Published online Dec 26, 2019. doi: 10.12998/wjcc.v7.i24.4327
Peer-review started: September 16, 2019
First decision: October 24, 2019
Revised: October 30, 2019
Accepted: November 15, 2019
Article in press: November 15, 2019
Published online: December 26, 2019
Processing time: 100 Days and 0 Hours
Cardiac perforation by a transvenous lead is an uncommon but serious complication. Delayed perforation, defined as migration and perforation of an implanted lead at least 1 mo after implantation, is exceedingly rare and prone to underdiagnosis, and its optimal management is currently unclear. We report an uneventful transvenous extraction of an active fixation lead that led to delayed perforation of the right atrium, pericardium, and lung, disclosed 2 mo after implantation.
A 61-year-old woman with atrial lead perforation was transferred to our center. She had a dual-chamber pacemaker with active fixation leads implanted 8 mo previously. At 2 mo after implantation, she complained of chest pain and hemoptysis. Chest computed tomography revealed atrial lead migration into the lung. No pericardial or pleural effusion was detected. She underwent transvenous lead extraction in the electrophysiology room with surgical backup. The percutaneous subxiphoid pericardial puncture was performed first, and a pigtail catheter was left in the pericardial sac throughout the procedure. Then, a new active fixation lead was implanted at a different site with less tension. After the active screw was retracted, the culprit atrial lead was explanted successfully with simple traction. There were no complications during or after the procedure. The patient recovered well and follow-up was uneventful.
Percutaneous management of perforated active fixation lead outside the pericardial sac under surgical backup is safe and effective.
Core tip: Delayed lead perforation is a rare complication but can be life-threatening. Surgical management is recommended by expert consensus. We describe a delayed lead perforation of the right atrium, pericardium, and lung, which was successfully managed by transvenous lead extraction followed by preoperative pericardial drainage.
- Citation: Zhou X, Ze F, Li D, Li XB. Percutaneous management of atrium and lung perforation: A case report. World J Clin Cases 2019; 7(24): 4327-4333
- URL: https://www.wjgnet.com/2307-8960/full/v7/i24/4327.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i24.4327
Delayed complications of pacemaker implantation that are well recognized include infection, lead malfunction, and subclavian vein thrombosis. Less well recognized as a late complication of device implantation is lead perforation. Delayed cardiac perforation (diagnosed 1 mo or later after implantation) by a pacemaker lead is a rare complication but can be life-threatening if misdiagnosed. The optimal management of this complication remains unclear. The traditional approach to correcting this problem has been an open surgical procedure, especially when the lead has migrated beyond the pericardial space or into other organs[1].
Here, we propose a percutaneous procedure as alternative management of delayed lead perforation that is less invasive. Our report describes a delayed perforation of the right atrium, pericardium, and lung tissue by an active fixation atrial lead, which was uneventfully managed by transvenous lead extraction with preoperative pericardial drainage.
A 61-year-old woman with a dual-chamber pacemaker implanted 8 mo previously was admitted to our hospital because of pleuritic right-sided chest pain and hemoptysis of 6 mo in duration.
The patient presented with repeated syncopal episodes and was diagnosed with intermittent complete heart block 8 mo prior, when a dual-chamber pacemaker with active fixation leads (atrial: St. Jude Medical, model no. 2088T; ventricular: St. Jude Medical, model no. 2088T) (St. Jude Medical, St. Paul, MN, United States) was implanted. At 6 mo prior to admission, the patient suffered pleuritic right-sided chest pain followed by hemoptysis. She was admitted to a local hospital where anti-infection treatment was administered, which was unsuccessful in relieving symptoms. Therefore, she was transferred to our department for further management.
The patient had no history of bronchiectasis, tuberculosis, or lung cancer.
The patient had no history of smoking or alcohol consumption, no additional family history, and no history of hereditary diseases.
The patient’s blood pressure was 135/80 mmHg, heart rate was 75 beats/min, temperature was 36.5°C, and breathing rate was 18/min. Further physical examination revealed no remarkable abnormality.
Laboratory examinations revealed elevated serum bilirubin (2.2 mg/dL). There were no other abnormal findings including routine blood tests, routine urine tests, routine fecal tests, occult blood tests, and blood biochemistry and infection indexes.
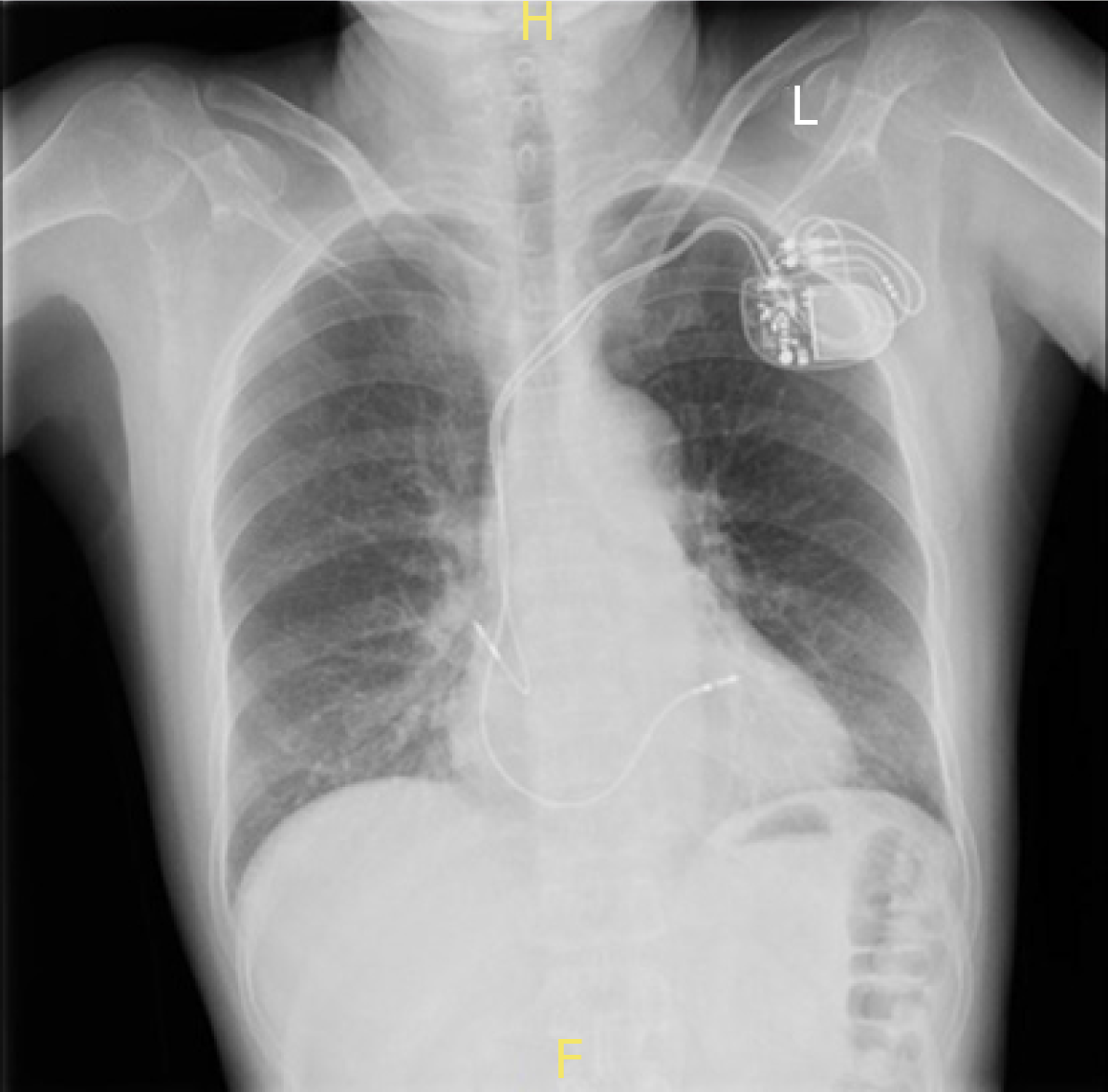
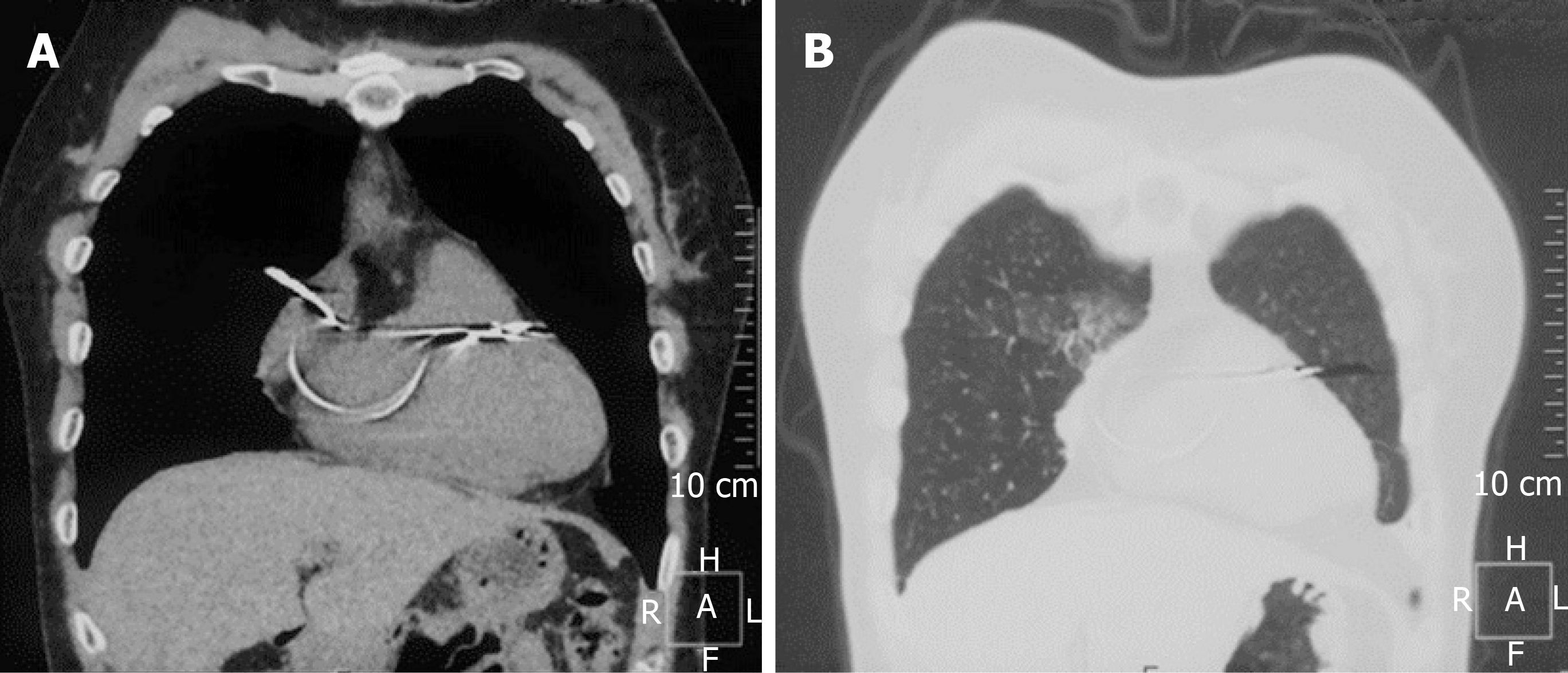
The chest X-ray showed the tip of the atrial lead appearing outside the cardiac silhouette (Figure 1). Chest computed tomography (CT) revealed the tip of the atrial lead migrating into the lung tissue with signs of local inflammation (Figure 2). No pericardial effusion was detected. Interrogation of the pacemaker revealed the failure of atrial pacing and sensing. Echocardiography indicated normal structure and function of the heart, and no signs of pericardial effusion were observed.
Delayed atrial lead perforation of the right atrium, pericardium, and lung tissue caused the symptoms.
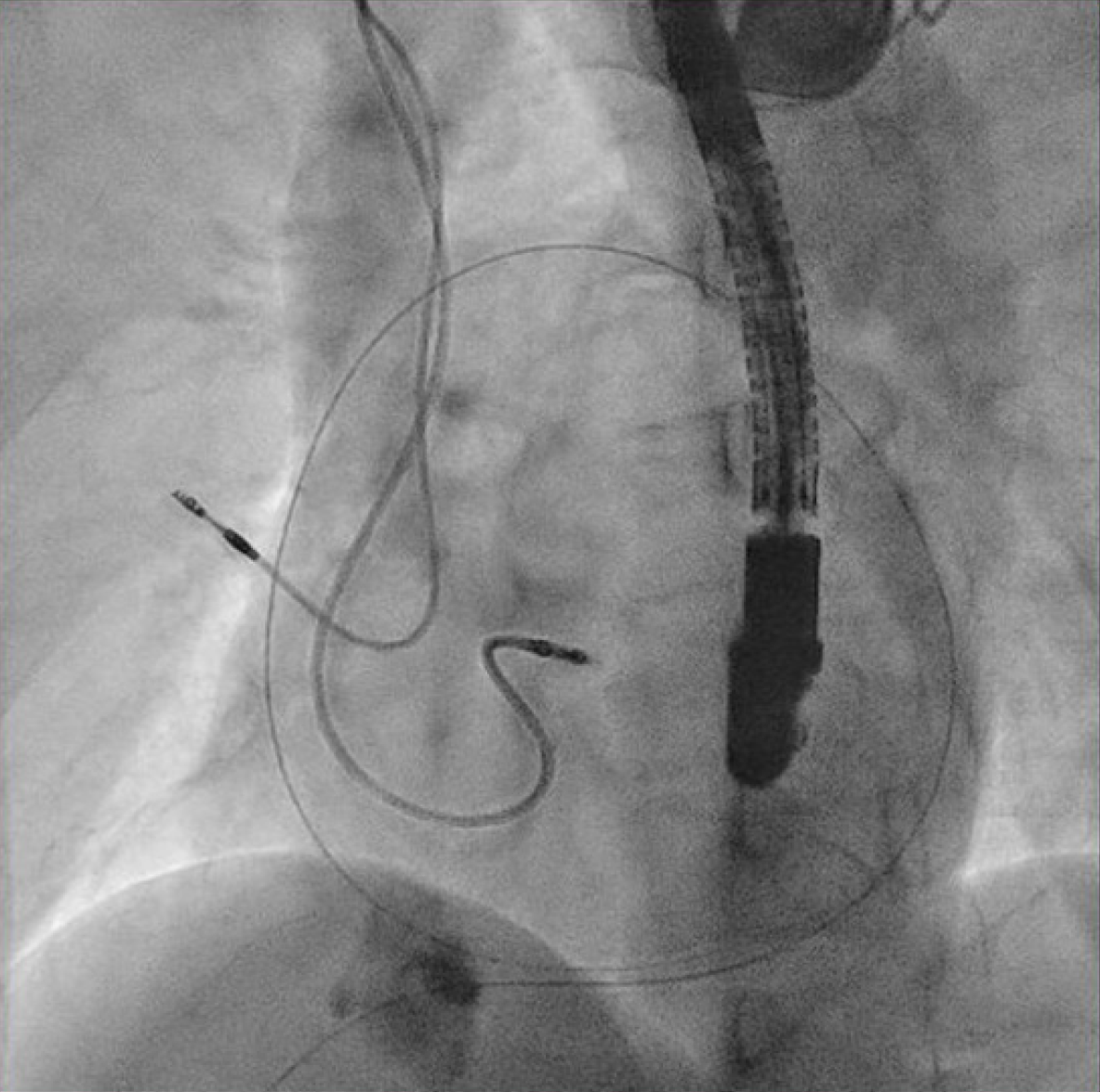
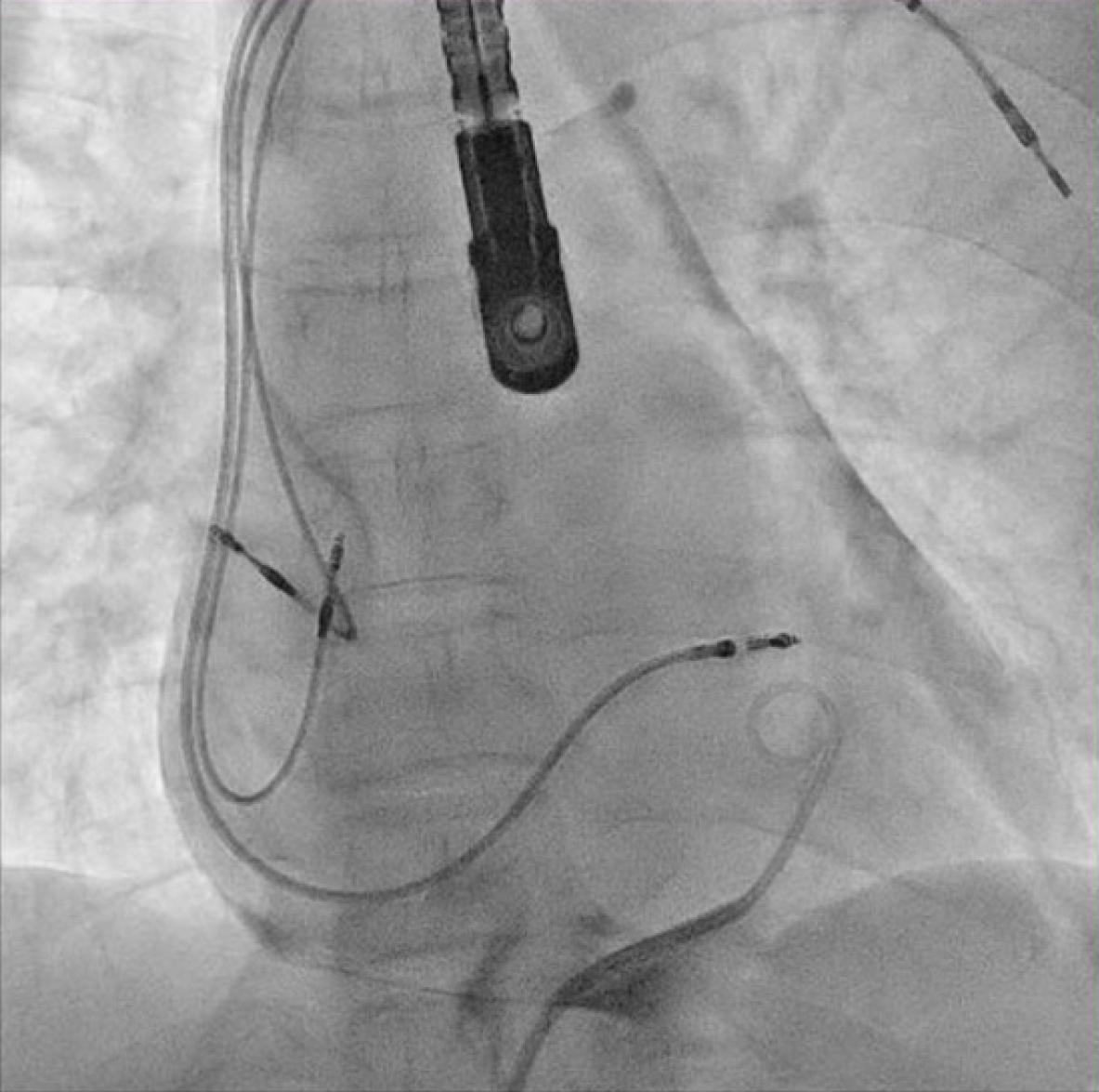
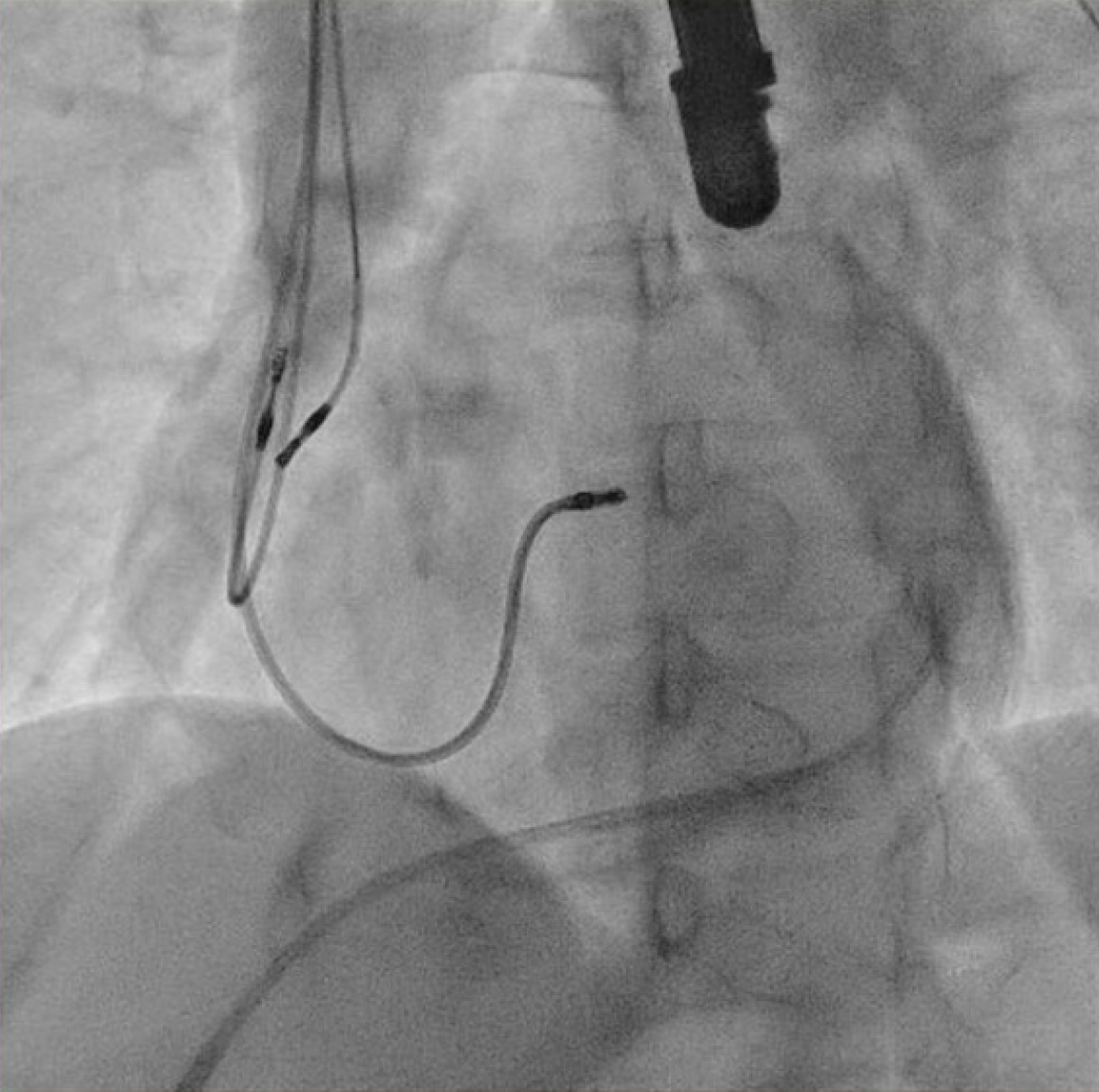
The patient underwent transvenous lead extraction performed in the electrophysiology room with surgical backup, under general anesthesia and transesophageal echocardiology (TEE) monitoring. First, a percutaneous subxiphoid pericardial puncture was carried out using the technique previously described (Figure 3)[2]. Subsequently, a pigtail catheter was inserted. The pocket was surgically explored and the atrial lead was freed from its extravascular adhesions. The terminal pin was then cut, and a standard stylet was inserted into the lead body. Before the extraction of the targeted atrial lead, a new atrial lead was implanted at the right atrial appendage (Figure 4) with less tension. The screw of the target atrial lead was then retracted, and simple traction was applied carefully under fluoroscopy (Figure 5). The culprit atrial lead was easily explanted.
The patient was hemodynamically stable and the TEE showed no pericardial effusion. Less than 10 mL of serosanguineous fluid was drained from the pigtail catheter. There were no complications, and she was admitted to the cardiac intensive care unit for close monitoring. Serial echocardiology showed no pericardial effusion, and the pigtail catheter was removed 1 d later. The patient was discharged 5 d after the procedure, and a repeat echocardiogram prior to discharge demonstrated no pericardial effusion. She had no chest pain or hemoptysis during 6 mo of follow-up. The chest X-ray was normal and the pacemaker functioned well.
Myocardial perforation is a serious but uncommon complication associated with the placement of a cardiac implantable electronic device. The reported incidence ranges from 0.1% to 0.8% for pacemaker leads and 0.6% to 5.2% for implantable cardioverter defibrillator leads[3]. Lead perforations are defined as acute, subacute, or delayed when they occur within 24 h, 1 mo, or more than 1 mo after implantation, respectively. Subacute or delayed lead perforations are fewer in number than acute lead perforations[4].
The most frequently reported risk factors of lead perforation are advanced age, low body mass index (BMI), female sex, active fixation lead, temporary pacing wires, and concomitant anticoagulation or steroid use[5]. Our patient was a 61-year-old woman with a BMI of 18.5 kg/m2. With regard to the mechanism, we speculated that a combination of exaggerated torsion of the atrial lead helix screw and placement of the active fixation lead on the lateral free wall allowed the perforation to occur. Therefore, in high-risk patients, preference should be given to placing the atrial lead on the septal wall of the right atrium, inside or at the base of the right atrial appendage, as we did in the present case.
Symptoms such as chest pain, dyspnea, syncope, and inappropriate shocks are important clues for reaching an accurate diagnosis. However, symptoms may vary widely, and asymptomatic cardiac perforations are not uncommon[6]. For equivocal cases, abnormal electrical parameters and abnormal signs on chest radiography or echocardiography may be helpful. Because visualization of the lead tip is the key component of the diagnosis of cardiac perforation, normal parameters do not exclude a perforation. Among the imaging tests, chest CT is currently considered the optimal imaging modality[7].
The optimal treatment of lead perforation remains unclear, and various strategies can be found in the literature. According to the 2017 Heart Rhythm Society expert consensus statement, lead extraction should be considered if a lead perforation causes pain, pericardial bleeding, or other complications[8]. Malfunction of the perforating lead is also an indication for extraction. However, in asymptomatic lead perforations with normal lead function, whether or not to extract remains controversial. Most authors agree that extraction is not mandatory, while some recommend the removal of the leads. Recently, a multicenter study with a 48-patient cohort concluded that, compared with early lead revision, conservative management of lead perforation is associated with more complications[9].
According to a consensus endorsed by the American Heart Association, surgical removal of the perforating leads should be the preferred strategy[1]. Alternatively, many authors have agreed that the transvenous extraction of a perforating lead with surgical backup is a safe and effective approach, especially for recent implantations. Using a stepwise lead extraction approach, a complete procedural success rate of 92%–96% has been reported[7,10,11]. Most of the perforating leads were removed by simple extraction because the dwelling time was usually not overlong. In the case of long implant time, advanced extraction tools may be employed.
In cases of lead perforation that has migrated beyond the pericardial space or into another cavity, surgical extraction seems to be the optimal option to repair the site of perforation and injury of the adjacent structures at the same time; the open surgery approach is more invasive and tends to have potential complications of sternotomy as well as long-term hospital stay. Our patient had a right atrium and lung perforation, while the culprit lead had an active fixation tip and the dwell time was not overly long (< 1 year), so less tissue was damaged during withdrawal. Moreover, there was no evidence of pericardial effusion or pneumothorax. Therefore, we proposed that the lead may be safely removed percutaneously, with transesophageal echocardiographic monitoring and cardiac surgical backup.
One potentially fatal complication during transvenous extraction of the perforated lead is pericardial tamponade. Huang et al[11] reported three cardiac tamponades that developed 1–4 d after percutaneous lead removal in a cohort of 31 patients. Laborderie et al[12] reported one case of cardiac tamponade among 10 patients with late right ventricle perforation (longest dwell time, 105 d) treated with percutaneous lead extraction by simple traction. However, the complication rate of pericardial bleeding was not high. Myocardial “self-sealing” properties, low pressure in the right heart chamber, and fibrous tissue formed at the perforating site may be beneficial to hemostasis of the perforated myocardium.
However, as is well known, the atrial myocardium is thinner and consists of fewer myocytes, and therefore has limited ability for spontaneous closure, especially when the pericardial sac is ruptured as in the present case. For this reason, we accessed the pericardial sac prior to the extraction procedure. In cases of pericardial bleeding or cardiac tamponade, aspiration through the pigtail catheter could relieve symptoms quickly and maintain hemodynamic stability.
Access to the pericardial space without pericardial effusion by the subxiphoid percutaneous approach has been widely used in pericardial ventricular tachycardia ablation procedures. Complication rates at experienced centers are acceptably low[13]. To the best of our knowledge, the use of this technique in transvenous lead extraction has not yet been reported.
Percutaneous management of active fixation lead perforation outside the pericardial sac under surgical backup is a safe and effective option, with preoperative pericardial puncture worthy of consideration in some high-risk cases.
Feng Ze performed the operation and edited the manuscript.
| 1. | Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, Crossley GH, Epstein LM, Friedman RA, Kennergren CE, Mitkowski P, Schaerf RH, Wazni OM; Heart Rhythm Society; American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009;6:1085-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 802] [Article Influence: 47.2] [Reference Citation Analysis (1)] |
| 2. | Sosa E, Scanavacca M, d'Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 667] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 3. | Khan MN, Joseph G, Khaykin Y, Ziada KM, Wilkoff BL. Delayed lead perforation: a disturbing trend. Pacing Clin Electrophysiol. 2005;28:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Velavan P, Chauhan A. An unusual presentation of delayed cardiac perforation caused by atrial screw-in lead. Heart. 2003;89:364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Sterliński M, Przybylski A, Maciag A, Syska P, Pytkowski M, Lewandowski M, Kowalik I, Firek B, Kołsut P, Religa G, Kuśmierczyk M, Walczak F, Szwed H. Subacute cardiac perforations associated with active fixation leads. Europace. 2009;11:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol. 2007;30:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Rajkumar CA, Claridge S, Jackson T, Behar J, Johnson J, Sohal M, Amraoui S, Nair A, Preston R, Gill J, Rajani R, Rinaldi CA. Diagnosis and management of iatrogenic cardiac perforation caused by pacemaker and defibrillator leads. Europace. 2017;19:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 8. | Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, Cha YM, Clancy J, Deharo JC, Ellenbogen KA, Exner D, Hussein AA, Kennergren C, Krahn A, Lee R, Love CJ, Madden RA, Mazzetti HA, Moore JC, Parsonnet J, Patton KK, Rozner MA, Selzman KA, Shoda M, Srivathsan K, Strathmore NF, Swerdlow CD, Tompkins C, Wazni O. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503-e551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 901] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 9. | Rav Acha M, Rafael A, Keaney JJ, Elitzur Y, Danon A, Shauer A, Taha L, Shechter Y, Bogot NR, Luria D, Ilan M, Singh SM, Mela T, Weisz G, Glikson M, Medina A. The management of cardiac implantable electronic device lead perforations: a multicentre study. Europace. 2019;21:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Migliore F, Zorzi A, Bertaglia E, Leoni L, Siciliano M, De Lazzari M, Ignatiuk B, Veronese M, Verlato R, Tarantini G, Iliceto S, Corrado D. Incidence, management, and prevention of right ventricular perforation by pacemaker and implantable cardioverter defibrillator leads. Pacing Clin Electrophysiol. 2014;37:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Huang XM, Fu HX, Zhong L, Osborn MJ, Asirvatham SJ, Sinak LJ, Cao J, Friedman PA, Cha YM. Outcomes of lead revision for myocardial perforation after cardiac implantable electronic device placement. J Cardiovasc Electrophysiol. 2014;25:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Laborderie J, Barandon L, Ploux S, Deplagne A, Mokrani B, Reuter S, Le Gal F, Jais P, Haissaguerre M, Clementy J, Bordachar P. Management of subacute and delayed right ventricular perforation with a pacing or an implantable cardioverter-defibrillator lead. Am J Cardiol. 2008;102:1352-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Sacher F, Roberts-Thomson K, Maury P, Tedrow U, Nault I, Steven D, Hocini M, Koplan B, Leroux L, Derval N, Seiler J, Wright MJ, Epstein L, Haissaguerre M, Jais P, Stevenson WG. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55:2366-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Choe YH, Jha NK S-Editor: Zhang L L-Editor: Filipodia E-Editor: Liu MY