Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.2009
Peer-review started: February 13, 2020
First decision: April 1, 2020
Revised: April 13, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: May 26, 2020
Processing time: 102 Days and 1.9 Hours
Pulmonary epithelioid hemangioendothelioma (P-EHE) is a rare disease. Thus far, consensus on a standard treatment for P-EHE has not been established given its low incidence worldwide. Apatinib combined with chemotherapy with doxorubicin/cyclophosphamide has been used as an effective combination treatment for human malignancies. However, the efficacy of this combination has not been reported in P-EHE cases.
We present the case of a 64-year-old woman with chest tightness, cough, and chest pain. Computed tomography showed multiple unresectable pulmonary nodules. She had been misdiagnosed with lung carcinoma and underwent gefitinib treatment at a hospital. Subsequently, the patient underwent a cardiothoracic surgery for further disease investigation. CD31, CD34, and Vimentin expression were detected in the resected nodule specimens by immunohistochemical analyses, and pathological analyses confirmed the diagnosis of P-EHE. Following this, four cycles of apatinib combined with chemotherapy with doxorubicin/cyclophosphamide were initiated. The patient demonstrated stabilization of multiple bilateral nodules and showed a dramatic improvement in the clinical presentation after combination treatment. The patient could not tolerate the side effects of chemotherapy. Therefore, she then continued apatinib monotherapy, which is ongoing to date. The patient was stable at the last follow-up after 24 mo.
Apatinib combined with chemotherapy with doxorubicin/cyclophosphamide may be an effective therapeutic option for P-EHE treatment.
Core tip: Pulmonary epithelioid hemangioendothelioma (P-EHE) is a rare disease. Patients with P-EHE demonstrate multiple unilateral or bilateral nodules that are often misdiagnosed as lung cancer or suspected to represent lung metastases. Meanwhile, currently, no standard treatment for this rare disease exists, and patients with P-EHE show a poor prognosis. Therefore, an effective therapeutic strategy for these patients is urgently required.
- Citation: Zhang XQ, Chen H, Song S, Qin Y, Cai LM, Zhang F. Effective combined therapy for pulmonary epithelioid hemangioendothelioma: A case report. World J Clin Cases 2020; 8(10): 2009-2015
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/2009.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.2009
Pulmonary epithelioid hemangioendothelioma (P-EHE) is a rare vascular tumor, which was first identified by Dail and Liebow in 1975 and was believed to be an intravascular bronchioloalveolar tumor of the lung[1]. Most patients with P-EHE show diverse clinical manifestations such as cough, chest pain, pleural effusion, and dyspnea, and the minority of patients are asymptomatic[2,3]. Multiple nodules in both lungs are usually identified on imaging, and unilateral P-EHE is often initially misdiagnosed as bronchogenic carcinoma[4]. Thus, immunohistochemical analysis is critical for the diagnosis of P-EHE. CD31, CD34, and Vimentin are vascular antigen markers that contribute to the diagnosis of P-EHE[5-7]. P-EHE accounts for 12% of all EHE cases and P-EHE prognosis is unpredictable; for patients with symptoms such as pleural effusion, chest pain, chest tightness, and cough, the prognosis is very poor, with a median survival time of 12 mo or less[3,8]. Meanwhile, no standard treatment for patients with these multiple unresectable lesions currently exists. In this study, we discuss the case of a 64-year-old patient with P-EHE, from P-EHE confirmation to effective combination therapy.
A 64-year-old woman was admitted to the Affiliated Hospital of Jiangnan University with obvious chest tightness, chest pain, and cough, and multiple pulmonary nodules were identified on imaging.
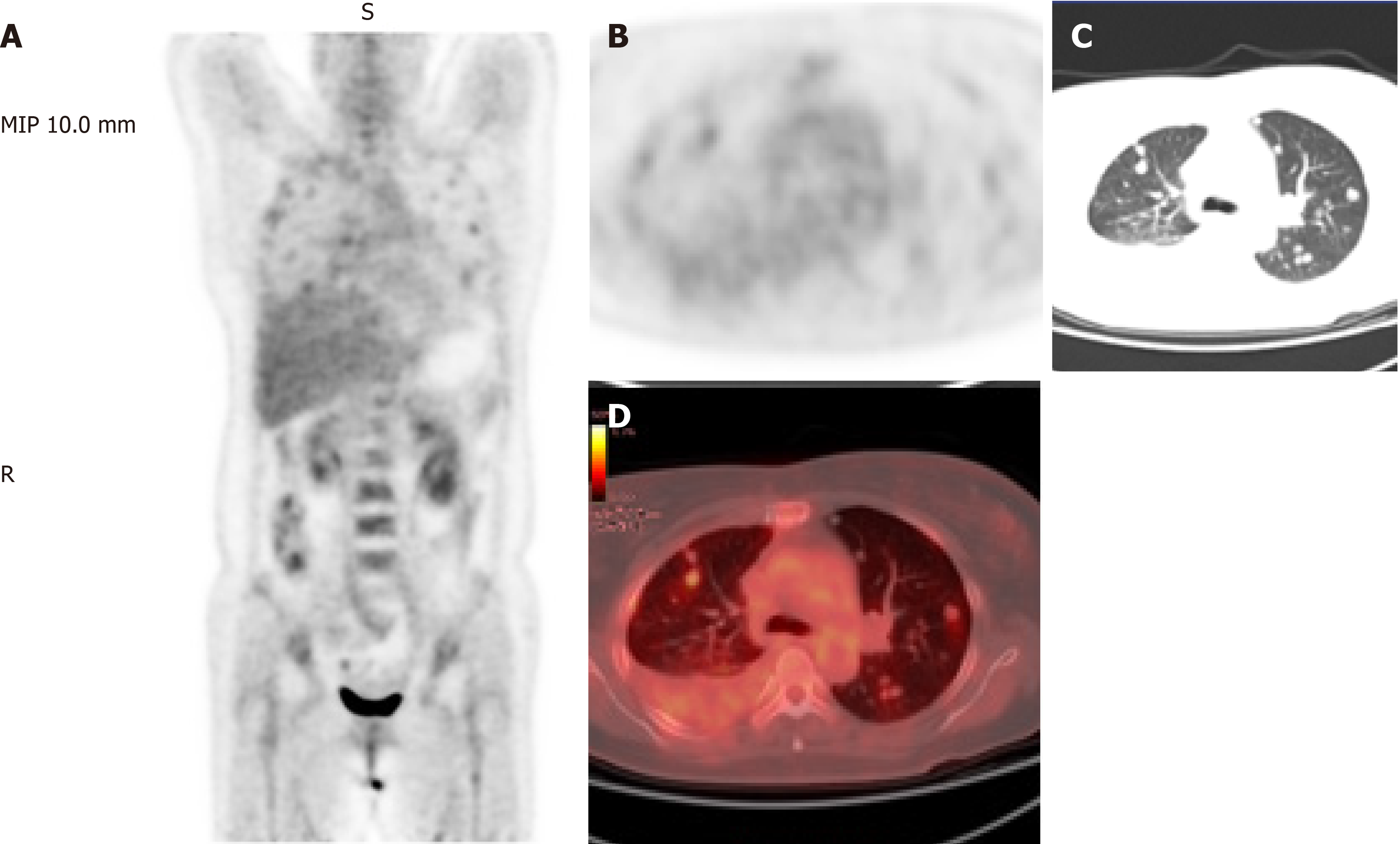
She previously visited another hospital with chest pain, chest tightness, and cough. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) showed abnormal FDG accumulation (Figure 1), and she was suspected to have lung metastasis. She was administered with gefitinib (250 mg/d) for 2 mo as treatment. However, her clinical manifestations persisted, and the number of pulmonary nodules did not decrease significantly.
Her medical history was unremarkable.
The patient had no family history of hereditary diseases or cancer.
On admission to our hospital, the body temperature of the patient was 37 °C with a pulse rate of 94 beats per minute (bpm); her respiratory rate was 25 breaths per minute and blood pressure was normal. The pain score was 8 on a scale of 1-10. She was slightly short of breath, and lung sounds were absent in the right middle and lower lung lobes. Her heart sounds were normal on auscultation.
Hematological analysis showed a white blood cell count of 11.9 × 109/L, with 75.8% of neutrophils; an erythrocyte sedimentation rate of 75 mm/H; and a C-reactive protein level of 59.17 mg/L. Carcinoembryonic antigen levels were normal.
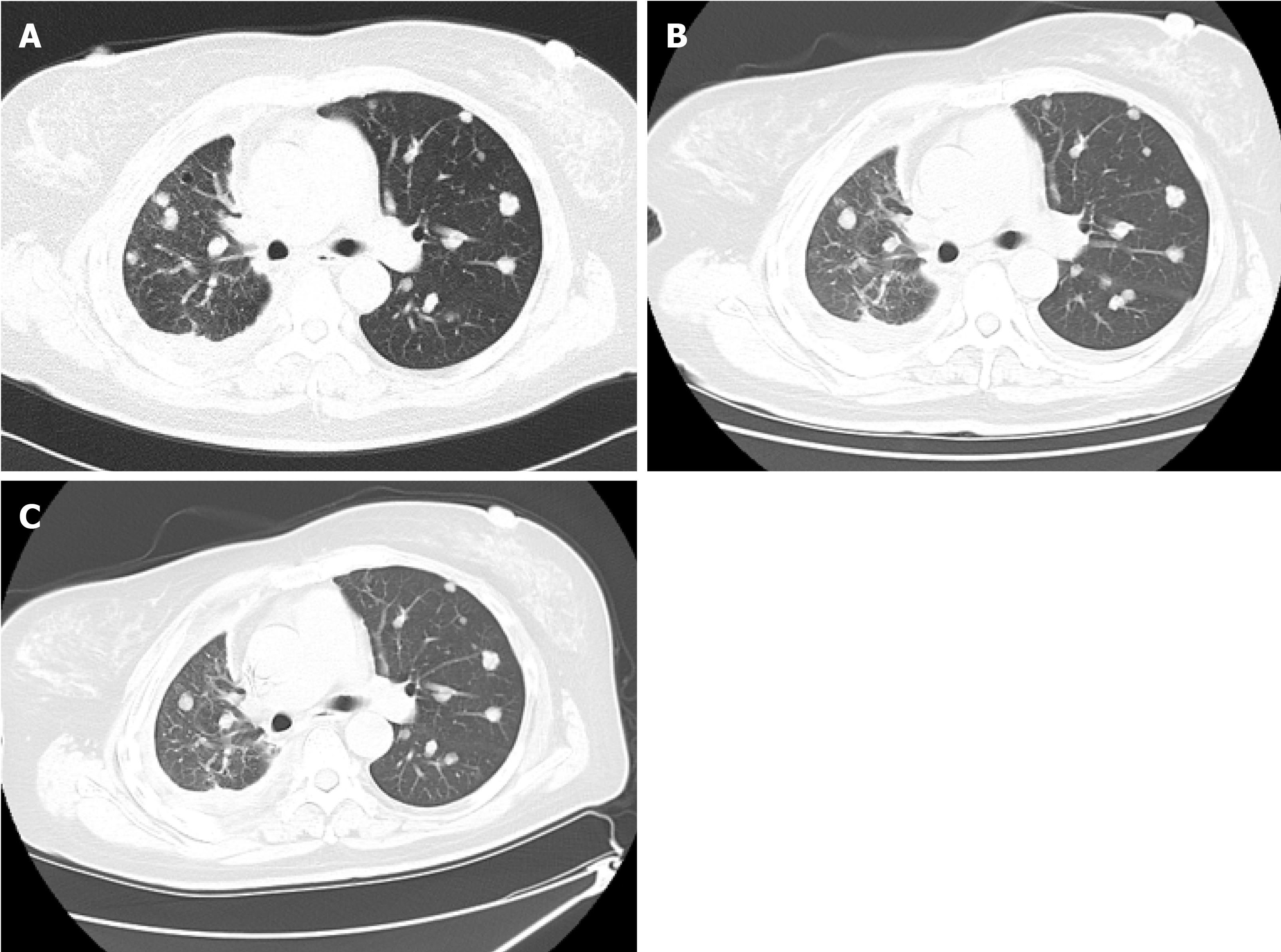
Lumbar CT showed diffusely scattered, high-density, non-calcified nodules, up to 13 mm in diameter, in both lungs. Pulmonary nodules were distributed along the tracheal vascular bundle, and the right lung had suffered serious damage with right pleural effusion (Figure 2A).
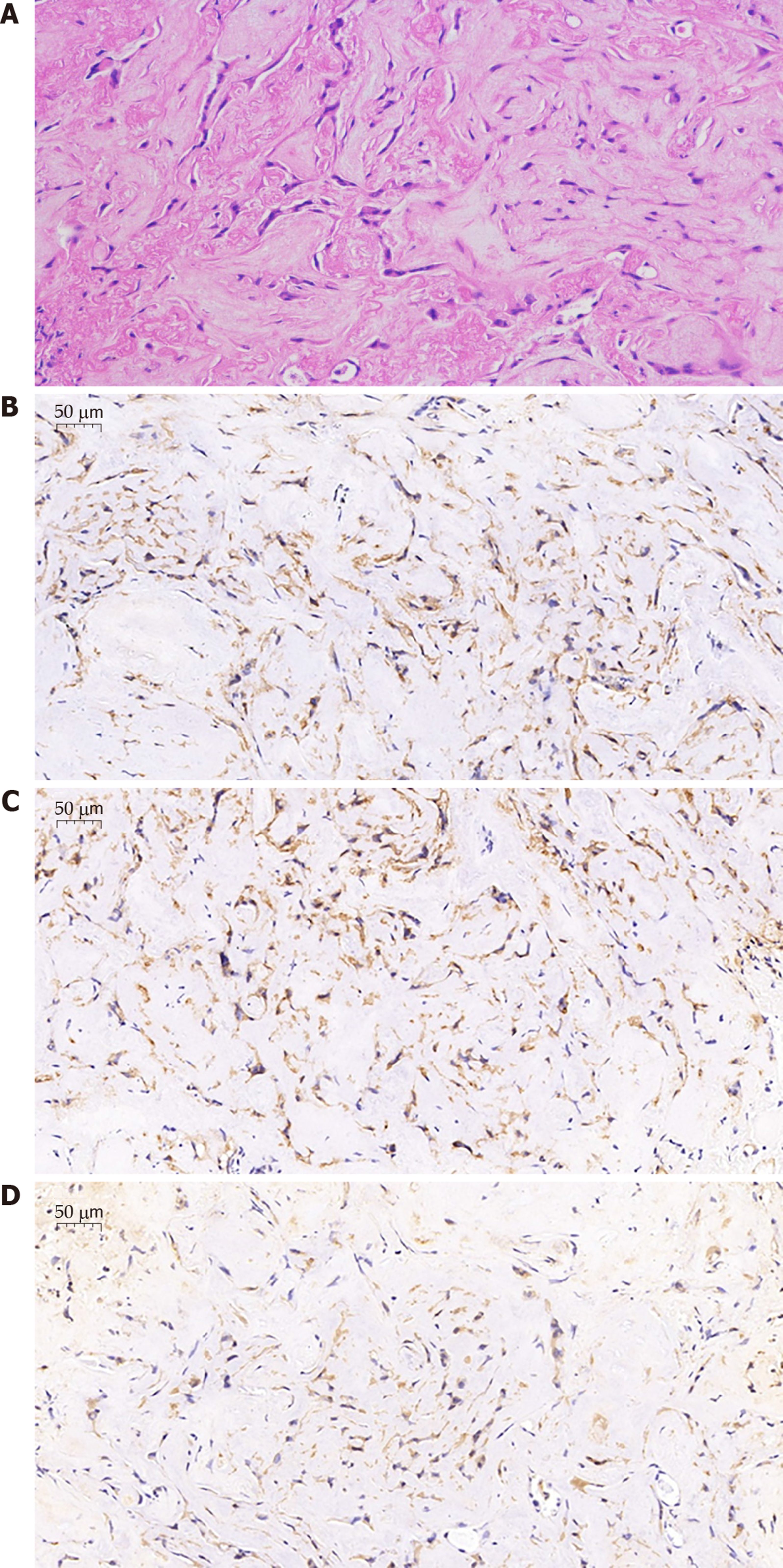
A biopsy of bronchial samples from the basal segment of the right lower lobe revealed collagen fibers and inflammatory cell infiltration. Further, alveolar lavage fluid was extracted and analyzed via high-throughput gene expression analysis for infectious pathogens, and Burkholderia and Propionibacterium genera were identified. Following this, the patient underwent a cardiothoracic surgery, wherein two nodules of the left upper lobe were removed for further investigation. Immunohistochemical analysis of these nodules revealed positive expression of CD31, CD34, and Vimentin (Figure 3).
A pathological examination confirmed the diagnosis of P-EHE.
The patient was first suspected of having a pulmonary infection based on the results of the high-throughput gene expression analysis of the alveolar lavage fluid; thus, she was treated with meropenem (2 g/q 8 h) for 14 d as per the Sanford Guide to Antimicrobial Therapy. Thoracic CT showed an only minor improvement in the multiple nodules and pleural effusion, following which, immunohistochemical staining of the resected nodule specimens confirmed the diagnosis of P-EHE. The subsequent therapeutic strategy included four cycles of apatinib combined with chemotherapy. Chemotherapy was started with doxorubicin (40 mg/m2; day 1) and cyclophosphamide (450 mg/m2; days 1-3), along with 250 mg daily dosage of apatinib.
The patient showed mild resolution of chest tightness and cough at 2 mo after two cycles of apatinib combined with chemotherapy. The clinical status of the patient showed a dramatic improvement with resolution of chest pain, chest tightness, and cough at 4 mo after four cycles of combination therapy. Finally, the patient became completely asymptomatic at 5 mo after four cycles of combination therapy. Meanwhile, the multiple pulmonary nodules were stable in both size and density on CT scan at the 8-month follow-up after combined treatment (Figure 2B). The patient was under the combined therapy for 4 mo; she had grade III-IV leukopenia and mild nausea after chemotherapy. She could not tolerate the side effects of chemotherapy and refused to continue the administration of doxorubicin + cyclophosphamide further. Thus, after 4 mo of apatinib combined with chemotherapy, she only continued apatinib monotherapy, which has been continued to date. The patient remained stable both in terms of lung nodule number and clinical status at the subsequent 2-year follow-ups (Figure 2C).
EHE demonstrates a low-to-intermediate grade malignancy and has metastatic potential with the lung and liver being the most commonly affected organs. EHE not only has composition of solid nests and short cords of epithelioid endothelial cells in myxohyaline stroma, but also shows the presence of increased mitotic activity and necrosis and greater nuclear atypia[8,9]. Tanas et al[10,11] reported that WWTR1/CAMTA1 gene fusion is a biological characteristic of P-EHE. Another hypothesis for this rare disease is that chronic Bartonella infection maybe have a causal relationship with EHE[12]. P-EHE with an epithelioid appearance has minor or nonspecific pulmonary clinical manifestations, but many patients are asymptomatic and bilateral multiple nodules are often incidentally observed by imaging. However, given its rare incidence, there is no consensus on P-EHE treatment; surgical resection should be performed if possible. A previous report describes a P-EHE case with multiple nodules wherein 32 pulmonary nodules were resected, and the patient was still alive 11 years after the surgery[13]. However, most patients with unresectable bilateral multiple nodules are usually treated with chemotherapy, anti-angiogenic treatment, or radiotherapy.
Several cases of chemotherapy treatment for unresectable P-EHE have been reported, showing variable efficacies. In a previous study, patients with P-EHE who underwent chemotherapy with carboplatin, paclitaxel, and bevacizumab showed short-term stabilization for 10 mo[14]. In another report, after the fourth cycle of chemotherapy with carboplatin, pemetrexed, and bevacizumab in a P-EHE patient, left pleural effusion was well-controlled with a 90% reduction[15]. Geramizadeh reported that a patient who received mesna, doxorubicin, ifosfamide, and dacarbazine (MAID regimen) showed long-term stability for more than 6 mo[16]. Further, a patient who underwent three cycles of chemotherapy with paclitaxel showed the involvement of other organ nodules such as mediastinal lymph nodes and liver nodules on CT imaging. The patient was alive for 7 mo after a confirmed diagnosis of P-EHE[17]. In another study, a P-EHE patient was treated with azathioprine and prednisone without any response. Thereafter, the patient did not undergo any further treatment, and showed no evidence of disease progression for 6 years[18]. Different chemotherapy regimens have demonstrated varying efficacies and periods of disease stability in P-EHE.
Vascular endothelial growth factor (VEGF) and VEGF receptor have been reported on P-EHE cells[19,20]. Apatinib, which selectively binds to VEGFR-2 as a tyrosine kinase inhibitor, exerts broad spectrum anti-tumor effects[21]. A P-EHE patient who received apatinib for a month showed dramatic improvements in clinical presentation and on CT imaging. However, the patient’s disease progressed, and his clinical status gradually aggravated after dose escalation; the patient survived for 6 mo[22]. Another patient was reported to receive pazopanib treatment and achieved disease stability for a period of 24 mo[23]. Thus, the use of VEGF inhibitors may be a reasonable therapeutic strategy for patients with P-EHE.
Radiation therapy is ineffective because of the slow growth of P-EHE, but is used as a symptomatic palliative treatment against pain caused by bone involvement in bone metastasis[24,25]. Sardaro et al[26] reported a patient with P-EHE presenting with vertebral metastasis (L3 and L4 vertebrae), wherein a course of radiotherapy with individual doses of 200 cGy/d for 5 d/wk was administered, and the patient’s lumbar pain was resolved.
Cyclophosphamide and doxorubicin have been used for lung carcinoma[27]. In our case, we used apatinib and four cycles of chemotherapy with doxorubicin and cyclophosphamide to treat P-EHE. The patient could not tolerate the side effects of chemotherapy, and only accepted apatinib treatment thereafter. CT scan showed the stabilization of multiple bilateral nodules in our patient, and a dramatic improvement in the clinical presentation was observed. The patient has been alive for more than 24 mo as of today.
We present the case of a patient with P-EHE who was treated with apatinib combined with chemotherapy (doxorubicin and cyclophosphamide). The patient demonstrated stabilization of bilateral multiple nodules and a dramatic improvement in the clinical presentation. This case presentation aids the efforts to find an effective treatment strategy for P-EHE.
| 1. | Corrin B, Manners B, Millard M, Weaver L. Histogenesis of the so-called "intravascular bronchioloalveolar tumour". J Pathol. 1979;128:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 96] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Amin RM, Hiroshima K, Kokubo T, Nishikawa M, Narita M, Kuroki M, Nakatani Y. Risk factors and independent predictors of survival in patients with pulmonary epithelioid haemangioendothelioma. Review of the literature and a case report. Respirology. 2006;11:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Haro A, Saitoh G, Tamiya S, Nagashima A. Four-year natural clinical course of pulmonary epithelioid hemangioendothelioma without therapy. Thorac Cancer. 2015;6:544-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Łochowski M, Rębowski M, Jesionek-Kupnicka D, Kozak J. Pulmonary epithelioid hemangioendothelioma imitating lung cancer. Kardiochir Torakochirurgia Pol. 2017;14:209-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Dutta R, Pal H, Garg G, Mohanty S. An Aggressive Large Epithelioid Hemangioendothelioma of the Anterior Mediastinum in a Young Woman. Korean J Thorac Cardiovasc Surg. 2018;51:419-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Sardaro A, Bardoscia L, Petruzzelli MF, Portaluri M. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev. 2014;8:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Chen Y, Khanna A, Chen JQ, Zhang HZ, Caraway NP, Katz RL. Cytologic features, immunocytochemical findings, and DNA ploidy in four rare cases of epithelioid hemangioendothelioma involving effusions. Cytojournal. 2018;15:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Lau K, Massad M, Pollak C, Rubin C, Yeh J, Wang J, Edelman G, Yeh J, Prasad S, Weinberg G. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140:1312-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 9. | Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 3305] [Article Influence: 330.5] [Reference Citation Analysis (0)] |
| 10. | Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, Flanagan J, Luo Y, Fenwick K, Natrajan R, Mitsopoulos C, Zvelebil M, Hoch BL, Weiss SW, Debiec-Rychter M, Sciot R, West RB, Lazar AJ, Ashworth A, Reis-Filho JS, Lord CJ, Gerstein MB, Rubin MA, Rubin BP. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011;3:98ra82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 11. | Tanas MR, Ma S, Jadaan FO, Ng CK, Weigelt B, Reis-Filho JS, Rubin BP. Mechanism of action of a WWTR1(TAZ)-CAMTA1 fusion oncoprotein. Oncogene. 2016;35:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Mascarelli PE, Iredell JR, Maggi RG, Weinberg G, Breitschwerdt EB. Bartonella species bacteremia in two patients with epithelioid hemangioendothelioma. J Clin Microbiol. 2011;49:4006-4012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Eguchi K, Sawafuji M. Surgical management of a patient with bilateral multiple pulmonary epithelioid hemangioendothelioma: report of a case. Surg Today. 2015;45:904-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Ye B, Li W, Feng J, Shi JX, Chen Y, Han BH. Treatment of pulmonary epithelioid hemangioendothelioma with combination chemotherapy: Report of three cases and review of the literature. Oncol Lett. 2013;5:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Kanemura S, Kuribayashi K, Moriya Y, Shimizu S, Tsujimura T, Nakano T. Pemetrexed for epithelioid haemangioendothelioma of the pleura. Respirol Case Rep. 2016;4:e00191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Geramizadeh B, Ziyaian B, Dehghani M, Tahmasebi K. Prolonged hemoptysis caused by primary pulmonary epithelioid hemangioendothelioma; a case report and review of the literature. Iran J Med Sci. 2014;39:223-227. [PubMed] |
| 17. | Calabrese C, Gilli M, De Rosa N, Di Crescenzo V, Zeppa P, Vitale C, Vatrella A. Role of FDG-PET scan in staging of pulmonary epithelioid hemangioendothelioma. Open Med (Wars). 2016;11:158-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 18. | Quiroz M, Undurraga Á, Moya R, Fernández C, Bezares K, Linacre V. [Lung epithelioid hemangioendothelioma: Report of one case]. Rev Med Chil. 2017;145:662-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Kim YH, Mishima M, Miyagawa-Hayashino A. Treatment of pulmonary epithelioid hemangioendothelioma with bevacizumab. J Thorac Oncol. 2010;5:1107-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Lopes T, Clemente S, Feliciano A, Lourenço I, Costa A, Gil Duarte J. [Pulmonary epithelioid hemangioendothelioma - rarity, diagnosis and treatment difficulties]. Rev Port Pneumol. 2009;15:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther. 2015;9:6075-6081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 22. | Zheng Z, Wang H, Jiang H, Chen E, Zhang J, Xie X. Apatinib for the treatment of pulmonary epithelioid hemangioendothelioma: A case report and literature review. Medicine (Baltimore). 2017;96:e8507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Semenisty V, Naroditsky I, Keidar Z, Bar-Sela G. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer. 2015;15:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Bagan P, Hassan M, Le Pimpec Barthes F, Peyrard S, Souilamas R, Danel C, Riquet M. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann Thorac Surg. 2006;82:2010-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Aquilina K, Lim C, Kamel MH, Marks CJ, O'Sullivan MG, Keohane C. Epithelioid hemangioendothelioma of the spine. Report of two cases. J Neurosurg Spine. 2005;3:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 26. | Sardaro A, Bardoscia L, Petruzzelli MF, Nikolaou A, Detti B, Angelelli G. Pulmonary epithelioid hemangioendothelioma presenting with vertebral metastases: a case report. J Med Case Rep. 2014;8:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Farago AF, Drapkin BJ, Lopez-Vilarino de Ramos JA, Galmarini CM, Núñez R, Kahatt C, Paz-Ares L. ATLANTIS: a Phase III study of lurbinectedin/doxorubicin versus topotecan or cyclophosphamide/doxorubicin/vincristine in patients with small-cell lung cancer who have failed one prior platinum-containing line. Future Oncol. 2019;15:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vidal EIO, Zhang K S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu MY