spherocytosis coexisting with UDP-glucuronosyltransferase deficiency highly suggestive of Crigler-Najjar syndrome type II. Yonsei Med J. 2011;52:369-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Ismail AQ, Gandhi A, El-Shimy N. Intractable neonatal jaundice due to hereditary spherocytosis and Gilbert's syndrome. BMJ Case Rep. 2011;2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Lee HJ, Moon HS, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY, Lee HY, Eu YJ. A case of concomitant Gilbert's syndrome and hereditary spherocytosis. Korean J Hepatol. 2010;16:321-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Lee MJ, Chang YH, Kang SH, Mun SK, Kim H, Han CJ, Kim J, Kang HJ. A Case of hereditary spherocytosis coexisting with Gilbert's syndrome. Korean J Gastroenterol. 2013;61:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Zhang HD, Ma YJ, Liu QF, Ye TZ, Meng FY, Zhou YW, Yu GP, Yang JP, Jiang H, Wang QS, Li GP, Ji YQ, Zhu GL, Du LT, Ji KM. Human erythrocyte lifespan measured by Levitt's CO breath test with newly developed automatic instrument. J Breath Res. 2018;12:036003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Weight LM, Byrne MJ, Jacobs P. Haemolytic effects of exercise. Clin Sci (Lond). 1991;81:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Strassburg CP. Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor syndrome). Best Pract Res Clin Gastroenterol. 2010;24:555-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Rodrigues C, Costa E, Vieira E, Santos R, De Carvalho J, Rocha-Pereira P, Santos-Silva A, Bronze-da-Rocha E. Bilirubin dependence on UGT1A1 polymorphisms, hemoglobin, fasting time and body mass index. Am J Med Sci. 2012;343:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Torres AK, Escartín N, Monzó C, Guzmán C, Ferrer I, González-Muñoz C, Peña P, Monzó V, Marcaida G, Rodríguez-López R. Genetic susceptibility to Gilbert's syndrome in a valencian population; efficacy of the fasting test. Rev Clin Esp. 2017;217:1-6. [PubMed] |
| 17. | Minucci A, Concolino P, Giardina B, Zuppi C, Capoluongo E. Rapid UGT1A1 (TA)(n) genotyping by high resolution melting curve analysis for Gilbert's syndrome diagnosis. Clin Chim Acta. 2010;411:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | McDonald GB, Evans AT, McCune JS, Schoch G, Ostrow JD, Gooley TA. Mortality outcomes after busulfan-containing conditioning treatment and haemopoietic cell transplantation in patients with Gilbert's syndrome: a retrospective cohort study. Lancet Haematol. 2016;3:e516-e525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Lankisch TO, Behrens G, Ehmer U, Möbius U, Rockstroh J, Wehmeier M, Kalthoff S, Freiberg N, Manns MP, Schmidt RE, Strassburg CP. Gilbert's syndrome and hyperbilirubinemia in protease inhibitor therapy--an extended haplotype of genetic variants increases risk in indinavir treatment. J Hepatol. 2009;50:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Mehra R, Murren J, Chung G, Smith B, Psyrri A. Severe irinotecan-induced toxicities in a patient with uridine diphosphate glucuronosyltransferase 1A1 polymorphism. Clin Colorectal Cancer. 2005;5:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Trompeter S, King M. Hereditary spherocytosis. Paediatr Child H (United Kingdom). 2019;29:359-364. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Bolton-Maggs PH, Stevens RF, Dodd NJ, Lamont G, Tittensor P, King MJ; General Haematology Task Force of the British Committee for Standards in Haematology. Guidelines for the diagnosis and management of hereditary spherocytosis. Br J Haematol. 2004;126:455-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Aiso M, Yagi M, Tanaka A, Miura K, Miura R, Arizumi T, Takamori Y, Nakahara S, Maruo Y, Takikawa H. Gilbert Syndrome with Concomitant Hereditary Spherocytosis Presenting with Moderate Unconjugated Hyperbilirubinemia. Intern Med. 2017;56:661-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Foulk WT, Butt HR, Owen CA, Whitcomb FF, Mason HL. Constitutional hepatic dysfunction (Gilbert's disease): its natural history and related syndromes. Medicine (Baltimore). 1959;38:25-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 85] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Powell LW, Hemingway E, Billing BH, Sherlock S. Idiopathic unconjugated hyperbilirubinemia (Gilbert's syndrome). A study of 42 families. N Engl J Med. 1967;277:1108-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 99] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Berk PD, Blaschke TF. Detection of Gilbert's syndrome in patients with hemolysis. A method using radioactive chromium. Ann Intern Med. 1972;77:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Cartei G, Chisesi T, Cazzavillan M, Barbui T, Battista R, Vianello Dri A, Dini E. Red blood cell survival and hyperbilirubinaemia in the Gilbert's syndrome. Folia Haematol Int Mag Klin Morphol Blutforsch. 1976;103:93-100. [PubMed] |
| 28. | Strocchi A, Schwartz S, Ellefson M, Engel RR, Medina A, Levitt MD. A simple carbon monoxide breath test to estimate erythrocyte turnover. J Lab Clin Med. 1992;120:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Furne JK, Springfield JR, Ho SB, Levitt MD. Simplification of the end-alveolar carbon monoxide technique to assess erythrocyte survival. J Lab Clin Med. 2003;142:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cure E, Iijima S S-Editor: Dou Y L-Editor: Filipodia E-Editor: Liu MY